Abstract
Female mice are protected from the cerebrovascular dysfunction induced by angiotensin II (AngII), an effect attributed to estrogen. We examined whether such cerebrovascular protection from AngII is related to the estrous cycle. Cerebral blood flow (CBF) was monitored by laser-Doppler flowmetry in anesthetized (urethane-chloralose) C57BL/6 female mice equipped with a cranial window. The phase of the estrous cycle was determined by vaginal smear cytology and plasma estrogen measurement. AngII (0.25 μg/Kg/min; i.v.; 30-45 min) elevated arterial pressure (15-20 mmHg) equally across the estrous cycle. However, in proestrus and estrus, phases in which estrogen is relatively high, AngII did not impair the in increase in the CBF induced by neural activity or by endothelium-dependent vasodilators (p>0.05 from vehicle). In contrast, in diestrus (lower estrogen) AngII induced a marked cerebrovascular dysfunction comparable to that of males. For example, the CBF response to whisker stimulation and to the endothelium-dependent vasodilator acetylcholine were attenuated by 41±12% and 49±12%, respectively (p<0.05; n=6/group). The protection from the cerebrovascular effects of AngII in proestrus was abolished by the estrogen receptor inhibitor ICI182,780. AngII also increased production of free radicals in cerebral blood vessels in diestrus (+116±13%; p<0.05), but not in proestrus and estrus (p>0.05 from control). Topical treatment with ICI182,780 reestablished AngII-induced oxidative stress in proestrus (p>0.05 from diestrus). We conclude that the protection from the neurovascular dysfunction induced by acute administration of AngII in females depends on the estrous cycle and may underlie the increased propensity to cerebrovascular damage associated with low estrogen states.
Keywords: functional hyperemia, endothelium-dependent relaxation, sex differences, reactive oxygen species, laser-Doppler flowmetry
INTRODUCTION
Hypertension is a major risk factor for stroke and dementia, devastating diseases related to the cerebrovascular damage induced by elevated blood pressure and its mediators1. Hypertension leads to hypertrophy and remodeling of cerebral blood vessels, and disrupts critical regulatory mechanisms of the cerebral circulation1. These alterations increase the susceptibility of the brain to vascular insufficiency and ischemic injury2. Pre-menopausal women are relatively spared from hypertension and its deleterious effects, a protection that is lost at menopause3. These observations have suggested that female reproductive hormones, estrogen in particular, protect women from cardiovascular diseases4. The finding that the cardiovascular risk of pre-menopausal women may vary cyclically across the menstrual cycle also suggests a role for ovarian hormones in cardiovascular diseases in women5, 6.
Sex differences have also been observed in animal models of hypertension7. In particular, the increase in blood pressure induced by chronic, but not acute, administration of the pressor peptide angiotensin II (AngII) is markedly attenuated in female animals7, 8. The attenuation is eliminated by ovariectomy and re-established by exogenous estrogen9. The deleterious effects of AngII on the regulation of the cerebral circulation are also sexually dimorphic8, 10. Thus, systemic administration of AngII to male mice disrupts the increase in cerebral blood flow (CBF) produced by neural activity or endothelium-dependent vasodilators11, 12. In contrast, young adult female mice (age 2-3 months) are spared from the cerebrovascular dysfunction induced by AngII, a protection abolished by ovariectomy and reinstated by estrogen8. However, because these studies were performed in random cycling females, a precise relationship between cerebrovascular protection and changes in hormonal levels during the estrous cycle could not be established. Furthermore, it is not known whether the mechanism of the protection in females is related to reduced vascular oxidative stress, a major causative factor in AngII-induced cerebrovascular dysfunction13.
We used acute administration of AngII in normally cycling female mice to determine whether the protection from the cerebrovascular effects of AngII is related to the estrous cycle. We found that AngII-induced cerebrovascular dysfunction and oxidative stress do not occur in female mice when plasma estrogens are high (proestrus and estrus), but only when they are low (diestrus). An estrogen receptor inhibitor blocked the reduction in oxidative stress and protection from cerebrovascular dysfunction. The data provide the first demonstration that the cerebrovascular effects of AngII are estrous cycle dependent and raise the possibility that the susceptibility to cerebrovascular injury in females varies across the estrous cycle.
METHODS
General surgical procedures
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Studies were conducted in young adult 2- to 3-month-old C57BL/6J female mice (weight: 20-25g; The Jackson Laboratory; Bar Harbor, ME). Mice were anesthetized with isoflurane (maintenance 2%) in oxygen, intubated, and artificially ventilated (SAR-830; CWE Inc.)11, 12. Femoral vessels were cannulated for recording mean arterial pressure (MAP), AngII administration, and for collection of blood samples at the conclusion of the experiment. Rectal temperature was maintained at 37°C. After surgery, anesthesia was maintained only with urethane (750 mg/kg; i.p.) and chloralose (50 mg/kg; i.p.)11, 12.
Monitoring of CBF
A portion of the parietal bone and underlying dura (2×2 mm) were removed, and a region of the cerebral cortex including the whisker-barrel area was superfused with a modified Ringer's solution (37°C; pH: 7.3 to 7.4)12. Relative CBF was monitored in the whisker barrel cortex with a laser-Doppler probe (Periflux System 5010, Perimed A.B.). The outputs of the flowmeter and blood pressure transducer were connected to a computerized data acquisition system (MacLab). CBF was expressed as percentage increases relative to the resting level. Zero values were obtained after the heart was stopped by an overdose of isoflurane.
Estrous stage and estrogen assay
The estrous cycle stage (proestrus, estrus and diestrus 2) was determined once a day by vaginal smear cytology14. Diestrus 2 rather than diestrus 1 (i.e., metestrus) was chosen to insure that mice were completely out of the estrus phase. For simplicity, we will use the term “diestrus” when referring to diestrus 2 throughout the paper. To avoid using pseudopregnant mice, only animals that showed 2 consecutive regular 4-5 days cycles were studied. Blood was collected from the femoral artery at the end of the experiment, usually at 12:00-1:00 PM. Plasma estradiol levels were measured with a commercially available ELISA (Bioquant, San Diego, CA, USA) as previously described8.
Experimental protocol
CBF experiments were performed without knowledge of the phase of the estrous cycle of the mice studied. CBF recordings were started after MAP and blood gases were in a steady state (Table S1; see the online data supplement at http://hyper.ahajournals.org). All pharmacological agents were dissolved in Ringer's solution. The cranial window was first superfused with Ringer (vehicle). The whisker-barrel cortex was activated for 60 seconds by stroking the contralateral facial whiskers11, and the evoked changes in CBF were recorded. Then, the endothelium-dependent vasodilators, calcium ionophore A23187 (3μM; Sigma), acetylcholine (Ach; 10μM; Sigma), bradykinin (BK; 50μM; Sigma), or the endothelium-independent vasodilator adenosine (50μM; Sigma) were superfused topically on the neocortex for 5 min. Agents were applied at concentrations previously determined to induce 50% of maximal responses15. After recording CBF responses, saline or AngII were infused intravenously. The AngII infusion was adjusted to elevate MAP by 15-20 mm Hg gradually over 10 to 15 minutes until a stable increase was obtained11, 12. At this time, the infusion rate was 0.25±0.02μg/kg/min, which produces elevations in plasma AngII within the upper range of those produced by endogenous activation of the renin-angiotensin system in rodents16. To avoid confounding effects on resting vascular tone, the concentration of AngII infused was chosen not to alter resting CBF and was identical to that used in previous studies8, 11, 12, 17, 18. CBF responses elicited by whisker stimulation, A23187, ACh, BK and adenosine were tested again after 30-45 minutes of AngII infusion. In some experiments, the high affinity estrogen receptor antagonist ICI182,78019 (10 μmol) or vehicle was topically applied to the neocortex during infusion of AngII or saline in proestrus females, and CBF responses tested 60 min later. The effect of ICI182,780 on CBF responses was investigated only in proestrus because plasma estrogen concentration is highest. The ICI182,780 concentration was selected based on published data20, 21. ICI182,780 was dissolved in dimethylsulfoxide (DMSO) and then diluted with Ringer to the desired concentration. The final DMSO concentration was <0.2%, which does not affect the cerebrovascular responses tested22.
Detection of ROS by hydroethidine□
Hydroethidine (HE) (Dihydroethidium; Molecular Probes, Eugene, OR; 2 μmol/L) in Ringer's solution was superfused on the somatosensory cortex for 60 minutes, as previously described23. Next, the brain was removed and frozen. Coronal brain sections (thickness 20 μm) were cut on a cryostat and collected at 100-μm intervals throughout the region exposed by the cranial window. Fluorescence intensity was measured as previously described12, 23. Briefly, brain sections were examined under a fluorescence microscope (Nikon, Melville, NY) equipped with an ethidium filter set (Chroma Technology, No. 31014). Images were acquired with a digital camera (Coolsnap; Roper Scientific, Trenton, NJ). Fluorescence intensity was assessed in the brain area superfused with HE. The analysis was performed in a blinded fashion using the IPLab software (Scanalytics, Fairfax, Va). Fluorescence intensities of all sections (20 per animal) were averaged and expressed as relative fluorescence units (RFU)23. The contralateral somatosensory cortex not superfused with HE served as control for background fluorescence. Reactive oxygen species (ROS) were assessed after intravenous infusion of saline or AngII in proestrus, estrus or diestrus. ROS also were assessed in proestrus mice treated with saline or AngII, with or without topical application of vehicle or ICI182,780.
Data analysis
Data are expressed as means ± SE. Multiple comparisons were evaluated by ANOVA and Tukey's test. Differences were considered statistically significant for P< 0.05.
RESULTS
Estrous cycle determination and estrogen assay
On vaginal smear cytology, proestrus was characterized by a predominance of round nucleated epithelial cells, estrus by a large number of cornified squamous epithelial cells, and diestrus by a predominance of small leucocytes14. The approximate length of the phases were: proestrus 1 day, estrus 2 days and diestrus 1.5 days24. In agreement with reports by others24 estradiol plasma levels (n=5/group) were higher in proestrus (39.3±2.6 pg/ml)(p<0.05 from estrus and diestrus; analysis of variance), intermediate in estrus (23.7±3.0)(p<0.05 from proestrus and diestrus), and lower in diestrus (11.6±3.5).
Effect of AngII on cerebrovascular responses during the estrous cycle
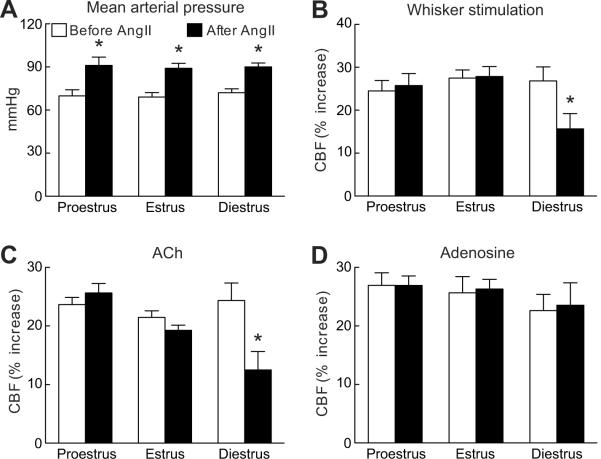
Relative CBF at baseline did not differ across the estrous cycle (proestrus: 22±3; estrus: 20±3; diestrus: 20±3 perfusion units; p>0.05; analysis of variance; n=6/group). Similarly, the CBF increase elicited by whisker stimulation, by the endothelium-dependent vasodilators A23187, ACh, and BK, or by the smooth muscle relaxant adenosine was comparable in across the cycle (fig. 1 and fig. S1; see the online data supplement at http://hyper.ahajournals.org; p>0.05; n=6/group). AngII produced comparable elevations in MAP during the different stages of the cycle (fig. 1A; p>0.05; n=6-7/group). However, AngII attenuated the CBF responses to whisker stimulation, A23187, ACh and BK in diestrus (fig. 1B-C and fig. S1; see the online data supplement at http://hyper.ahajournals.org; p<0.05), but not in proestrus and estrus (fig. 1B-C and fig. S1; see the online data supplement at http://hyper.ahajournals.org; p>0.05). AngII did not alter the CBF response to adenosine (fig. 1D; p>0.05). The attenuation in cerebrovascular responses in diestrus was comparable to that observed in male mice or in ovariectomized females8.
Figure 1.
Cerebrovascular effect of acute i.v. administration of AngII in cycling female mice. Effect of AngII on MAP (A) and on the CBF increase produced by whisker stimulation (B), ACh (C), or adenosine (D). *p<0.05 from respective control before AngII; analysis of variance and Tukey's test; n=6/group.
Effect of the estrogen receptor inhibitor ICI182,780 on the cerebrovascular dysfunctions induced by AngII
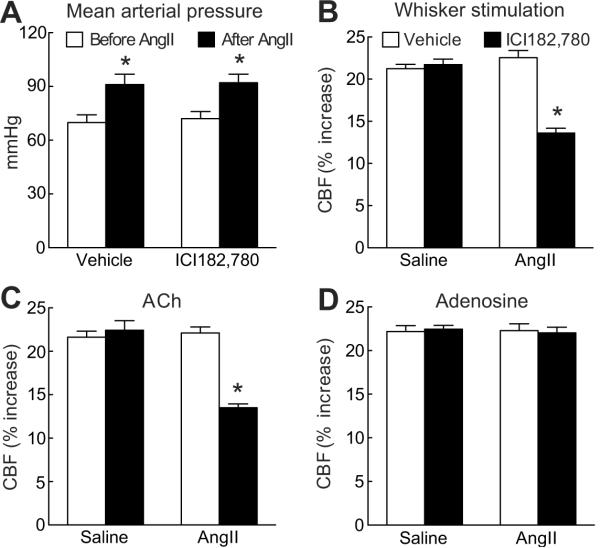
To determine whether the lack of sensitivity to the cerebrovascular effects of AngII in proestrus was related to estrogen we treated mice with the estrogen receptor antagonist ICI182,780. In proestrus, neocortical application of ICI182,780 did not affect relative CBF in the resting state (vehicle=22±4; ICI182,780=22±3 perfusion units; p>0.05; n=5/group) or the CBF responses tested (p>0.05; n=5/group) (fig. 2 and fig. S2; see the online data supplement at http://hyper.ahajournals.org). During AngII infusion, ICI182,780 did not alter the increase in MAP, but it abolished the protection from the AngII-induced cerebrovascular dysfunction observed in proestrus (fig. 2 and fig. S2; see the online data supplement at http://hyper.ahajournals.org; p<0.05; n=5/group).
Figure 2.
Cerebrovascular effect of AngII in proestrus mice treated with the estrogen receptor inhibitor ICI182,780 (10μM; neocortical application). Effect of AngII on MAP (A) and on the CBF increase produced by whisker stimulation (B), ACh (C), or adenosine (D). *p<0.05 from vehicle; analysis of variance and Tukey's test; n=5/group.
Effect of AngII on ROS production
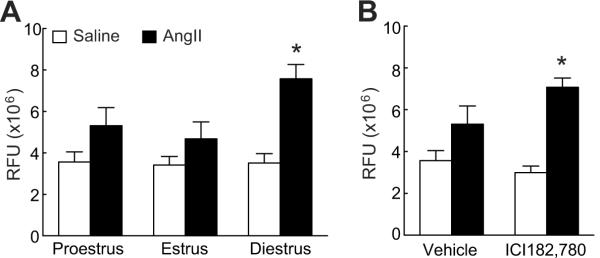
ROS are involved in the deleterious effect of AngII on the cerebral circulation11-13. To determine whether the differences in the cerebrovascular responses to AngII during the estrous cycle were related to oxidative stress we compared ROS production in proestrus, estrus and diestrus. As illustrated in fig. 3A, AngII increased ROS more in diestrus than in proestrus or estrus (p<0.05 from diestrus; n=5/group). The increase in ROS did not reach statistical significance in proestrus and estrus (p>0.05). In proestrus, when AngII-induced ROS production was attenuated (fig. 3A), neocortical application of ICI182,780 abolished such attenuation and re-established the ROS increase to the levels comparable to those observed in diestrus (p>0.05 from diestrus; p<0.05 from vehicle; fig. 3B).
Figure 3.
AngII-induced ROS production in cycling female mice (A) and in proestrus mice topically treated with ICI182,780 (B). *p<0.05 from vehicle; analysis of variance and Tukey's test; n=5/group.
DISCUSSION
We investigated whether the cerebrovascular dysfunction induced by AngII is related to the phases of the estrous cycle. We found that AngII elevates MAP equally across the estrous cycle, but that its cerebrovascular effects are only observed in diestrus (low plasma estrogen) and not in proestrus and estrus (high and intermediate plasma estrogen). To determine whether the reduced susceptibility to the cerebrovascular effects of AngII in proestrus was attributable to estrogen, we used an estrogen receptor inhibitor. We found that ICI182,780 eliminated the protection from the cerebrovascular dysfunction induced by AngII observed in proestrus. Furthermore, to determine whether the estrous cycle dependence of the cerebrovascular effects of AngII was related to changes in ROS, we investigated ROS production across the estrous cycle. Consistent with the cerebrovascular data, we found that AngII-induced ROS production was attenuated in proestrus and estrus, and was increased in diestrus. These observations unveil a previously unrecognized estrous cycle dependence of the cerebrovascular dysfunction induced by AngII, a phenomenon attributable to variations in AngII-induced ROS production throughout the estrous cycle.
Exclusion of potential sources of artifacts
The differences in the cerebrovascular susceptibility to AngII observed in the present study cannot be attributed to variations in body temperature or arterial blood gases because these parameters were carefully controlled and did not differ among the groups of mice studied. Similarly, the difference in the cerebrovascular responses cannot be due to differences in baseline cerebrovascular reactivity across the estrous cycle because the increase in CBF induced by whisker stimulation, ACh, BK, A23187 and adenosine were comparable in proestrus, estrus and diestrus. This finding is consistent with studies in rat the tail artery or aorta in which vascular reactivity was not influenced by the estrous cycle25, 26. Finally, the reversal the protection from the cerebrovascular dysfunction induced by AngI induced by ICI182,780 in proestrus cannot be attributed to an indiscriminate worsening of vascular responses because this agent did not affect resting cerebrovascular reactivity.
Estrous cycle dependence of the cerebrovascular effects of AngII
We previously reported that the cerebrovascular effects of acute and chronic (7 days) AngII administration are not observed in random cycling females8. The vascular protection was abolished by ovariectomy and re-established by administration of estradiol8, raising the possibility that estrogen was involved in the effect. Consistent with this hypothesis, we found that the protection from the cerebrovascular effects of AngII is observed in proestrus and estrus, when plasma estrogen is higher, and not in diestrus, when estrogen levels are lowest. Because the neurovascular dysfunction is not observed in proestrus and estrus, female mice are protected for most of the duration of the estrous cycle. This observation may explain why in previous studies by us and others, random cycling females were found to be protected from the cerebrovascular and hypertensive effects of AngII 8-10. It remains to be established whether the cerebrovascular effects of chronic administration of AngII or other pressor agents are also cycle dependent.
Estrous cycle dependence of AngII induced ROS production
ROS play a major role in the vascular effects of AngII27. In the model used in the present study, the increase in ROS induced by AngII occurs only in cerebral blood vessels and, in males, is mediated by NADPH oxidase via activation of AT1 receptors12. Therefore, we investigated whether the estrous cycle dependence of the cerebrovascular dysfunction elicited by AngII is related to changes in vascular oxidative stress. We found that the increase in ROS production evoked by AngII was present in diestrus, when cerebrovascular reactivity is impaired. However, AngII-induced ROS production was attenuated in proestrus and estrus, when cerebral vessels are protected from the deleterious effects of AngII. In proestrus, when estrogen levels are highest, ICI180,780 enhanced ROS production to levels comparable to those seen in diestrus, indicating that estrogen may have a role in the attenuation of ROS production. These findings indicate that the protection from the cerebrovascular effects of AngII observed in proestrus and estrus is related to estrogen-induced attenuation of ROS production. However, because the estrogen receptor inhibitor ICI180,780 is not subtype specific, the estrogen receptor subtype(s) (α or β), involved in this effect remains to be determined. Although estrogen receptors are well known to fluctuate in neurons during the estrous cycle28, it has not been established whether cycle dependent fluctuations also occur cerebrovascular estrogen receptors29.
Our data suggest that the cerebrovascular effects of AngII depend on the stage of the estrous cycle. Other biological actions of AngII are also estrous cycle dependent. For example, the dipsogenic effect of centrally administered AngII is attenuated in proestrus and estrus, and more marked in diestrus30. Considering that the dipsogenic effect of AngII requires ROS production in the subfornical organ31, this observation is consistent with our finding that the ROS production induced by AngII also depends on the estrous cycle. Therefore, the estrous cycle may modulate of ROS production not only in cerebral blood vessels, but also in the subfornical organ, suggesting a broader role of reproductive hormones in the biological effects of AngII.
Mechanisms of the neurovascular protection
The mechanisms of estrogen-dependent variation in ROS production induced by AngII in females remain to be established. Cerebral blood vessels of female rats have lower ROS production than those of males, a reduction abolished by ovariectomy and reinstated by estradiol administration29. Expression of vascular AT1 receptors, which are coupled to ROS production, as well as the balance between AT1 and AT2 receptors, could also be modulated by sex hormones32. In addition, estrogen increases the production of vasoprotective agents, such as prostacyclin and nitric oxide, which could counteract the deleterious effects of AngII29. Direct antioxidant effects of estrogen and/or modulation of antioxidant defenses could play a also role33. For example, the brain activity of the superoxide scavenging enzyme Mn-superoxide dismutase is higher in proestrus than in diestrus34.
Perspective
Although the incidence of cardiovascular and cerebrovascular diseases is in pre-menopausal women is relatively low, the risk for myocardial ischemia in women with coronary artery diseases fluctuates with the phases of the estrous cycle, being highest when estrogen is low5. Furthermore, the cardiovascular responses to acute stress in African-American women are enhanced when estrogen is low6. To our knowledge, no evidence of estrous cycle-dependent variations in the susceptibility to acute stroke has been thus far provided. Nevertheless, the present data raise the possibility that the susceptibility to cerebrovascular events in women may depend on the phase of the estrous cycle. This hypothesis is supported by the observations that focal cerebral ischemia in rat produces larger infarcts in diestrus than in proestrus35 and that cerebral microcirculatory flow and thrombotic tendency is highest in diestrus36. Therefore, our data suggest that the vulnerability of the female brain to vascular injury may vary according to the phases of the estrous cycle, as it is well established to occur in other neurological diseases, including epilepsy, migraine, and multiple sclerosis37-39.
Conclusions
We have demonstrated that the cerebrovascular dysfunction induced by AngII varies across the estrous cycle. Thus, the dysfunction is not observed in proestrus and estrus, but is present in diestrus. The protection from the cerebrovascular actions of AngII in proestrus is abolished by the non-selective estrogen receptor antagonist ICI182,780, suggesting the involvement of estrogen in the mechanisms of the protection. Like the cerebrovascular effects, AngII-induced ROS production is also estrous cycle dependent, being maximal in diestrus and attenuated in proestrus and estrus. ICI182,780 enhances the ROS increase in proestrus, attesting to a role of estrogen receptors also in the AngII-induced ROS generation. These findings, collectively, unveil a previously unrecognized dependence of the cerebrovascular effects of AngII on the estrous cycle, and raise the possibility that the vulnerability of the female brain to cerebrovascular injury varies cyclically across the estrous cycle.
Supplementary Material
Acknowledgments
Sources of funding Supported by HL18974. Costantino Iadecola. is the recipient of a Javits award from NIH/NINDS.
Footnotes
Conflict of interest None for all authors.
REFERENCES
- 1.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlof B. Prevention of stroke in patients with hypertension. Am J Cardiol. 2007;100:17J–24J. doi: 10.1016/j.amjcard.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Howard VJ. Distribution of stroke: Heterogeneity of stroke by age, race and sex. In: Moore JP, Choi DW, Grotta JC, Weir B, Wolf PA, editors. Stroke: Pathophysiology, diagnosis, and management. Churchill Livingstone; New York: 2004. pp. 3–12. [Google Scholar]
- 4.Huang A, Kaley G. Gender-specific regulation of cardiovascular functions:Estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd GW, Patel NR, McGing E, Cooper AF, Brennand-Roper D, Jackson G. Does angina vary with the menstrual cycle in women with premenopausal coronary artery disease? Heart. 2000;84:189–192. doi: 10.1136/heart.84.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills P, Ziegler M, Nelesen R, Kennedy B. The effects of the menstrual cycle, race, and gender on adrenergic receptors and agonists. Clin Pharmacol Ther. 1996;60:99–104. doi: 10.1016/S0009-9236(96)90172-1. [DOI] [PubMed] [Google Scholar]
- 7.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- induced hypertension. Braz J Med Biol Res. 2007;40:727–734. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- 8.Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol. 2008;294:H156–163. doi: 10.1152/ajpheart.01137.2007. [DOI] [PubMed] [Google Scholar]
- 9.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: New insights from genetic models. J Cereb Blood Flow Metab. 2006;26:449–455. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- 11.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through nadph-oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 12.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 13.Faraci FM. Reactive oxygen species: Influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Turner C, Bagnara J. General endocrinology. wb saunders; philadelphia: 1971. [Google Scholar]
- 15.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. Sod1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 16.Mann JF, Johnson AK, Ganten D. Plasma angiotensin II: Dipsogenic levels and angiotensin-generating capacity of renin. Am J Physiol. 1980;238:R372–377. doi: 10.1152/ajpregu.1980.238.5.R372. [DOI] [PubMed] [Google Scholar]
- 17.Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2003;285:H1890–1899. doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- 18.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 19.Wakeling A, Bowler J. Ici 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol. 1992;43:173–177. doi: 10.1016/0960-0760(92)90204-v. [DOI] [PubMed] [Google Scholar]
- 20.Xue B, Hay M. 17beta-estradiol inhibits excitatory amino acid-induced activity of neurons of the nucleus tractus solitarius. Brain Res. 2003;976:41–52. doi: 10.1016/s0006-8993(03)02629-5. [DOI] [PubMed] [Google Scholar]
- 21.Dhandapani K, Wade F, Mahesh V, Brann D. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: Involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 22.Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- 23.Park L, Anrather J, Zhou P, Frys K, Wang G, Iadecola C. Exogenous nadph increases cerebral blood flow through nadph oxidase-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:1860–1865. doi: 10.1161/01.ATV.0000142446.75898.44. [DOI] [PubMed] [Google Scholar]
- 24.Spencer J, Waters E, Milner T, McEwen B. Estrous cycle regulates activation of hippocampal akt, lim kinase, and neurotrophin receptors in c57bl/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Duckles SP. Influence of gender on vascular reactivity in the rat. J Pharmacol Exp Ther. 1994;268:1426–1431. [PubMed] [Google Scholar]
- 26.Stallone J, Crofton JT, Share L. Sexual dimorphism in vasopressin-induced contraction of rat aorta. Am J Physiol. 1991;260:H453–H458. doi: 10.1152/ajpheart.1991.260.2.H453. [DOI] [PubMed] [Google Scholar]
- 27.Selemidis S, Sobey C, Wingler K, Schmidt H, Drummond G. Nadph oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Milner T, Drake C, Lessard A, Waters E, Torres-Reveron A, Graustein B, Mitterling K, Frys K, Iadecola C. Angiotensin II-induced hypertension differentially affects estrogen and progestin receptors in central autonomic regulatory areas of female rats. Exp Neurol. 2008;212:393–406. doi: 10.1016/j.expneurol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: Multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 30.Findlay AL, Fitzsimons JT, Kucharczyk J. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol. 1979;82:215–225. doi: 10.1677/joe.0.0820215. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for rac1-dependent nadph oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 32.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates at1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 33.Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on nadph-oxidase activity, expression, and function in the cerebral circulation: Role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 34.Pajović S, Nikezić G, Martinović J. Effects of ovarian steroids on superoxide dismutase activity in the rat brain. Experientia. 1993;15:73–75. doi: 10.1007/BF01928794. [DOI] [PubMed] [Google Scholar]
- 35.Carswell HV, Anderson NH, Morton JJ, McCulloch J, Dominiczak AF, Macrae IM. Investigation of estrogen status and increased stroke sensitivity on cerebral blood flow after a focal ischemic insult. J Cereb Blood Flow Metab. 2000;20:931–936. doi: 10.1097/00004647-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Ono H, Sasaki Y, Bamba E, Seki J, Giddings JC, Yamamoto J. Cerebral thrombosis and microcirculation of the rat during the oestrous cycle and after ovariectomy. Clin Exp Pharmacol Physiol. 2002;29:73–78. doi: 10.1046/j.1440-1681.2002.03600.x. [DOI] [PubMed] [Google Scholar]
- 37.Pozzilli C, Falaschi P, Mainero C, Martocchia A, D'Urso R, Proietti A, Frontoni M, Bastianello S, Filippi M. Mri in multiple sclerosis during the menstrual cycle: Relationship with sex hormone patterns. Neurology. 1999;53:622–624. doi: 10.1212/wnl.53.3.622. [DOI] [PubMed] [Google Scholar]
- 38.Zupanc M, Haut S. Epilepsy in women: Special considerations for adolescents. Int Rev Neurobiol. 2008;83:91–111. doi: 10.1016/S0074-7742(08)00005-6. [DOI] [PubMed] [Google Scholar]
- 39.Martin V, Lipton R. Epidemiology and biology of menstrual migraine. Headache. 2008;48(suppl 3):S124–S130. doi: 10.1111/j.1526-4610.2008.01310.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.