Abstract
Diabetes mellitus (DM) is an emerging chronic health condition of developed and developing countries. We conducted a retrospective cohort study of patients with active, culture-confirmed tuberculosis (TB) in Maryland to determine the impact of DM on TB treatment outcomes. Of 297 TB patients, 42 (14%) had DM. Patients with diabetes had 2.0 times higher odds of death than patients without diabetes (95% confidence interval [CI] 0.74–5.2, P = 0.18). Adjusting for human immunodeficiency virus (HIV), age, weight, and foreign birth, the odds of death were 6.5 times higher in patients with diabetes than patients without diabetes (95% CI 1.1–38.0, P = 0.039). In pulmonary TB patients, time to sputum culture conversion was longer in patients with diabetes than patients without diabetes (median 49 versus 39 days, P = 0.09). Two-month culture conversion proportions were similar (70% and 69%). Treatment failure occurred in 4.1% of patients without diabetes and 6.7% of patients with diabetes (P = 0.51). In conclusion, DM was a risk factor for death in Maryland TB patients. There was a trend toward increased time to culture conversion; two-month culture conversion proportions, however, were similar.
INTRODUCTION
Over the past several decades, tuberculosis (TB) incidence has declined in industrialized countries, but incidence has risen in countries with high rates of infection with human immunodeficiency virus (HIV).1 At the same time, the prevalence of diabetes mellitus (DM) is soaring globally, fueled by the obesity epidemic.2 In the United States, 23.1% of individuals over 60 years of age are diabetic, and DM is the seventh leading cause of death.3 In developing countries, the number of individuals with DM is expected to increase from 84 million people in 1995 to 228 million people by 2025.4 In a recent study in Taiwan, diabetes was the most common underlying co-morbidity in patients with culture-confirmed TB, present in 21.5% of patients. 5 With the convergence of the TB and DM epidemics, co-affliction with the two diseases is on the rise.6
A number of case-control studies have shown that the relative odds of developing TB is higher in patients with diabetes than non-diabetics, with odds ratios (OR) ranging from 2.44 to 8.33.7-10 Cohort studies involving thousands of patients and a recent 1.7 million participant meta-analysis provide further convincing evidence that TB is more common in patients with diabetes,11-15 especially in those with poor glycemic control.14 Diabetes has been associated with increased risk of TB treatment failure or relapse,8,16 and diminished 2-month and 6-month culture conversion rates.17,18 Diabetes mellitus has been associated with increased risk of all-cause mortality in TB patients19 and, more specifically, with death related to pulmonary TB.20
We conducted a retrospective study to determine the prevalence of DM among TB patients, and to determine and compare treatment outcomes among TB patients with versus without DM.
METHODS
Study design and patient population
This retrospective cohort study included all patients with culture-confirmed TB diagnosed in 2004 and 2005 in Montgomery County, Prince George's County, and Baltimore City in Maryland. Collectively, these three counties include 80% of the state's incident TB cases. Tuberculosis cases were identified via routine surveillance data (TB Information Management System, or TIMS). Patients were excluded from the study if they were not alive at the time of TB diagnosis, initiated TB treatment outside of Maryland, or died before information regarding diabetes status could be obtained. Analyses of time to sputum culture conversion included only those patients with a baseline diagnostic sputum culture positive for Mycobacterium tuberculosis plus at least one additional sputum submitted for mycobacterial culture. Sputum cultures were collected under program conditions, and frequency of sampling varied but was at least monthly. For each identified TB case, information about demographics, TB treatment course, medical history, cavitary disease status, and outcomes were abstracted from both public health clinic charts and the TIMS database. Data were recorded on a study form and entered into a computer database. The study was approved by Institutional Review Boards of the Johns Hopkins Schools of Public Health and Medicine and the Maryland Department of Health and Mental Hygiene.
Study definitions and data collection
Patients were considered to be diabetic if they had baseline diagnosis of DM, were taking oral hypoglycemics, or had a non-fasting glucose measurement of greater than 200 recorded in public health clinic charts. Human immunodeficiency virus status was determined using chart documentation of serologic testing. Causes of death were determined by review of death certificates.
Tuberculosis treatment was in accordance with current guidelines.21 Directly observed therapy (DOT) was provided to patients by the respective counties' health department staff members throughout the treatment course.
Outcomes
The primary study outcome was all-cause mortality during TB treatment, comparing patients with diabetes to patients without diabetes. In the subset of pulmonary TB patients with growth of M. tuberculosis in baseline sputum cultures, secondary outcomes included proportion of patients with sputum culture conversion to negative by completion of 2 months of treatment; treatment failure; time to sputum culture negativity; and acquired resistance by M. tuberculosis isolates to any anti-tuberculosis drug. Time to sputum culture negativity was defined as days from treatment initiation date to date of first of three negative sputum cultures. Treatment failure was defined by a sputum culture positive for M. tuberculosis at or after completion of 4 months of treatment.
Statistical analysis
To compare demographic and clinical characteristics of TB patients with and without DM, we used Pearson's χ2 or Fisher's exact tests for categoric variables and student's t tests or Mann-Whitney U tests for continuous variables. For multivariable analyses, initial criteria for selection of factors to be considered for inclusion in the models were based on factors known in the TB literature to be risk factors for increased mortality or for delays in clearance. To compare mortality between the two groups, univariate and multivariate logistic regression was used. Covariates in mortality analyses included factors known to be risk factors for death from TB, including HIV status, weight, and age, as well the predictor shown in our data to be strongly associated with improved outcomes, foreign birth.22,23 To compare proportions of patients with and without DM with sputum culture conversion by 2 months of treatment, we used multiple logistic regression, stratifying by cavitary disease status, as the relationship between 2-month culture conversion and diabetes varied by cavitary disease status in our data, and adjusting for Hispanic ethnicity. To evaluate our logistic regression models, we used Hosmer-Lemeshow goodness of fit tests. To compare time to culture conversion, log-rank and stratified log-rank tests were performed. For those individuals who did not convert their sputum cultures to negative, their data was right-censored and included in the survival analysis. A two-tailed alpha level of 0.05 was used to determine statistical significance. Statistical analyses were performed using STATA software, version 10.0 (StataCorp LP, College Station, TX).
RESULTS
There were 419 reported cases of incident TB during the study period in the study jurisdictions. Of these, 413 were alive at the time of TB diagnosis. Five patients died before initiation of treatment or began treatment outside of Maryland and were excluded from the study. Of these, 299 had culture-confirmed TB and were followed for all their treatment in the state of Maryland. Of the 299, 2 died within 4 days of diagnosis, and their diabetes status could not be ascertained, so they were excluded. For the 297 patients included in the study, Table 1 shows the baseline demographic and clinical characteristics, stratified by diabetes status. Of these 297 patients, 42 (14%) were diabetic. Patients with diabetes more commonly had pulmonary disease (90% versus 75%, P = 0.029), were older (mean age 57 versus 40 years of age, P < 0.01), and were slightly heavier than those without DM (mean weight 146 versus 135 pounds, P = 0.051). Of the 297 patients, 228 had pulmonary TB; among these 228, presence of cavitary disease was similar in patients with diabetes and patients without diabetes (39% versus 37%, P = 0.88).
Table 1.
Baseline demographic and clinical characteristics of 297 patients with culture-confirmed tuberculosis, stratified by diabetes status
| Characteristic | Patients without diabetes (N = 255) |
Patients with diabetes (N = 42) |
P value |
|---|---|---|---|
| Male gender (n, %) | 130 (51) | 27 (64) | 0.11 |
| Race (n, %) | |||
| Asian | 72 (21) | 13 (26) | 0.37 |
| Black | 206 (58) | 24 (48) | |
| White | 77 (22) | 13 (26) | |
| Hispanic ethnicity (n, %) | 39 (15) | 7 (17) | 0.82 |
| HIV-positive* (n, %) | 44 (20) | 4 (11) | 0.19 |
| Foreign-born (n, %) | 178 (70) | 28 (67) | 0.68 |
| Site of disease (n, %) | |||
| Pulmonary | 146 (57) | 33 (79) | 0.024 |
| Extrapulmonary | 65 (25) | 4 (10) | |
| Both | 44 (17) | 5 (12) | |
| Cavitary disease† (n, %) | 71 (37) | 15 (39) | 0.88 |
| Smear positivity†† (n, %) | 105 (55) | 23 (61) | 0.70 |
| Dialysis (n, %) | 5 (2.0) | 3 (7.1) | 0.22 |
| Age (mean, [SD]) | 39.8 (18) | 56.5 (15) | < 0.01 |
| Weight (SD)§ | 135.1 (32) | 146.1 (37) | 0.051 |
N = 253.
N = 228 (pulmonary only).
N = 208 (sputum culture positive patients with smear collected).
N = 285.
Death
Twenty-six (9%) of the 297 study patients died of TB or other causes while undergoing TB treatment. In bivariate analyses, patients with DM had an odds of death that was 2.0 times higher than patients without DM (OR 95% confidence interval [CI] 0.74–5.2, P = 0.18). Death was more common in patients with HIV or whose HIV status was unknown, those born in the United States, and those with pulmonary involvement ( Table 2 ). Being US-born was strongly associated with likelihood of death (OR 7.50, 95% CI 3.03–18.6, P < 0.001). For each one-year increase in age, odds of death were 6% higher (95% OR 1.04–1.09, P < 0.01), and for each one-pound increase in weight, odds of death were 1% lower (OR 95% CI 0.97–1.0, P = 0.08). Because HIV was such a strong risk factor for death, HIV status was included in our multivariate model, despite the fact that HIV status was known for only 250 of the subjects. After adjusting for HIV status, age, weight, and foreign birth, the odds of death were 6.5 times higher in patients with diabetes than patients without diabetes (OR 95% CI 1.1–38.0, P = 0.039). Of the diabetic TB patients, 9.5% died within one month after starting TB treatment, compared with 3.5% of patients without diabetes (P = 0.09). Causes of death are listed in Table 3.
Table 2.
Comparison of proportion of patients who died during tuberculosis (TB) therapy by demographic or clinical characteristics
| Characteristic (N = 297) | Number of deaths (%) | P value |
|---|---|---|
| Diabetes | ||
| Yes | 6 (14.3) | 0.17 |
| No | 20 (7.8) | |
| Gender | 0.61 | |
| Male | 15 (9.6) | |
| Female | 11 (7.9) | |
| Race | ||
| Asian | 4 (5.6) | 0.51 |
| Black | 17 (10.5) | |
| White | 5 (7.8) | |
| Ethnicity | 0.39 | |
| Hispanic | 2 (4.4) | |
| Non-Hispanic | 24 (9.6) | |
| HIV | < 0.001 | |
| Positive | 5 (10.4) | |
| Negative | 6 (2.9) | |
| Unknown | 15 (34.1) | |
| US-born | < 0.001 | |
| Yes | 19 (20.9) | |
| No | 7 (3.4) | |
| Site of disease | 0.013 | |
| Pulmonary | 25 (11.0) | |
| Extrapulmonary only | 1 (1.5) | |
| Dialysis | 0.22 | |
| Yes | 2 (25.0) | |
| No | 24 (8.3) |
Table 3.
Causes of death among Maryland tuberculosis (TB) cases, by diabetes status
| Patients without diabetes (N = 19) | Patients with diabetes (N = 6) |
|---|---|
| Tuberculosis | |
| Pulmonary TB (3) | Pulmonary TB (2) |
| Pulmonary and CNS TB (1) | Miliary TB (1) |
| AIDS with disseminated TB (3) | |
| Pulmonary TB and pulmonary embolus (1) | |
| Diabetes | |
| N/A | Hypoglycemia (1) |
| Infection | |
| Community-acquired pneumonia (1) | Klebsiella pneumoniae septicemia in setting of end stage renal disease (1) |
| Pneumonia with sepsis (1) | Pneumonia with renal failure (1) |
| Pneumonia with sepsis complicated by chronic obstructive pulmonary disease (1) | |
| Cancer | |
| Lung cancer (2) | None |
| Metastatic lung cancer with brain metastases plus TB (1) | |
| Colon cancer with colon perforation and shock (1) | |
| Metastatic renal cell carcinoma with renal failure complicated by upper gastrointestinal bleed (1) | |
| Other | |
| Myocardial infarction (1) | None |
| Parkinson's disease plus TB (1) | |
| Chronic obstructive pulmonary disease (1) |
CNS = central nervous system; AIDS = acquired immunodeficiency syndrome.
Acquired drug resistance
Drug susceptibility testing results were available for a pre-treatment M. tuberculosis isolate for all 297 culture-positive patients. There was no difference in the proportion of patients with baseline drug resistance, comparing patients with diabetes to patients without diabetes (14.3% versus 15.3%, P = 0.87), so differences in drug resistance patterns were unlikely to have contributed to mortality differences between the two groups. Among the 26 patients who died, none had multidrug-resistant (MDR) TB. Of 89 study patients who also had drug susceptibility testing performed on an isolate obtained during treatment, 19 had an isolate that was resistant to one or more anti-tuberculosis agents. However, all resistance was present at baseline, and none was acquired during the study.
Sputum culture conversion
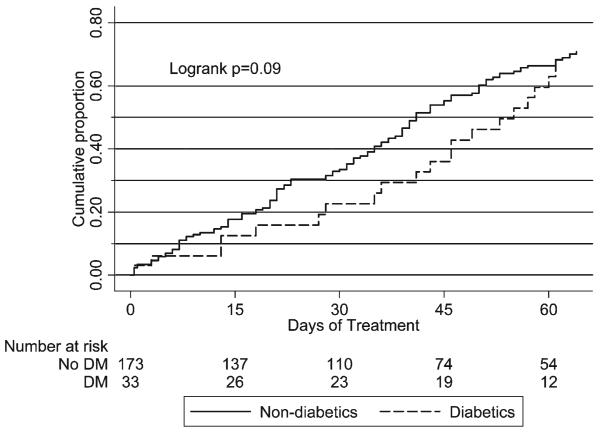
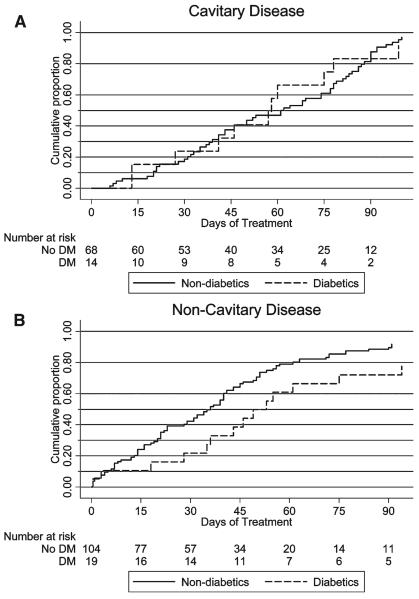
Of 228 patients with pulmonary TB, 208 had baseline positive sputum cultures; of the 208, one elderly patient with dementia declined subsequent sputum collection. Among the 207 patients with culture-confirmed pulmonary TB and at least one follow-up culture, the median time to sputum culture conversion among patients with diabetes was 49 days versus 39 days among patients without diabetes, but this difference did not reach statistical significance (P = 0.09 by log-rank test) (Figure 1). In an analysis evaluating time to culture conversion stratified by cavitary disease status, mean time to culture conversion was longer in patients with diabetes than patients without diabetes among those with noncavitary disease (P = 0.05) but similar in those with cavitary disease (P = 0.88); however, overall time to culture conversion did not differ between patients with diabetes and patients without diabetes (P = 0.14 by stratified log-rank test) ( Figure 2 ).
Figure 1.
Kaplan-Meier survival analysis showing days to sputum culture conversion in patients with culture-positive pulmonary TB, comparing patients with diabetes to patients without diabetes.
Figure 2.
Kaplan-Meier survival analysis showing days to sputum culture conversion in patients with cavitary (panel A) and noncavitary (panel B) pulmonary TB, comparing patients with diabetes to patients without diabetes.
Sixty-five percent (134/207) of the patients with culture-confirmed pulmonary TB converted their sputum to negative by 2 months, 59 (29%) still had positive sputum cultures at or beyond 2 months of treatment, and 14 (7%) were missing these data (12 of the 14 died before 60 days of therapy). Of the 193 whose 2-month sputum culture conversion status was known, the proportion of patients with diabetes and patients without diabetes who converted their sputum culture to negative by 2 months was 70% and 69%, respectively (P = 0.94). Of those with cavitary disease, 55% converted their sputum by 2 months, compared with 78% of those with noncavitary disease (P = 0.001) (Table 4). Asians had a 2-month culture conversion proportion of 77%, whereas blacks had a proportion of 72%, and whites 54% (P = 0.05). Of white subjects, those of Hispanic ethnicity had a 50% 2-month culture conversion proportion, whereas non-Hispanics had a proportion of 73%. In multivariate analysis, among those with noncavitary disease, patients with diabetes had a 61% lower odds of converting their sputum by 2 months than patients without diabetes, after adjusting for Hispanic ethnicity and age, but this difference was not statistically significant (P = 0.12); among those with cavitary disease, 2-month culture conversion proportions were not different (P = 0.18). Treatment failure was seen in 4.1% of patients without diabetes and 6.7% of patients with diabetes (P = 0.51). In a multivariate model, odds of treatment failure were 59% higher in patients with diabetes than patients without diabetes after adjusting for cavitary status, ethnicity, and age, but this association was not statistically significant (P = 0.34)
Table 4.
Comparison of proportion of patients with pulmonary tuberculosis (TB) who converted their sputum cultures to negative within 2 months after initiation of TB therapy by demographic or clinical characteristics
| Characteristic (N = 193) | 2-month sputum culture conversion (N[%]) |
P value |
|---|---|---|
| Diabetes | 0.94 | |
| Yes | 21 (70) | |
| No | 113 (69) | |
| Gender | 0.53 | |
| Male | 73 (68) | |
| Female | 61 (72) | |
| Race | 0.052 | |
| Asian | 30 (77) | |
| Black | 83 (72) | |
| White | 21 (54) | |
| Ethnicity | 0.014 | |
| Hispanic | 17 (52) | |
| Non-Hispanic | 117 (73) | |
| HIV* | 0.59 | |
| Positive | 29 (73) | |
| Negative | 89 (68) | |
| US-born | 0.92 | |
| Yes | 40 (69) | |
| No | 94 (70) | |
| Cavitary disease | 0.001 | |
| Yes | 42 (55) | |
| No | 91 (78) | |
| Hemodialysis | 0.31 | |
| Yes | 4 (100) | |
| No | 129 (69) |
N = 171.
DISCUSSION
In this community-based study, we found that diabetes was a relatively common comorbid condition in patients with active TB, and diabetes was independently associated with an increased risk of death in patients undergoing treatment of TB. After adjusting for other covariates, the odds of death were over six times higher in patients with diabetes than patients without diabetes, though confidence intervals were wide. There was a trend toward earlier culture conversion in patients without diabetes compared with patients with diabetes, especially among patients with noncavitary culture positive pulmonary TB; however, 2-month culture conversion proportions and odds of treatment failure were similar.
To our knowledge, our study was the first cohort study designed to directly evaluate the relationship between diabetes and TB treatment outcomes in an industrialized country, where management of DM and adherence to DOT should not be hindered by limitations of resources. Our results corroborate those seen in developing and developed country settings. In Egypt, in one case-control study, diabetes conferred a 9-fold increased risk of treatment failure, and this risk increased with adjustment for adherence.16 Similarly, in the Congo, treatment failure or death from respiratory failure or diabetic coma was seen in 41% of patients with diabetes but only 13% of nondiabetics.8 In a study in Indonesia with high adherence rates, 6-month sputum culture results were positive in 22% of patients with diabetes and 6.9% of nondiabetics.17 In one study in Baltimore, Maryland, evaluating multiple potential risk factors for poor TB treatment outcomes, the hazards ratio for all-cause mortality in patients with diabetes as compared with patients without diabetes was 6.7 after adjusting for renal failure, chronic obstructive pulmonary disease (COPD), HIV status, and age.19
It has been shown that the severity of diabetes affects the risk of developing active TB. In one large cohort study, for example, diabetes was associated with an increase in the risk of active pulmonary TB only in those with a hemoglobin A1c greater than 7%.14 Similarly, the incidence of active TB is higher in insulin-dependent diabetes than non-insulin dependent diabetes.24,25 The patients classified as diabetic in our study included a heterogeneous mix: 10 had insulin-dependent diabetes mellitus (IDDM); 18 were receiving oral diabetic medications at baseline; 4 were insulin-requiring type II patients with diabetes; 3 had a pre-treatment diagnosis of diabetes in the medical records; 1 had prednisone-induced hyperglycemia; and 6 were classified as patients with diabetes based on hyper-glycemia (undiagnosed diabetes or temporal hyperglycemia). Glycemic control was not systematically assessed, so the relationship between severity of diabetes and TB outcomes could not be evaluated in this retrospective study. Of note, it is not clear if treatment outcomes are affected by glycemic control and whether tight diabetes control would positively impact treatment outcomes in those who already have active TB. In one study that evaluated TB treatment outcomes in patients with diabetes, of those who died, 60% had complicated DM.8 In addition, it is not known whether increased mortality in patients with diabetes with TB is secondary to DM-related comorbidities or to increased TB severity, although in one recent study, TB-related death was nearly three times as common in patients with diabetes as in patients without diabetes.20 In this study, information regarding the cause of death in patients with diabetes was collected from death certificates, limiting our ability to fully assess the role of DM in the patients' clinical course. Nonetheless, mortality in patients with diabetes was secondary to TB or other infections in all cases but one, in which hypoglycemia was listed as the cause of death. Patients without diabetes on the other hand, died of a broader array of disease processes. It will be important to understand whether or not aggressive management of DM can improve TB treatment outcomes, perhaps by diminishing risk of concurrent infection or by decreasing TB severity.26,27 If so, diabetes management should be a standard part of TB care.
In this study, among patients with culture-positive pulmonary TB, there was a trend toward increased time to culture conversion in patients with diabetes, especially those with noncavitary disease, but the proportion of patients who converted their sputum culture to negative by 2 months was similar. In one recent study involving 469 patients in Texas, an evaluation using survival techniques showed that the median time to culture conversion was statistically significantly longer in patients with diabetes by 5 days.28 In addition, a study in Indonesia showed that, although culture conversion proportions were similar in patients with diabetes and patients without diabetes at 2 months (17.1% and 18.3%, respectively), of those tested at the end of 6 months of treatment, 22.2% of patients with diabetes compared with 9.6% of non-diabetics had positive cultures.17 If it is true that patients with diabetes with pulmonary TB take longer to convert their sputum cultures to negative, this delay could impact transmission, risk of relapse, and, consequently, appropriate treatment duration. A systematic evaluation of relapse rates, comparing patients with diabetes to patients without diabetes would shed light on the clinical consequences of delay in sputum conversion and the potential benefit of prolonged treatment duration in patients with diabetes, particularly if, as our data suggest, time to culture conversion among patients with diabetes is increased. The unexpected finding of prolonged time to culture conversion in patients with diabetes with noncavitary disease, discovered by visual inspection of Kaplan–Meier curves and evaluated in a subgroup analysis, should be confirmed prospectively.
Several mechanisms have been explored for increased development of TB or increased severity of TB in patients with diabetes. In studies of dogs with pancreatectomy, tuber-culous lesions contain higher bacillary counts, suggesting that the direct effects of hyperglycemia or insulinopenia contributed to diminished TB control.29 Indirect effects on immune function likely also play a role. Patients with diabetes may have impaired chemotaxis of monocytes,30 diminished activation of alveolar macrophages,31 diminished interferon (IFN)-Υ levels,32,33 or altered innate and type I cytokine expression.34 Pharmacologic interactions between rifampin and diabetic agents and decreased absorption of rifampin by patients with diabetes further impacts concurrent treatment of the two diseases.35,36
Our study has several limitations. First, because this was a retrospective cohort study, sputum samples were not systematically collected on a weekly basis, which would have enhanced our ability to determine exact time to sputum culture conversion. To ensure that there was no bias resulting from differences in frequency of sputum collection between patients with diabetes and patients without diabetes, we compared the rates of sputum collection for the two groups and found that the mean rate of culture collection was the same (5.6 versus 5.4 samples per month, respectively, P = 0.89). Second, because HIV is a strong risk factor for death in patients with TB, we included HIV status in our multivariable model despite the fact that 44 patients did not have their HIV status recorded, resulting in a smaller sample size for this analysis. It is important to note the considerable uncertainty around the point estimates for mortality in the univariate and multivariable analyses; the potential for residual confounding resulting from unmeasured factors exists and would likely be better addressed in a prospective study. Third, though we would like to have collected data on relapse, an important TB treatment outcome, the Centers for Disease Control and Prevention (CDC) TB surveillance system used by the state did not routinely track information about recurrent disease in the year after treatment, the time period when the majority of relapses occur. Last, the number of deaths in Maryland TB patients was low; a larger cohort with higher absolute mortality would provide improved statistical power to evaluate TB outcomes in diabetic patients.
In conclusion, in our TB patient population, diabetes was a relatively common morbidity, and it had a negative impact on treatment outcome. Mortality was higher in TB patients with diabetes compared with patients without diabetes, particularly in the first month of treatment. TB patients should be queried about diabetes, which should be attentively managed if present. Enhanced medical vigilance, especially during the early part of TB treatment, is warranted.
Acknowledgments
We thank Thomas Walsh, Karla Alwood, and Akintoye Adelakun, who provided clinical care; Lynn Federline, Sherry Johnson, and Yvonne Richards who facilitated medical record reviews; and Dipti Shah, coordinator of the Public Health Applications for Student Experience (PHASE) program at the Maryland Department of Health and Mental Hygiene.
Financial support: This study was supported by grants from the National Institutes of Health including 5 T32 GM066691 to K.D., K23AI51528 to S.D., and K01AI066994 to J.G.
Contributor Information
Kelly E. Dooley, Divisions of Clinical Pharmacology and Infectious Diseases, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Osler 527, Baltimore, MD 21287, Tel: 410-955-3100, Fax: 410-614-9978, kdooley1@jhmi.edu..
Tania Tang, Johns Hopkins University Bloomberg School of Public Health, 615 N. Wolfe Street, Box 119, Baltimore, MD 21205, Tel: 510-381-3623, Fax: c/o Jonathan Golub 410-955-0740, ttang@jhsph.edu..
Jonathan E. Golub, Infectious Diseases, Johns Hopkins University School of Medicine, and Johns Hopkins University School of Public Health, Center for Tuberculosis Research, CRB II 1M.07, 1550 Orleans Street, Baltimore, MD 21231, Tel: 443-287-2969, Fax: 410-955-0740, jgolub@jhmi.edu.
Susan E. Dorman, Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School of Medicine, Center for Tuberculosis Research, CRBII 1M.08, Tel: 410-955-1755, Fax: 410-955-0740, dsusan1@jhmi.edu.
Wendy Cronin, Division of TB Control, Refugee and Migrant Health, Maryland Department of Health and Mental Hygiene, 201 W. Preston Street, Room 307-A, Baltimore, MD 21201, Tel: 410-767-6693, Fax: 410-669-4215, croninw@dhmh.state.md.us..
REFERENCES
- 1.World Health Organization . Global Tuberculosis Control: Surveillance, Planning, Financing. WHO; Geneva: 2008. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.National Diabetes Statistics 2007 Available at: http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed February 4, 2009.
- 4.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Lee LN, Hsueh PR. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777–783. [PubMed] [Google Scholar]
- 6.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, Unwin N. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coker R, McKee M, Atun R, Dimitrova B, Dodonova E, Kuznetsov S, Drobniewski F. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ. 2006;332:85–87. doi: 10.1136/bmj.38684.687940.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mboussa J, Monabeka H, Kombo M, Yokolo D, Yoka-Mbio A, Yala F. Course of pulmonary tuberculosis in diabetics. Rev Pneumol Clin. 2003;59:39–44. [PubMed] [Google Scholar]
- 9.Shetty N, Shemko M, Vaz M, D'Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. 2006;10:80–86. [PubMed] [Google Scholar]
- 10.Jabbar A, Hussain SF, Khan AA. Clinical characteristics of pulmonary tuberculosis in adult Pakistani patients with coexisting diabetes mellitus. East Mediterr Health J. 2006;12:522–527. [PubMed] [Google Scholar]
- 11.Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76:529–533. doi: 10.1016/0962-8479(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 12.Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55:19–26. doi: 10.1002/art.21705. [DOI] [PubMed] [Google Scholar]
- 13.Dyck RF, Klomp H, Marciniuk DD, Tan L, Stang MR, Ward HA, Hoeppner VH. The relationship between diabetes and tuberculosis in Saskatchewan: comparison of registered Indians and other Saskatchewan people. Can J Public Health. 2007;98:55–59. doi: 10.1007/BF03405386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, Law WS, Tam CM, Chan CK, Chang KC. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–1494. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 15.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morsy AM, Zaher HH, Hassan MH, Shouman A. Predictors of treatment failure among tuberculosis patients under DOTS strategy in Egypt. East Mediterr Health J. 2003;9:689–701. [PubMed] [Google Scholar]
- 17.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, Nelwan RH, Parwati I, van der Meer JW, van Crevel R. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–435. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 18.Guler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–235. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 19.Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis. 2002;34:752–759. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 20.Wang CS, Yang CJ, Chen HC, Chuang SH, Chong IW, Hwang JJ, Huang MS. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–210. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA, American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 22.Sterling TR, Zhao Z, Khan A, Chaisson RE, Schluger N, Mangura B, Weiner M, Vernon A, Tuberculosis Trials Consortium Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis. 2006;10:542–549. [PubMed] [Google Scholar]
- 23.Wang CS, Chen HC, Yang CJ, Wang WY, Chong IW, Hwang JJ, Huang MS. The impact of age on the demographic, clinical, radiographic characteristics and treatment outcomes of pulmonary tuberculosis patients in Taiwan. Infection. 2008;36:335–340. doi: 10.1007/s15010-008-7199-8. [DOI] [PubMed] [Google Scholar]
- 24.Olmos P, Donoso J, Rojas N, Landeros P, Schurmann R, Retamal G, Meza M, Martinez C. Tuberculosis and diabetes mellitus: a longitudinal-retrospective study in a teaching hospital. Rev Med Chil. 1989;117:979–983. [PubMed] [Google Scholar]
- 25.Swai AB, McLarty DG, Mugusi F. Tuberculosis in diabetic patients in Tanzania. Trop Doct. 1990;20:147–150. doi: 10.1177/004947559002000402. [DOI] [PubMed] [Google Scholar]
- 26.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 27.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 28.Restrepo BI, Fisher-Hoch SP, Smith B, Jeon S, Rahbar MH, McCormick JB, Nuevo Santander Tuberculosis Trackers Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79:541–544. [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbach MM, Klein SJ, Deskowitz M. Experimental diabetes and tuberculosis in the dog. Am Rev Tuberc. 1935;32:665. [Google Scholar]
- 30.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- 31.Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis. 1999;79:235–242. doi: 10.1054/tuld.1998.0167. [DOI] [PubMed] [Google Scholar]
- 32.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, Saito A. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, Cortes-Penfield N, McCormick JB. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivisto KT. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001;69:400–406. doi: 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 36.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, van der Ven AJ, Danusantoso H, Aarnoutse RE, van Crevel R. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43:848–854. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]