Abstract
Palladium-catalyzed reactions of γ-hydroxy internal acyclic alkenes with aryl bromides afford 2,1′-disubstituted tetrahydrofurans in good yields with diastereoselectivities of 3-5:1. The analogous transformations of substrates bearing internal cyclic alkenes afford fused bicyclic and spirocyclic tetrahydrofuran derivatives in good yields with excellent diastereoselectivities (>20:1). A series of deuterium labeling experiments indicate that the origin of the modest diastereoselectivity in reactions of acyclic internal alkene substrates likely derives from a series of reversible β-hydride elimination and σ-bond rotation processes that occur following a rare intramolecular syn-alkene insertion into an intermediate Pd(Ar)(OR) complex. In addition, these studies shed light on the chemoselectivity of insertion, suggesting that the alkene inserts into the Pd-O bond in preference to the Pd-C bond.
Introduction
The synthesis of substituted tetrahydrofurans remains a topic of considerable interest due to the prevalence of these structures in natural products and other biologically active compounds.1,2 Although a considerable amount of effort has been devoted to the development of efficient and stereoselective methods for the construction of these molecules, there are still few existing methods that allow for intramolecular closure of a tetrahydrofuran ring with concomitant formation of both a carbon-carbon bond and a stereocenter at the C1′-carbon adjacent to the ring.3
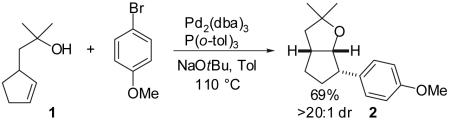
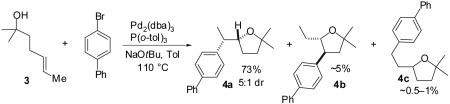
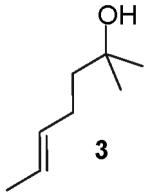
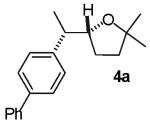
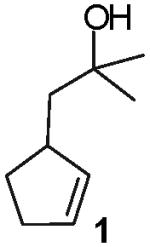
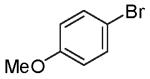
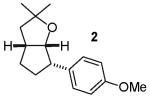
We recently reported a new method for the stereoselective synthesis of tetrahydrofurans from reactions of aryl or vinyl bromides with γ-hydroxyalkenes.4,5,6,7,8,9 These reactions lead to the formation of both a C-O and a C-C bond in a single step, and transformations of substrates bearing substituents at the 1- or 3-position afford trans-2,5- or trans-2,3-disubstituted tetrahydrofurans with diastereoselectivities of up to ≥20:1.4,5 Our preliminary studies indicated that reactions of substrates bearing internal alkenes afford products that derive from syn-addition of the aryl group and the oxygen atom across the double bond.4 For example, treatment of internal cyclic alkene 1 with 4-bromoanisiole in the presence of NaOtBu and a Pd2(dba)3/P(o-tol)3 catalyst provided bicyclic product 2 in 69% yield with >20:1 diastereoselectivity (eq 1). The analogous coupling of acyclic internal alkene substrate 3 with 4-bromobiphenyl afforded tetrahydrofuran 4a in 73% yield with modest (5:1) diastereoselectivity for the product of syn-addition. Curiously, the latter reaction also provided a small amount of the regioisomeric 3-aryl-2-ethyltetrahydrofuran 4b and a trace amount of 4c.10
 |
(1) |
 |
(2) |
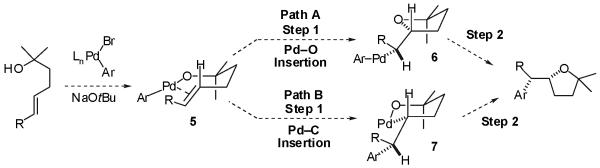
On the basis of the observed product stereochemistry, we proposed that the mechanism of these reactions likely proceeds via an unusual intramolecular insertion of an alkene into either the Pd-O bond of a Pd(Ar)(OR) intermediate 5 followed by C-C bond-forming reductive elimination of the resulting complex 6 (Scheme 1, Path A), or by alkene insertion into the Pd-C bond of 5 followed by C-O bond-forming reductive elimination from intermediate 7 (Scheme 1, Path B).4,11,12 We were unable to differentiate between these two possibilities, as either pathway could potentially afford the observed major product. However, we felt this mechanistic scenario was rather intriguing, as each of these two possible mechanisms involved one step with very little literature precedent. For example, documented cases of alkene insertion into late transition metal-oxygen bonds (Path A, step 1) are exceptionally rare,13,14 and only one example of alkene insertion involving a late metal complex bearing both a metal-carbon and a metal-alkoxide bond had been previously reported.14 The formation of sp3 carbon-oxygen bonds via reductive elimination reactions from late transition metal complexes (Path B, step 2) is also very rare, and known examples involve metals in high oxidation states such as Ni(III),15 and Pt(IV).16 Reductive eliminations from Pd(II) complexes that form sp3 C-O bonds have not been reported.17,18
Scheme 1.
The mechanistic hypothesis for tetrahydrofuran formation described above also posed a challenging question. In the absence of competing pathways or side reactions, the mechanism shown in Scheme 1 predicts that the tetrahydrofuran formation should occur with high diastereoselectivity for products resulting from syn-addition across the double bond. Thus, we were puzzled by the observation that although cycloalkenes were converted to tetrahydrofurans with high stereoselectivity (>20:1), transformations of acyclic internal alkenes proceeded with modest stereoselectivity (ca. 5:1).19 These results prompted us to devise new experiments, which ultimately provided insights into both the origin of the differences in stereoselectivity as well as information concerning the chemoselectivity (Pd-O vs Pd-C) of the alkene insertion into complex 5.
In this Article we present the results of deuterium labeling studies that are most consistent with a mechanism involving selective intramolecular insertion of the alkene into the Pd-O bond of an intermediate Pd(Ar)(OR) complex followed by C-C bond-forming reductive elimination (Scheme 1, Path A). These experiments also suggest the modest stereoselectivity observed in reactions of acyclic internal alkene substrates arises from reversible β-hydride elimination and σ-bond rotation processes that occur after the alkene insertion step but prior to reductive elimination. We also describe studies on the scope and limitations of these transformations, and their application to the synthesis of a variety of monocyclic, bicyclic, and spirocyclic tetrahydrofuran derivatives.
Results
Stereoselective Synthesis of 1′-Substituted Tetrahydrofurans from γ-Hydroxy Internal Acyclic Alkenes
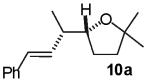
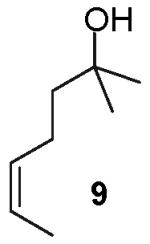
As described above, our preliminary experiments indicated that E-alkene 3 was effectively transformed to 2,1′-disubstituted tetrahydrofuran derivatives upon treatment with an aryl or vinyl bromide in the presence of NaOtBu and a palladium catalyst.20 In order to further probe the scope and the stereospecificity of this methodology, Z-alkene substrate 9 was prepared and treated with three different aryl bromides. As shown in Table 1, the transformations proved to be stereospecific, and in all reactions the major product diastereomer resulted from syn-addition of the aryl group and the heteroatom across the C-C double bond. As observed in transformations of the E-alkene substrate 3, reactions of Z-alkene 9 also afforded 3-aryl-2-ethyltetrahydrofuran side products with structures analogous to 4b shown above (eq 2), albeit in greater amounts (ca. 10-25%) than were formed in similar reactions of 3 (ca. 5-12%). In addition, small amounts of arenes that derive from reduction of the aryl bromide substrates are also observed in reactions of both 3 and 9.21
Table 1. Reactions of Substrates Bearing Acyclic Internal Alkenes(a).
| entry | alcohol | R-Br | product | dr(b) | a:b(c) | yield(d) |
|---|---|---|---|---|---|---|
| 1 |
|
|
0%(e) | |||
| 2 |
|
|
|
5:1 (5:1) |
20:1 (20:1) |
73% |
| 3 |
|
|
5:1 (5:1) |
20:1 (20:1) |
55% | |
| 4 |
|
|
6:1 (3:1) |
12:1 (10:1) |
79% | |
| 5 |
|
|
10:1 (5:1) |
10:1 (7:1) |
78% | |
| 6 |
|
|
|
5:1 (5:1) |
12:1 (8:1) |
71% |
| 7 |
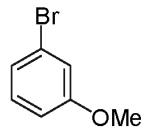
|
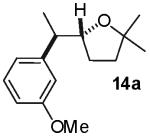
|
7:1 (5:1) |
6:1 (4:1) |
78% | |
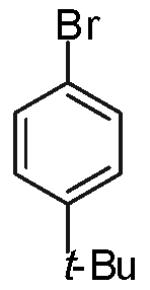
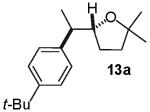
| 8 |
|
|
3:1 (2:1) |
6:1 (3:1) |
50% |
Conditions: 1.0 equiv alcohol, 2.0 equiv ArBr, 2.0 equiv NaOtBu, 1 mol % Pd2(dba)3, 4 mol % P(o-tol)3, toluene(0.125 M), 110 °C.
Diastereomeric ratios are given for the pure, isolated products. The numbers in parentheses represent the diastereomeric ratios observed in the crude reaction mixture. In some cases the diastereomeric ratio improved upon purification as the minor diastereomer was partially separable by chromatography.
Regioisomer ratios (a:b; see eq 2) are given for the pure, isolated products. The numbers in parentheses represent the regioisomer ratios observed in the crude reaction mixture. In some cases the regioisomer ratio improved upon purification as the minor regioisomer was partially separable by chromatography. Regioisomer c was not detected by 1H NMR analysis of crude reaction mixtures or isolated products, but may be present in small amounts (≤2%).
Yields represent average isolated yields for two or more experiments.
Reduction of the aryl bromide was observed.
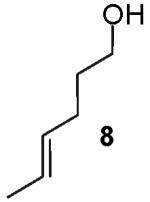
Transformations of substrates bearing internal alkenes required higher temperatures (110 °C) than analogous transformations of substrates bearing terminal alkenes (65 °C),4,5 and gem-disubstitution of the C-1 position was necessary for successful conversion of internal alkene substrates to tetrahydrofuran products. For example, the reaction of 4-hexen-1-ol (8) with 4-bromobiphenyl provided only products derived from oxidation/reduction processes (Table 1, entry 1).
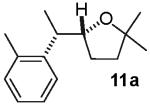
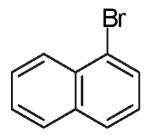
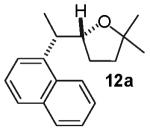
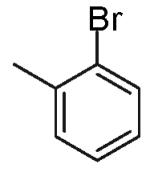
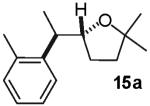
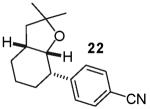
The reactions examined in these studies typically afforded products with c.a. 5:1 diastereoselectivity when unhindered aryl bromides were employed as the coupling partners. However, in some cases the steric properties of the aryl bromide had an impact on diastereoselectivity. For example, the reaction of 3 with 2-bromotoluene gave 11a with 3:1 diastereoselectivity as judged by 1H NMR analysis of the crude reaction mixture; upon purification 11a was obtained in 79% isolated yield and 6:1 dr (entry 4).22 The analogous reaction of 9 with 2-bromotoluene afforded 15a with 2:1 crude dr; the pure product was subsequently obtained in 50% yield as a 3:1 mixture of diastereomers (entry 8).22 In contrast, the reaction of 3 with 1-bromonaphthalene afforded 12a with 5:1 crude dr; 12a was isolated in 78% yield as a 10:1 mixture of diastereomers.22 Increased amounts of regioisomer formation were also observed in reactions of sterically hindered aryl bromides (entries 4, 5, and 8).
Stereoselective Synthesis of Tetrahydrofurans from γ-Hydroxy Internal Cyclic Alkenes
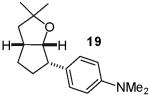
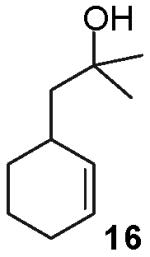
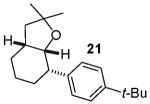
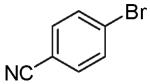
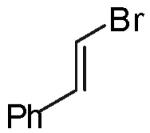
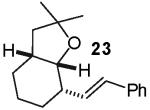
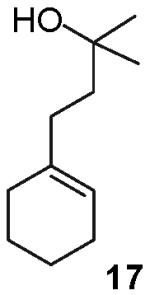
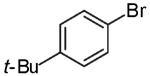
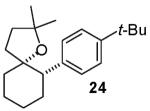
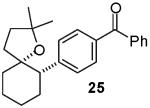
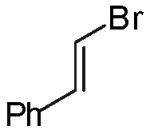
In contrast to the transformations of acyclic internal alkenes described above, reactions of substrates bearing cyclic alkenes proceeded with generally high (>20:1) levels of diastereoselectivity and did not afford mixtures of regioisomers (Table 2). For example, treatment of cyclopentene-derived substrate 1 with N,N-dimethyl-4-bromoaniline in the presence of NaOtBu and a catalytic amount of Pd2(dba)3/P(o-tol)3 afforded a 53% yield of 19 as a single diastereomer (entry 2), and the related coupling of cyclohexene derivative 16 with 1-bromo-4-tert-butylbenzene afforded 21 in 66% yield and >20:1 dr (entry 4). Reactions of cyclohexene derived substrates were typically faster than transformations involving the analogous cyclopentene derivatives. For example, the coupling of 1 with 4-bromoanisole (entry 1) required 2 days at 110 °C to reach completion, whereas the related reaction of 16 with 1-bromo-4-tert-butylbenzene (entry 4) was complete after only 2 h. Moreover, although the stereoselective synthesis of spirocyclic tetrahydrofuran 24 from cyclohexene derivative 17 was achieved in 61% yield (>20:1 dr; entry 7), efforts to transform the analogous cyclopentene-containing substrate 18 only afforded products resulting from Heck-arylation of the alkene (entry 10). As was observed in transformations involving acyclic internal alkene substrates, the major products produced in reactions of cycloalkene substrates derive from syn addition of the aryl group and the oxygen across the olefin.
Table 2. Reactions of Substrates Bearing Cyclic Internal Alkenes.
| entry | alcohol | R2-Br | product | dr | yield(b) |
|---|---|---|---|---|---|
| 1 |
|
|
|
>20:1 | 69% |
| 2 |
|
|
>20:1 | 53% | |
| 3(c) |
|
|
>20:1 | 78% | |
| 4 |
|
|
|
>20:1 | 66% |
| 5(c) |
|
|
>20:1 | 56% | |
| 6 |
|
|
>20:1 | 61% | |
| 7 |
|
|
|
>20:1 | 61% |
| 8 |
|
|
>20:1 | 33% | |
| 9 |
|
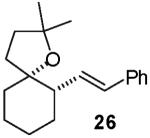
|
>20:1 | 83% | |
| 10 |
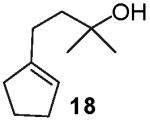
|
|
0%(d) |
Conditions: 1.0 equiv alcohol, 2.0 equiv ArBr, 2.0 equiv NaOtBu, 2.5 mol % Pd2(dba)3, 10 mol % P(o-tol)3, toluene (0.125 M), 110 °C.
Yields represent average isolated yields for two or more experiments.
Xantphos (5 mol %) was used in place of P(o-tol)3.
Heck-type products were observed.
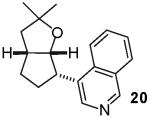
As noted above, the main side products observed in these reactions are small amounts of dehalogenated arenes resulting from reduction of the aryl bromide.21 In some cases, transformations of β-bromostyrene (entry 6) or electron-deficient aryl bromides (entries 5 and 8) proceeded in lower yield than related reactions of electron-neutral aryl halides due to competing O-vinylation or O-arylation of the alcohol substrate. The reaction of the heteroaryl halide 4-bromoisoquinoline was very slow and proceeded with low conversion when a catalyst comprised of Pd2(dba)3/P(o-tol)3 was employed. However, use of the chelating ligand xantphos23 for this transformation afforded 20 in good yield (78%) with excellent diastereoselectivity (entry 3). Xantphos also provided better results than P(o-tol)3 for the reaction of 16 with 4-bromobenzonitrile (entry 5).
Deuterium Labeling Experiments
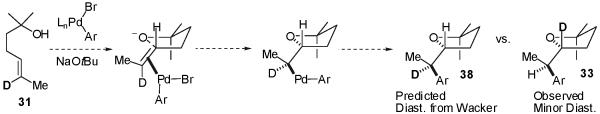
As noted above, the mechanistic hypothesis outlined in Scheme 1 cannot, in itself, account for the formation of diastereomers formally resulting from anti-addition of the oxygen and the arene across the double bond of acyclic internal alkenes. However, we felt that these minor diastereomers could potentially arise either via alkene isomerization, competing Wacker-type anti-alkoxypalladation,3 or competing β-hydride elimination processes that occur post-alkene insertion (see below). It seemed likely that a series of deuterium labeling experiments could potentially differentiate between these three scenarios, as formation of the minor diastereomer via post-insertion β-hydride elimination processes would lead to positional scrambling of the label, whereas the other two possibilities would be expected to proceed with positional retention of the deuterium atom (see discussion below). Thus, a series of deuterated substrates were prepared and converted to tetrahydrofurans under our standard reaction conditions.
As shown below, tertiary alcohol substrate 27 bearing a deuterium atom at the 5-position was treated with 4-bromobiphenyl in the presence of NaOtBu and catalytic Pd2(dba)3/P(o-tol)3 to afford a 79:17:4 mixture of the two diastereomers 28 and 29 along with the regioisomeric side product 30 (eq 3). Interestingly, although the major product diastereomer 28 was deuterated at the expected 2-position, the minor diastereomer 29 was formed as a mixture of two isotopomers; the major isotopomer contained a hydrogen atom at C2 and a deuterium atom at C1′. The regioisomer 30 was deuterated solely at the 1′-position. All products contained a single deuterium atom; products bearing zero or two deuterium atoms were not detected.24 Unreacted starting material that was isolated from a reaction stopped at partial conversion (37%) was deuterated exclusively at the 5-position; deuterium migration in the starting material was not observed.
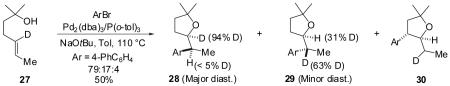 |
(3) |
Treatment of the analogous substrate bearing a deuterium atom at C-6 (31) with 4-bromobiphenyl under identical conditions afforded a 75:20:5 ratio of the two diastereomeric tetrahydrofurans 32 and 33, along with the 3-aryl-2-ethyltetrahydrofuran regioisomer 30. The outcome of this transformation was similar to that described above, with the major diastereomer bearing the deuterium atom at the expected C1′ position, the minor diastereomer bearing the majority of the deuterium label at C2, and the
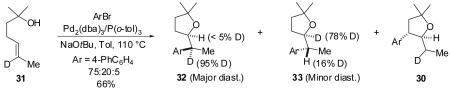 |
(4) |
regioisomer deuterated exclusively at C1′ (eq 4). As above, unreacted starting material isolated from a reaction stopped at partial conversion (63%) was deuterated only at C-6.
The reaction between substrate 34 bearing a vinyl-CD3 group in place of the vinyl CH3-group and 4-bromobiphenyl afforded a mixture of tetrahydrofurans 35 and 36 along with 3-aryl-2-ethyltetrahydrofuran 37 in a 71:23:6 ratio (eq 5). In contrast to the reactions of 27 and 31 described above, no migration of deuterium was observed in the reaction of 34.
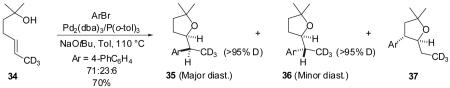 |
(5) |
Discussion
Synthetic Scope and Limitations
Treatment of γ-hydroxy internal alkenes with aryl bromides in the presence of NaOtBu and a Pd-catalyst affords substituted tetrahydrofuran products in moderate to good yields with diastereoselectivities ranging from 3-5:1 (acyclic alkenes) to >20:1 (cyclic alkenes). Two bonds and two contiguous stereocenters are formed in a single step, and the major products derive from syn-addition of the oxygen atom and the arene across the double bond. These reactions are operationally simple, and can be employed for the synthesis of a range of monocyclic, spirocyclic and fused bicyclic products. The starting materials, reagents, and catalysts are readily available, and can be weighed and handled in air. These reactions are effective with a variety of different aryl/vinyl halide coupling partners, including aryl halides bearing electron-donating, electron-withdrawing, or ortho-substituents, although the best chemical yields are obtained with electron-neutral or electron-rich aryl bromides. The use of heteroaryl bromides is also feasible, provided that the chelating phosphine ligand xantphos is used in place of P(o-tol)3.
In contrast to related reactions of γ-hydroxy terminal alkenes, which are effective for a broad array of primary, secondary, and tertiary alcohol substrates, the transformations described in this Article are currently limited to tertiary alcohols. Reactions involving internal alkene substrates also require higher reaction temperatures (110 °C), and/or catalyst loadings (2-5 mol% Pd) than are employed with substrates bearing terminal alkenes (65 °C, 2 mol % Pd). However, despite these limitations, most reactions are complete in a few hours.25
The reactions of substrates bearing tethered acyclic internal alkenes are stereospecific, and reactions of both E- and Z-alkene substrates proceed with similar diastereoselectivities. Mixtures of tetrahydrofuran regioisomers are obtained in these transformations with regioselectivities from 3-20:1. Highest regioselectivities are observed in reactions of unhindered aryl bromides, and regioselectivities appear to be generally higher with E-alkene substrates. In all cases examined, excellent selectivity is obtained for 5-exocyclization; tetrahydropyran derivatives resulting from 6-endocyclization are not observed.
Transformations of substrates bearing cycloalkenes proceed with uniformly high diastereoselectivity and regioselectivity (>20:1). Reactions of cyclohexene derivatives proceed more rapidly than the analogous reactions of cyclopentene derivatives. Cyclizations of trisubstituted internal alkenes proceed in good yield for cyclohexene-containing substrates, but provide only Heck-type products when cyclopentene derivatives are employed as starting materials.
Mechanism and Origin of Moderate Diastereoselectivity In Reactions of Internal Acyclic Alkene Substrates
In our preliminary disclosure of the Pd-catalyzed carboetherification of γ-hydroxy alkenes we suggested that the reactions likely proceed via an intramolecular alkene insertion into an intermediate Pd(Ar)(OR) complex via an organized cyclic transition state (Scheme 1).4 This mechanism accounts for the observed syn-addition of the oxygen atom and the arene across the C-C double bond, and is also consistent with the high stereoselectivity for the formation of trans-2,5- and trans-2,3-disubstituted
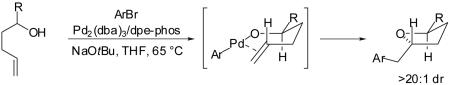 |
(6) |
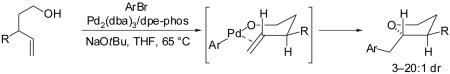 |
(7) |
tetrahydrofurans from 1- and 3-substituted γ-hydroxy terminal alkenes (eq 6-7).4,5 Other plausible mechanistic pathways were ruled out on the basis of the observed stereoselectivity and the high regioselectivity (5-exo addition vs. 6-endo addition) of these transformations,11 although our preliminary data could not distinguish between the two possible pathways (Pd-C vs. Pd-O bond insertion) depicted in Scheme 1.
In the absence of competing pathways or other side reactions the mechanistic hypothesis outlined in Scheme 1 suggests that very high stereoselectivity for syn-addition should be observed. This prediction holds true for reactions of cyclic alkene substrates, which proceed with >20:1 syn-diastereoselectivity, but transformations of acyclic internal alkenes afford 3-5:1 mixtures of syn- and anti-addition products. The origin of the modest stereoselectivity for syn-addition observed with acyclic internal alkene substrates could potentially arise from three possible scenarios. One possibility would involve rapid E/Z alkene interconversion under the reaction conditions, as syn-addition across the different alkene isomers would afford different product diastereomers. However, three observations suggest that this scenario is unlikely. First of all, the reactions are stereospecific, and both the E- and the Z-alkene isomers are transformed with ∼5:1 diastereoselectivity for syn-addition across either the E- or the Z-double bond (with simple p-substituted aryl bromide coupling partners). Additionally, retention of alkene geometry is observed when starting materials are isolated from reactions that are monitored and stopped at partial conversion. Finally, the observation that transformations of 27 and 31 bearing deuterium atoms at the C-5 or C-6 positions provide products in which deuterium migration is observed in the minor diastereomer but not the major diastereomer (eq 3-4) is also not consistent with loss of selectivity via E/Z isomerization.
It is also possible that the modest diastereoselectivity in these transformations could arise from two competing mechanisms, the Wacker-type anti-addition3 and the syn-insertion of the alkene into the Pd(Ar)(OR) complex, operating simultaneously at different rates. These competing pathways would provide opposite diastereomers, and should both be stereospecific. However, this scenario does not account for the observed migration of deuterium depicted in eq 3-4. As shown in Scheme 2, the Wacker mechanism should lead to products resulting from anti-addition with the deuterium label located on the same carbon that was labeled in the starting material. For example, the expected diastereomer that would be formed by Wacker-type reaction of 31 would be 38 (deuterated at C1′) rather than the observed product 33 (deuterated at C2). Thus, it does not appear that competing Wacker-type addition is the predominant pathway for formation of the minor diastereomer.
Scheme 2.
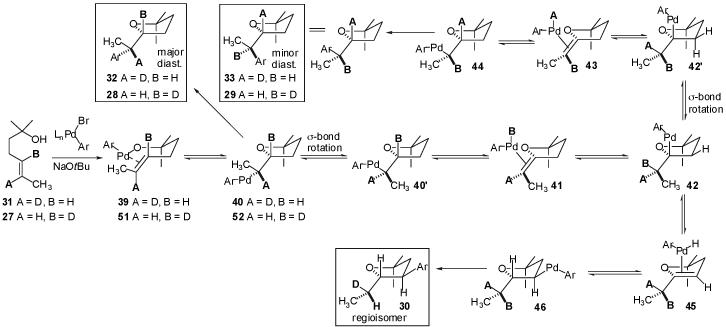
A third possible mechanism that accounts for formation of the minor diastereomer with deuterium migration and also for formation of regioisomer 30 (deuterated at C1′) is shown in Scheme 3.26 Treatment of 31 with a Pd(0) catalyst, an aryl bromide, and NaOtBu could afford palladium(aryl)(alkoxide) complex 39.17 Syn-insertion of the alkene into the Pd-O bond would provide 40,13,14 which could undergo C-C bond-forming reductive elimination to produce the observed major diastereomer with the deuterium label at C1′ (32).27 Alternatively, rotation about the C2-C1′ sigma bond of 40 followed by syn-β-hydride elimination (via conformer 40′) would provide palladium(aryl)(hydride)(alkene) complex 41. Reinsertion of the alkene into the Pd-H bond with opposite regiochemistry would give 42,28 which could then undergo another C2-C1′ sigma bond rotation and syn-β-deuteride elimination (via conformer 42′) to give 43. Reinsertion of the alkene into the Pd-D bond with reversed regiochemistry would provide 44, which could undergo C-C bond-forming reductive elimination to produce the observed minor diastereomer with the deuterium label at C2 (33). Alternatively, intermediate 42 could undergo syn-elimination of the C-3 β-hydrogen followed by regiochemically reversed reinsertion into the Pd-H bond and subsequent C-C bond-forming reductive elimination from 46 to give regioisomeric product 30. The observation that deuterium scrambling in the minor diastereomer is not complete (78% D at C2; 84% H at C1′)24 could be due to a small amount of diastereomer formation via the competing Wacker-type pathway described above (Scheme 2).29
Scheme 3.
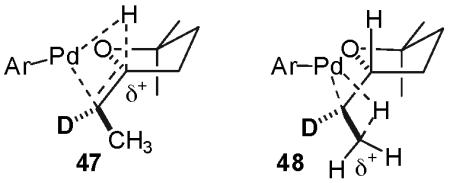
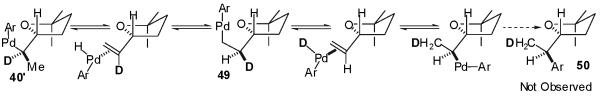
Although, in principle, β-hydride elimination of 40′ could also occur from the C2′-methyl group, this could be expected to be considerably slower than the observed elimination from the C2-H (adjacent to the THF-oxygen atom). The transition state for a β-hydride elimination process leading from an alkylpalladium complex to a palladium(hydride)(alkene) complex is believed to contain a significant developing positive charge on the carbon from which the hydride departs.30 The developing positive charge could be stabilized by the adjacent THF oxygen in transition state 47 (C-2 β-hydride elimination), whereas in transition state 48 (C2′-methyl β-hydride elimination) the positive charge would be relatively unstable due to its location on a methyl carbon. Thus, transition state 47 should be considerably lower in energy than 48. If isomerization occurs via reversible β-hydride elimination from the C2′-methyl group, the minor diastereomer would be expected to contain at least partial deuterium incorporation at the C2′-methyl group (Scheme 4, 50), which is not observed by 2H NMR analysis of the product mixture.
Scheme 4.
The results of the additional deuterium labeling experiments described in eq 3 and eq 5 are also consistent with this mechanistic proposal. As shown in Scheme 3, treatment of 27 with 4-bromobiphenyl in the presence of NaOtBu and a Pd(0) complex could provide Pd(Ar)(OR) intermediate 51. Intramolecular alkene insertion into the Pd-O bond of 51 followed by reductive elimination from 52 would afford major diastereomer 28 bearing the deuterium label at C2. From intermediate 52, a series of σ-bond rotations and reversible β-hydride or β-deuteride elimination reactions analogous to those described above would afford minor diastereomer 29 or regioisomer 30 bearing the deuterium at C1′. As noted above, the incomplete scrambling of the deuterium in 29 may be due, in part, to the formation of a small amount of the minor diastereomer via competing Wacker-type cyclization (Scheme 2).29
The transformation described in eq 5 is also consistent with product formation via intramolecular alkene insertion into the Pd-O bond of an intermediate Pd(Ar)(OR) complex. Based on the mechanistic hypothesis outlined in Scheme 3, the transformation of CD3-labeled substrate 34 would be expected, as observed, to afford both the major (35) and the minor (36) diastereomers with no migration of deuterium.
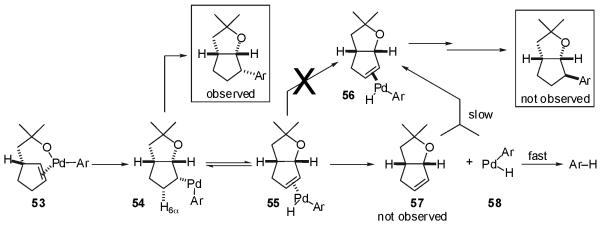
The mechanistic hypothesis described in Scheme 3 can also be used to explain the large difference in diastereoselectivity observed in reactions of cyclic alkenes as compared to acyclic internal alkenes. As shown in Scheme 5, intramolecular insertion of the cycloalkene into the Pd-O bond of palladium(aryl)(alkoxide) intermediate 53 derived from cyclic olefin substrate 1 would afford 54. Complex 54 contains a single β-hydrogen atom with a syn-relationship to the metal (H6α). Although H6α could potentially undergo β-hydride elimination to afford 55, the Pd(Ar)(H) complex cannot be transferred to the opposite face of the alkene to afford 56 through a mechanism analogous to that described in Scheme 3; there is no pathway that would lead to a σ-alkyl palladium complex similar to 42 that could undergo a C-C bond rotation as depicted above (Scheme 3, 42 → 42′). In principle, the alkene 57 could be displaced from the metal and then reassociate on the opposite alkene face to afford 56. However, the intermediate LnPd(Ar)(H) complex 58 would be likely to undergo unimolecular C-H bond-forming reductive elimination at a much faster rate than bimolecular alkene coordination and insertion into the Pd-H bond. Thus, the mechanism outlined above predicts, as observed, that cyclic alkenes should be transformed with high levels of diastereoselectivity. The origins of the diminished stereo- and regioselectivity observed in reactions of sterically hindered aryl bromides with acyclic internal alkenes is not entirely clear, but may arise from changes in the relative rates of C-C bond-forming reductive elimination vs. β-hydride elimination, or from shifts in the equilibria shown in Scheme 3.
Scheme 5.
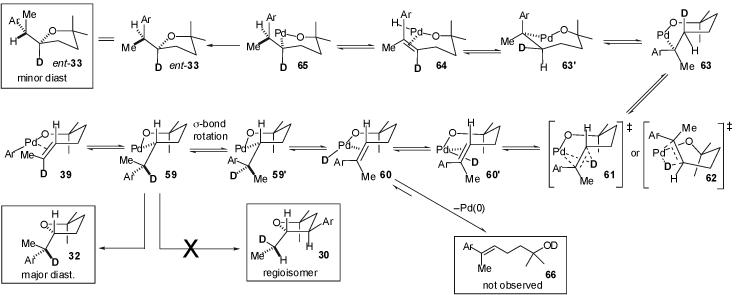
The outcome of the deuterium labeling experiments described in eq 3-5 can also be used to evaluate the potential viability of a mechanism involving insertion into the Pd-C bond rather than the Pd-O bond of an intermediate Pd(Ar)(OR) complex (e.g. 39). As shown in Scheme 6, intramolecular alkene insertion into the Pd-C bond of 39 (derived from substrate 31 as in Scheme 3) would afford palladium(alkyl)(alkoxide) 59. In principle this intermediate Pd(II) complex could undergo an unprecedented sp3C-O bond-forming reductive elimination17,18 to afford the observed major diastereomer 32 bearing a deuterium atom at C1′. However, carbopalladation reactions in mechanistically related Heck reactions are believed to be irreversible,31,32,33 thus conversion of 59 to observed regioisomer 30 is unlikely. Moreover, there is no plausible pathway that could lead to the formation of 30 from 39 via either endo- or exo-carbopalladation of the alkene.
Scheme 6.
Although a mechanism could be postulated for the formation of the observed minor diastereomer 33 bearing a C2-deuterium atom from 59, several of the required steps appear to be rather implausible. The pathway for conversion of 59 to 33 would involve initial rotation about the C2-C1′ bond followed by β-hydride elimination from conformer 59′ to afford Pd(deuterio)(alkoxide) 60. Although related Pd(H)(OMe) complexes have been described,34 they are known to undergo facile O-H bond forming reductive elimination reactions to generate alcohols, and the formation of Pd(H)(OMe) complexes via O-H bond oxidative addition is thermodynamically unfavorable.34 Reductive elimination of potential intermediate 60 would generate Heck-type product 66, which is not observed in significant amounts (<2%).
Conversion of potential intermediate 60 to the observed minor diastereomer 33 would require 7-endo deuteriopalladation either via twisted transition state 6135 or via eclipsed transition state 62 to afford 63.36 Intramolecular alkene insertion reactions of Pd(II) complexes generally favor 6-exocyclization over 7-endocyclization,31 with the latter transformations extremely rare for Pd-C insertion processes31,37 and, to the best of our knowledge, unknown for Pd-H or Pd-D insertion processes. If this sequence were to occur, 63 could, in principle, undergo selective β-hydride elimination of the endocyclic hydrogen atom (via conformation 63′) in preference to β-elimination of one of the exo-methyl hydrogen atoms to afford palladium(hydrido)alkoxide 64.38 This type of endo β-hydride elimination is thermodynamically favorable relative to competing exo-β-hydride elimination, but is often kinetically slow.38 From complex 64, alkene insertion into the Pd-H bond followed by sp3C-O bond-forming reductive elimination from 65 would provide observed minor diastereomer 33.
Although we cannot unambiguously rule out the mechanistic scenario outlined in Scheme 6, this pathway cannot account for the observed formation of regioisomer 30. In addition, this scenario requires several steps that would proceed via high-energy transition states (e.g. 61 or 62) to occur in preference to alternative pathways that appear to be lower in energy (e.g. 60 → 66). Thus, product formation via this mechanism seems unlikely. In contrast, the mechanistic pathway involving intramolecular insertion of the alkene into the Pd-O bond outlined in Scheme 3 accounts for the formation of all observed products. Most steps invoked in this pathway are well established (e.g. σ-bond rotation, β-hydride elimination, and C-C bond forming reductive elimination), with the exception of the alkene insertion into the Pd-O bond, which, although rare, is not entirely unprecedented.13,14
Conclusions
In conclusion, our studies on the Pd-catalyzed carboetherification of internal alkenes suggest that the predominant mechanistic pathway for tetrahydrofuran formation likely involves a rare syn-insertion of an alkene into the Pd-O bond of an intermediate palladium(aryl)(alkoxide). These studies also indicate that the moderate diastereoselectivity observed in reactions of substrates bearing acyclic internal alkenes is likely due to stereochemical scrambling via β-hydride elimination/reinsertion and σ-bond rotation processes that occur after the alkene insertion step. This pathway for stereochemical scrambling is not accessible when substrates bearing cyclic internal alkenes are employed, and high diastereoselectivity for syn addition is observed.
The transformations described in this Article represent an operationally simple and straightforward means for the stereoselective construction of 2,1′-disubstituted tetrahydrofurans, 6-substituted octahydrocyclopenta[b]furans, 7-substituted octahydrobenzofurans, and 6-substitued-1-oxaspiro[4.5]decanes. The reactions are stereospecific, and provide good yields with a variety of different aryl and vinyl bromide coupling partners. Further studies to improve scope of these transformations and the diastereoselectivity in reactions of acyclic internal alkene substrates are currently underway.
Experimental39
General Procedure for Palladium Catalyzed Reactions of γ-Hydroxy internal alkenes with Aryl Bromides
A flame dried or oven dried Schlenk tube equipped with a magnetic stirbar was cooled under a stream of argon or nitrogen and charged with Pd2(dba)3 (4.6 mg, 0.005 mmol), P(o-tol)3 (6.1 mg, 0.02 mmol), sodium tert-butoxide (96 mg, 1.0 mmol), and the aryl or vinyl bromide (1.0 mmol). The tube was purged with Ar followed by the addition of toluene (2 mL), the alcohol substrate (0.5 mmol), and additional toluene (2 mL). The reaction mixture was heated to 110°C with stirring until the alcohol substrate had been completely consumed as judged by GC analysis. The reaction mixture was cooled to rt and saturated aqueous NH4Cl (2 mL) and ethyl acetate (10 mL) were added. The layers were separated and the aqueous layer was extracted with ethyl acetate (2 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, decanted, and concentrated in vacuo. The crude material was purified by flash chromatography on silica gel.
(±)-(1′S,5R)-5-[1′-(4-Phenyl)phenylethyl]-2,2-dimethyltetrahydrofuran (4a)
Reaction of 64 mg (0.5 mmol) of trans-2-methyl-5-hepten-2-ol with 4-bromobiphenyl (223 mg, 1.0 mmol) following the general procedure afforded 100 mg (71 %) of the title compound as a colorless oil. This material was obtained as a 5:1 mixture of diastereomers and contained ∼5% of 4b+4c as judged by 1H NMR analysis; data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.60-7.57 (m, 2 H), 7.53-7.51 (m, 2H), 7.44-7.40 (m, 2 H), 7.34-7.32 (m, 3 H), 4.19-4.15 (m, 1 H), 2.97-2.91 (m, 1 H), 1.92-1.86 (m, 1 H), 1.79-1.72 (m, 1 H), 1.69-1.62 (m, 1 H), 1.61-1.54 (m, 1 H), 1.30 (d, J = 7.0 Hz, 3 H), 1.213 (s, 3 H), 1.210 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 143.3, 141.2, 138.9, 128.7, 128.6, 127.0, 126.9, 126.7; 82.6, 80.6, 43.9, 38.5, 28.7, 28.6, 28.0, 16.5; IR (film) 1515, 1059 cm-1. Anal calcd for C20H24O: C, 85.67; H, 8.63. Found: C, 85.29; H, 8.31.
Supplementary Material
Acknowledgment
The authors thank the NIH-NIGMS (GM-076150) and the University of Michigan for financial support of this work. JPW acknowledges the Camille and Henry Dreyfus Foundation for a New Faculty Award, and Research Corporation for an Innovation Award. The authors also thank Amgen, 3M, and Eli Lilly for additional unrestricted support.
Footnotes
The yields reported in the experimental section and the supporting information describe the result of a single experiment, whereas the yields reported in eq 1-2 and Tables 2-3 are average yields of two or more experiments. Thus, the yields reported in the supporting information may differ from those shown in eq 1-2 and Tables 2-3.
References and Notes
- (1).(a) Faul MM, Huff BE. Chem. Rev. 2000;100:2407–2474. doi: 10.1021/cr940210s. [DOI] [PubMed] [Google Scholar]; (b) Alali FQ, Liu X-X, McLaughlin JL. J. Nat. Prod. 1999;62:504–540. doi: 10.1021/np980406d. [DOI] [PubMed] [Google Scholar]; (c) Hou X-L, Yang Z, Wong HNC. Prog. Heterocycl. Chem. 2002;14:139–179. [Google Scholar]; (d) Ward RS. Nat. Prod. Rep. 1999;16:75–96. [Google Scholar]; (e) Nicotra F. Top. Curr. Chem. 1997;187:55–83. [Google Scholar]
- (2).(a) Elliott MC. J. Chem. Soc., Perkin Trans. 1. 2002:2301–2323. [Google Scholar]; (b) Harmange JC, Figadere B. Tetrahedron: Asymmetry. 1993;4:1711–1754. [Google Scholar]
- (3).(a) Semmelhack MF, Bodurow C. J. Am. Chem. Soc. 1984;106:1496–1498. [Google Scholar]; (b) Semmelhack MF, Kim C, Zhang N, Bodurow C, Sanner M, Dobler W, Meier M. Pure Appl. Chem. 1990;62:2035–2040. [Google Scholar]; (c) Semmelhack MF, Epa WR. Tetrahedron Lett. 1993;34:7205–7208. [Google Scholar]
- (4).A portion of this work has been previously communicated. See: Wolfe JP, Rossi MA. J. Am. Chem. Soc. 2004;126:1620–1621. doi: 10.1021/ja0394838.
- (5).For detailed studies on the scope and limitations of reactions of γ-hydroxy terminal alkenes, see: Hay MB, Hardin AR, Wolfe JP. J. Org. Chem. 2005;70:3099–3107. doi: 10.1021/jo050022+.
- (6).For related transformations of γ-aminoalkenes that afford nitrogen heterocycles see: Ney JE, Wolfe JP. Angew. Chem. Int. Ed. 2004;43:3605–3608. doi: 10.1002/anie.200460060. Ney JE, Wolfe JP. J. Am. Chem. Soc. 2005;127:8644–8651. doi: 10.1021/ja0430346. Beaudoin Bertrand M, Wolfe JP. Tetrahedron. 2005;61:6447–6459. Lira R, Wolfe JP. J. Am. Chem. Soc. 2004;126:13906–13907. doi: 10.1021/ja0460920. Yang Q, Ney JE, Wolfe JP. Org. Lett. 2005;7:2575–2578. doi: 10.1021/ol050647u.
- (7).(a) For related transformations of γ-alkylidene malonates that afford carbocycles see: Balme G, Bouyssi D, Lomberget T, Monteiro N. Synthesis. 2003:2115–2134. Balme G, Bouyssi D, Faure R, Gore J, Van Hemelryck B. Tetrahedron. 1992;48:3891–3902.
- (8).For related transformations of γ-hydroxy allenes or alkynes see: Kang S-K, Baik T-G, Kulak AN. Synlett. 1999:324–326. Walkup RD, Guan L, Mosher MD, Kim SW, Kim YS. Synlett. 1993:88–90. Luo FT, Schreuder I, Wang RT. J. Org. Chem. 1992;57:2213–2215.
- (9).For related heteroannulations of internal alkynes with o-haloanilines, o-halophenols, and related compounds see: Larock RC, Yum EK, Doty MJ, Sham KKC. J. Org. Chem. 1995;60:3270–3271.
- (10).The structure of this regioisomer had not been assigned at the time of our initial communication of these studies (reference 4). We have included the characterization data for this compound in the Supporting Information of this article. In addition, a trace amount (ca. 0.5-1.0 %) of regioisomer 4c was detected upon isolation of 4b by preparative HPLC. This regioisomer was not present in sufficient amount to detect by 1H NMR analysis of the mixture of 4a-c.
- (11).Trost has noted the formation of a tetrahydrofuran side product in the Heck arylation of an enyne bearing a secondary OH group and suggested a mechanism of intermolecular carbopalladation to account for its formation. See: Trost BM, Pfrengle W, Urabe H, Dumas J. J. Am. Chem. Soc. 1992;114:1923–1924.
- (12).Two other possible mechanisms were ruled out on the basis of product stereochemistry. Product formation via a Wacker-type mechanism in which the alkene was activated for nucleophilic attack of the tethered alkene was discounted as this pathway would afford products resulting from anti addition of the oxygen and the arene. Product formation via an intermolecular Heck-type carbopalladation followed by C-O bond-forming reductive elimination was ruled out on the basis of the high regioselectivity for five-membered ring formation and also based on the fact that alcohols substituted at C-1 provided trans-2,5-disubstituted tetrahydrofurans with excellent (>20:1) diastereoselectivity. Intermolecular carbopalladation reactions of terminal alkenes typically proceed with only 5-7:1 regioselectivity, and it is unlikely that high stereoselectivity for the formation of trans-2,5-disubstituted products would be obtained if the stereochemistry determining event was an intermolecular reaction that occurred two atoms away from the substrate stereocenter. For further discussion, see references 4 and 5.
- (13).At the time of our preliminary communication no examples of syn-alkene insertions into Pd-O bonds had been reported. However, in elegant recent studies, Hayashi has provided evidence for the syn-insertion of an alkene into a Pd-O bond in the conversion of an o-allylphenol derivative to a tetrahdrodibenzofuran. See: Hayashi T, Yamasaki K, Mimura M, Uozumi Y. J. Am. Chem. Soc. 2004;126:3036–3037. doi: 10.1021/ja031946m.
- (14).Bryndza has described the stoichiometric insertion of tetrafluoroethylene into the Pt-O bond of (dppe)Pt(Me)(OMe). See: Bryndza HE. Organometallics. 1985;4:406–408. Bryndza HE, Calabrese JC, Wreford SS. Organometallics. 1984;3:1603–1604.
- (15).(a) Matsunaga PT, Hillhouse GL, Rheingold AL. J. Am. Chem. Soc. 1993;115:2075–2077. [Google Scholar]; (b) Koo K, Hillhouse GL. Organometallics. 1998;17:2924–2925. [Google Scholar]
- (16).(a) Dick AR, Kampf JW, Sanford MS. Organometallics. 2005;24:482–485. [Google Scholar]; (b) Williams BS, Goldberg KI. J. Am. Chem. Soc. 2001;123:2576–2587. doi: 10.1021/ja003366k. [DOI] [PubMed] [Google Scholar]; (c) Stahl SS, Labinger JA, Bercaw JE. Angew. Chem. Int. Ed. 1998;37:2180–2192. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2180::AID-ANIE2180>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- (17).For examples of sp2C-O bond-forming reductive elimination from Pd(II) aryl alkoxide complexes see: Widenhoefer RA, Buchwald SL. J. Am. Chem. Soc. 1998;120:6504–6411. and references cited therein. Mann G, Hartwig JF. J. Am. Chem. Soc. 1996;118:13109–13110.
- (18).For recent examples of catalytic reactions that may involve sp3C-O bond forming reductive elimination from Pd(IV), see: Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542–9543. doi: 10.1021/ja046831c.
- (19).Both mechanistic pathways (A and B) would be expected to provide high selectivity for syn addition products unless other competing mechanistic pathways are operating simultaneously.
- (20).The relative stereochemistry of the tetrahydrofuran products was assigned based on comparison of 1H and 13C NMR chemical shifts to related compounds of known configuration and/or by 1H NMR nOe experiments. See the Supporting Information for complete details.
- (21).Unarylated tetrahydrofuran or dihydrofuran products were not observed in these reactions. However, these potential side products would be quite volatile, and may be difficult to detect by GC analysis of crude reaction mixtures.
- (22).In some instances diastereomeric ratios improved upon purification as the two product diastereomers were partially separable by silica gel chromatography.
- (23).Xantphos = 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene.
- (24).Monodeuteration was confirmed by MS analysis of the product mixture. The %deuterium incorporation was determined by 1H and 2H NMR spectroscopy and was reproducible within ±3% over two runs. Although the sum of the %deuterium at C-2 and C1′ is <100% for the minor diastereomers shown in eq 3-5, this is likely due to small errors associated with NMR measurement and integration.
- (25).Reactions of cyclopentene derivatives require longer reaction times (ca. 48 hours).
- (26).For an example of stereochemical inversion in an isolated palladium complex that proceeds through a similar mechanism, see: Burke BJ, Overman LE. J. Am. Chem. Soc. 2004;126:16820–16833. doi: 10.1021/ja045047p.
- (27).Carbon-carbon bond-forming reductive elimination is believed to occur with retention of configuration. See: Milstein D, Stille JK. J. Am. Chem. Soc. 1979;101:4981–4991.
- (28).For related examples of stereospecific deuterium migration via reversible β-hydride/deuteride elimination processes, see: Qian H, Widenhoefer RA. J. Am. Chem. Soc. 2003;125:2056–2057. doi: 10.1021/ja0293002. (b) Reference 13.
- (29).The reaction of 31 with 4-bromobiphenyl shown in eq 4 afforded the minor diastereomer 33 with ∼80% deuterium scrambling, whereas the analogous reaction of 27 (eq 3) provided the minor diastereomer 29 with ∼60% deuterium scrambling. This small difference was reproducible, and is not believed to be due simply to experimental error. The origin of this difference is not clear. However, it is possible this difference could be due to a kinetic isotope effect, which would slow the rate of C-2 β-deuteride elimination from 52 (Scheme 3). This could lead to competing β-hydride elimination from the CH3 group and partial formation of the minor diastereomer with no isotopic scrambling through a mechanism similar to that shown in Scheme 4. The isolation of a trace amount of minor regioisomer 4c (eq 2), which likely arises from reductive elimination of an intermediate similar to 49 (Scheme 4) suggests this pathway may be viable, albeit a minor contributor to the overall product distribution. One might anticipate that the influence of kinetic isotope effects on other β-hydride elimination/reinsertion steps could lead to other changes in product distribution. However, efforts to interpret possible isotope effects in this reaction are complicated by the fact that primary deuterium kinetic isotope effects for syn-β-hydride elimination have been reported to range from ∼1.3 to >5, and appear to be dependent on both catalyst and substrate structure. Thus, it is not inconceivable that the magnitude of kinetic isotope effects in this system could depend on the position of the deuterium atom in any given intermediate. For lead references, see: Mueller JA, Goller CP, Sigman MS. J. Am. Chem. Soc. 2004;126:9724–9734. doi: 10.1021/ja047794s. Steinhoff BA, Stahl SS. Org. Lett. 2002;4:4179–4181. doi: 10.1021/ol026988e. Lloyd-Jones GC, Slatford PA. J. Am. Chem. Soc. 2004;126:2690–2691. doi: 10.1021/ja039349n.
- (30).Mueller JA, Sigman MS. J. Am. Chem. Soc. 2003;125:7005–7013. doi: 10.1021/ja034262n. [DOI] [PubMed] [Google Scholar]
- (31).(a) Gibson SE, Middleton RJ. Contemp. Org. Synth. 1996;3:447–471. [Google Scholar]; (b) Link JT. Org. React. 2002;60:157–534. [Google Scholar]; (c) Negishi E. -i., Coperet C, Ma S, Liou S-Y, Liu F. Chem. Rev. 1996;96:365–393. doi: 10.1021/cr950020x. [DOI] [PubMed] [Google Scholar]
- (32).Perch NS, Widenhoefer RA. J. Am. Chem. Soc. 2004;126:6332–6346. doi: 10.1021/ja049806f. [DOI] [PubMed] [Google Scholar]
- (33).Previously described examples of β-aryl elimination from (phenethyl)Pd(II) complexes typically involve cationic complexes with no syn-β-hydrogens. Analogous intermediates bearing β-hydrogen atoms appear to undergo β-hydride elimination more rapidly than β-aryl elimination. See: Campora J, Gutierrez-Puebla E, Lopez JA, Monge A, Palma P, del Rio D, Carmona E. Angew. Chem. Int. Ed. 2001;40:3641–3644. doi: 10.1002/1521-3773(20011001)40:19<3641::aid-anie3641>3.0.co;2-9. Catellani M, Frignani F, Rangoni A. Angew. Chem. Int. Ed. 1997;36:119–122. Catellani M, Fagnola MC. Angew. Chem. Int. Ed. 1994;33:2421–2422. Most examples of β-alkyl elimination from Pd(II) intermediates involve strained ring systems. See: Matsumura S, Maeda Y, Nishimura T, Uemura S. J. Am. Chem. Soc. 2003;125:8862–8869. doi: 10.1021/ja035293l. Wang X, Stankovich SZ, Widenhoefer RA. Organometallics. 2002;21:901–905. and references cited therein.
- (34).Perez PJ, Calabrese JC, Bunel EE. Organometallics. 2001;20:337–345. [Google Scholar]
- (35).Related carbopalladation reactions of alkenes are believed to proceed via transition states in which the Pd-C bond is eclipsed with the C-C double bond; twisted transition states in which the Pd-C and C=C bonds are perpendicular are believed to be energetically unfavorable. See: Overman LE. Pure. Appl. Chem. 1994;66:1423–1431. and references cited therein. Insertion of ethylene into Pt-H bonds is also believed to require a coplanar, eclipsed orientation of the alkene and the platinum hydride. See: Thorn DL, Hoffmann R. J. Am. Chem. Soc. 1978;100:2079–2090.
- (36).Eclipsed transition state 62 would afford the enantiomer of 63 (ent-63). The mechanistic scheme for conversion of ent-63 to 33 is analogous to that shown for the conversion of 63 to ent-33, and has been omitted for clarity.
- (37).Gibson SE, Guillo N, Middleton RJ, Thuilliez A, Tozer MJ. J. Chem. Soc., Perkin Trans. 1. 1997:447–456. and references cited therein.
- (38).(a) Osakada K, Doh M-K, Ozawa F, Yamamoto A. Organometallics. 1990;9:2197–2198. [Google Scholar]; (b) Zhang L, Zetterberg K. Organometallics. 1991;10:3806–3813. [Google Scholar]; (c) Newkome GR, Puckett WE, Gupta VK, Formczek FR. Organometallics. 1983;2:1247–1249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.