Abstract
An effective total synthesis of the marine sponge cytotoxin (+)-psymberin [irciniastatin (1)] has been achieved. Highlights of the strategy include a Diels-Alder reaction between a bissiloxy diene and an allene to construct the aromatic ring, a boron-mediated aldol to elaborate the C(15–17) all syn stereo-triad, catalytic reagent control to set the C(8, 9, 11 and 13) stereogenic centers of the tetrahydropyran core, and a late-stage Curtius rearrangement to install the sensitive N,O-aminal moiety. The synthesis proceeds with a longest linear sequence of 21 steps from commercially a v a2,2i-diml ethayl-1b,3-plropaenediol.
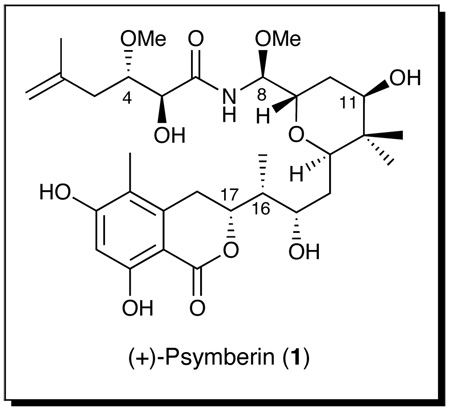
In 2004, the laboratories of Pettit and Crews independently disclosed the isolation of ircinistatin A1 and psymberin (1),2 respectively from the marine sponges Ircinia ramose and Psammocinia sp. A closely related compound, irciniastatin B, possessing a carbonyl moiety at C(11) was also reported by the Pettit group. From the outset, irciniastatin A and psymberin appeared to be constitutionally equivalent based on high resolution mass spectrometry, in conjunction with the 1D and 2D NMR data. The absolute configuration of psymberin was assigned by the Crews group based on a combination of CD studies along with the assumed analogy to the structure of pederin.3 Neither group however assigned the relative stereogenecity at C(4); also recorded were conflicting stereochemical assignments for the C(8) N,O-aminal. Importantly, both isolates displayed significant cancer cell growth inhibitory activity against a wide variety of human cancer cell lines. The potent cytotoxicity data, in conjuction with the conflicting and incomplete stereochemical assignments, quickly drew the attention of the synthetic community. In 2005, Williams and Kiren4 assigned the C(3,4) stereorelationship as anti based on a model compound synthesis, in conjuction with detailed NMR comparisons.2 Shortly thereafter, the DeBrabander group announced completion of the first total synthesis of (+)-psymberin, complete with construction of the four possible C(4)-C(8) epimers, which not only completed the structural assignment, including absolute configuration, but also confirmed that irciniastatin A and psymberin (1) were in fact one and the same.5 Related synthetic work followed quickly,6 with a formal synthesis in 20077 and a second total synthesis reported by the Schering-Plough group,8 the latter exploiting a novel (diacetoxyiodo)benzene-mediated cyclization to construct the central tetrahydropyran core.
We also were intrigued with (+)-psymberin (1) as a potential new cancer therapeutic lead. Herein we report our efforts recently culminating in an effective total synthesis of (+)-1. Central to our synthetic plan was the use of catalytic reagent control to set the stage for an eventual structure-activity study to define the structural elements required for biological activity.9
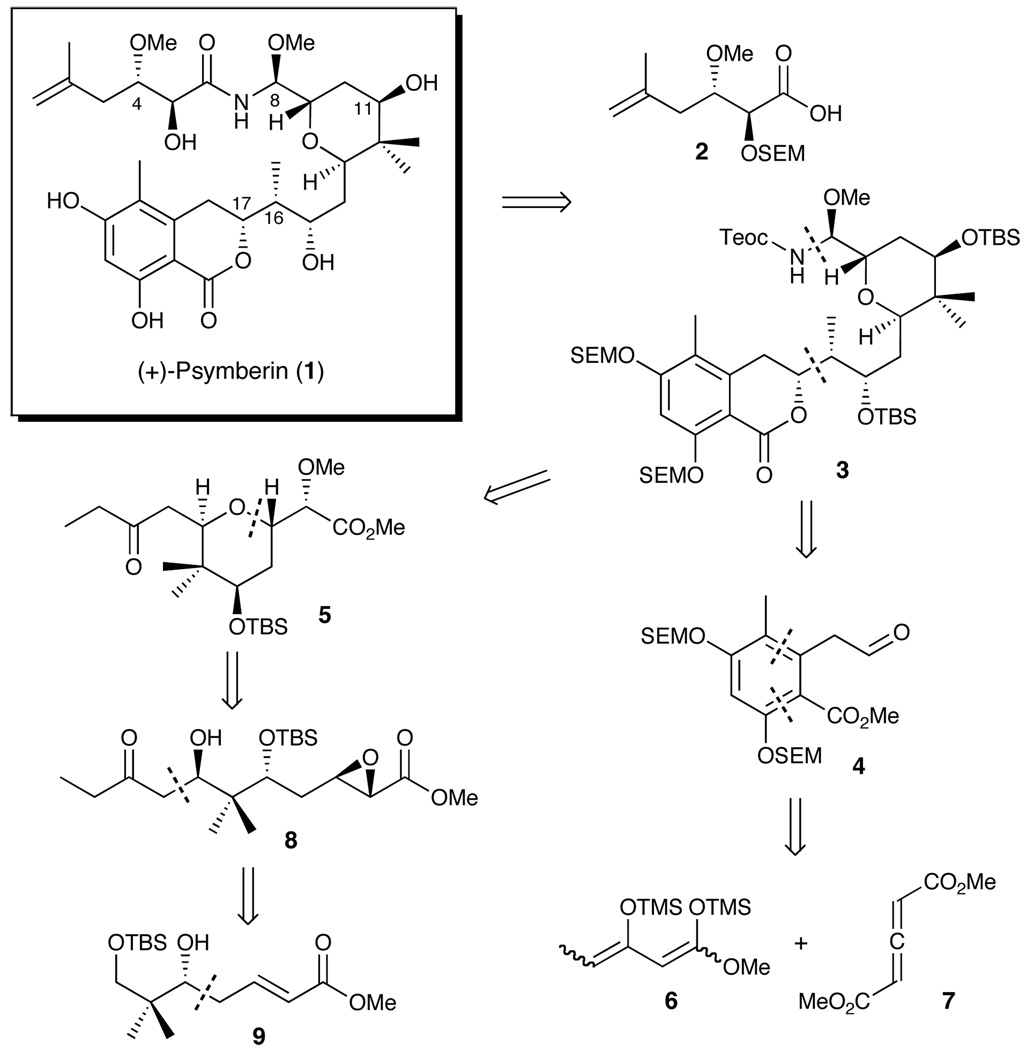
With this overview in mind, disconnection of the amide bond in (+)-1 (Scheme 1), as with the earlier reported syntheses, 5,7,8 leads to a side chain acid (2) and amide coupling precursor (3), the latter bearing a Teoc-protected N,O-aminal. We envisioned that elaboration of the N,Oaminal could be achieved in a highly stereoselective fashion exploiting a late-stage Curtius rearrangement similar to that developed and employed in our total syntheses of (+)- dactylolide and (+)-zampanolide.10 Further disconnection at the C(16,17) bond leads to aldehyde 4 and 2,6-transtetrahydropyran 5, which would be joined via a 1,4- substrate controlled boron-mediated aldol reaction to control the configurations of the C(16,17) stereogenic centers. In the forward sense, access to aldehyde 4 would entail a Diels-Alder reaction between bis-siloxydiene 611 and allene 7.12 The requisite 2,6-trans-tetrahydropyran (5) in turn would derive via cyclization of a epoxy alcohol linear precursor (cf. 8). From the strategic perspective, installation of the four requisite stereogenetic centers at C(8, 9, 11 and 13) would take advantage of catalytic reagent control, beginning with an asymmetric vinylogous Mukaiyama aldol reaction13 to furnish 9. The clear advantage of this tactic, if successful, would be rapid access to a wide variety of stereochemically diverse congeners, simply by changing the enantiomer of the catalyst, thereby avoiding significant strategy redesign to access analogues for the prospective structure-activity relationship study.9
Scheme 1.
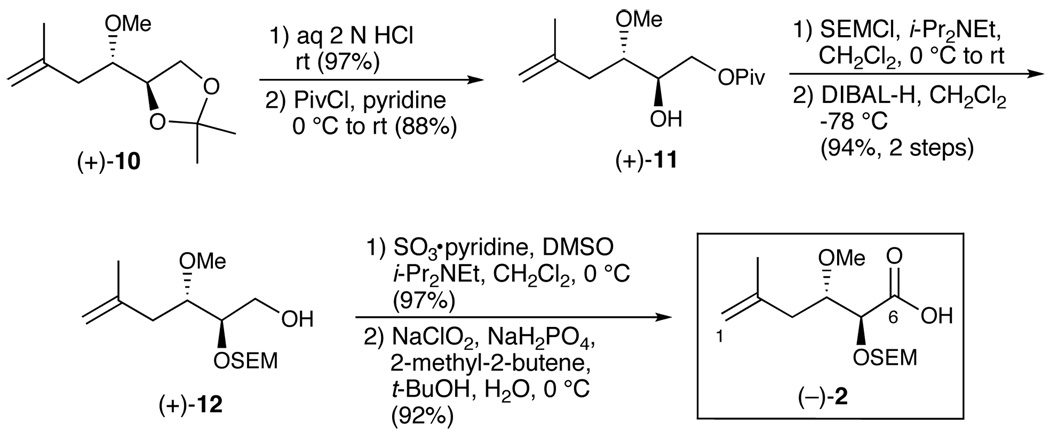
We began with the synthesis of side chain acid 2 employing known methyl ether (+)-10,5 which was constructed in two steps with an overall yield of 57% yield (dr > 20:1) from commercially available (+)-isopropylidene glyceraldyde (Scheme 2). The acetonide was then removed with aqueous hydrochloric acid, followed by masking of the primary alcohol as the pivalate ester (+)-11; the yield for the two steps was 85%. Subsequent protection of the secondary alcohol as the SEM ether, followed by DIBAL-H reduction of the pivalate ester furnished primary alcohol (+)-12, which upon a two-step Parikh-Doering14/Pinnick15 oxidation provided the C(1–6) side chain acid (−)-2 in 89% yield.
Scheme 2.
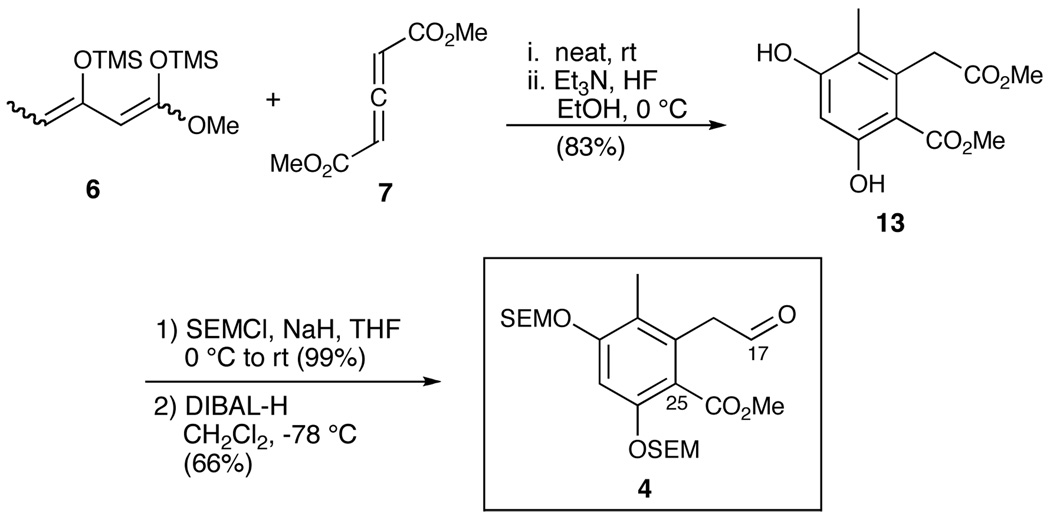
Construction of the C(17–25) aryl aldehyde 4 began with the proposed Diels-Alder reaction between 1,3-bis(trimethylsiloxy)-1,3-diene 611 and dimethyl-1,3-allene-dicarboxlate 7,12 followed by treatment with HF•NEt3 to effect aromatization leading to known homophthalate 1 316 (Scheme 3). The two phenolic hydroxyls were then protected as SEM ethers; selective reduction of the alkyl ester in the presence of the benzylic ester completed construction of aldehyde 4. The overall yield for the three-step sequence was 55%.
Scheme 3.
With the side chain and aryl fragments in hand, we turned attention to the central fragment, tetrahydropyran 5. Beginning with commercially available 2,2-dimethyl-1,3-propanediol (14), mono-protection as the TBS ether, followed by Parikh-Doering oxidation of the free hydroxyl provided aldehyde 15 in 85% yield (Scheme 4). A vinylogous Mukaiyama aldol reaction promoted by oxazaborilidinone employing silyl ketene acetal 1613 and aldehyde 15 then set the C(11) configuration, the first of the catalytic reagent controlled reactions. The yield was 66%. More importantly, a single (R) isomer resulted as determined by the Mosher’s ester analysis.17,18
Scheme 4.
Alcohol (+)-9 was next protected as the TBS ether and then subjected to DIBAL-H to provide the corresponding allylic alcohol. Sharpless asymmetric epoxidation19 furnished β-epoxide (+)-17 in 88% yield (β:α = 13:1). Without separation, a one-step TEMPO oxidation20 to furnish the carboxylic acid, followed by methylation with TMSCHN2 led to epoxy ester (+)-18, as a mixture of epoxides. Selective removal of the primary TBS group in the presence of the secondary TBS group utilizing buffered HF•pyridine provided alcohol (+)-19; removal of the minor epoxide diastereomer (dr > 20:1) by flash chromatography now proved straightforward. Parikh-Doering oxidation of the neopentyl alcohol then completed construction of aldehyde (+)-20 in 95% yield.
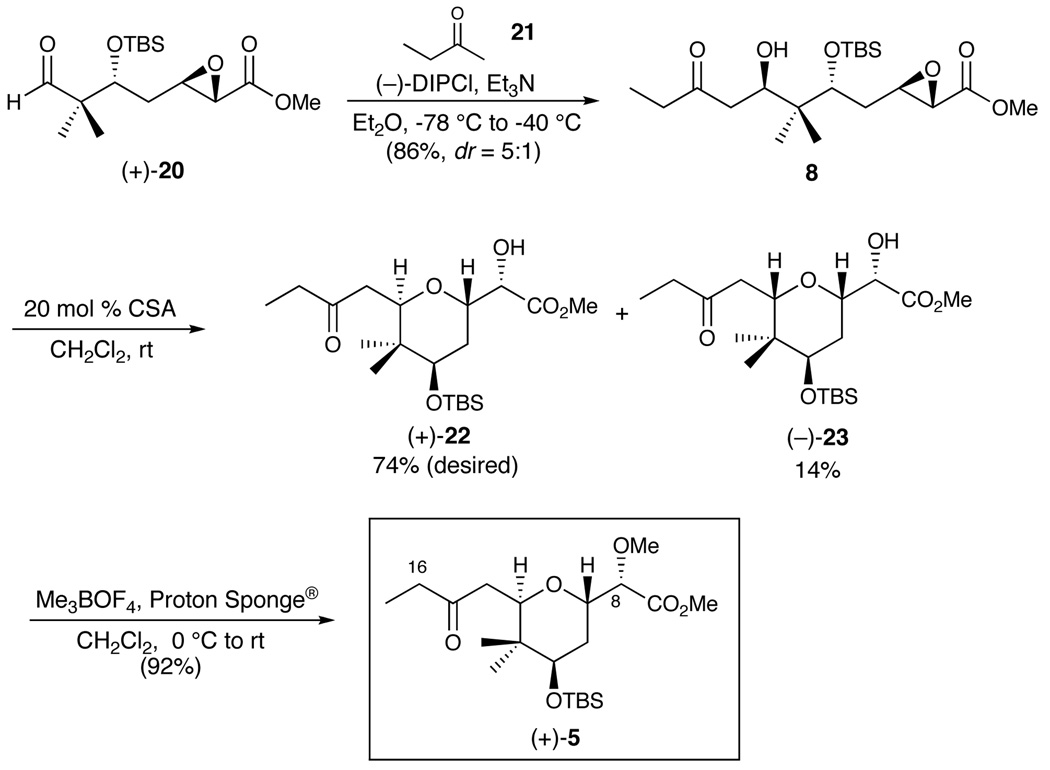
Final elaboration of 8, the requisite cyclization precursor, entailed treatment of 2-butanone (21) with (−)-DIPCl and Et3N according to conditions developed by Paterson,21,22 followed by addition of aldehyde (+)-20; cyclization precursor (8) was obtained as an inseparable mixture of diastereomers (5:1) in 86% yield (Scheme 5). Treatment with camphorsulfonic acid then led to the desired 2,6-trans-tetrahydropyran (+)-22 in 74%, in conjunction with 14% of the cis-congener (−)-23, the latter resulting from cyclization of the undesired C(13) α-hydroxyl epimer formed during the Paterson aldol reaction. Separation of (+)-22 and (−)-23 was now possible by flash chromatography on silica gel. Of special note, the cyclization proceeded exclusively via the 6-exotet pathway, without intervention of 7-endotet manifold, as determined by 1H NMR analysis. Since both processes are viewed as “allowed” vis-à-vis Baldwin rules,23 the six-membered ring transition state, in conjunction with the electron-withdrawing nature of the methyl ester that destabilizes cationic character at the α-position, conspire to favor reaction at the β-position to generate the required tetrahydropyran ring system. Methylation of the resultant secondary hydroxyl utilizing trimethyloxonium tetrafluoroborate with Proton Sponge® as base then furnished (+)-5, the 2,6-trans-tetrahydropyran fragment, in 92% yield.
Scheme 5.
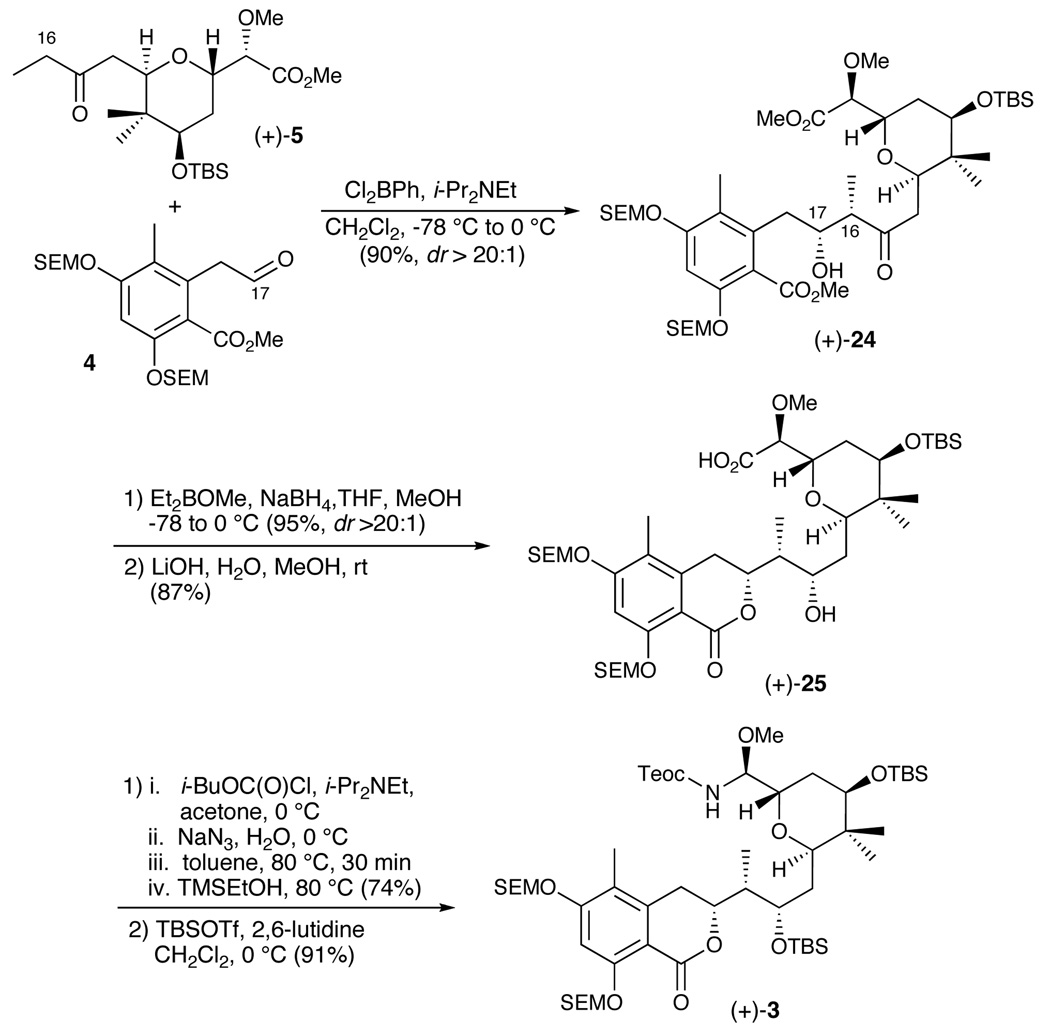
Turning to the union of ketone (+)-5 with aldehyde 4, the Z-boron enolate of (+)-5 was generated exploiting the rarely used dichlorophenylborane.24 Addition of aldehyde 4 at −78 °C provided the desired syn-aldol product (+)-24 in 90% yield (dr > 20:1), taking advantage of 1,4 substrate stereoinduction25 (Scheme 6). Treatment of the latter with diethylmethoxyborane and sodium borohydride resulted in chelation-controlled reduction26 of the ketone to provide the 1,3-syn diol in 95% yield (dr > 20:1), which upon exposure to lithium hydroxide in wet methanol effected hydrolysis of the methyl ester, with concomitant lactonization to provide dihydroisocoumarin (+)-25; the yield was 87%.
Scheme 6.
We next called upon the Curtius rearrangement tactic developed during the total syntheses of zampanolide and dactylolide to set the configuration of the C(8) stereogenic center.10 Pleasingly, this protocol, employing 2-trimethylsilylethanol to capture the intermediate isocyanate, provided the Teoc-protected N,O-aminal in 74% yield. Final protection of the free C(15) secondary alcohol as the TBS ether completed construction of the amide coupling precursor (+)-3.
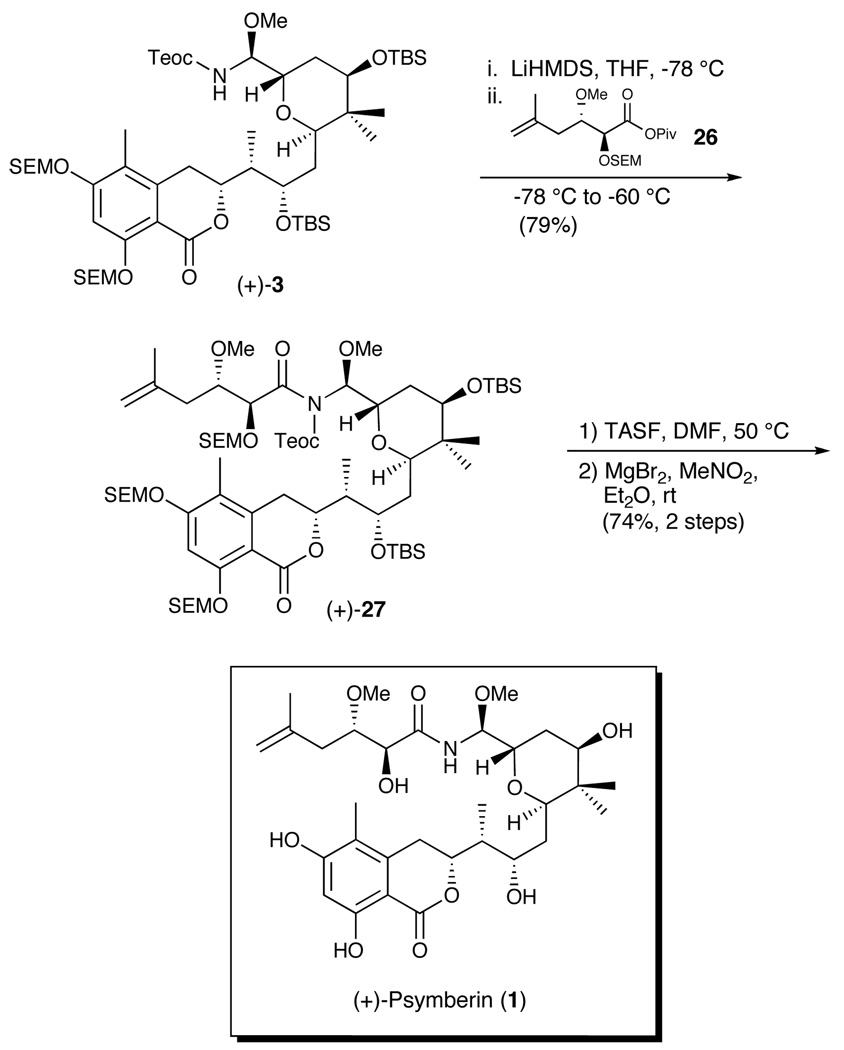
Union of (+)-3 with the side chain acid (−)-2 was not without considerable difficulty (Scheme 7). However, after extensive experimentation, we discovered that deprotonation of the Teoc-protected amine (+)-3, employing LiHMDS, followed by addition of the side chain acid activated as the pivalate mixed anhydride (26), provided amide (+)-27 comprising the full carbon skeleton of (+)-psymberin; the yield was 79%. Next, treatment with TASF in DMF at 50 °C, employing conditions developed during an advanced model study used to devise the optimized conditions for the amide coupling, as well as to address the stability of the N,O-aminal moiety, resulted in two major products. The first proved to be (+)-psymberin (1), with the second still bearing a SEM group on one of the phenolic oxygens. Treatment of the latter with magnesium bromide cleanly provided (+)-psymberin (1) in a combined 74% yield for the two steps.
Scheme 7.
The spectral data (1H and 13C NMR) for synthetic (+)-psymberin were in complete agreement with the corresponding data for natural (+)-psymberin (1) reported by Pettit, as well as the spectral data from the DeBrabander laboratory.
In summary, an enantioselective total synthesis of (+)-psymberin has been achieved in 21 steps (longest linear sequence). The central goal of this synthetic venture was to construct the 2,6-trans-tetrahydropyran utilizing catalytic reagent control to install the requisite stereogenicity within the tetrahydropyran core, thereby permitting potential future access to a series of stereochemically defined congeners without major changes to the synthetic sequence. Work focusing on the synthesis of analogues using this catalytic reagent control approach is currently ongoing in our laboratory. Finally, application of the Curtius rearrangement protocol developed in our laboratory for the synthesis of α–methoxy acids permitted late-stage introduction of the labile N, O -aminal in a highly diastereoselective fashion.
Supplementary Material
Spectroscopic and analytical data for all new compounds, as well as experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
Financial support was provided by the National Institute of Health (National Cancer Institute) through Grant No. CA-19033, and to J.A.J. by Merck & Co., Inc. through the MMD-Ph.D. program. In addition, we thank Professor Cheon-Gyu Cho (Hanyang University, Seoul, Korea) and Won-Suk Kim (University of Pennsylvania) for their exploratory studies on (+)-psymberin.
References
- 1.Pettit GR, Xu JP, Chapuis JC, Pettit RK, Tackett LP, Doubek DL, Hooper JNA, Schmidt JM. J. Med. Chem. 2004;47:1149. doi: 10.1021/jm030207d. [DOI] [PubMed] [Google Scholar]
- 2.Cichewicz RH, Valeriote FA, Crews P. Org. Lett. 2004;6:1951. doi: 10.1021/ol049503q. [DOI] [PubMed] [Google Scholar]
- 3.Furusaki A, Watanabe T, Matsumoto T, Yanagiya M. Tetrahedron Lett. 1968;9:6301. [Google Scholar]
- 4.Williams LJ, Kiren S. Org. Lett. 2005;7:2905. doi: 10.1021/ol0508375. [DOI] [PubMed] [Google Scholar]
- 5.Jiang X, Garcia-Fortanet J, DeBrabander JK. J. Am. Chem. Soc. 2005;127:11254. doi: 10.1021/ja0537068. [DOI] [PubMed] [Google Scholar]
- 6.Rech JC, Floreancig PE. Org. Lett. 2005;7:5175. doi: 10.1021/ol0520267. [DOI] [PubMed] [Google Scholar]
- 7.Shangguan N, Kiren S, Williams LJ. Org. Lett. 2007;9:1093. doi: 10.1021/ol063143k. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Shao N, Palani A, Aslanian R, Buevich A. Org. Lett. 2007;9:2597. doi: 10.1021/ol071068n. [DOI] [PubMed] [Google Scholar]
- 9.Smith AB, III, Walsh SP, Frohn M, Duffey MO. Org. Lett. 2005;7:139. doi: 10.1021/ol047792c. [DOI] [PubMed] [Google Scholar]
- 10.Smith AB, III, Safonov IG, Corbett RM. J. Am. Chem. Soc. 2002;124:11102. doi: 10.1021/ja020635t. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Suzuki S, Tsuji J. Chem. Lett. 1978:649. [Google Scholar]
- 12.Bryson TA, Dolak TM. Org. Synth. 1977;57:62. [Google Scholar]
- 13.Simsek S, Horzella M, Kalesse M. Org. Lett. 2007;9:5637. doi: 10.1021/ol702640w. [DOI] [PubMed] [Google Scholar]
- 14.Parikh JR, Doering WE. J. Am. Chem. Soc. 1967;89:5505. [Google Scholar]
- 15.Bal BS, Childers WE, Pinnick HW. Tetrahedron. 1981;37:2091. [Google Scholar]
- 16.Langer P, Kracke B. Tetrahedron Lett. 2000;41:4545. [Google Scholar]
- 17.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092. [Google Scholar]
- 18.Hoye TR, Jeffrey CS, Shao F. Nat. Protoc. 2007;2:2451. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- 19.Katsuki T, Sharpless KB. J. Am. Chem. Soc. 1980;102:5974. [Google Scholar]
- 20.Zhao MM, Li J, Mano E, Song ZJ, Tschaen DM. Org. Synth. 2005:81. [Google Scholar]
- 21.Paterson I, Goodman JM. Tetrahedron Lett. 1989;30:997. [Google Scholar]
- 22.Paterson I, Goodman JM, Anne Lister M, Schumann RC, McClure CK, Norcross RD. Tetrahedron. 1990;46:4663. [Google Scholar]
- 23.Baldwin JE. J. Chem. Soc., Chem. Commun. 1976:734. [Google Scholar]
- 24.Hamana H, Sasakura K, Sugasawa T. Chem. Lett. 1984;10:1729. [Google Scholar]
- 25.Evans DA, Calter MA. Tetrahedron Lett. 1993;34:6871. [Google Scholar]
- 26.Chen K-M, Hardtmann GE, Prasad K, Repic O, Shapiro MJ. Tetrahedron Lett. 1987;28:155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectroscopic and analytical data for all new compounds, as well as experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.