Abstract
Concurrent with increasing prostate cancer incidence and declining prostate cancer mortality in the United States, the prevalence of obesity has been increasing steadily. Several studies have reported that obesity is associated with increased risk of high-grade prostate cancer and prostate cancer mortality, and it is thus likely that the increase in obesity has increased the burden of prostate cancer. In this study, we assess the potential effect of increasing obesity on prostate cancer incidence and mortality. We first estimate obesity-associated relative risks of low- and high-grade prostate cancer using data from the Prostate Cancer Prevention Trial. Then, using obesity prevalence data from the National Health and Nutrition Examination Survey and prostate cancer incidence data from the Surveillance, Epidemiology, and End Results program, we convert annual grade-specific prostate cancer incidence rates into incidence rates conditional on weight category. Next, we combine the conditional incidence rates with the 1980 prevalence rates for each weight category to project annual grade-specific incidence under 1980 obesity levels. We use a simulation model based on observed survival and mortality data to translate the effects of obesity trends on prostate cancer incidence into effects on disease-specific mortality. The predicted increase in obesity prevalence since 1980 increased high-grade prostate cancer incidence by 15.5% and prostate cancer mortality by between 7.0% (under identical survival for obese and nonobese cases) and 23.0% (under different survival for obese and nonobese cases) in 2002. We conclude that increasing obesity prevalence since 1980 has partially obscured declines in prostate cancer mortality.
Introduction
Prostate cancer is the most common non-skin cancer in American men, with ~219,000 new cases diagnosed in 2007 (1). From 1992 to 2004, prostate cancer death rates in the United States dropped by a staggering 35% (2), most likely due to a combination of increased prostate-specific antigen (PSA) screening and advances in prostate cancer treatment practices (3, 4). For example, data from Austria, the United States, and the United Kingdom show that populations with high PSA screening rates have lower prostate cancer mortality rates than populations with low uptake of screening (5, 6). In addition to trends in treatment and screening practices, it is important to consider trends in population-level risk exposures such as obesity.
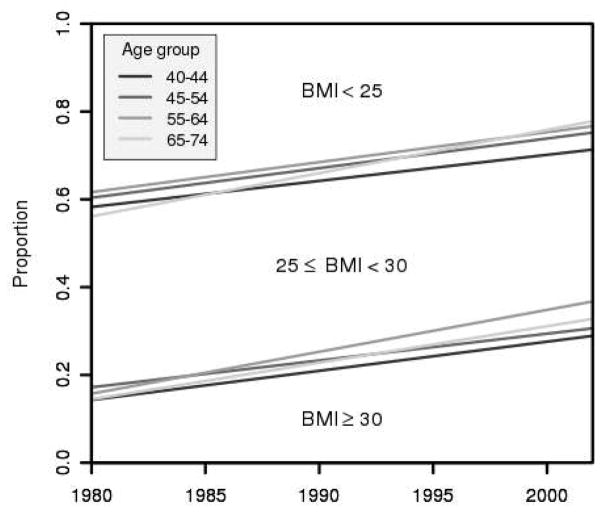
Between 1980 and 2002, obesity [defined as body mass index (BMI) ≥ 30 kg/m2] prevalence rates in men ages 40 to 74 years more than doubled in the United States, from 15% to 32% (7). In contrast, overweight (25 ≤ BMI < 30) prevalence rates remained relatively constant at around 44% over this time period (see Fig. 1; ref. 7). Obesity has been associated with a greater incidence of high-grade prostate cancer (8–11), with poorer disease-specific survival (12) and clinical outcomes after cancer treatment (13), and with worse other-cause survival (14).
Figure 1.
NHANES weight trends by age group. Prevalence proportions partition the population in each year into weight categories BMI < 25, 25 ≤ BMI < 30, and BMI ≥ 30.
The association between obesity and high-grade prostate cancer is biologically plausible because obesity is associated with marked alterations in the serum concentrations of numerous hormones such as estrogen, testosterone, insulin, insulin-like growth factor 1, all of which have been linked to prostate cancer, and leptin, which has been associated with high-grade prostate cancer (15–18). Obesity is also associated with increased levels of several biomarkers related to inflammation, including interleukin 6 and tumor necrosis factor-α (19). A number of publications have shown that chronic inflammation is associated with proliferative inflammatory lesions that may be precursors of prostate tumors (20–22).
The association between obesity and low-grade prostate cancer is less clear. Although some studies have also linked obesity with a modestly reduced incidence of low-grade disease (11, 23), others have found no association (8, 24, 25). Several studies have found that obese men have very slightly decreased PSA levels (26–28) and enlarged prostates (29, 30). Thus, obese men with prostate cancer may be less likely to be referred to biopsy, and obese men receiving biopsies may be more likely to receive false-negative results (31). However, there is much uncertainty about whether these potential diagnostic biases could substantially affect rates of low-grade disease.
Given that obesity is associated with worse high-grade incidence and survival, and given that its protective effects for low-grade cancer are likely modest, the fact that mortality has declined despite increasing obesity suggests that even greater gains could have been seen had obesity rates remained constant over time. In this study, we investigate the extent to which the increasing prevalence of obesity has increased prostate cancer incidence and mortality in the United States. Our investigation uses data on grade-specific disease incidence, the annual prevalence of obesity in the United States, and information from the Prostate Cancer Prevention Trial (PCPT) on the link between obesity and grade-specific incidence. With this information, we estimate the grade-specific prostate cancer incidence that would have been observed had the prevalence of obesity remained constant between 1980 and 2002 and compare projected and observed trends. We then use survival data to translate the difference between the grade-specific incidence curves under projected and observed trends into the effect of the observed increase in obesity on age-adjusted mortality.
Materials and Methods
Our method to project grade-specific prostate cancer incidence had overweight and obese prevalence rates remained constant between 1980 and 2002 consists of two components: (a) calculating grade-specific incidence rates from 1980 to 2002 conditional on weight category (healthy, overweight, and obese) and (b) the conditional grade-specific incidence rates on weight category together with 1980 prevalence rates of each weight category to project overall, or unconditional, grade-specific incidence rates under 1980 obesity levels. Both components rely on weight trend data, patterns of prostate cancer incidence, and relative risks of low- and high-grade cancer associated with being overweight and obese.
Trends in Overweight and Obese Prevalence Rates in the United States
Overweight and obese prevalence rates among American males aged 40 to 74 y between 1980 and 2002 were obtained from National Health and Nutrition Examination Survey (NHANES) public use data files.1 NHANES has collected data on the health and nutritional status of adults and children in the United States since the 1960s. The NHANES survey questions are administered to a nationally representative sample of individuals, and the survey results are extrapolated to produce estimates for the general population. NHANES did not collect data on men age 75 y or older until 1988; therefore our analysis is limited to men ages 40 to 74 y. NHANES defines healthy weight as BMI < 25, overweight as 25 ≤ BMI < 30, and obese as BMI ≥ 30. NHANES publishes age-specific data on population overweight and obese prevalence rates for 1976 to 1980, 1988 to 1994, and 1999 to 2002. We assumed that the NHANES results pertain to the midpoints of the survey years and used linear interpolation to impute prevalence rates for interim years.
Prostate Cancer Incidence Trends
Prostate cancer incidence rates between 1980 and 2002 were obtained from the core 9 population-based cancer registries contributing data to the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute in this time period (32). We did not include available data from later years due to changes in SEER coding practices for prostate cancer grade beginning in 2003. Annual grade-specific incidence for men ages 40 to 74 y was calculated as cases per 100,000 men using SEER*Stat software.2 Incidence rates were age-adjusted to the 2000 US standard population using seven 5-y age groups (ages 40–44 y, …, 70–74 y). SEER grade categories well-differentiated and moderately differentiated were grouped into a single category for low-grade cancer and poorly differentiated and undifferentiated were grouped into a single category for high-grade cancer. Cases with unknown grade were distributed into low- and high-grade categories in proportion to the relative number of known low- and high-grade cases in each year and age group.
Relative Risks of Low- and High-Grade Prostate Cancer for Overweight and Obese Men
Table 1 summarizes studies of the risk of high-grade prostate cancer associated with overweight and obese weight categories: there are five cohort studies (10, 11, 24, 25, 33), one case-control study (8), and one cohort study nested within a randomized controlled trial (23). These studies used a variety of definitions of high-grade prostate cancer and obesity, yet most reported at least some increased risk for high-grade prostate cancer associated with the highest category of BMI. To obtain risk estimates appropriate for weight categories consistent with NHANES definitions and grade categories consistent with SEER, we reanalyzed data from the PCPT.
Table 1.
Previous studies of BMI and risk of high-grade prostate cancer
| Authors | Population | Study design | BMI measurement | Definition of high grade | Risk measure (95% CI) |
|---|---|---|---|---|---|
| MacInnis et al. 2003 | 16,336 men aged 27–75 participating in MCCS | Prospective cohort | BMI < 25, 25 ≤ BMI < 30, and BMI ≥ 30; also used quartiles of fat mass | Gleason score 8–10 or metastatic | RR = 2.2 (1.2–4.1) for BMI ≥ 30 vs BMI < 25 |
| RR = 1.1 (0.6–1.9) for 25 ≤ BMI < 30 vs BMI < 25 adjusted for age, birthplace, education | |||||
| Dal Maso et al. 2004 | 1294 cases and 1451 controls aged 46–74 in Italy | Hospital-based case-control | Quartiles of BMI taken near diagnosis, recollection at age 30, lifetime lowest. | Gleason score 7–10 | OR = 1.61 (1.13–2.28) for BMI ≥ 28.41 vs BMI < 24.22 |
| OR = 1.57 (1.11–2.22) for 26.18 ≤ BMI < 28.41 vs BMI < 24.22 adjusted for age, location, education, family history, physical activity | |||||
| Gong et al. 2006 | 10,258 men in PCPT | Randomized controlled trial | BMI < 25, 25 ≤ BMI < 27, 27 ≤ BMI < 30, BMI ≥ 30 measured 1 year post randomization | Gleason score 7–10, Gleason score 8–10 | OR = 1.29 (1.01–1.67) for BMI ≥ 30 vs BMI < 25, Gleason 7–10 |
| OR = 1.78 (1.10–2.87) for BMI ≥ 30 vs BMI < 25, Gleason 8–10 adjusted for age, race, treatment, diabetes, family history | |||||
| Rodriguez et al. 2007 | 69,991 men in CPS II | Prospective Cohort | BMI < 25, 25 ≤ BMI < 27.5, 27.5 ≤ BMI < 30, 30 ≤ BMI < 35, BMI ≥ 35 measured at enrollment | Gleason score 8–10 and local-regional stage (“High grade”); distant stage or unknown stage but prostate cancer listed as primary cause on death certificate (“Metastatic”) | High grade |
| RR = 1.22 (0.96–1.55) for BMI ≥ 30 vs BMI < 25 | |||||
| RR = 1.23 (1.00–1.53) for 27.5 ≤ BMI < 30 vs BMI < 25 | |||||
| Metastatic | |||||
| RR = 1.54 (1.06–2.23) for BMI ≥ 30 vs BMI < 25 | |||||
| RR = 1.14 (0.79–1.63) for 27.5 ≤ BMI < 30 vs BMI < 25 adjusted for age, race, education, family history, total calorie intake, smoking, PSA history, diabetes, physical activity | |||||
| Giovannucci et al. 2007 | 51,529 men in HPFS | Prospective cohort | BMI 21–22.9, 23–24.9, 25–27.4, 27.5–29.9, > 30 measured at baseline | Gleason score 7–10 | RR = 1.07 (0.73–1.55) for BMI ≥ 30 vs BMI < 21 |
| RR = 1.02 for BMI 27.5–29.9 vs BMI < 21 | |||||
| RR = 1.03 for BMI 25–27.4 vs BMI < 21 adjusted for age, time period, BMI at age 21, height, smoking, activity level, family history, diabetes, race, and dietary measures | |||||
| Littman et al. 2007 | 34,754 men in VITAL | Prospective cohort | BMI < 25, 25 ≤ BMI < 30, BMI ≥ 30 measured at baseline | Gleason score 8–10 or regional/distant stage | HR = 1.3 (0.89–1.9) for 25 ≤ BMI < 30 vs BMI < 25 |
| HR = 1.1 (0.71–1.8) for BMI ≥ 30 vs BMI < 25 adjusted for age, family history, race | |||||
| Pischon et al. 2008 | 148,372 men in EPIC | Prospective cohort | BMI < 25, 25 ≤ BMI < 30, BMI ≥ 30 measured at baseline | Gleason score 7–10, Gleason score 8–10 | HR = 1.09 (0.90–1.31) for 25 ≤ BMI < 30 vs BMI < 25 |
| HR = 1.08 (0.83–1.41) for BMI ≥ 30 vs BMI < 25 adjusted for smoking, education, alcohol consumption, height, and physical activity. |
Abbreviations: MCCS, Melbourne Collaborative Cohort Study; PCPT, Prostate Cancer Prevention Trial; CPS, Cancer Prevention Study; HPFS, Health Professionals Follow-up Study; VITAL, Vitamins and Lifestyle Study; EPIC, European Prospective Investigation into Cancer and Nutrition; RR, relative risk; OR, odds ratio.
The PCPT was a randomized controlled trial conducted to investigate the efficacy of finasteride as a chemopreventive agent for prostate cancer. The trial enrolled 18,880 healthy men to receive either placebo or finasteride and provided annual prostate cancer screening for up to 7 y of follow up. Our analysis considers the 911 participants diagnosed with prostate cancer after for-cause biopsy (i.e., biopsy triggered by suspicious PSA or digital rectal examinaton results) relative to the 9,347 participants who underwent end-of-study biopsy and therefore have known disease status. These definitions of case and control populations circumvent a potential problem in this dataset if associations of obesity with cases diagnosed without cause (i.e., detections among end-of-study biopsies) differed from those diagnosed under standard clinical practice. A prior analysis of these data (23) demonstrated that men with BMI ≥ 30 had a 78% increased risk of high-grade prostate cancer (Gleason grade 8–10) compared to men with BMI < 25. In contrast, BMI ≥ 30 was associated with an 18% decreased risk of low-grade prostate cancer (Gleason score 2–7) compared to BMI < 25.
To use risk estimates appropriate for NHANES weight categories, we reanalyzed the PCPT data considered in Gong et al. (23). First, we constructed NHANES weight categories w = 1, 2, 3 for healthy weight (BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI ≥ 30). We then estimated grade-specific relative risks for categories 2 and 3 relative to 1 via Poisson regression modeling of individual-level observations, adjusting for age, race, family history of prostate cancer, diabetes status, and finasteride arm. The Poisson regressions yielded relative risks rgw for overweight low-grade (rL2), overweight high-grade (rH2), obese low-grade (rL3), and obese high-grade (rH3) prostate cancer incidence relative to healthy weight men.
Grade-Specific Incidence Trends Conditional on Weight Categories
We used the estimated relative risks to obtain annual grade-specific prostate cancer incidence conditional on weight category. By the law of total probability, the unconditional incidence rate for grade g and year y given age group a can be written as follows:
| (A) |
where Ig(y | a, w) denotes the incidence rate for grade g, year y, age group a, and weight category w and Py(w | a) represents the prevalence of weight category w in year y given age group a (with Σw Py (w | a) = 1). Using the relative risks rgw for g = L, H and w = 2, 3 estimated from the PCPT data we have:
We can now replace the terms for overweight and obese incidence in (1) with healthy weight incidence scaled by the risk of disease for individuals in these weight categories relative to healthy weight individuals. Consequently, for each grade g, year y, and age group a we have one equation in one unknown and can solve equation (A) for healthy weight incidence. With this solution, we immediately obtain incidence for overweight and obese individuals using the estimated relative risks.
Projecting Grade-Specific Incidence Trends under 1980 Obesity Levels
To project grade-specific incidence assuming that overweight and obese prevalence rates had remained constant at 1980 levels, we use the conditional grade-specific incidence rates computed above together with prevalence rates observed in 1980 as follows to approximate the expected incidence by grade, year, and age group:
Here, the notation ~ designates unconditional grade-specific incidence under constant 1980 overweight and obese prevalence rates. Comparing Ig(y | a) with Ĩg(y | a) allows us to estimate the effect of the observed rise in obesity in the United States on prostate cancer incidence. To quantify the uncertainty in our estimates due to uncertainty in the estimated relative risks, we also estimate the effect of increasing obesity on high-grade prostate cancer incidence using end points of the 95% confidence intervals (CIs) for the relative risks.
As a by-product of this calculation, we can estimate obesity distributions among prostate cancer cases by grade, year, and age group using Bayes theorem:
where Py(w | a, g) is the proportion of cases diagnosed in year y, age group a, and grade g who fall into weight category w. This ability to classify the low- and high-grade cases by obesity status at diagnosis is useful when we translate the effect of obesity on incidence into its effect on mortality.
Effect on Mortality
To translate the effect on incidence into the effect on mortality, we use a microsimulation model of prostate cancer and other-cause death given grade-specific incidence patterns. The model first generates populations to match case counts by age group, year, and grade corresponding to observed and projected incidence rates Ig (y | a) and Ĩg(y | a). Each case is assigned a disease-specific and other-cause survival time from the date of diagnosis. Both survival times are allowed to depend on obesity status at diagnosis, which is assigned based on obesity distributions obtained as described above.
We consider two prostate cancer survival hazard ratios for obese men: hpc = 1 (no effect of obesity on disease-specific survival) and hpc = 2.64 (obesity adversely affects disease-specific survival). These hazard ratios represent the instantaneous risk of death in obese versus nonobese prostate cancer cases. The latter hazard ratio was estimated by Gong et al. (34) for men aged 40 to 64 y, but we assume this for ages 65 to 74 y as well. This estimate is remarkably similar to that reported by Ma et al. (35), who found a hazard ratio of 2.66 for obese versus nonobese prostate cancer cases in the Physicians Health Study. Analogous to our method for obtaining incidence rates by weight category using relative risks, we partition SEER cause-specific survival curves by age and year of diagnosis into weight category-specific survival curves by noting that each corresponding hazard function is a weighted combination of the hazard functions for obese and nonobese with the weights given by the obesity distributions. For any hazard ratio of prostate cancer death, we can therefore solve for the survival among nonobese cases and use the hazard ratio to obtain survival among obese cases. The obese and nonobese cause-specific survival curves are derived under observed obesity trends in the population. The simulation model uses these curves to produce mortality projections corresponding to grade-specific incidence under both observed weight trends and under constant 1980 prevalence rates.
Similarly, we consider two other-cause survival hazard ratios for obese men: hoc = 1 (no effect of obesity on other-cause survival) and ( ) = (1.4, 1.2, 1.1) for age groups 40 to 54 y, 55 to 64 y, and 65 to 74 y (obesity adversely affects other-cause survival). The latter set was estimated by Davis et al. (36). We assume that all-cause mortality hazard ratios are adequate approximations of other-cause mortality hazard ratios.
Each case in the model is assigned a date of death given by the minimum of the dates of cause-specific and other-cause death; cause of death is assigned accordingly. The model tabulates prostate cancer deaths by grade at diagnosis and age and year at death. The difference between the prostate cancer deaths under observed and constant 1980 weight trends each year is age-adjusted and subtracted from observed mortality to project mortality trends had BMI prevalence rates remained at 1980 levels. Differences between the observed and projected mortality counts are inflated to the US population to estimate the number of excess deaths nationally due to observed increases in BMI in the population. To limit random variation due to the simulation model, results of 50 independent runs are averaged to produce the final results.
To validate our model projections, we compare incidence-based mortality since 1980 (i.e., prostate cancer deaths among cases diagnosed after 1980 as a percentage of the population) observed in SEER and corresponding incidence-based mortality projected under observed obesity trends. This provides an opportunity to check that the overall number of deaths produced by the model reasonably approximates that observed in practice.
Results
Table 2 reports cross-tabulation of Gleason grades and BMI categories for PCPT cases. The BMI distributions did not differ significantly between cases and controls. Table 2 also reports the estimated relative risks for overweight and obese men relative to healthy weight men by grade for all participants and for the placebo arm only. Considering data from all participants, we found that obesity (BMI ≥ 30) was associated with a non-significant decreased risk of low-grade prostate cancer. In contrast, obesity was associated with a significant 79% increased risk of high-grade prostate cancer. Consistent with Gong et al. (23), we found that the higher risk of low- and high-grade prostate cancer for obese men was similar across study arms. They defined low-grade prostate cancer to consist of Gleason scores 6 and below and found a significant reduction in the risk of these tumors among obese men. We combined Gleason 7 with lower grade tumors for consistency with SEER data and to sidestep changes in grading practices over time that have resulted in a considerable shift from lower to higher grades within this group (37).
Table 2.
Cross-tabulation of grade and BMI categories in PCPT data and estimated relative risks
| Cross-tabulation of grade and BMI categories in PCPT data | |||||||
|---|---|---|---|---|---|---|---|
| BMI < 25 | 25 ≤ BMI < 30 | BMI ≥ 30 | Total | ||||
| N | (%) | N | (%) | N | (%) | ||
| Cases | |||||||
| Low grade | 213 | (26.6) | 420 | (52.3) | 169 | (21.1) | 802 |
| High grade | 23 | (21.1) | 49 | (45.0) | 37 | (33.9) | 109 |
| Controls | 2,376 | (25.4) | 4,789 | (51.2) | 2,182 | (23.3) | 9,347 |
| Relative risks for overweight and obese men by arm and grade | |||||||
| BMI < 25 | 25 ≤ BMI < 30 | BMI ≥ 30 | |||||
| Reference | RR (95% CI) | RR (95% CI) | Ptrend | ||||
| P | P | ||||||
| All participants | |||||||
| Low grade | 1.00 | 1.01 (0.86–1.18) | 0.93 (0.76–1.13) | ||||
| 0.92 | 0.45 | 0.47 | |||||
| High grade | 1.00 | 1.13 (0.69–1.85) | 2.00 (1.19–3.38) | ||||
| 0.62 | 0.01 | 0.01 | |||||
| Placebo arm only | |||||||
| Low grade | 1.00 | 0.96 (0.79–1.18) | 0.89 (0.70–1.14) | ||||
| 0.70 | 0.36 | 0.37 | |||||
| High grade | 1.00 | 1.29 (0.59–2.80) | 1.75 (0.74–4.15) | ||||
| 0.53 | 0.20 | 0.20 | |||||
NOTE: Low grade is defined as Gleason score 2–7 and high grade is defined as Gleason score 8–10. Relative risks are adjusted for age, race, family history of prostate cancer, diabetes status, and PCPT study arm.
Figure 2 plots the observed incidence of low- and high-grade disease together with the projected incidence given 1980 overweight and obese prevalence rates using relative risks from our reanalysis of PCPT data with 95% confidence limits. Results indicate that age-adjusted low-grade incidence would have been 280.8 (95% CI, 271.1–291.5) instead of the observed 277.1 cases per 100,000 men, high-grade incidence would have been 50.1 (95% CI, 45.9–55.7) instead of the observed 57.8 cases per 100,000 men, and all-grade incidence would have been 330.8 (95% CI, 317.1–347.2) instead of the observed 334.9 cases per 100,000 men in 2002. In other words, the increase in obesity is estimated to have produced a 1.3% decrease in age-adjusted low-grade incidence (95% CI, 4.9% decrease to 2.2% increase), a 15.5% increase in age-adjusted high-grade incidence (95% CI, from 3.9% increase to 25.9% increase), and a 0.7% increase in age-adjusted all-grade incidence (95% CI, 3.2% decrease to 4.4% increase) in 2002. See Table 3.
Figure 2.
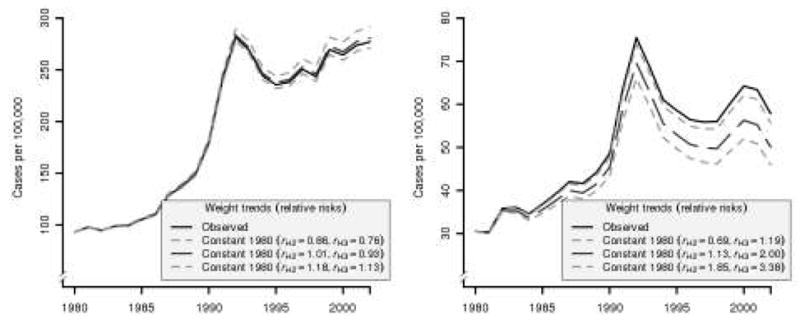
Projected impact of increasing obesity on age-adjusted low-grade (left) and high-grade (right) prostate cancer incidence for men aged 40–75. Projections assume constant 1980 obesity prevalence rates and are based on relative risks re-estimated using PCPT data. 95% confidence limits are based on 95% confidence limits for estimated relative risks.
Table 3.
Projected effect of increasing obesity on grade-specific prostate cancer incidence and overall mortality among men ages 40–75 y in 2002
| Effect on incidence per 100,000 men | ||||||
|---|---|---|---|---|---|---|
| Ages (y) | Low grade |
High grade |
||||
| Observed | Projected | %Δ | Observed | Projected | %Δ | |
| 40–44 | 7.7 | 7.8 | −1.0 | 0.9 | 0.8 | 12.0 |
| 45–49 | 37.1 | 37.4 | −0.9 | 5.5 | 5.0 | 11.1 |
| 50–54 | 131.5 | 132.8 | −0.9 | 23.7 | 21.3 | 11.1 |
| 55–59 | 324.3 | 329.4 | −1.5 | 58.3 | 50.0 | 16.6 |
| 60–64 | 522.8 | 531.0 | −1.5 | 101.6 | 87.1 | 16.6 |
| 65–69 | 783.7 | 793.6 | −1.3 | 173.2 | 149.8 | 15.6 |
| 70–74 | 884.5 | 895.7 | −1.3 | 212.2 | 183.5 | 15.6 |
| Adjusted | 277.1 | 280.8 | −1.3 | 57.8 | 50.1 | 15.5 |
| Effect on mortality per 100,000 men | ||||||
| Ages (y) | Observed | Projected |
||||
| General | %Δ | BMI-specific | %Δ | |||
| 40–44 | 0.2 | 0.2 | 11.1 | 0.1 | 58.1 | |
| 45–49 | 0.8 | 0.7 | 7.5 | 0.6 | 28.2 | |
| 50–54 | 2.4 | 2.2 | 8.2 | 1.9 | 25.6 | |
| 55–59 | 7.3 | 6.7 | 9.1 | 5.3 | 37.1 | |
| 60–64 | 21.2 | 19.5 | 8.5 | 17.1 | 23.7 | |
| 65–69 | 47.0 | 43.4 | 8.4 | 38.5 | 22.2 | |
| 70–74 | 102.1 | 96.7 | 5.6 | 84.1 | 21.5 | |
| Adjusted | 16.9 | 15.8 | 7.0 | 13.7 | 23.0 | |
NOTE: Projected incidence rates assume constant 1980 obesity prevalence rates and are based on relative risks re-estimated using PCPT data. Projected mortality rates use projected incidence and selected cause-specific and other-cause survival hazard ratios for obese men. General survival uses hazard ratios hpc = hoc = 1 for obese men, whereas BMI-specific survival uses hpc = 2.64 and ( ) = (1.4, 1.2, 1.1) for obese men in age groups 40–54, 55–64, and 65–74 y.
Under equal risks of prostate cancer and other-cause death for obese men, model projections under observed obesity trends validate well, with small (< 5%) mean relative errors across years for all age groups. The model projects increasing additional deaths attributable to increasing obesity in all age groups, with 70% of these deaths among men ages 65 to 74 y. Totaling across years from 1980 to 2002, we estimate that increasing obesity could account for 5,687 of the observed 245,158 prostate cancer deaths in the United States during this interval. After age adjusting and converting to rates, we estimate that in 2002 the observed prostate cancer death rate was 7.0% higher than would have been expected had obesity prevalence remained constant at 1980 levels (95% CI, 0.4% lower to 11.5% higher).
Under higher risks of prostate cancer and other-cause death for obese men, model projections under observed obesity trends again validate well, with small (< 8%) mean relative errors across years for all age groups. The model projects increasing additional deaths attributable to increasing obesity in all age groups, again with 70% of these deaths among men ages 65 to 74 y. Summing over 1980 to 2002, we estimate that increasing obesity may be responsible for 19,370 of the observed 245,158 prostate cancer deaths in the United States in this interval. Age adjusting and converting to rates, we estimate that in 2002 the observed prostate cancer death rate was 23.0% higher than would have been expected had obesity prevalence remained constant at 1980 levels (95% CI, 15.8% higher to 29.3% higher). Figure 3 illustrates the net effect on age-adjusted mortality rates under the two assumptions of risks of prostate cancer and other-cause death for obese men.
Figure 3.
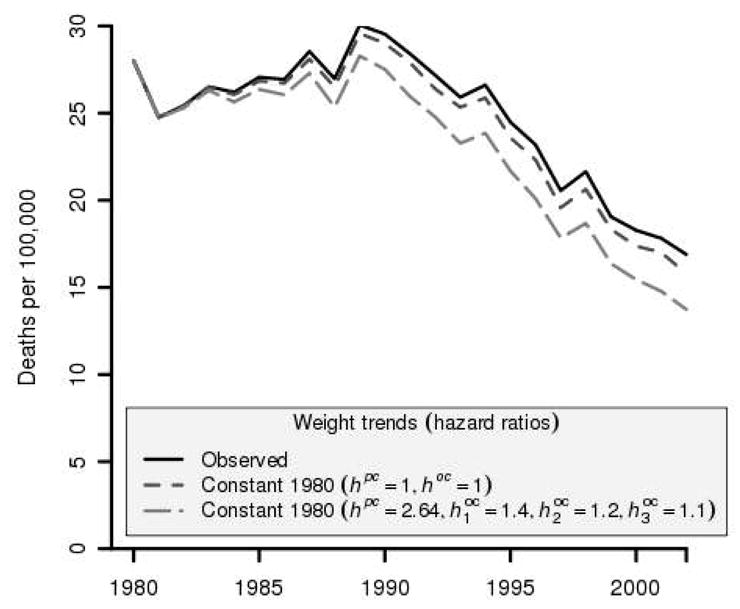
Projected impact of increasing obesity on prostate cancer mortality among men aged 40–75. Projections are based on relative risks re-estimated using PCPT data and selected cause-specific and other-cause survival hazard ratios. General survival uses hazard ratios hpc = hoc = 1 for obese men while BMI-specific survival uses hpc = 2.64 and ( ) = (1.4, 1.2, 1.1) for obese men in age groups 40–54, 55–64, and 65–74.
Discussion
The consequences of the obesity epidemic in the United States are far-reaching and, in the case of diseases like cardiac disease and diabetes, well studied. In recent years, evidence linking obesity with adverse outcomes in prostate cancer has accumulated, but the likely population effects have not been quantified. We used NHANES data on overweight and obesity prevalence rates in conjunction with disease incidence, survival, and mortality data from SEER to quantify how prostate cancer trends have been affected by the rise in obesity in this country. We estimated that rising BMI levels since 1980 may have decreased low-grade incidence by 1.3% and increased high-grade incidence by 15.5%. In addition, we estimated that these trends may have increased prostate cancer deaths by between 7.0% (under obesity-independent disease-specific and other-cause survival rates) and 23.0% (under different survival for obese and nonobese cases) in 2002. Our findings suggest that, despite the dramatic declines in prostate cancer mortality observed since 1992, deaths from the disease might have declined even further had obesity prevalence rates not simultaneously increased.
This study has several limitations. Although NHANES is an excellent source of population-based obesity data, the NHANES survey was conducted intermittently between 1980 and 2002, and data were pooled over several years. We interpolated overweight and obesity levels for years with no survey data, assuming that overweight and obesity levels followed linear trends in the interim. In addition, our computations of grade-specific incidence given weight category in a given year and our estimates of the obesity-associated relative risks of low- and high-grade disease are based on current obesity status and do not take into account obesity history or duration. Although it is likely that the risk of prostate cancer at any given age depends on risk factors accumulated over several years, neither the NHANES data on obesity prevalence nor the PCPT data on risk of disease associated with obesity provide information on individual obesity histories. Our mortality simulation model allows obese and nonobese men to have different risks of prostate cancer death, but the magnitude of the increase in risk due to obesity is still uncertain. Some studies do not find a significant increase in risk, and, although a number of studies have found a positive association, only two (34, 35) provide estimates of the relative risk. Because the effect on mortality is highly dependent on how obesity affects the risk of prostate cancer death over and above its effect on high-grade incidence, it will be important to refine the estimate of the obesity-associated risk of prostate cancer death provided as input to the model as more information becomes available. Thus, the uncertainty inherent in our mortality estimates is greater than what is reflected in CIs and may be reduced as more specific model inputs become available.
In conclusion, current evidence indicates that trends in obesity have likely increased the incidence of high-grade prostate cancer over time, with a nontrivial effect on prostate cancer mortality through 2002. We conclude that in the absence of increasing prevalence of obesity, the decline in prostate cancer mortality in the United States would have been noticeably more pronounced than was observed. This analysis underscores the complexity of the determinants of prostate cancer incidence and mortality trends and shows that it is likely that these trends depend on factors beyond screening and treatment.
Acknowledgments
Grant support: Research supported by National Cancer Institute/NIH U01-CA88160 and DOD/DAMD W81XWH-06-1-0296.
Footnotes
References
- 1.Ries L, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda: National Cancer Institute; 2007. [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Mortality - All COD, Public-Use With State, Total U.S. (1969–2005), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released. 2008 April; ( www.seer.cancer.gov) Underlying mortality data provided by NCHS ( www.cdc.gov/nchs)
- 3.Albertsen PC. The prostate cancer conundrum. J Natl Cancer Inst. 2003;95:930–1. doi: 10.1093/jnci/95.13.930. [DOI] [PubMed] [Google Scholar]
- 4.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175–81. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartsch G, Horninger W, Klocker H, et al. Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int. 2008;101:809–16. doi: 10.1111/j.1464-410X.2008.07502.x. [DOI] [PubMed] [Google Scholar]
- 6.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol. 2008;9:445–52. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. National Health and Nutrition Examination Survey. Home Page: http://www.cdc.gov/nchs/nhanes.htm.
- 8.Dal Maso L, Zucchetto A, La Vecchia C, et al. Prostate cancer and body size at different ages: an Italian multicentre case-control study. Br J Cancer. 2004;90:2176–80. doi: 10.1038/sj.bjc.6601859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165:1271–9. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:63–9. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 12.Kristal AR, Gong Z. Obesity and prostate cancer mortality. Future Oncol. 2007;3:557–67. doi: 10.2217/14796694.3.5.557. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuyama H, Wallner KE, Merrick GS. Treatment of prostate cancer in obese patients. Oncology (Williston Park) 2006;20:1191–7. discussion 1198:1206–1208. [PubMed] [Google Scholar]
- 14.Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. Am J Public Health. 2004;94:1486–9. doi: 10.2105/ajph.94.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sher D, Mantzoros C, Jacobus S, Regan M, Lee G, Oh W. Absence of relationship between steroid hormone levels and prostate cancer tumor grade. Urology. 2008 doi: 10.1016/j.urology.2008.07.068. in press. [DOI] [PubMed] [Google Scholar]
- 16.Roddam AW, Allen NE, Appleby P, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149:461–71. W83–8. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saglam K, Aydur E, Yilmaz M, Goktas S. Leptin influences cellular differentiation and progression in prostate cancer. J Urol. 2003;169:1308–11. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 18.Amling CL. Relationship between obesity and prostate cancer. Curr Opin Urol. 2005;15:167–71. doi: 10.1097/01.mou.0000165550.94663.fb. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 20.Nelson WB, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172(5 Pt 2):S6–11. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- 21.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebbeck TR, Rennert H, Walker AH, et al. Joint effects of inflammation and androgen metabolism on prostate cancer severity. Int J Cancer. 2008;123:1385–9. doi: 10.1002/ijc.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 24.MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:1417–21. [PubMed] [Google Scholar]
- 25.Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. 2000;10:361–9. doi: 10.1016/s1047-2797(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 26.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001–2004 Cancer. Epidemiol Biomarkers Prev. 2007;16:70–6. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 27.Barqawi AB, Golden BK, O’Donnell C, Brawer MK, Crawford ED. Observed effect of age and body mass index on total and complexed PSA: analysis from a national screening program. Urology. 2005;65:708–12. doi: 10.1016/j.urology.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 28.Rundle A, Neugut AI. Obesity and screening PSA levels among men undergoing an annual physical exam. Prostate. 2008;68:373–80. doi: 10.1002/pros.20704. [DOI] [PubMed] [Google Scholar]
- 29.Fowke JH, Motley SS, Cookson MS, et al. The association between body size, prostate volume and prostate-specific antigen. Prostate Cancer Prostatic Dis. 2007;10:137–42. doi: 10.1038/sj.pcan.4500924. [DOI] [PubMed] [Google Scholar]
- 30.Freedland SJ, Terris MK, Platz EA, Presti JC., Jr Body mass index as a predictor of prostate cancer: development versus detection on biopsy. Urology. 2005;66:108–13. doi: 10.1016/j.urology.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Freedland SJ, Platz EA, Presti JC, Jr, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500–4. doi: 10.1016/S0022-5347(05)00162-X. discussion 504. [DOI] [PubMed] [Google Scholar]
- 32.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2006 Sub (1973–2005), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission ( www.seer.cancer.gov)
- 33.Pischon T, Boeing H, Weikert S, et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3252–61. doi: 10.1158/1055-9965.EPI-08-0609. [DOI] [PubMed] [Google Scholar]
- 34.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis MA, Neuhaus JM, Moritz DJ, Lein D, Barclay JD, Murphy SP. Health behaviors and survival among middle-aged and older men and women in the NHANES I Epidemiologic Follow-up Study. Prev Med. 1994;23:369–76. doi: 10.1006/pmed.1994.1051. [DOI] [PubMed] [Google Scholar]
- 37.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–53. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]