Abstract
In an effort to probe whether the metal content of metallo-β-lactamase L1 is affected by metal ion bioavailability, L1 was over-expressed as mature protein (M-L1) and full-length (FL-L1) analogs, and the analogs were characterized with metal analyses, kinetics, and EPR spectroscopy. FL-L1, containing the putative leader sequence, was localized in the periplasm of E. coli and shown to bind Zn(II) preferentially. The metal content of FL-L1 could be altered if the enzyme was over-expressed in minimal medium containing Fe and Mn, and surprisingly, an Fe-binding analog was obtained. On the other hand, M-L1, lacking the putative leader sequence, was localized in the cytoplasm of E. coli and shown to bind various amounts of Fe and Zn(II), and like FL-L1, the metal content of the resulting enzyme could be affected by the amount of metal ions in the growth medium. L1 was refolded in the presence of Fe, and a dinuclear Fe-containing analog of L1 was obtained, although this analog is catalytically-inactive. EPR spectra demonstrate the presence of an antiferromagnetically-coupled Fe(III)Fe(II) center in Fe-containing L1 and suggests the presence of a Fe(III)Zn(II) center in M-L1. Metal analyses on the cytoplasmic and periplasmic fractions of E. coli showed that the concentration of metal ions in the periplasm is not tightly controlled and increases as the concentration of metal ions in the growth medium increases. In contrast, the concentration of Zn(II) in the cytoplasm is tightly-controlled while that of Fe is less so.
Bacterial resistance to β-lactam containing antibiotics such as penicillins, cephalosporins, and carbapenems is most often accomplished by expression of β-lactamases, which hydrolyze the C-N bond of these antibiotics (1-4). A majority of these β-lactamases utilize an active site serine group for the nucleophilic attack on the β-lactam carbonyl, and the serine β-lactamases have been studied extensively for many years (4). On the other hand, one class (Class B) of β-lactamases utilizes a metal-assisted hydrolysis pathway to inactivate β-lactam containing antibiotics, and these enzymes are called metallo-β-lactamases (mβl’s) (1, 2, 5-7). The mβl’s have been further divided into subgroups based on sequence identity, Zn(II) content, substrate preference, and biochemical properties. Subgroup B1 enzymes require 2 Zn(II) ions for full catalytic activity, exhibit kinetic preference for penicillins as substrates, exhibit >23% sequence identity toward other subgroup B1 members, and are represented by mβl’s CcrA from Bacteroides fragilis, BcII from Bacillus cereus, and IMP-1 from various sources (1, 5). Subgroup B2 enzymes require only 1 Zn(II) ion for full catalytic activity, preferentially hydrolyze carbapenems, exhibit 11% sequence identity with the subgroup B1 enzymes, and are represented by mβl’s ImiS from Aeromonas sobria and CphA from Aeronomas hydrophila (1, 5). Subgroup B3 enzymes require 2 Zn(II) ions for full activity, exhibit a kinetic preference for penicillins, contains only 9 conserved residues with the subgroup B1 enzymes, and are represented by mβl’s L1 from Stenotrophomonas maltophilia and FEZ-1 from Legionella gormanii (1, 5). Extensive structural and mechanistic studies have been reported on certain mβl’s, and several studies reporting non-clinical inhibitors have been reported.
β-Lactam containing antibiotics are antibacterial because these drugs inactivate transpeptidase, which is an enzyme that catalyzes the crosslinking of peptidoglycan building blocks to form part of the bacterial cell wall (4). In order to interact with transpeptidase in Gram negative bacteria, β-lactam containing antibiotics must be able to cross the outer membrane and be present in the periplasm (8). Likewise, β-lactamases must be exported into the extracellular space in Gram positive bacteria or into the periplasm in Gram negative bacteria in order to interact and inactivate the β-lactam containing antibiotics. To mimic the physiological situation, most recombinant mβl’s are expressed into the periplasm of E. coli by the addition of fusion tags or leader sequences, and most of the resulting recombinant enzymes have been shown to bind Zn(II) after isolation (1, 5, 7).
Despite significant amino acid sequence divergence, mβl’s contain an αββα motif, and the Zn(II) ions bind in a pocket contained in the ββ interface (1, 5, 7). For the B1 and B3 mβl’s, one of the Zn(II) ions binds to a site (called Zn1 site), in which three histidine residues and the bridging hydroxide serve as metal binding ligands. In all three classes of mβl’s, Zn(II) binds to a site (called Zn2 site) made up of one aspartic acid, one histidine, one histidine/cysteine, the bridging hydroxide, and a terminally-bound water molecule. Previous modeling, mechanistic, and structural studies have suggested that the β-lactam carbonyl interacts with the Zn(II) in the Zn1 site (or residues in the Zn1 site for the B2 mβl’s), while the lone pair electrons on the β-lactam nitrogen coordinate metal ion in the Zn2 site (9-13).
The αββα tertiary fold motif is called the metallo-β-lactamase fold, and there is an increasing number of proteins that contain this motif, including rubredoxin oxidoreductase (ROO), glyoxalase II, arylsulfatase, cAMP phosphodiesterase, and tRNA maturase (5). The most common metal ion found in these proteins is Zn(II); however, glyoxalase II has been reported to bind Fe, Zn, and Mn (14-16), and ROO has been reported to be a dinuclear Fe protein (17). In addition, Vila and coworkers recently reported that metallo-β-lactamase GOB from E. meningoseptica when over-expressed as a GST-fusion construct and folded in the cytoplasm of E. coli is isolated containing various amounts of Zn(II) and iron (18). These results suggest that the localization where a protein is folded (cytoplasm or periplasm) and the bioavailability of the metal ions in that location play a role in which metal ions bind to recombinant proteins. To test this suggestion, we constructed an over-expression plasmid for full-length L1 that contains the S. maltophilia leader sequence (FL-L1) and a plasmid containing mature L1 that lacked the S. maltophilia leader sequence (M-L1). The resulting over-expression plasmids were used to produce L1, and the FL-L1 and M-L1 were characterized using metal analyses, steady-state kinetics, and spectroscopic studies.
Material and Methods
Cloning
Forward primer, 5′-AAAAA CAT ATG GCC GAA GTA CCA CTG CCG C, and reverse primer, 5′-AAAAA AAG CTT AGC GGG CCC CG, were purchased from Integrated DNA Technologies. The PCR reactions were carried out with the following conditions: 95 °C, 90 sec; 95 °C, 30 sec, 55.5 °C, 30 sec, 72 °C, 60 sec; 25 cycles. The gel-purified PCR product and pET26b (Novagen) plasmid were digested with NdeI and HindIII according to manufacturer’s instructions. The gene that encodes for L1 was ligated into pET26b, and the resulting plasmid (pM-L1) was transformed into E. coli DH5α and confirmed with DNA sequencing. The pM-L1 plasmid was transformed into E. coli BL21(DE3)pLysS cells, and the resulting cells were used for over-expression studies.
Antimicrobial susceptibility assay
The in vitro activity of ampicillin against L1 (FL-L1, full-length L1) and truncated-L1 (M-L1, mature L1) that was over-expressed in E. coli BL21(DE3) cells was determined by a disk diffusion susceptibility test. Bacterial cultures were grown to mid-log phase, and protein production was induced by making the cultures 1 mM in IPTG. Cultures were then grown approximately 3 hrs, and disk diffusion testing was performed by the NCCLS methodology on Mueller-Hinton agar (Becton-Dickinson) plates (National Committee for Clinical Laboratory, 1997, Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A6. National Committee for Clinical Laboratory Standards, Wayne, PA, USA). Culture plates were incubated for 16 hrs, and the zone diameter sizes were measured. Periplasmic and cytoplasmic accumulation of L1 in both cultures was confirmed by fractionating cytoplamsic and periplasmic components using a PeriPreps™ periplasting kit (Epicentre, Madison, WI).
Over-expression and purification of L1
A 50 mL overnight preculture of BL21(DE3) E. coli cells containing the pET26b-based plasmid that encodes for M-L1 or FL-L1 was used to innoculate 4 X 1L flasks of LB or minimal medium, and the resulting culture was grown at 37 °C with shaking until the culture reached an optical density at 600 nm of 0.6-0.8. The cultures were then cooled to 15 °C for 30 minutes, made 0.5 mM in IPTG, and shaken overnight at 15 °C (roughly 16 hours). For cultures to which metal ions were added, the metal ions were added at the same time as IPTG was added. The culture was centrifuged for 15 minutes (8200 × g), and the resulting cell pellet was resuspended in 50 mM Hepes, pH 6.0 (buffer A). The cells were lysed by passing the resuspended cells through a French Press three times at a pressure of 1000-1500 psi. After removal of insoluble components by centrifugation (25 minutes at 23,400 × g), the supernatant was dialyzed versus buffer A overnight. The dialyzed protein solution was centrifuged (25 min at 23,400 × g) and subjected to FPLC as previously reported (19). The FPLC fractions were analyzed using SDS PAGE gels, and protein bands thought to contain L1 were subjected to in-gel trypsin digestions and peptide identifications using MALDI-TOF mass spectrometry, as previously reported (20).
Preparation of metal-free (apo) L1
A concentrated solution of L1 (∼ 0.3 mM) was dialyzed against 4 × 1 L of 50 mM Hepes, pH 7.0, containing 10 mM 1,10-phenanthroline and then dialysed against 6 × 1 L of 50 mM Hepes, pH 7.0. The metal content of the resulting sample was ascertained by ICP-AES, as previously reported (21). The sample was stored in a -80 °C freezer.
In vitro unfolding and refolding of L1
Apo-L1 (2 ml, 100 M) was unfolded in 18 ml of 6 M guanidinium chloride (Gdn-HCl). The sample was incubated on ice for 1 hour and then dialyzed versus 1 L of 50 mM Hepes, pH 7.0, containing no added metal, 50 μM Fe(II), Mn(II), or Zn (II) or Fe(II) and Zn(II). Refolded L1 was further dialyzed versus 5 × 1L of Chelex-treated, 50 mM Hepes, pH 7.0, to remove any unbound metals and remaining Gdn-HCl. The resulting solution was centrifuged (25 min at 23,400 × g) to remove any precipitates.
Steady-state kinetics
Steady-state kinetic studies were performed on an Agilent 8453A UV-Vis diode array spectrophotometer at 25 °C using nitrocefin as the substrate and 50 mM cacodylate, pH 7.0, as the buffer.
EPR spectroscopy
EPR spectra were recorded using a Bruker E600 EleXsys spectrometer equipped with an Oxford Instruments ESR900 helium flow cryostat and ITC503 temperature controller, and an ER4116DM cavity operating at 9.63 GHz in perpendicular mode. Other recording parameters are given in the figure legends. Quantitation of signals was carried out by double integration of spectra recorded under non-saturating conditions at 10-12 K. A 2 mM Cu(II)-EDTA standard in Hepes, pH 7.5 recorded at 60 K, 50μW was used. Integration limits and correction factors for S = 1/2, and S = 5/2 signals where D is assumed to be small compared to temperature, as employed elsewhere 22) and recently described explicitly by Bou-Abdallah and Chasteen and references therein (23, 24).
Metal content in cytoplasm and periplasm of E. coli cells
E. coli BL21 cells were cultured in M9 minimal medium containing no added metal ions or 100 μM Zn(II), Fe(II), or Mn(II) until reaching an optical density at 600 nm of 1.0. The cells were collected by centrifugation (8 min at 6000 × g). The resulting cell pellets were washed twice with 5 mL of PBS (137 mM NaCl; 2.7 mM KCl; 1.5 mM KH2PO4; 7.7 mM Na2HPO4; pH 7.4), and cytoplasmic fractions were separated from periplasmic components of the cell using a PeriPrepsTM periplasting kit (Epicentre, Madison, WI) according to manufacturer’s instructions. The metal content of the cytoplamsic/periplasmic fractions of the cells was measured using ICP-AES, as previously reported (21).
Results
S. maltophilia leader sequence leads to L1 being exported to and folded in the periplasm of E. coli
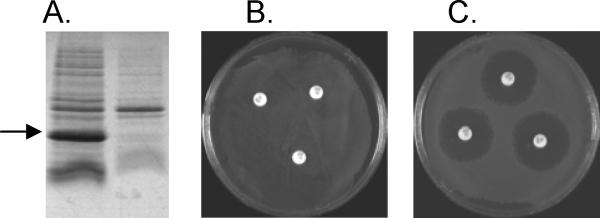
The gene for metallo-β-lactamase L1 from pathogen Stenotrophomonas maltophilia encodes for a 290 amino acid protein that contains a 21 amino acid (MRSTLLAFALAVALPAAHTSA) leader sequence (25), which presumably targets the protein for export into and folding in the periplasm, and a 269 amino acid mature peptide containing the N-terminus of AEVPLPQ (19). To determine if the leader sequence from S. maltophilia is recognized by E. coli and used to target L1 for export into the periplasm, we generated a pET26b-based over-expression plasmid containing the L1 gene lacking the leader sequence (M-L1) and used this plasmid to over-express L1. The over-expression plasmid containing the gene for L1 and the leader sequence (FL-L1) has previously been reported (19) and was used as a control in these studies. Both over-expression plasmids were transformed into E. coli BL21(DE3)pLysS cells, and M-L1 and FL-L1 were over-expressed. The periplasmic fraction of each cell culture was obtained using the PeriPreps™ periplasting kit according to manufacturer’s instructions. An SDS-PAGE gel (Figure 1A), followed by in-gel trypsin digestions/peptide identifications with MALDI-TOF MS, demonstrated that L1 was only found in the sample that was produced from the over-expression plasmid containing the L1 gene and the leader sequence. The over-expression plasmid containing the gene for L1 without the leader sequence (M-L1) produced no L1 in the periplasm of E. coli. SDS-PAGE gels of the boiled cell fractions from the cultures demonstrated that L1 was over-expressed at similar levels in both cultures (data not shown). This result demonstrates that the S. maltophilia leader sequence can be recognized by E. coli and directs L1 for export in the periplasm.
Figure 1.
Localization of L1 produced in cells that contain the L1 gene and the leader sequence (FL-L1) and the L1 gene without the leader sequence (M-L1). (A) SDS-PAGE gel of periplasmic fractions of (left) E. coli cells containing gene for FL-L1 and (right) E. coli cells containing gene for M-L1. Arrow marks the band for L1. (B and C) Antibiotic selection assay. (B) E. coli cells on LB-kanamycin plate containing gene for L1 and leader sequence. (C) E. coli cells on LB-kanamycin plate containing gene for L1 without leader sequence. The white dots are the disks containing 10 μg ampicillin.
To confirm this result, an antibiotic sensitivity assay was conducted. E. coli cells containing the over-expression plasmids for M-L1 (Figure 1B) and FL-L1 (Figure 1C) were plated on LB plates containing kanamycin. Disks containing 10 μg ampicillin were placed on the plates, and the petri dishes were incubated at 37 °C for 16 hours. The E. coli cells containing the gene for FL-L1 in pET26b grew well in the presence of ampicillin; however, those cells containing the gene for M-L1 in pET26b did not grow in the area near the disk containing ampicillin. Since ampicillin imparts its antibacterial activity in the periplasm, this result demonstrates that E. coli containing the gene for FL-L1 exports L1 into the periplasm of the cell, while E. coli containing the gene for M-L1 does not.
Characterization of M-L1 and FL-L1
M-L1 and FL-L1 were over-expressed and purified as described by Crowder et al. (19). As previously reported (19), purified FL-L1, which was over-expressed in LB medium, contained 1.9 equivalents of Zn(II) and exhibited steady-state kinetic constants of Km = 4 μM and kCat = 26 s-1 when using nitrocefin as the substrate (Table 1). In contrast, M-L1, which was over-expressed in LB medium, bound 0.7 eq. of Fe and 0.6 eq. of Zn(II) (Table 2). This enzyme exhibited a kcat of 10 s-1 and a Km of 1.0 μM when using nitrocefin as substrate. Clearly, the metal content of L1 is greatly affected by where the protein is localized.
Table 1.
Steady-state kinetic and metal content data for FL-L1. Substrate used in the kinetic studies was nitrocefin, and kinetic studies were conducted as described in Materials and Methods
| Enzyme | kcat (s-1) | Km (μM) | Metal content (eq) |
|---|---|---|---|
| FL-L1 w/ Mna | 13 ± 1 | 5 ± 1 | 0.3Mn/0.4Fe/0.6Zn(II) |
| FL-L1 w/ Zn(II)a | 28 ± 2 | 6 ± 1 | 0.1Fe/1.9Zn(II) |
| FL-L1 w/ Fea | 3.6 ± 0.1 | 6 ± 1 | 0.9Fe/0.3Zn(II) |
| FL-L1 in LB mediumb | 26 ± 1 | 4 ± 1 | 1.9 ± 0.1 Zn(II) |
| FL-L1 in minimal mediumb | 10 ±1 | 4 ± 1 | 0.4Fe/0.3Zn(II) |
L1 was over-expressed in minimal medium containing 50 μM of the indicated metal ion as described in Materials and Methods.
L1 was over-expressed in minimal or LB medium without adding any additional metal ions.
Table 2.
Steady-state kinetic and metal content data for L1 folded in the cytoplasm (M-L1). Substrate used in the kinetic studies was nitrocefin, and kinetic studies were conducted as described in Materials and Methods
| Enzyme | kcat (s-1) | Km (μM) | Metal content (eq) |
|---|---|---|---|
| M-L1 w/ Mn(II)a | 4.2 ± 0.4 | 2.1 ± 0.9 | 0.4 Mn; 0.4Zn; 0.4 Fe |
| M-L1 w/ Zn(II)a | 21 ± 1 | 7.0 ± 1.1 | 0.3 Fe ; 1.2 Zn(II) |
| M-L1 w/ Fe(II)a | <0.1 | N/A | 1.5 Fe; 0.1 Zn(II) |
| M-L1 in minimal mediumb | <0.1 | N/A | 0.2 Mn;0.7 Fe;0.1 Zn(II) |
| M-L1 in LB mediumb | 10 ± 1 | 1.0 ± 0.2 | 0.7 Fe; 0.6 Zn(II) |
L1 was over-expressed in minimal medium containing 50 μM of the indicated metal ion as described in Materials and Methods.
L1 was over-expressed in minimal or LB medium without adding any additional metal ions.
To probe further the relationship where a protein is localized and the resulting metal content of the purified protein, FL-L1 and M-L1 were over-expressed in minimal medium in the presence of iron, zinc, or manganese. As a control, the proteins were also over-expressed in minimal medium that had no added metal ions. When the two proteins were over-expressed in minimal medium in the absence of added metal ions, FL-L1 contained 0.4 eq. Fe and 0.3 eq. Zn(II) and exhibited a kcat of 10 s-1 and a Km of 4 μM (Table 1); while M-L1 contained 0.2 eq. Mn, 0.7 eq. of Fe, and 0.1 eq. Zn(II) and exhibited no measurable catalytic activity (Table 2). From minimal medium containing 50 μM Zn(II), FL-L1 contained 1.9 eq. Zn(II) and 0.1 eq. Fe and exhibited a kcat of 28 s-1 and a Km of 6 μM(Table 1), while M-L1 contained 0.3 eq. Fe and 1.2 eq. Zn(II) and exhibited a kcat of 21 s-1 and a Km of 7 μM (Table 2). From minimal medium that was made 50 μM in Fe(II), FL-L1 contained 0.9 eq. Fe and 0.3 eq. Zn(II) and exhibited a kcat of 3.6 s-1 and a Km of 6 μM (Table 1), while M-L1 contained 1.5 eq. Fe and 0.1 eq. Zn(II) and exhibited no measureable catalytic activity (Table 2). Lastly from minimal medium containing 50 μM Mn, FL-L1 contained 0.3 eq. Mn, 0.4 eq. Fe, and 0.6 eq. Zn(II) and exhibited a kcat of 13 s-1 and a Km of 5 μM (Table 1), while M-L1 contained 0.4 eq. Mn, 0.4 eq. Zn(II), and 0.4 eq. Fe and exhibited a kcat of 4.2 s-1 and a Km of 2.1 μM (Table 2). mβl L1 can bind a number of different metal ions, and the final metal content depends greatly on whether the protein is exported and localized in the periplasm or whether it is localized in the cytoplasm.
Refolding L1 in the presence of different divalent metal ions
The biological incorporation of metal ions experiments described above suggest that the bioavailability of metal ions has a large effect on the metal content of L1 after purification. Even though the steady-state kinetic studies demonstrated that L1 localized in the cytoplasm does have catalytic activity when bound to Zn(II), it is possible that the different metal content of the M-L1 and FL-L1 may be due to different folding mechanisms in the periplasm and cytoplasm. In an effort to probe whether bioavailability of metal ions does in fact affect metal content, in vitro unfolding/refolding experiments were conducted.
Purified, apo-L1 was unfolded in the presence of 6M Gdn-HCl and refolded in the presence of 50 μM Fe(II), Mn(II), Zn(II), and Fe(II)/Zn(II) (Table 3). L1 refolded in the presence of Zn(II) was shown to bind 2 equivalents of Zn(II) and exhibited steady-state kinetic constants of kcat = 37 ± 1 s-1 and Km = 3.5 ± 0.2 μM when using nitrocefin as substrate (Table 3). These values are very similar to those of FL-L1 (Table 1). L1 refolded in the presence of Fe(II) resulted in an protein that binds 2 equivalents of iron; however, the protein exhibited no catalytic activity. L1 refolded in the presence of Mn(II) did not bind its full complement of metal (only 0.2 equivalents), and this protein also did not exhibit any catalytic activity. Since both Zn(II) and Fe appear to bind well to L1, we conducted a competition study in which L1 (20 μM) was refolded in the presence of equimolar amounts (50 μM) of Fe(II) and Zn(II). The resulting enzyme was shown to bind 1.5 equivalents of Zn(II) and 0.4 equivalents of Fe and exhibited steady-state kinetic constants of kcat = 28 ± 1 s-1 and Km of 3.0 ± 0.2 μM. This result demonstrates that L1 “prefers” Zn(II) binding but can also bind Fe.
Table 3.
L1 refolded in the presence of Fe(II), Zn(II), and Mn(II). Substrate used in the kinetic studies was nitrocefin, and kinetic studies were conducted as described in Materials and Methods
| Enzyme refolded | kcat (s-1) | Km> (μM) | Metal content (eq) |
|---|---|---|---|
| w/ Zn(II) | 37 ± 1 | 3.5 ± 0.2 | 2.0 ± 0.1 Zn |
| w/ Fe(II) | < 0.1 | N/A | 2.0 ± 0.1 Fe |
| w/ Mn(II) | < 0.1 | N/A | 0.20 ± 0.05 Mn |
| w/ Zn(II) + Fe(II) |
28 ± 1 | 3.0 ± 0.2 | 1.5 ± 0.1 Zn 0.4 ± 0.1 Fe |
EPR studies on Fe-containing forms of L1
EPR spectra of as-isolated M-L1, containing 0.7 eq. Fe and 0.6 eq. Zn(II), exhibited typical temperature-dependent features at geff values of 9.3 (745 G; 74.5 mT) and 4.3 (1616 G; 161.6 mT) due to transitions in the ground state and middle Kramers’ doublets, respectively, of S = 5/2 Fe(III) (Figure 2A). An additional signal was observed with geff < 2.0 and was assigned to an Fe(II)Fe(III) species on the basis of the similarity of the signal to one from glyoxalase 2-5 (16). Similar signals have been reported for uteroferrin and mammalian purple acid phosphatases (26, 27). This signal in M-L1 exhibited a complex temperature and power dependence and was optimally developed at 10 - 12 K. Difference analysis indicated that the g < 2 signal was due to at least two discrete but extremely similar species with slightly different spin Hamiltonian and relaxation parameters, due either to discrete [Fea(II)Feb(III)] and [Fea(III)Feb(II)] species or to structural microheterogeneity. EPR of L1 that was refolded in the presence of Fe(II) showed the same sets of S = ½ and S = 5/2 signals but in very different proportions (Figure 2B). Because of the complex temperature dependence of the S = 1/2 signal and because of the inherent complexity of the S = 5/2 “geff = 4.3” system (28), results of attempts at quantitation should be treated with some caution; for signals of the quality obtained, an error of ± 5 % in spin concentration is expected and an additional error due to uncertainty in the respective zero-field splittings in the species is estimated be ± 5 %, leading to an overall error of ± 10 %. Nevertheless, the results of quantitation are enlightening as a comparison tool. The EPR signal from as-isolated M-L1, containing both Fe and Zn(II), was estimated to be due to a 45 ± 5 % contribution of the Fe(III) signal and a 55 ± 5 % contribution of the Fe(II)Fe(III) signal. In contrast, L1 refolded in the presence of iron exhibited a spectrum with > 90 % spin density due to the Fe(II)Fe(III) signal and only 6-8 % due to isolated S = 5/2 Fe(III). The total spin density of the refolded L1 corresponded to 0.8 eq. spins and, therefore, suggests that 0.8 × 90% ∼ 70 % of the molecules contained an Fe(II)Fe(III) center. This, in turn, suggests an overall Fe content of ∼ 1.55 ± 0.15 Fe/mol, which is in very good agreement with the analytical value of 1.5 eq. Fe/mol. Efforts to collect 1H NMR spectra of the iron-containing L1 samples have not been successful as the proteins are not stable and precipitate during acquisition times.
Figure 2.
(A) EPR spectra of as-isolated M-L1 recorded at 5.6 K, 63 mW (filled line), 12 K, 63 mW (dotted line) and 12 K, 10 mW (dashed line). Various regions of the spectrum are shown to highlight the different behaviors as a function of temperature and microwave power. (B) EPR spectrum of as-isolated L1, ‘FeZn-L1’ (dotted line, 12 K, 10 mW), and that of L1 refolded in the presence of iron, ‘Fe-L1’ (filled line; 10 mW, 10 K).
Zn(II) and Fe content in E. coli
The studies above strongly suggest that the metal content of L1 is determined by the bioavailability of Fe and Zn(II) where the protein is folded/localized. In an effort to evaluate the amount of Fe and Zn(II) in the periplasm and cytoplasm of E. coli cells, we conducted metal analyses on the periplasmic and cytoplasmic fractions of E. coli cells grown in minimal medium containing no additional metal ions and containing 100 μM Zn(II) or Fe. The amount of Zn(II) and Fe in the soluble portion of the cytoplasm of E. coli cells cultured in the absence of added metal ions is ca. 45 μM for both metal ions. If the cells are cultured in minimal medium containing 100 μM Zn(II) or Fe, the amount of Zn(II) in the cytoplasm remains at 45 μM, while the amount of Fe increases 3-fold to about 153 μM. In the soluble periplasmic fractions, the amount of Zn(II) and Fe from cells cultured in the absence of added metal ion is < 1 μM. In the soluble periplasmic fraction of cells cultured in the presence of Zn(II) and Fe, the amount of Zn(II) increased >10-fold to 1.23 mM, while the amount of Fe increased >20-fold to 2.83 mM. The bioavailability of Zn(II) in the periplasm and the preference for Zn(II) binding to FL-L1 leads then to the preparation of ZnZn-L1 when the protein is over-expressed in medium containing enough Zn(II). In the absence of enough Zn(II), Fe can bind to L1 (in the cytoplasm and in the samples over-expressed with added Fe).
Discussion
While the number of Zn(II) ions physiologically bound to mβl’s is under debate (29-33), there is universal agreement that Zn(II) is the metal ion bound to these enzymes in vivo. Nonetheless, several groups have reported spectroscopic studies using Co(II)-and Cd(II)-analogs (29, 34-39) (both metal ions are excellent surrogates of Zn(II)) of several mβl’s, and early papers on several mβl’s reported activation of apo-enzymes by manganese, iron, and other metal ions (40, 41). A recent paper by Vila and coworkers reports the binding of Fe to GOB-1 from E. meningoseptica, although the resulting enzyme was catalytically-inactive (33). Page and coworkers recently reported that manganese-substituted BcII exhibited catalytic activity (42). Since mβl’s are enzymes that confer resistance to antimicrobial agents, it seems reasonable that the activation of these enzymes by different metal ions, particularly in environments that lack sufficient quantities of Zn(II), would be beneficial to the organism. There certainly is precedence in the literature for Zn(II) binding sites in Zn(II)-metalloenzymes being able to bind a number of different metal ions (14, 15), and a large of number of studies on metal-substituted aminopeptidase from A. proteolytica have been reported (43-47). Nonetheless, it is not clear that many of these metal-substituted enzymes are physiologically-relevant. In the present study, we were interested in determining whether the metal content of mβl L1 is affected by where the protein is folded/metallated/localized.
We, therefore, prepared over-expression constructs that contained the full length gene for L1 (leader sequence plus gene for L1, FL-L1) or that contained only the gene for L1 (no leader sequence, M-L1) and used these plasmids to over-express L1. Our first task was to determine where L1 was localized in E. coli after protein production, and our data clearly show that L1 produced from the FL-L1 plasmid is exported into the periplasm, while L1 produced from the M-L1 plasmid is in the cytoplasm. The over-expression and localization of L1 in the cytoplasm without a fusion peptide/protein is the first example of a mature mβl being produced and localized in the cytoplasm. Biochemical analyses of the two enzymes when over-expressed in rich medium clearly show that the two enzymes are different, with FL-L1 binding only Zn(II) and M-L1 binding nearly equal amounts of Fe and Zn(II). This latter result is similar to results previously reported for recombinant glyoxalase II, which is a member of the β-lactamase superfamily of proteins (14, 15) and is over-expressed and localized in the cytoplasm of E. coli.
The export of L1 into the periplasm could be accomplished by at least two different pathways. The first pathway is the TAT system in E. coli, which transfers fully-folded proteins from the cytoplasm into the periplasm (48). This system requires the presence of an Arg rich sequence in the protein that serves as a signaling sequence. Given that L1 does not have this Arg-rich sequence (25) and that M-L1 and FL-L1 bind different metal ions, it is highly unlikely that L1 is transported as a folded, metallated protein via the TAT system. The second major transport system is the Sec system, which has been studied in detail (49). The Sec system exports unfolded proteins, and therefore once in the periplasm, the periplasmic protein must be folded by folding proteins in the periplasm. Moreover, periplasmic proteins must be metallated in the periplasm, and the metal content data presented in this work supports this pathway in use by L1.
To further probe how the metal content of L1 is affected by where the protein is folded and metallated, we over-expressed and purified M-L1 and FL-L1 from minimal medium containing Fe, Zn(II), Mn, or Fe/Zn(II). The resulting proteins contained different amounts of metal ions and exhibited different kinetic properties. The amount of metal available for metallation of L1 is determined by the amount of the metal in the growth medium, no matter if the protein is folded in the periplasm or cytoplasm. While the increase in bioavailability of a metal ion in the periplasm as the metal concentration of the medium goes up was expected, the increase in bioavailable metal in the cytoplasm was unexpected. All cells have elaborate homeostatic pathways that presumably maintain metal ion concentrations in a very narrow range of concentrations (50-52). For example, the cytoplasmic concentrations of Zn(II) are maintained by importers ZnuABC and ZupT and exporters ZitB and ZntA (53). Metal analyses of the soluble periplasmic and cytoplasmic fractions of E. coli demonstrate that the concentration of metal ions in both fractions increase with increasing levels (only slightly so for Zn(II)) of the metal ion in the growth medium. The relatively higher increase in cytoplasmic Fe levels may be due to the cell’s better ability to store Fe rather than Zn(II) in bacterioferritin (54). The preparation of L1 that was folded and metallated in the cytoplasm contains a different metal content, and this result indicates that extreme caution should be exercised when recombinant metalloproteins are over-expressed. Ideally, over-expression constructs should be made so that bacterial metalloproteins will be over-expressed and folded in the same place in E. coli as they are folded in the original organism. When over-expressing eukaryotic metalloproteins in E. coli, over-expression constructs should be designed to fold/metallate the protein in the cytoplasm, periplasm, and/or possible in the extracellular environment (using a pelB leader for example) so that the possibility of different metal content of the resulting protein can be evaluated.
The over-expression of M-L1 in the cytoplasm yielded an analog of L1 that contains nearly equimolar concentrations of Fe and Zn(II). Given the tri-histidine site in the Zn1 site of L1 (13), it was predicted that this enzyme was a FeZn-analog of L1; however, EPR studies demonstrated that there is a mixture of metal centers in this sample including a spin-coupled Fe(III)Fe(II) center, a Fe(III)Zn(II) center, and a Zn(II)Zn(II) center. Our ability to refold L1 in the presence of Zn(II) and/or Fe allowed for us to obtain metal-enriched forms of L1. These data clearly show that the FeFe-analog of L1 is inactive, possibly due to Asp120 bridging the metal centers (55, 56), which is probably required for the observed antiferromagnetic coupling between the Fe ions (57),and not being available to form an essential hydrogen bond with the bridging hydroxide (55). It is not clear from our data whether the Fe(III)Zn(II)-analog of L1 is catalytically-active, but the refolding of L1 in the presence of equimolar concentrations of Fe and Zn(II) clear shows a preference for Zn(II) binding to L1. Future studies will address whether the Fe(III)Zn(II) analog of L1 is catalytically-active.
Taken together, this work demonstrates that the metal content of L1 depends strongly on bioavailability of metal ions where the protein is folded. This result will aid in the preparation of metal-substituted metalloproteins to study the structure/function of these proteins.
Footnotes
References
- 1.Crowder MW, Spencer J, Vila AJ. Metallo-β-lactamases: Novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 2006;39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 2005;18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgopapadakou NH. β-Lactamase inhibitors: evolving compounds for evolving resistance targets. Expert Opin. Investig. Drugs. 2004;13:1307–1318. doi: 10.1517/13543784.13.10.1307. [DOI] [PubMed] [Google Scholar]
- 4.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 5.Bebrone C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 2007;74:1686–1701. doi: 10.1016/j.bcp.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Toney JH, Moloughney JG. Metallo-β-lactamase inhibitors: promise for the future? Curr. Opin. Invest. Drugs. 2004;5:823–826. [PubMed] [Google Scholar]
- 7.Heinz U, Adolph HW. Metallo-β-lactamases: two binding sites for one catalytic metal ion? CMLS, Cell. Mol. Life Sci. 2004;61:2827–2839. doi: 10.1007/s00018-004-4214-9. [DOI] [PubMed] [Google Scholar]
- 8.Knowles JR. In: Enzyme Inhibitors. Brodbeck U, editor. Verlag Chemie; Weinheim: 1980. pp. 163–167. [Google Scholar]
- 9.Park H, Brothers EN, Merz KM. Hybrid QM/MM and DFT investigations of the catalytic mechanism and inhibition of the dinuclear zinc metallo-β-lactamase CcrA from Bacteroides fragilis. J. Am. Chem. Soc. 2005;127:4232–4241. doi: 10.1021/ja042607b. [DOI] [PubMed] [Google Scholar]
- 10.Rasia RM, Vila AJ. Mechanistic study of the hydrolysis of nitrocefin mediated by B. cereus metallo-β-lactamase. ARKIVOC. 2003;3:507–516. [Google Scholar]
- 11.Oelschlaeger P, Schmid RD, Pleiss J. Insight into the mechanism of the IMP-1 metallo-β-lactamase by molecular dynamics simulations. Protein Engineering. 2003;16:341–350. doi: 10.1093/protein/gzg049. [DOI] [PubMed] [Google Scholar]
- 12.Suarez D, Brothers EN, Merz KM. Insights into the structure and dynamics of the dinuclear zinc β-lactamase site from Bacteroides fragilis. Biochemistry. 2002;41:6615–6630. doi: 10.1021/bi0121860. [DOI] [PubMed] [Google Scholar]
- 13.Ullah JH, Walsh TR, Taylor IA, Emery DC, Verma CS, Gamblin SJ, Spencer J. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 angstrom resolution. J. Mol. Biol. 1998;284:125–136. doi: 10.1006/jmbi.1998.2148. [DOI] [PubMed] [Google Scholar]
- 14.Schilling O, Wenzel N, Naylor M, Vogel A, Crowder M, Makaroff C, Meyer-Klaucke W. Flexible metal binding of the metallo-β-lactamase domain: Glyoxalase II incorporates iron, manganese, and zinc in vivo. Biochemistry. 2003;42:11777–11786. doi: 10.1021/bi034672o. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel NF, Carenbauer AL, Pfiester MP, Schilling O, Meyer-Klaucke W, Makaroff CA, Crowder MW. The binding of iron and zinc to glyoxalase II occurs exclusively as di-metal centers and is unique within the metallo-β-lactamase family. J. Biol. Inorg. Chem. 2004;9:429–438. doi: 10.1007/s00775-004-0535-2. [DOI] [PubMed] [Google Scholar]
- 16.Marasinghe GPK, Sander IM, Bennett B, Periyannan G, Yang KW, Makaroff CA, Crowder MW. Structural studies on a mitochondrial glyoxalase II. J. Biol. Chem. 2005;280:40668–40675. doi: 10.1074/jbc.M509748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes CM, Silva G, Oliveira S, LeGall J, Liu MY, Xavier AV, Rodrigues-Pousada C, Teixeira M. Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J. Biol. Chem. 1997;272:22502–22508. doi: 10.1074/jbc.272.36.22502. [DOI] [PubMed] [Google Scholar]
- 18.Moran-Barrio J, Gonzalez JM, Lisa MN, Costello AL, Peraro MD, Carloni P, Bennett B, Tierney DL, Limansky AS, Viale AM, Vila AJ. The metallo-β-lactamase GOB Is a mono-Zn(II) enzyme with a novel active site. J. Biol. Chem. 2007;282:18286–18293. doi: 10.1074/jbc.M700467200. [DOI] [PubMed] [Google Scholar]
- 19.Crowder MW, Walsh TR, Banovic L, Pettit M, Spencer J. Overexpression, purification, and characterization of the cloned Metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicro. Agents Chemo. 1998;42:921–926. doi: 10.1128/aac.42.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigdel TK, Cilliers R, Gursahaney PR, Crowder MW. Fractionation of soluble proteins in Escherichia coli using DEAE-, SP-, and phenyl sepharose chromatographies. J. Biomol. Tech. 2004;15:199–207. [PMC free article] [PubMed] [Google Scholar]
- 21.Crowder MW, Maiti MK, Banovic L, Makaroff CA. Glyoxalase II from A. thaliana requires Zn(II) for catalytic activity. FEBS Lett. 1997;418:351–354. doi: 10.1016/s0014-5793(97)01416-6. [DOI] [PubMed] [Google Scholar]
- 22.Purpero VM, Moran GR. Catalytic, noncatalytic, and inhibitory phenomena: kinetic analysis of (4-hydroxyphenyl)pyruvate dioxygenase from Arabidopsis thaliana. Biochemistry. 2006;45:6044–6055. doi: 10.1021/bi052409c. [DOI] [PubMed] [Google Scholar]
- 23.Bou-Abdallah F, Chasteen ND. Spin concentration measurements of high-spin (g ’=4.3) rhombic iron(III) ions in biological samples: theory and application. J. Biol. Inorg. Chem. 2008;13:15–24. doi: 10.1007/s00775-007-0304-0. [DOI] [PubMed] [Google Scholar]
- 24.Aasa R, Vanngard T. EPR signal intensity and powder shapes: A reexamination. J. Mag. Res. 1975;19:308–315. [Google Scholar]
- 25.Walsh TR, Hall L, Assinder SJ, Nichols WW, Cartwright SJ, MacGowan AP, Bennett PM. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Crowder MW, Vincent JB, Averill BA. Electron paramagnetic resonance studies on the high-salt form of bovine spleen purple acid phosphatase. Biochemistry. 1992;31:9603–9608. doi: 10.1021/bi00155a012. [DOI] [PubMed] [Google Scholar]
- 27.David SS, Que L. Anion binding to uteroferrin. Evidence for phosphate coordination to the iron(III) ion of the dinuclear active site and interaction with the hydroxo bridge. J. Am. Chem. Soc. 1990;112:6455–6463. [Google Scholar]
- 28.Copik AJ, Waterson S, Swierczek SI, Bennett B, Holz RC. Both nucleophile and substrate bind to the catalytic Fe(II)-center in the type II methionyl aminopeptidase from Pyrococcus furiosus. Inorg. Chem. 2005;44:1160–1162. doi: 10.1021/ic0487934. [DOI] [PubMed] [Google Scholar]
- 29.Badarau A, Damblon C, Page MI. The activity of the dinuclear cobalt-β-lactamase from Bacillus cereus in catalysing the hydrolysis of β-lactams. Biochem. J. 2007;401:197–203. doi: 10.1042/BJ20061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badarau A, Page MI. Enzyme deactivation due to metal ion dissociation during turnover of the cobalt-β-lactamase catalyzed hydrolysis of β-lactams. Biochemistry. 2006;45:11012–11020. doi: 10.1021/bi0610146. [DOI] [PubMed] [Google Scholar]
- 31.Wommer S, Rival S, Heinz U, Galleni M, Frere JM, Franceschini N, Amicosante G, Rasmussen B, Bauer R, Adolph HW. Substrate-activated zinc binding of metallo-β-lactamases-Physiological importance of the mononuclear enzymes. J. Biol. Chem. 2002;277:24142–24147. doi: 10.1074/jbc.M202467200. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez JM, Medrano Martin FJ, Costello AL, Tierney DL, Vila AJ. The Zn2 position in metallo-β-lactamases is critical for activity: a study on chimeric metal sites on a conserved protein scaffold. J. Mol. Biol. 2007;373:1141–1156. doi: 10.1016/j.jmb.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Moran-Barrio J, Gonzalez JM, Lisa MN, Costello AL, Dal Peraro M, Carloni P, Bennett B, Tierney DL, Limansky AS, Viale AM, Vila AJ. The metallo-β-lactamase GOB is a mono-Zn(II) enzyme with a novel active site. J. Biol. Chem. 2007;282:18286–18293. doi: 10.1074/jbc.M700467200. [DOI] [PubMed] [Google Scholar]
- 34.Periyannan G, Costello AL, Tierney DL, Yang KW, Bennett B, Crowder MW. Sequential binding of cobalt(II) to metallo-β-lactamase CcrA. Biochemistry. 2006;45:1313–1320. doi: 10.1021/bi051105n. [DOI] [PubMed] [Google Scholar]
- 35.Crawford PA, Yang KW, Sharma N, Bennett B, Crowder MW. Spectroscopic studies on cobalt(II)-substituted metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Biochemistry. 2005;44:5168–5176. doi: 10.1021/bi047463s. [DOI] [PubMed] [Google Scholar]
- 36.Paul-Soto R, Zeppezauer M, Adolph HW, Galleni M, Frere JM, Carfi A, Dideberg O, Wouters J, Hemmingsen L, Bauer R. Preference of Cd(II) and Zn(II) for the two metal sites in Bacillus cereus β-lactamase II: A perturbed angular correlation of β-rays spectroscopic study. Biochemistry. 1999;38:16500–16506. doi: 10.1021/bi9911381. [DOI] [PubMed] [Google Scholar]
- 37.Paul-Soto R, Bauer R, Frere JM, Galleni M, Meyer-Klaucke W, Nolting H, Rossolini GM, de Seny D, Hernandez-Villadares M, Zeppezauer M, Adolph HW. Mono-and binuclear Zn2+ β-lactamase. J. Biol. Chem. 1999;274:13242–13249. doi: 10.1074/jbc.274.19.13242. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Benkovic SJ. Purification, characterization, and kinetic studies of soluble Bacteroides fragilis metallo-β-lactamase. J. Biol. Chem. 1998;273:22402–22408. doi: 10.1074/jbc.273.35.22402. [DOI] [PubMed] [Google Scholar]
- 39.Paul-Soto R, Hernandez-Valladares M, Galleni M, Bauer R, Zeppezauer M, Frere JM, Adolph HW. Mono-and binuclear Zn-β -lactamase from Bacteroides fragilis: Catalytic and structural roles of the zinc ions. FEBS Lett. 1998;438:137–140. doi: 10.1016/s0014-5793(98)01289-7. [DOI] [PubMed] [Google Scholar]
- 40.Saino Y, Kobayashi F, Inoue M, Mitsuhashi S. Purification and properties of inducible penicillin β-lactamase isolated from Pseudomonas maltophilia. Antimicro. Agents Chemo. 1982;22:564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K, Fujii T, Okamoto R, Inoue M, Mitsuhashi S. Biochemical properties of β-lactamase produced by Flavobacterium odoratum. Antimicro. Agents Chemo. 1985;27:612–614. doi: 10.1128/aac.27.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badarau A, Page MI. The variation of catalytic efficiency of Bacillus cereus metallo-β-lactamase with different active site metal ions. biochemistry. 2006;45:10654–10666. doi: 10.1021/bi060934l. [DOI] [PubMed] [Google Scholar]
- 43.Bennett B, Antholine WE, D’Souza VM, Chen GJ, Ustinyuk L, Holz RC. Structurally distinct active sites in the copper(II)-substituted aminopeptidases from Aeromonas proteolytica and Escherichia coli. J. Am. Chem. Soc. 2002;124:13025–13034. doi: 10.1021/ja026341p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett B, Holz RC. Spectroscopically distinct cobalt(II) sites in heterodimetallic forms of the aminopeptidase from Aeromonas proteolytica: Characterization of substrate binding. Biochemistry. 1997;36:9837–9846. doi: 10.1021/bi970735p. [DOI] [PubMed] [Google Scholar]
- 45.Bienvenue DL, Mathew RS, Ringe D, Holz RC. The aminopeptidase from Aeromonas proteolytica can function as an esterase. J. Biol. Inorg. Chem. 2002;7:129–135. doi: 10.1007/s007750100280. [DOI] [PubMed] [Google Scholar]
- 46.D’Souza VM, Swierczek SI, Cosper NJ, Meng L, Ruebush S, Copik AJ, Scott RA, Holz RC. Kinetic and structural characterization of manganese(II) loaded methionyl aminopeptidase. Biochemistry. 2002;41:13096–13105. doi: 10.1021/bi020395u. [DOI] [PubMed] [Google Scholar]
- 47.D’souza VM, Bennett B, Copik AJ, Holz RC. Divalent metal binding properties of the methionyl aminopeptidase from Escherichia coli. Biochemistry. 2000;39:3817–3826. doi: 10.1021/bi9925827. [DOI] [PubMed] [Google Scholar]
- 48.Gohlke U, Pullan L, McDevitt CA, Porcelli I, de Leeuw E, Palmer T, Saibil HR, Berks BC. The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc. Nat. Acad. Sci. 2005;102:10482–10486. doi: 10.1073/pnas.0503558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Driessen AJM, Fekkes P, van der Wolk JPW. The Sec system. Curr. Opin. Microbiol. 1998;1:216–222. doi: 10.1016/s1369-5274(98)80014-3. [DOI] [PubMed] [Google Scholar]
- 50.Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 51.Moore CM, Helmann JD. Metal ion homeostasis in Bacillus subtilis. Curr. Opin. Microbiol. 2005;8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Solioz M, Stoyanov JV. Copper homeostasis in Enterococcus hirae. FEMS Microbiol. Lett. 2003;27:183–195. doi: 10.1016/S0168-6445(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 53.Chimienti F, Aouffen M, Favier A, Seve M. Zinc homeostasis-regulating proteins: New drug targets for triggering cell fate. Curr.Drug Targets. 2003;4:323–338. doi: 10.2174/1389450033491082. [DOI] [PubMed] [Google Scholar]
- 54.Andrews SC. Iron storage in bacteria. Adv. Microb. Physiol. 1998;40:281–351. doi: 10.1016/s0065-2911(08)60134-4. [DOI] [PubMed] [Google Scholar]
- 55.Garrity JD, Carenbauer AL, Herron LR, Crowder MW. Metal binding Asp-120 in Metallo-β-lactamase L1 from Stenotrophomonas maltophilia plays a crucial role in catalysis. J. Biol. Chem. 2004;279:920–927. doi: 10.1074/jbc.M309852200. [DOI] [PubMed] [Google Scholar]
- 56.Crisp J, Conners R, Garrity JD, Carenbauer AL, Crowder MW, Spencer J. Structural basis for the role of Asp-120 in metallo-β-lactamases. Biochemistry. 2007;46:10664–10674. doi: 10.1021/bi700707u. [DOI] [PubMed] [Google Scholar]
- 57.Vincent JB, Olivier-Lilley GL, Averill BA. Proteins containing oxo-bridged dinuclear iron centers: A bioinorganic perspective. Chem. Rev. 1990;90:1447–1467. [Google Scholar]