Abstract
Orally active dual μ-/δ-opioid receptor antagonist, H-Dmt-Tic-Lys-NH-CH2-Ph (MZ-2) was applied to study body weight gain, fat content, bone mineral density, serum insulin, cholesterol and glucose levels in female ob/ob (B6.V-Lep<ob>/J homozygous) and lean wild mice with or without voluntary exercise on wheels for three weeks, and during a two week post-treatment period under the same conditions. MZ-2 (10 mg/kg/day, p.o.) exhibited the following actions: (1) reduced body weight gain in sedentary obese mice that persisted beyond the treatment period without effect on lean mice; (2) stimulated voluntary running on exercise wheels of both groups of mice; (3) decreased fat content, enhanced bone mineral density (BMD), and decreased serum insulin and glucose levels in obese mice; and (4) MZ-2 (30 μM) increased BMD in human osteoblast cells (MG-63) comparable to naltrexone, while morphine inhibited mineral nodule formation. Thus, MZ-2 has potential application in the clinical management of obesity, insulin and glucose levels, and the amelioration of osteoporosis.
Keywords: obesity, ob/ob mice, bone mineral density, insulin, glucose, Dmt-Tic pharmacophore, dual μ-/δ-opioid receptor antagonist
1. Introduction
The opioid system is one of the key homeostatic mechanisms participating in the development of obesity by affecting the neural reward system through feeding behavior and body weight regulation (Atkinson, 1987; Berestov, 1983; Cota et al., 2006; Kas et al., 2004; Khawaja et al., 1989; Tabarin et al., 2005; Yeomans and Gray, 2002). While opioid agonists increase food intake and body weight gain (Fields, 2007; McCormack and Denbow, 1989), these endpoints are decreased by various opioid antagonists (Cole et al., 1997; Jarosz and Metzger, 2002; Levine et al., 1991; Recant et al., 1980; Shaw et al., 1991), indicating that opioid antagonists may have a potential role in the management of obesity.
Among the multiplicity of opioid receptors, the μ-opioid (MOP) receptor appears to be a key element in the neuronal reward pathway within the central nervous system responsible for craving and addiction to various drugs, such as morphine and its derivatives, as well as alcohol and excess food consumption (Avena et al., 2008; Wang et al., 2006). This observation was verified by MOP receptor knockout mice which had decreased food anticipation (Kas et al., 2004; Papaleo et al., 2007; Tabarin et al., 2005) and a reduced response to the anorectic effects of opioid antagonists (Zhang et al., 2006). These data suggest that MOP receptor is a responsible factor in the reward pathway for feeding behavior in mammals. Furthermore, MOP receptor knockout mice have higher body weight than control animals, which may be a result of the up-regulation of neuropeptide Y expression as a compensatory pathway (Han et al., 2006). Studies using positron emission tomography in obese individuals showed reductions in striatal dopamine D2 receptors similar to that observed in drug addicted subjects, indicating that overeating in obese individuals shares similarities with the loss of control and compulsive drug taking behavior observed in drug-addicted subjects (Wang et al., 2004).
In our efforts in developing opioid antagonists that elicit a reduction in and impinge upon addiction, withdrawal and the development of tolerance, we described an effective dual functioning μ-/δ-opioid (MOP/DOP) receptor antagonist H-Dmt-Tic-Lys-NH-CH2-Ph (MZ-2) (Balboni et al., 2006). MZ-2 inhibited analgesia and tolerance to morphine in mice after intracerebroventricular, subcutaneous and oral administration (Jinsmaa et al., 2008). In the present study we investigated whether a single oral dose of MZ-2 (10 mg/kg/day) can elicit an effect on obesity in ob/ob mice, a well established in vivo model system (Recant et al., 1980), in comparison to wild type lean mice. Female mice were used because obesity is more prevalent in female than male population (Gellner and Domschke, 2008; Ogden et al., 2006). Using an exercise paradigm, the mice were divided into two groups: one with voluntary access to exercise wheels and another without the wheels as representations of physically active and sedentary life styles, respectively. Each group was administered MZ-2 or saline orally for three weeks and body weight gain, body fat content and bone mineral density were recorded, and then housed for additional two weeks without treatment but measuring the same endpoints.
2. Materials and methods
2.1. Chemicals
Minimal Essential Medium (MEM) with Earle's salt and non-essential amino acids was from Invitrogen Corp., (Carlsbad, CA, USA), penicillin-streptomycin solution, l-glutamine, sodium pyruvate, alizarin red, morphine sulphate pentahydrate, β-glycerophosphate, dexamethasone, and 2-phospho-l-ascorbic acid from Sigma (Louis, MO, USA), AlamarBlue solution from Biosource International Inc. (Camarillo, CA, USA), Insulin (Mouse) Ultrasensitive EIA Kit from Alpco Diagnostics (Salem, NH, USA), fetal bovine serum from HyClone (Logan, UT, USA), and a protease inhibitor cocktail from Roche Diagnostics (Indianapolis, IN, USA). Naltrexone hydrochloride was purchased from Tocris Bioscience (Ellsville, MO, USA). The reagents for determination of cholesterol, glucose and triglycerides in serum were from Olympus America Inc., (Melville, NY, USA), and the reagents for determination of HDL, LDL, and free fatty acids were from The Genzyme Corporation (Cambridge, MA, USA). MZ-2 (H-Dmt-Tic-Lys-NH-CH2-Ph) was synthesized as described earlier (Balboni et al., 2006).
2.2. Animals
Five week old female ob/ob (B6.V-Lep<ob>/J homozygous) and age matched lean control mice (C57BL/6) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Upon arrival to our facility mice were housed one per cage in a 12 h light/dark cycle and temperature-controlled room with free access to food and water. Animals were acclimatized for one week before starting the experiments. All animal procedures were carried out according to protocols approved and on file by the Animal Care and Use Committee (ACUC) at the National Institute of Environmental Health Sciences.
2.3. Monitoring of body weight, food intake and physical activity of animals
Animals were divided into two groups, one with sedentary life style and the other with voluntary access to exercise wheels. Animals were treated orally either with MZ-2 (10 mg/kg) dissolved in saline or with saline alone (vehicle group) once daily for three weeks one half hour before the dark cycle (19:00 h). Each treatment group consisted of 5 animals. Body weight and food consumption were recorded daily before treatment. Voluntary exercise on wheels was recorded automatically with the use of stainless steel rodent activity wheels (34.5 cm diameter) (Mini Mitter, Bend, OR, USA). The wheel formed a portion of the lid of the cage; the other portion contained a food hopper and water bottle holder. Mice were placed on wheels for one week before the experiment began in order to acclimate them to this novelty in their housing regime. Revolutions of wheels were counted by magnetic switches connected to the Vital View 3000 data acquisition system (Mini Mitter, Bend, OR, USA). Wheel revolution counts were collected every 5 minutes 24 hours a day for the entire duration of the experiment (five weeks). Wheel activity data were exported into MS Excel spreadsheet program for data reduction and analysis.
2.4. Determination of body composition and bone mineral density
Body composition and bone mineral density were measured three times during the course of the study: before dosing, at the termination of drug administration (three weeks), and at the end of study (five weeks). Measurements were based on dual-energy X-ray absorptiometry (DEXA) with the use of PIXImus2 Mouse Densitometer (GE Medical Systems, Madison, WI). Prior to measurements, mice were anesthetized with isoflurane gas. Bone mineral density (g/cm2) was measured from a region of interest (ROI) that encompassed the entire body without the head. At the end of the study mice were euthanized with CO2, and blood was taken for analyses.
2.5. Analyses of serum
Serum insulin levels were determined with the use of Insulin (Mouse) Ultrasensitive EIA Kit (Alpco Diagnostics, Salem, NH, USA), following the manufacturers protocol. Assays of serum glucose, triglycerides, cholesterol, HDL, LDL, and free fatty acids were performed in duplicates on an AU400e Clinical Chemistry analyzer (Olympus America Inc. Irving, TX, USA).
2.6. Cell culture
The MG-63, human osteoblast-like cells, (CRL-1427) were obtained from the ATCC (Manassas, VA, USA). MG-63 cells were cultured as monolayers in MEM with Earle's salt, non-essential amino acids, 2 mM l-glutamine and 1 mM sodium pyruvate, supplemented with10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The effect of 30 μM MZ-2, morphine or naltrexone on cell proliferation was determined with the use of AlamarBlue assay (Nakayama et al., 1997). Cells were seeded in 96 well plates (5 × 104cells/well in 0.1 ml medium) and grown overnight as described above. The medium was changed with fresh medium containing test compounds and cells were incubated for 24 hours. Then 10 μl of the AlamarBlue solution was added to each well and after 1 h incubation fluorescence (λEx 530 nm, λEm 590 nm) was measured with the use of Microplate Fluorescence Reader FL600 (BioTek Instruments, Winooski, VT, USA). In order to measure the effect of the compounds on mineral nodule formation, cells were seeded in 24 well plates (2.5−3 × 105cells/well) and grown for one day. When cells reached ∼70% confluency, they were treated with an osteogenic medium [β-glycerophosphate (2mM), dexamethasone (10 nM), 2-phospho-l-ascorbic acid (50 μM)] with or without MZ-2, morphine or naltrexone (30 μM) for 30 days. Medium was changed every 2−3 days. Effect of compounds on bone nodule formation was determined by alizarin red staining, a common histochemical technique used to detect calcium deposits in mineralized tissues and cultures (Lievremont et al., 1982). Medium was aspirated, cells were washed with phosphate buffered saline, fixed with 70% ethanol and stained with 1% alizarin red for 30 min following washing with demineralized water, and viewed under a light microscope. Quantitative analysis of mineralization was performed according to (Gregory et al., 2004) with a minor modification. Briefly, cells stained with alizarin red were dispersed in 10% acetic acid and transferred into 2 ml tubes. After heating for 10 min at 85°C, cell dispersion was cooled on ice and centrifuged at 15,000×g for 15 min. Aliquots of supernatants were transferred to a new tubes and pH was adjusted to 4.1−4.5 with 10% ammonium hydroxide. Optical density of solution was measured at 405 nm and amount of alizarin red bound was calculated from standard curve obtained with the use of standard alizarin red solutions.
2.7. Statistical analysis
Results were expressed as the means ± S.E.M. Statistical analysis of body weight gain was performed with repeated measures ANOVA. Other outcomes were analyzed with the use of one way ANOVA. Recognizing that the number of statistically significant results increases with the number of tests, even in the absence of real differences between the groups, and noting that our interest was in comparisons between pairs of groups, we applied the Tukey method, which is specially designed to control the experiment-wise type I error rate for set of pairwise comparisons. The differences between groups were considered statistically significant at P < 0.05.
3. Results
3.1. Effect of MZ-2 on body weight gain and body fat
During the observation time encompassing 5 weeks, MZ-2 (10 mg/kg, p.o.) had no effect on body weight gain in obese mice which had access to exercise wheels (Fig. 1A); in contrast, it decreased this parameter in sedentary obese mice (Fig. 1B). Whereas a two week time lag was necessary to observe statistically significant effects in MZ-2 treated obese mice, decreased body weight gain continued beyond the three week treatment period. After discontinuing oral administration of MZ-2, the difference between saline and MZ-2 groups diminished gradually and eventually became non significant; nonetheless, a distinct trend was maintained during the two week post MZ-2 treatment period. In lean control mice, administration of MZ-2 failed to have any effect on body weight gain independent of access to the exercise wheel (Fig. 1C,D).
Figure 1.
Effect of MZ-2 (10 mg/kg/day, p.o.) on body weight gain in ob/ob and lean control mice. A: ob/ob mice with access to wheels; B: sedentary ob/ob mice; C: control mice with access to wheels; D: control mice without access to wheels. Mice were treated once per day, daily for three weeks and monitored for additional two weeks after treatment. Results are presented as mean ± S.E.M. (n = 5 per group). There are no statistically significant differences in weight gain between MZ-2- and saline-treated obese mice on wheels (panel A: P > 0.05) and lean mice (panel C and D: P > 0.05); MZ-2 treated sedentary obese mice gained weight significantly less than saline treated group (panel B: P < 0.001) by Tukey's test following repeated measures ANOVA (F7,279 = 159.7, P < 0.0001).
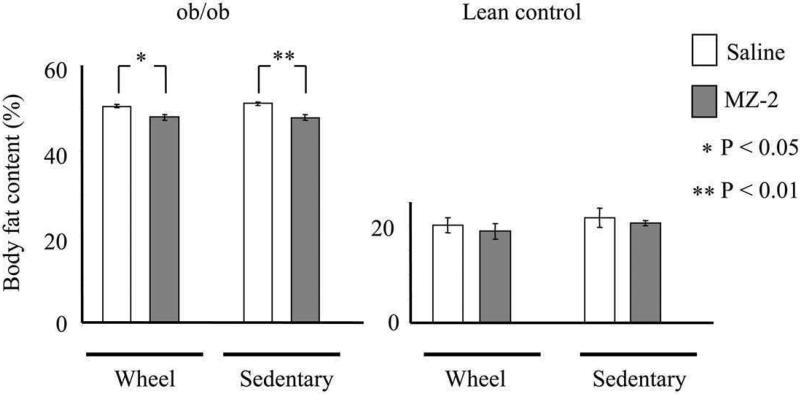
The obese mice treated with MZ-2 also had significantly lower body fat content than those receiving saline (Fig. 2A) in both sedentary and exercising animals (50.5 ± 0.40% in saline versus 47.0 ± 0.7% in MZ-2-treated group on wheels and 51.2 ± 0.5% versus 47.7 ± 0.6% in sedentary saline- and MZ-2-treated mice, respectively). Control mice had much lower body fat content (∼20%) than their obese counterparts (∼50%). In control mice with MZ-2 there was a non significant tendency to decrease body fat content (20.2 ± 1.5% in saline- versus 19.0 ± 1.6% in MZ-2-treated group on wheels and 21.82 ± 1.9% versus 20.7 ± 0.6% in sedentary saline- and MZ-2-treated groups, respectively) (Fig. 2B).
Figure 2.
Effect of MZ-2 (10 mg/kg/day, p.o.) on total body fat content in ob/ob (A) and lean control mice (B) after five week experimental period. Results are presented as mean ± S.E.M. (n = 5 per group). (*) Denotes body fat contents that are significantly different from saline-treated mice by Tukey's test (** P < 0.01, * P < 0.05) following ANOVA (F7,39 = 659.1; P < 0.0001).
3.2. Effect of MZ-2 on food intake
Obese mice consumed noticeably more food than control mice over a 24 h period (Fig. 3), but MZ-2 had no significant impact on food intake; nonetheless, obese mice with access to wheels consumed slightly less food (6.4 ± 0.40 g in saline, and 6.0 ± 0.48 g in MZ-2 group) than sedentary animals (6.6 ± 0.2 g and 6.4 ± 0.2 g in saline and MZ-2 groups, respectively). While these differences were not statistically significant, it should be noticed that a tendency occurred toward a lower food intake in MZ-2 groups (Fig. 3A). In contrast, lean mice with access to wheels had a higher food intake (4.7 ± 0.12 g and 4.5 ± 0.28 g in exercising saline and MZ-2 groups, respectively) compared to sedentary control mice independent of the treatment, (3.98 ± 0.14g and 4.02 ± 0.17g in sedentary saline and MZ-2 groups, respectively) (Fig. 3B). Similar to obese mice, MZ-2 was without a statistically significant effect on 24 h food intake in lean control mice.
Figure 3.
Effect of MZ-2 (10 mg/kg/day, p.o.) on 24 h food intake in ob/ob (A) and lean control mice (B) during treatment period. Results are presented as mean ± S.E.M. (n = 5 per group). There are no statistically significant differences in food intake between MZ-2- and saline-treated mice (P > 0.05); obese mice consume significantly more food than lean mice (P < 0.001) by Tukey's test following ANOVA (F7,39 = 22.49; P < 0.0001).
3.3. Effect of MZ-2 on voluntary wheels exercise
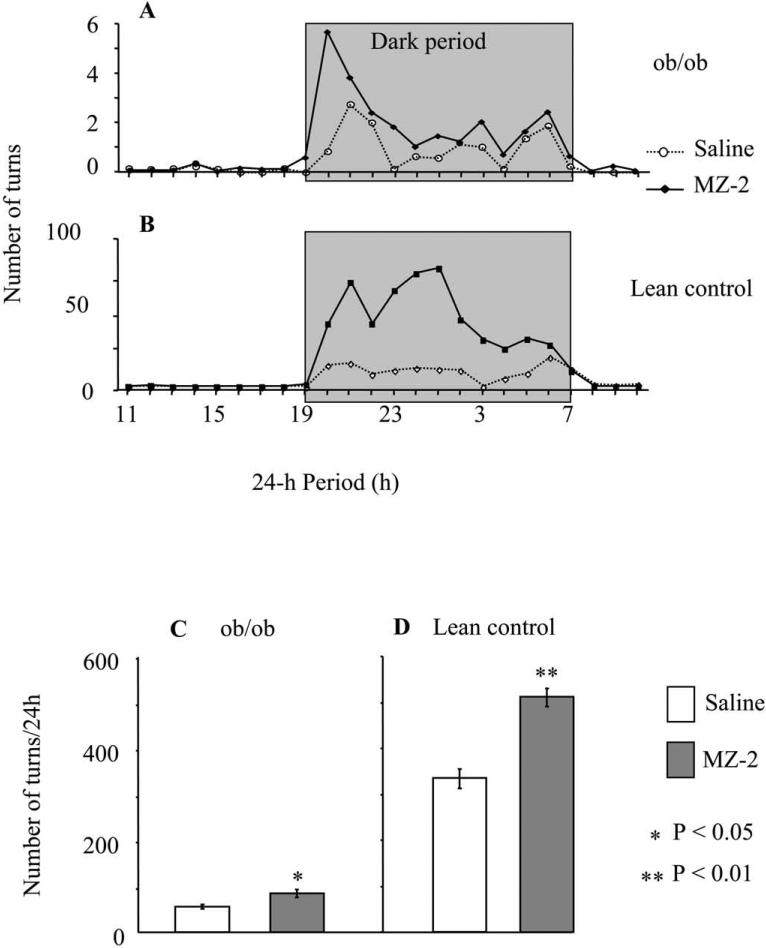
Obese mice exercised on wheels much less than the lean control animals (Fig 4). As expected, a vast amount of data on the physical activity for all animals was recorded during the nocturnal period, the normal time for peak activity behaviour for mice (Fig 4A,B). Interestingly, MZ-2 significantly stimulated voluntary wheel exercise in both obese and lean control mice; however, that increase was much more pronounced in lean mice (334 ± 22 turns/24 h in saline group versus 512 ± 20 turns/24 h in MZ-2 group) in comparison to obese mice (25 ± 2 turns/24 h in saline group versus 38 ± 4 turns/24 h in MZ-2 group). Furthermore, the increased activity was observed only during first two hours of the nocturnal cycle for obese mice, whereas the effect lasted through 10 h of the dark cycle for the controls (Fig. 4A,B). Differences in voluntary wheel exercise between MZ-2 and saline groups diminished after discontinuation of the treatment in both obese and control mice. Although a trend toward higher voluntary activity of the MZ-2 groups during post-treatment period was observed, the differences between groups were not statistically significant (data not shown).
Figure 4.
Effect of MZ-2 (10 mg/kg/day, p.o.) on voluntary wheel exercise of ob/ob and lean control mice. A and B: Activity pattern during a 24-h-period of ob/ob (A) and control mice (B). Gray box indicates the 12-h dark cycle period. C and D: Average activity of ob/ob (C) and control mice (D) during treatment period. Results are presented as mean ± S.E.M. (n = 5 per group). (*) Denotes number of turns that are significantly different from saline-treated mice by Tukey's test (** P < 0.01, * P < 0.05) following ANOVA (F3,99 = 1521, P < 0.0001).
3.4. Effect of MZ-2 on serum insulin level
As anticipated, the obese mice exhibited significantly higher serum insulin levels (approximately 20-fold) compared to lean controls (Fig. 5), but both lean and obese mice exercising on wheels had much lower insulin levels than sedentary animals. MZ-2 had tendency to decrease insulin levels in obese sedentary mice (23.6 ± 7.7 ng/ml in saline versus 21.3 ± 2.8 ng/ml in MZ-2 treated mice), and substantially decreased the insulin level in obese mice exercising on wheels by 58% (from 12.4 ± 3.7 ng/ml in saline treated group to 5.2 ± 0.28 ng/ml). MZ-2 was without effect on insulin levels in lean mice independent of the life style (0.64 ± 0.05 ng/ml in saline and 0.68 ± 0.13 ng/ml in MZ-2 treated mice on wheels, and 1.09 ± 0.3 in saline and 1.12 ± 0.17 ng/ml in MZ-2 treated sedentary mice).
Figure 5.
Effect of MZ-2 (10 mg/kg/day, p.o.) on serum insulin level in ob/ob (A) and lean control mice (B) after five week experimental period. Results are presented as mean ± S.E.M. (n = 5 per group). (*) Denotes insulin levels that are significantly different from saline-treated mice by Tukey's test (* P < 0.05) following ANOVA (F7,39 = 44.79, P < 0.0001).
3.5. Effect of MZ-2 on blood chemistry
Table 1 summarizes the effect of MZ-2 on blood chemistry. MZ-2 significantly decreased serum glucose levels in sedentary obese mice (649 ± 35 mg/dl in saline- versus 447 ± 89 mg/dl in the MZ-2-group). Serum glucose levels were also lower in exercising obese mice (432 ± 108 mg/dl in saline- versus 412 ± 96.7 mg/dl in the MZ-2-group), however, this difference was not statistically significant. MZ-2 did not have any effect on serum glucose level of sedentary control mice (262.6 ± 31.7 mg/dl in saline versus 266.7 ± 23.1 mg/dl in the MZ-2 group), whereas it decreased glucose level in animals exercising on wheels (343.8 ± 38.8 mg/dl in saline versus 288.6 ± 47.7 mg/dl in the MZ-2 group). Furthermore, MZ-2 did not exert any effect on serum triglyceride (TG) and nonesterified fatty acid (NEFA) content in ob/ob mice having access to exercising wheels. These parameters were decreased in sedentary ob/ob mice and lean control mice independently of life style. MZ-2 had a following effect on serum cholesterol profiles: in ob/ob mice MZ-2 had a tendency to increase total serum cholesterol (207 ± 14 mg/dl in saline versus 246 ± 48mg/dl in MZ-2 exercising mice and 248 ± 33 mg/dl in saline versus 258 ± 20 mg/dl in MZ2 sedentary mice), decrease the level of LDL (19.8 ± 3 mg/dl in saline versus 17.6 ± 2.9 mg/dl in MZ-2 exercising mice and 21.4 ± 5.1 mg/dl in saline versus 22.3 ± 1.7 mg/dl in MZ2 sedentary mice), and increase HDL (92.4 ± 6.6 mg/dl in saline versus 119.7 ± 12.8 mg/dl in MZ-2 exercising mice and 122.2 ± 12.6 mg/dl in saline versus 130 ± 12.4 mg/dl in MZ2 sedentary mice), while in lean control mice MZ-2 decreased total cholesterol and both LDL and HDL (Table 1). Although effects of MZ-2 on cholesterol levels were not statistically significant, they resulted in an increase of the HDL/LDL ratio, and this was especially pronounced in animals having access to exercise wheels. In ob/ob mice this ratio amounted to 4.67 (saline) versus 6.80 (MZ-2) and in lean mice 4.90 versus 5.31 in saline and MZ-2 group, respectively.
Table 1.
Effect of MZ-2 on serum chemistry in ob/ob and lean mice. Results are presented as mean ± SD (n = 5 per group)
| Cholesterol (total) (mg/dl) | LDL (mg/dl) | HDL (mg/dl) | HDL/LDL | TG (mg/dl) | NEFA (mM/l) | Glucose (mg/dl) | |
|---|---|---|---|---|---|---|---|
| ob/ob + wheel | |||||||
| Saline | 207±14 | 19.8±3 | 92.4±6.6 | 4.67 | 90.6±20.9 | 2.02±0.4 | 432±108 |
| MZ-2 | 246±48 | 17.6±2.9 | 119.7±12.8 | 6.80 | 90.2±15.1 | 2.06±0.4 | 412±97.6 |
| ob/ob sedentary | |||||||
| Saline | 248±33 | 21.4±5.1 | 122.2±12.6 | 5.71 | 130.5±25.5 | 3.02±0.6 | 649±35 |
| MZ-2 | 258±20 | 22.3±1.7 | 130±12.4 | 5.84 | 122.3±45 | 2.7±0.8 | 447±89 |
| Control + wheel | |||||||
| Saline | 86±9.7 | 10±2.1 | 49±5.3 | 4.90 | 101.6±14.9 | 1.74±0.2 | 343.8±38.8 |
| MZ-2 | 78±4.2 | 8.4±1.1 | 44.6±5.1 | 5.31 | 86.75±12.1 | 1.44±0.3 | 288.6±47.7 |
| Control sedentary | |||||||
| Saline | 86±7.7 | 8.4±1.5 | 47.4±3.6 | 5.64 | 89.4±13 | 1.2±0.2 | 262.6±31.7 |
| MZ-2 | 81±6.7 | 9.7±2.1 | 44.4±5.6 | 4.58 | 62±7.9 | 0.92±0.2 | 266.7±23.1 |
3.6. Effect of MZ-2 on bone mineral density
Lean mice had higher total bone mineral density (BMD) than obese mice (Fig. 6A). In animals having access to wheels, MZ-2 did not have a significant effect on BMD. However, MZ-2 increased BMD in sedentary animals (Fig. 6A), both ob/ob (42.85 ± 1.02 mg/cm2 in saline group versus 45.44 ± 1.4 mg/cm2 in MZ-2 group) and lean controls (48.70 ± 1.28 mg/cm2 in saline group versus 51.68 ± 0.98 mg/cm2 in MZ-2 group). This effect was especially noticeable when analysing the increase in BMD (Fig. 6B). During the 5 week experimental period, the BMD of all treatment groups increased. Ob/ob mice, treated with MZ-2 had a greater increase in BMD than saline treated groups. This effect however, was statistically significant only in sedentary obese animals (6.15 ± 0.69 mg/cm2 in saline group versus 8.74 ± 1.00 mg/cm2 in MZ-2 group). In lean mice having access to exercise wheels exercise alone increased significantly BMD in saline group (10.66 ± 0.69 mg/cm2) as compared to saline treated sedentary mice (8.12 ± 0.9 mg/cm2). In these mice MZ-2 did not have any additional effect on BMD (10.72 ± 1.00 mg/cm2). In lean sedentary animals, MZ-2 treatment caused a statistically significant increase (23%) of bone mineral density (10.56 ± 0.51 mg/cm2) as compared to saline (8.12 ± 0.9 mg/cm2) reaching the increase in BMD values of lean exercising animals.
Figure 6.
Effect of MZ-2 (10 mg/kg/day, p.o.) on bone mineral density (A) and increase of bone mineral density (B) in ob/ob and control mice after five week experimental period. Results are presented as mean ± S.E.M. (n = 5 per group). (*) Denotes BMD increases that are significantly different from saline-treated mice by Tukey's test (* P < 0.05) following ANOVA (F7,39 = 15.93, P < 0.0001).
3.7. Effect of MZ-2 on proliferation and mineralization of human osteoblastic MG-63 cells
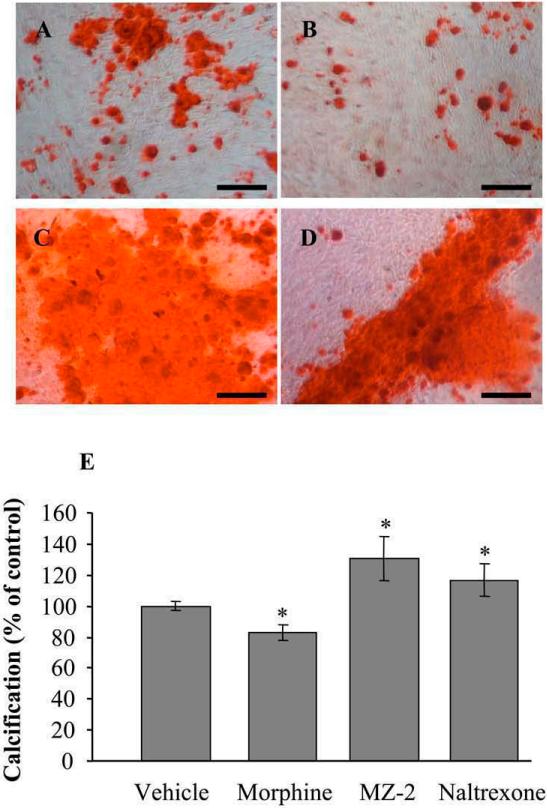
None of the opioid compounds exerted an effect on cell proliferation of MG-63 cells (data not shown). In contrast, treatment with 30 μM MZ-2 and the non-selective opioid antagonist naltrexone for 30 days significantly increased mineralization of MG-63 cells in culture (Fig. 7), while morphine significantly decreased their formation in comparison to medium treated cells (Fig. 7).
Figure 7.
Effect of 30 μM each morphine, MZ-2 and naltrexone on bone nodule formation in MG-63 cells. Panels A-D: Alizarin red staining of MG-63 cells treated with vehicle (A), morphine (B), MZ-2 (C) and naltrexone (D). Scale bar = 250 μ. E, Quantitative analysis of mineralization. Results are presented as mean ± S.E.M. (n = 5). (*) Denotes calcification % that are significantly different from vehicle treated cells by Tukey's test (* P < 0.05) following ANOVA (F3,19 = 24.0, P < 0.0001).
4. Discussion
In the present study the effect of the Dmt-Tic pharmacophoric opioid MZ-2, an orally active dual MOP/DOP receptor antagonist (Balboni et al., 2006), was manifested in ameliorated body weight gain, body fat, decreased serum insulin and glucose levels, and increased BMD in obese mice. The increase of BMD in vivo was further supported by the increased deposition of calcium-containing nodules in human osteoblast MG-63 cells in vitro. These data support the notion that an inhibitory action of the neural reward system, which contains MOP receptor as an integral component, can alleviate some of the prevalent factors involved in obesity.
In view of the fact that obese mice have increased brain levels of the opioid agonists β-endorphin and [Met5]enkephalin (Khawaja et al., 1989); it is conceivable that administration of an opioid antagonist would necessarily compete with these opioids before exerting recognizable effects. In fact, the administration of MZ-2 to obese and control mice exerted differential effects on body weight gain that depended on the treatment group; it significantly decreased weight gain in sedentary obese mice, without an apparent effect in comparable lean animals or obese mice that exercised. When comparing food intake in sedentary and exercising obese mice, there were no significant differences between groups. Obese mice having access to exercise wheels consumed not significantly lower amount of food than their sedentary counterparts despite increased energy expenditure related to exercise. Consequently, weight gain of exercizing obese mice treated with saline was slower than saline treated obese sedentary mice. Saline treated exercising obese mice had body weight gain similar to the sedentary obese MZ-2 group. Lack of decrease of body weight gain in obese animals on wheels upon treatment with MZ-2 may be due to exercise-induced release of endogenous opioids which reduced effect of MZ-2. In the case of lean mice, those on exercising wheels consumed more food than sedentary animals, but gained weight to similar extent as sedentary group. This suggests that higher food intake in lean exercising mice was utilized by increased fuel requirement due to exercise related higher energy expenditure. The effect of MZ-2 to reduce body weight gain over the relatively short experimental period of 3 weeks in sedentary obese mice cannot be solely explained by its effect on food intake, although MZ-2 exhibited a tendency to decrease food consumption during a 24 h period in both obese and control mice. Nonetheless, it is quite conceivable that this effect might gradually become additive over a period of weeks to months, and should be explored under other experimental paradigms covering longer time periods. Furthermore, while MZ-2 failed to demonstrate an effect on body weight gain in lean mice independent of life style, MZ-2 increased voluntary running on wheels of both obese and lean mice. Therefore, we speculate that the effect of MZ-2 on reduced body weight gain in sedentary obese mice might be related to the increase of overall physical activity similar to that observed with voluntary activity on wheels that lead to an increase in energy expenditure. In relation to decrease of body fat, it is known that important determinants of fat oxidation are both diet and exercise. The intensity of exercise is the most important factor. Fat oxidation rates are highest at low to moderate exercise intensities and decrease when the intensity of exercise becomes high (Achten and Jeukendrup, 2004; Knechtle, 2002). This phenomenon may explain higher decrease of body fat in sedentary obese mice than in exercising obese mice. In our experimental setting we can not exclude differences in body water content between treatment groups. We used DEXA for measurement of body composition. Body water content is not a direct measure in this technique, but it can be calculated by multiplying the lean body mass by a constant factor of 0.742. It is also known that the differences in tissue hydration can lead to erroneous body fat and lean tissue mass determination by DEXA (Pietrobelli et al., 1998).
Increased voluntary running on wheels in both obese and lean mice by MZ-2 conflict with previous observations in that opioid antagonists were observed to either have no effect or decreased voluntary running in laboratory animals (Carey et al., 1981; Sisti and Lewis, 2001). The differences, however, are attributable to different experimental paradigms, the experimental animal used and the antagonist administered. In previous studies in which animals had limited access to exercise wheels, the effects of the antagonist depended on the length of time during the exercise period; i.e., naloxone inhibited voluntary running in rats being on exercise wheels for one hour (Sisti and Lewis, 2001), but was ineffective in rats having access to wheels for three hours (Carey et al., 1981). Behavioral observations with naloxone (30 mg/kg) and naltrexone (10 and 30 mg/kg) specifically inhibited linear locomotion in mice within 15 min following administration in activity chambers without running wheels (Ukai and Kameyama, 1985a; b). Our studies permitted mice an unlimited access to exercise wheels during the entire study, providing them the freedom to choose any type of activity (running on wheels, moving within cage, grooming, resting, feeding, or drinking); we observed that MZ-2 significantly stimulated voluntary running during the first two hours of the nocturnal cycle in obese and for ten hours in lean mice. The observed difference in the effect on voluntary wheel running between MZ-2 and naloxone or naltrexone may also be related to the specific pharmacological activities among these compounds. Naloxone and naltrexone are primarily antagonists at μ- and κ-opioid receptors, and to a lesser extent at δ-opioid receptors (Peng et al., 2007), while MZ-2 is a mixed μ-/δ-opioid receptor antagonist without interaction at κ-opioid receptors (Balboni et al., 2006). Moreover, both naloxone and naltrexone exhibit a propensity toward inverse agonism (Marczak et al., 2007) that would tend to skew the observational data seen with these compounds.
Voluntary running on exercise wheels significantly decreased serum insulin levels in both ob/ob and control mice as well as serum glucose level in ob/ob mice. These data are consistent with reports that aerobic exercise decreases insulin level and improves insulin resistance in human (Jurimae et al., 1989). Treatment with MZ-2 of ob/ob mice had an additive effect on insulin and glucose levels, which is probably mediated through increased physical activity.
Regarding blood chemistry, analysis of blood samples taken at the end of drug administration would be more informative. However, we did not draw blood samples at that time point due to inherent problems with healing of even negligible wounds in the obese mice. Taking this later aspect into account, to obtain precise information about effect of MZ-2 on blood chemistry, additional groups of animals would be required. Limited in the number of exercise wheels available and trying to reduce number of animals used, we decided to determine whether removal of the compound will continue to influence body weight gain and voluntary activity on wheels. Blood chemistry two weeks after discontinuation of dosing can be considered a derivative of the potential long term “remaining or lingering” effect of the action by MZ-2.
Patients undergoing opioid agonist treatment were observed to have increased risk of fractures and symptoms of osteoporosis (Ensrud et al., 2003; Shorr et al., 1992; Vestergaard et al., 2006), since these drugs promote osteoporosis by inhibiting either the production of sex steroids (Daniell, 2002) or by a direct influence on bone formation. Considering that opioid receptors are expressed in bons and joints (Elhassan et al., 1998; Perez-Castrillon et al., 2000) and that opioid agonists inhibit growth of osteoblasts and osteocalcin production in cell cultures, it is sufficient to explain their effect on bone deterioration in vivo. On the other hand, naloxone increased osteogenesis in chick embryos (Liskov et al., 2005). Treatment with MZ-2 of both obese and lean mice provided a positive effect on bone mineral density. While MZ-2 did not further increase the effect of exercise on BMD in lean mice, it increased BMD in sedentary mice to the level of the exercising group. Absence of an effect in exercising lean mice may be related to limitations in an availability to increase BMD; i.e., the wheel running itself increased bone mineral density to its peak maximum (Hagihara et al., 2005; Newhall et al., 1991). In view of the fact that both MZ-2 and naloxone increased, while morphine decreased calcification of human osteoblast MG-63 cells in culture, the observed increase of BMD in ob/ob and lean mice upon MZ-2 treatment might be related to a direct effect of opioids on bone formation.
In the present study we have chosen female mice in order to develop an experimental animal model to address the prevalence of obesity in women in general population (Gellner and Domschke, 2008; Ogden et al., 2006). In future studies we plan to investigate the contribution of the female neuroendocrine system on the same endpoints as described here.
In conclusion, the pharmacological profile of MZ-2, the dual MOP/DOP receptor antagonist suggests that this compound may have a promissing clinical potential to manage some important aspects attributable to obesity.
Acknowledgements
This work was supported in part by the Intramural Research Program of the NIH and NIEHS, and in part by the University of Cagliari and the University of Ferrara. The authors would like to thank Dr. Boris Risek for his comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work was presented during International Narcotics Research Conference, Charleston, SC, USA, July 13−18, 2008.
References
- Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition (Burbank, Los Angeles County, Calif. 2004;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Atkinson RL. Opioid regulation of food intake and body weight in humans. Fed Proc. 1987;46:178–182. [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni G, Onnis V, Congiu C, Zotti M, Sasaki Y, Ambo A, Bryant SD, Jinsmaa Y, Lazarus LH, Trapella C, Salvadori S. Effect of lysine at C-terminus of the Dmt-Tic opioid pharmacophore. J Med Chem. 2006;49:5610–5617. doi: 10.1021/jm060741w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berestov LA. [Endogenous morphines--possible role in the pathogenesis of exogenous-constitutional obesity]. Ter Arkh. 1983;55:131–134. [PubMed] [Google Scholar]
- Carey MP, Ross JA, Enns MP. Naloxone suppresses feeding and drinking but not wheel running in rats. Pharmacol Biochem Behav. 1981;14:569–571. doi: 10.1016/0091-3057(81)90318-x. [DOI] [PubMed] [Google Scholar]
- Cole JL, Berman N, Bodnar RJ. Evaluation of chronic opioid receptor antagonist effects upon weight and intake measures in lean and obese Zucker rats. Peptides. 1997;18:1201–1207. doi: 10.1016/s0196-9781(97)00074-0. [DOI] [PubMed] [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3:377–384. doi: 10.1054/jpai.2002.126790. [DOI] [PubMed] [Google Scholar]
- Elhassan AM, Lindgren JU, Hultenby K, Bergstrom J, Adem A. Methionine-enkephalin in bone and joint tissues. J Bone Miner Res. 1998;13:88–95. doi: 10.1359/jbmr.1998.13.1.88. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, Hanlon JT, Nevitt MC, Whooley MA. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163:949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Gellner R, Domschke W. [Epidemiology of obesity]. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2008;79:807–810. 812–806, 818. doi: 10.1007/s00104-008-1534-6. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Hagihara Y, Fukuda S, Goto S, Iida H, Yamazaki M, Moriya H. How many days per week should rats undergo running exercise to increase BMD? J Bone Miner Metab. 2005;23:289–294. doi: 10.1007/s00774-005-0601-z. [DOI] [PubMed] [Google Scholar]
- Han W, Hata H, Imbe H, Liu QR, Takamatsu Y, Koizumi M, Murphy NP, Senba E, Uhl GR, Sora I, Ikeda K. Increased body weight in mice lacking mu-opioid receptors. Neuroreport. 2006;17:941–944. doi: 10.1097/01.wnr.0000221829.87974.ad. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Metzger BL. The effect of opioid antagonism on food intake behavior and body weight in a biobehavioral model of obese binge eating. Biol Res Nurs. 2002;3:198–209. doi: 10.1177/10900402003004005. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Marczak ED, Balboni G, Salvadori S, Lazarus LH. Inhibition of the development of morphine tolerance by a potent dual mu-/delta-opioid antagonist, H-Dmt-Tic-Lys-NH-CH(2)-Ph. Pharmacol Biochem Behav. 2008;90:651–657. doi: 10.1016/j.pbb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurimae T, Viru A, Karelson K, Smirnova T. Biochemical changes in blood during the long and short triathlon competition. J Sports Med Phys Fitness. 1989;29:305–309. [PubMed] [Google Scholar]
- Kas MJ, van den Bos R, Baars AM, Lubbers M, Lesscher HM, Hillebrand JJ, Schuller AG, Pintar JE, Spruijt BM. Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci. 2004;20:1624–1632. doi: 10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- Khawaja XZ, Bailey CJ, Green IC. Central mu, delta, and kappa opioid binding sites, and brain and pituitary beta-endorphin and met-enkephalin in genetically obese (ob/ob) and lean mice. Life Sci. 1989;44:1097–1105. doi: 10.1016/0024-3205(89)90337-8. [DOI] [PubMed] [Google Scholar]
- Knechtle B. Exercise intensity and fat burning-theoretical principles and practical considerations. Praxis. 2002;91:915–919. doi: 10.1024/0369-8394.91.21.915. [DOI] [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ, Zimmerman DM. Central administration of the opioid antagonist, LY255582, decreases short- and long-term food intake in rats. Brain Res. 1991;566:193–197. doi: 10.1016/0006-8993(91)91698-z. [DOI] [PubMed] [Google Scholar]
- Lievremont M, Potus J, Guillou B. Use of alizarin red S for histochemical staining of Ca2+ in the mouse; some parameters of the chemical reaction in vitro. Acta Anat (Basel) 1982;114:268–280. doi: 10.1159/000145596. [DOI] [PubMed] [Google Scholar]
- Liskov AV, Solnyshkova TG, Frolov BA, Pavlovichev SA. Effect of naloxone hydrochloride on osteogenesis in chick embryos. Bull Exp Biol Med. 2005;139:331–333. doi: 10.1007/s10517-005-0286-2. [DOI] [PubMed] [Google Scholar]
- Marczak ED, Jinsmaa Y, Li T, Bryant SD, Tsuda Y, Okada Y, Lazarus LH. [N-allyl-Dmt1]-endomorphins are micro-opioid receptor antagonists lacking inverse agonist properties. J Pharmacol Exp Ther. 2007;323:374–380. doi: 10.1124/jpet.107.125807. [DOI] [PubMed] [Google Scholar]
- McCormack JF, Denbow DM. Ingestive responses to mu and delta opioid receptor agonists in the domestic fowl. Br Poult Sci. 1989;30:327–340. doi: 10.1080/00071668908417154. [DOI] [PubMed] [Google Scholar]
- Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997;204:205–208. doi: 10.1016/s0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Newhall KM, Rodnick KJ, van der Meulen MC, Carter DR, Marcus R. Effects of voluntary exercise on bone mineral content in rats. J Bone Miner Res. 1991;6:289–296. doi: 10.1002/jbmr.5650060311. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999−2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone, and naloxone at mu, delta, and kappa opioid receptors. J Med Chem. 2007;50:2254–2258. doi: 10.1021/jm061327z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Castrillon JL, Olmos JM, Gomez JJ, Barrallo A, Riancho JA, Perera L, Valero C, Amado JA, Gonzalez-Macias J. Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology. 2000;72:187–194. doi: 10.1159/000054586. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998;274:E808–816. doi: 10.1152/ajpendo.1998.274.5.E808. [DOI] [PubMed] [Google Scholar]
- Recant L, Voyles NR, Luciano M, Pert CB. Naltrexone reduces weight gain, alters “beta-endorphin”, and reduces insulin output from pancreatic islets of genetically obese mice. Peptides. 1980;1:309–313. doi: 10.1016/0196-9781(80)90008-x. [DOI] [PubMed] [Google Scholar]
- Shaw WN, Mitch CH, Leander JD, Mendelsohn LG, Zimmerman DM. The effect of the opioid antagonist LY255582 on body weight of the obese Zucker rat. Int J Obes. 1991;15:387–395. [PubMed] [Google Scholar]
- Shorr RI, Griffin MR, Daugherty JR, Ray WA. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47:M111–115. doi: 10.1093/geronj/47.4.m111. [DOI] [PubMed] [Google Scholar]
- Sisti HM, Lewis MJ. Naloxone suppression and morphine enhancement of voluntary wheel-running activity in rats. Pharmacol Biochem Behav. 2001;70:359–365. doi: 10.1016/s0091-3057(01)00624-4. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ, Coscina DV, Rousset S, Redonnet A, Parker GC, Inoue K, Ricquier D, Penicaud L, Kieffer BL, Koob GF. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes. 2005;54:3510–3516. doi: 10.2337/diabetes.54.12.3510. [DOI] [PubMed] [Google Scholar]
- Ukai M, Kameyama T. Multi-dimensional analyses of behavior in mice treated with naltrexone. Physiol Behav. 1985a;34:311–313. doi: 10.1016/0031-9384(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Ukai M, Kameyama T. Naloxone specifically blocks the linear locomotion in mice. Brain Res. 1985b;328:378–380. doi: 10.1016/0006-8993(85)91053-4. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260:76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Frassetto A, Huang RR, Lao JZ, Pasternak A, Wang SP, Metzger JM, Strack AM, Fong TM, Chen RZ. The mu-opioid receptor subtype is required for the anorectic effect of an opioid receptor antagonist. Eur J Pharmacol. 2006;545:147–152. doi: 10.1016/j.ejphar.2006.06.069. [DOI] [PubMed] [Google Scholar]