Abstract
We present here a patient with end stage renal failure who received two weeks antimalarial prophylaxis at full dose leading to life threatening toxicity with severe acute megaloblastic anaemia, symptomatic pancytopenia and exfoliative dermatitis. Prompt recognition and treatment can rapidly reverse these fatal effects but more importantly, education of patients before travel is imperative in preventing such events.
Keywords: antimalarials, end stage renal failure, exfoliative dermatitis, megaloblastic anaemia, pancytopenia
Chloroquine and proguanil, used alone or together, are the most commonly utilised drugs in prophylaxis against malaria. It is advised that prophylaxis must start 1 week before travel into an endemic area and should be continued for 4 weeks after return. The recommended dose of chloroquine for an adult is 300 mg once weekly and proguanil 200 mg once daily.1
A 39‐year‐old Asian man with end stage renal failure of 18 months duration, secondary to congenital single kidney and hypertension, was hospitalised on his return from holiday to India. Initially on haemodialysis, he had been self‐administering peritoneal dialysis for 1 year. Seven days before travelling to Delhi and 17 days before admission, he started taking Avloclore (chloroquine phosphate, AstraZeneca) 500 mg once a week and Paludrine (proguanil, AstraZeneca) 200 mg daily which is recommended as the appropriate antimalarial prophylaxis when travelling to South Asia.
On admission, he complained of a 2 week history of progressive lethargy, rash and increased pigmentation over the limbs, bruising, epistaxis and bleeding gums. On examination he looked pale, had mucositis, glossitis and gum bleeding; there were widespread petechiae and urticarial rash over his limbs and trunk, his upper and lower limbs were pigmented, and areas of desquamation were seen in the extremities (fig 1).
Figure 1 Areas of desquamation seen in the extremities: (A) lower arm and (B) lower leg. Informed consent was obtained for publication of this figure.
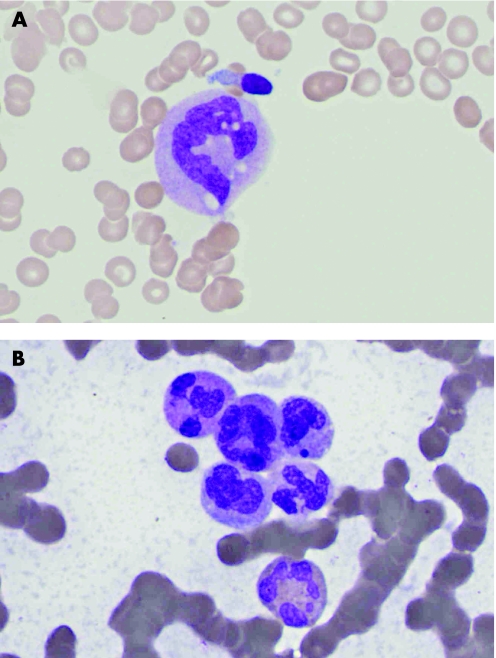
Blood analysis (table 1) revealed a pancytopenia. Serum B12 and red cell folate were within normal range. The peripheral blood film did not show evidence of myelodysplasia or leukaemic blasts. The presence of giant metamyelocytes was highly suggestive of acute megaloblastic anaemia (fig 2A). The bone marrow aspirate (fig 2B) and trephine biopsy confirmed the diagnosis: there was reduced cellularity, notably reduced erythropoiesis, with severe megaloblastic changes, reduced granulopoiesis with hypersegmented eosinophils and giant metamyelocytes, and an absence of megakaryocytes.
Table 1 Results of blood analysis.
| Test | Day 1 | Day 9 | Normal range | |
|---|---|---|---|---|
| Full blood count | Haemoglobin | 10.7 | 9.4 | 13–18 g/dl |
| Mean cell volume | 88 | 88 | 77–97 fl | |
| White cell count | 0.4 | 6.2 | 4–11.0×109/l | |
| Neutrophils | 0.1 | 3.4 | 2.0–7.5×109/l | |
| Lymphocytes | 0.3 | 1.6 | 1.5–4.5×109/l | |
| Monocytes | 0.0 | 0.7 | 0.2–0.8×109/l | |
| Eosinophils | 0.0 | 0.5 | 0.1–0.4×109/l | |
| Basophils | 0.0 | 0.0 | 0.0–0.01×109/l | |
| Platelets | 10 | 100 | 150–400×109/l | |
| Reticulocyte % | 0.3 | 0.5–2.5% | ||
| Red cell folate | >610 | 160–640 ng/l | ||
| Vitamin B12 | 178 | 150–900 ng/l | ||
Figure 2 Microscopic examination revealed the presence of (A) giant metamyelocytes in the blood film and (B) hypersegmented granulocytes in the bone marrow aspirate.
On the day following his admission antimalarials were discontinued and subcutaneous vitamin B12 and intravenous folinic acid were started. The patient continued to have intermittent epistaxis and gum bleeding and required packed red cell and platelets transfusions. His admission was further complicated by a lobar pneumonia, for which he received intravenous broad spectrum antibiotics. He made a rapid recovery and by day 9 his haematological parameters had reversed (table 1), and the rash and hyperpigmentation have resolved after several months.
Discussion
Fifty per cent of chloroquine, and 70% of proguanil, are excreted by the kidneys. It is therefore recommended that doses should be lowered in patients with renal impairment. Chloroquine should be reduced to no more than 50 mg once daily in patients with a glomerular filtration rate (GFR) of 10–20 ml/min; it is contraindicated in patients with a GFR <10 ml/min. Proguanil should be administered at a dose of 50 mg once weekly in patients with creatinine clearance <10 ml/min.1 Haemodialysis and peritoneal dialysis are of no value in chloroquine overdose as the drug extensively binds to tissues2 and there is evidence that proguanil is not haemodialysed.3 No information on their clearance by peritoneal dialysis is available.
Chloroquine at the dose used in malaria prophylaxis is well tolerated. Mild gastrointestinal disturbance, headache, dizziness, pruritus and rashes have been commonly reported and appear to be more common in patients of African origin.2 At higher doses, and with chronic use in treatment of autoimmune disorders, agranulocytosis, thrombocytopenia and aplastic anaemia are well recognised, and exfoliative dermatitis, Steven‐Johnson syndrome and hepatitis have been noted,1 as seen in our patient. Retinopathy is a less frequent, but serious, complication. There are no reports in the literature of such complications occurring at the doses used for prophylaxis against malaria.
Proguanil, on the other hand, is the best tolerated antimalarial drug. There are, however, several case reports of proguanil induced acute megaloblastic anaemia in patients with renal failure and/or on dialysis with a similar clinical and haematological presentation to our patient.3,4,5 As with our patient, these patients had taken a short prophylactic course of proguanil at full dose and had presented with symptomatic pancytopenia with characteristic changes in the bone marrow. The bone marrow of patients with long standing renal impairment was hypocellular.4 Withdrawal of the offending drug and administration of folic/folinic acid led to the rapid recovery of haematological indices. End stage renal failure in our patient had resulted in high plasma concentration levels of both drugs, which induced marrow, skin and mucosal surface toxicity. Proguanil is the main offender in causing acute megaloblastic anaemia and pancytopenia, as well as contributing to dermatitis. Once the drug was stopped and folinic acid commenced, all haematological parameters in our case had reversed by day 9. However, the patient's dermatitis took several months to settle. Chloroquine's half‐life in a patient with normal creatinine clearance has been estimated at 30–60 days. It is probable that chloroquine added to the skin and mucosal toxicity.
Cells that undergo a rapid turnover, such as hematopoietic and enteric lining cells, are especially sensitive to any interference with DNA synthesis. Vitamin B12 and folic acid are required to synthesise DNA. Although the exact steps remain elusive, it is known that the transfer of a methyl group from methyl‐tetrahydrofolate (methyl‐THF) via vitamin B12 to homocysteine to form methionine has two important effects: it reduces the plasma concentration of homocysteine which is probably toxic to endothelial cells; and, perhaps more importantly, it results in the demethylation of methyl‐THF which is a critical step in DNA synthesis since it is THF—the reduced form of folate—that forms the substrate for purine synthesis and therefore DNA synthesis.6,7,8
Proguanil is a weak inhibitor and its metabolite, cycloguanil, is a strong inhibitor of dihydrofolate reductase, the enzyme responsible for THF formation, and is a rate limiting step that ultimately enables DNA synthesis. Its affinity to the plasmodial enzyme is 100–1000 times greater than to the human, hence in the context of normal renal elimination, no significant toxicity is observed. Folinic acid was given to the patient as this bypasses the block in folate metabolism resulting from the proguanil induced inhibition of dihydrofolate reductase. The mechanism of action of chloroquine is not fully understood and several theories exist. Chloroquine is an alkali and may interfere with cellular maturation by raising lysosomal pH, which leads to the loss of lysosomal function.9 Another explanation is that chloroquine interposes itself into double‐stranded DNA and inhibits both DNA and RNA synthesis, showing preference for the malarial parasite genome.10
Conclusion
This case highlights the potential life‐threatening toxicity of antimalarials, in particular, proguanil, when used in patients with renal impairment. With the recent advances in renal replacement therapy, dialysis patients are increasingly able to travel to areas where malaria is endemic. Increasing awareness of the risks of antimalarial prophylaxis in this setting is still required in spite of several case reports over the last two decades highlighting the risks. Strict policies on drug prescribing and dose reduction for patients with renal impairment should be set in place. A point for consideration could be that such patients ought to be folate replete before the use of antimalarials, and oral prophylaxis with folinic acid may be of use in this setting.
Acknowledgements
We would like to thank the patient for his permission to publish this case.
Footnotes
Competing interests: None declared
Contributors: NT, SA and SGA wrote the paper, MJ prepared the slides, WM, JDC and DAL looked after the patient during his admission. SA is the guarantor
References
- 1.Anon British National Formulary. London: British Medical Association, Royal Pharmaceutical Society of Great Britain, 2007
- 2.Davies D.et al eds. Davies's textbook of adverse drug reactions, 5th ed. London: Chapman & Hall Medical 1998
- 3.Tattersall J E, Greenwood R N, Baker R I.et al Proguanil poisoning in a haemodialysis patient. Clin Nephrol 198728104. [PubMed] [Google Scholar]
- 4.Boots M, Phillips M, Curtis J R. Megaloblastic anaemia and pancytopenia due to proguanil in patients with chronic renal failure. Clin Nephrol 198218106–108. [PubMed] [Google Scholar]
- 5.Sirsat R A, Dasgupta A. Haematological complications of proguanil in a patient with chronic renal failure. Nephron 199775108. [DOI] [PubMed] [Google Scholar]
- 6.Allen R H, Stabler S P, Savage D G.et al Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 199391334. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Pruthi R K. The biochemical basis of cobalamin deficiency. Mayo Clin Proc 199469181. [DOI] [PubMed] [Google Scholar]
- 8.Anthony C A. Megaloblastic anemias. In: Hoffman R, Benz EJ, Shattil SJ, et al eds. Hematology: basic principles and practice, 2d ed. New York: Churchill Livingston 1995552
- 9.Krogstad D J, Schlesinger P H. Acid vesicle function, intracellular pathogens, and the action of chloroquine against Plasmodium falciparum. N Engl J Med 1987317512–519. [DOI] [PubMed] [Google Scholar]
- 10.Meshnick S R. Chloroquine as intercalator: a hypothesis revived. Parasitology Today 1990677–79. [DOI] [PubMed] [Google Scholar]