Abstract
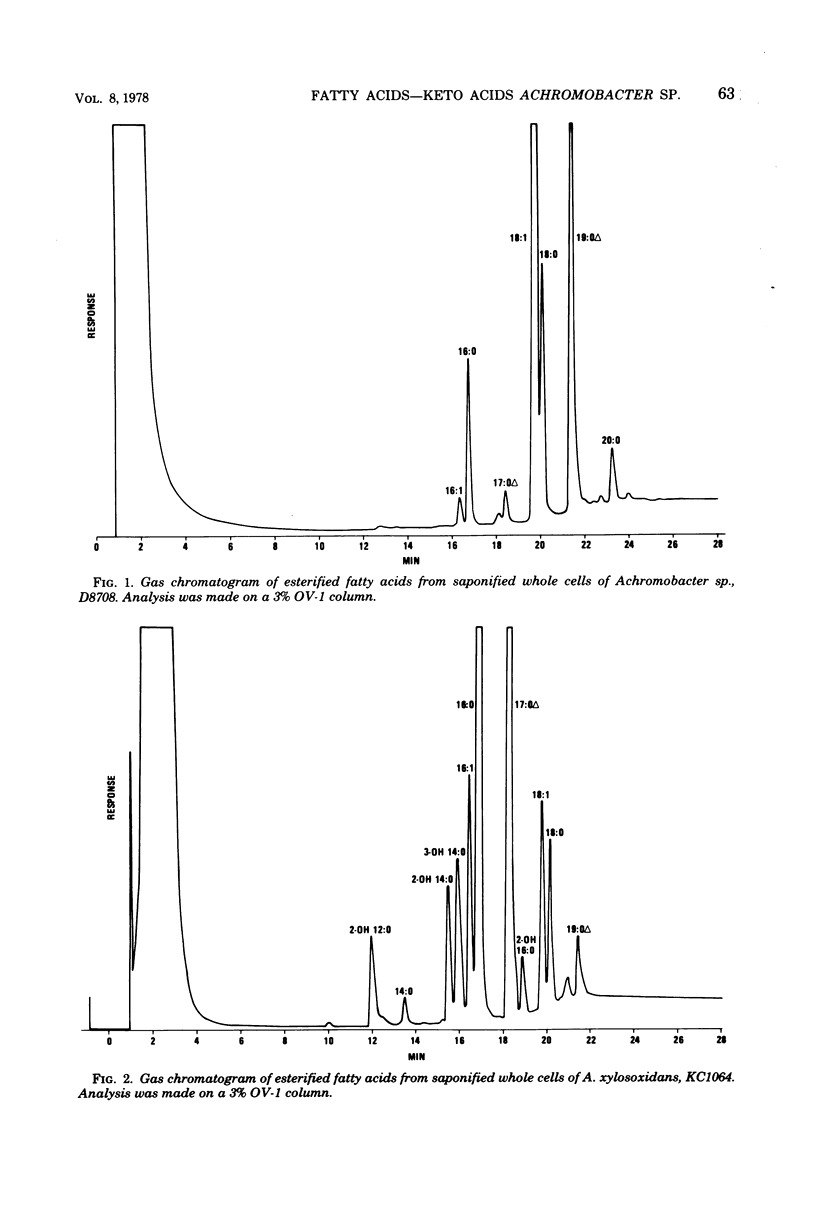
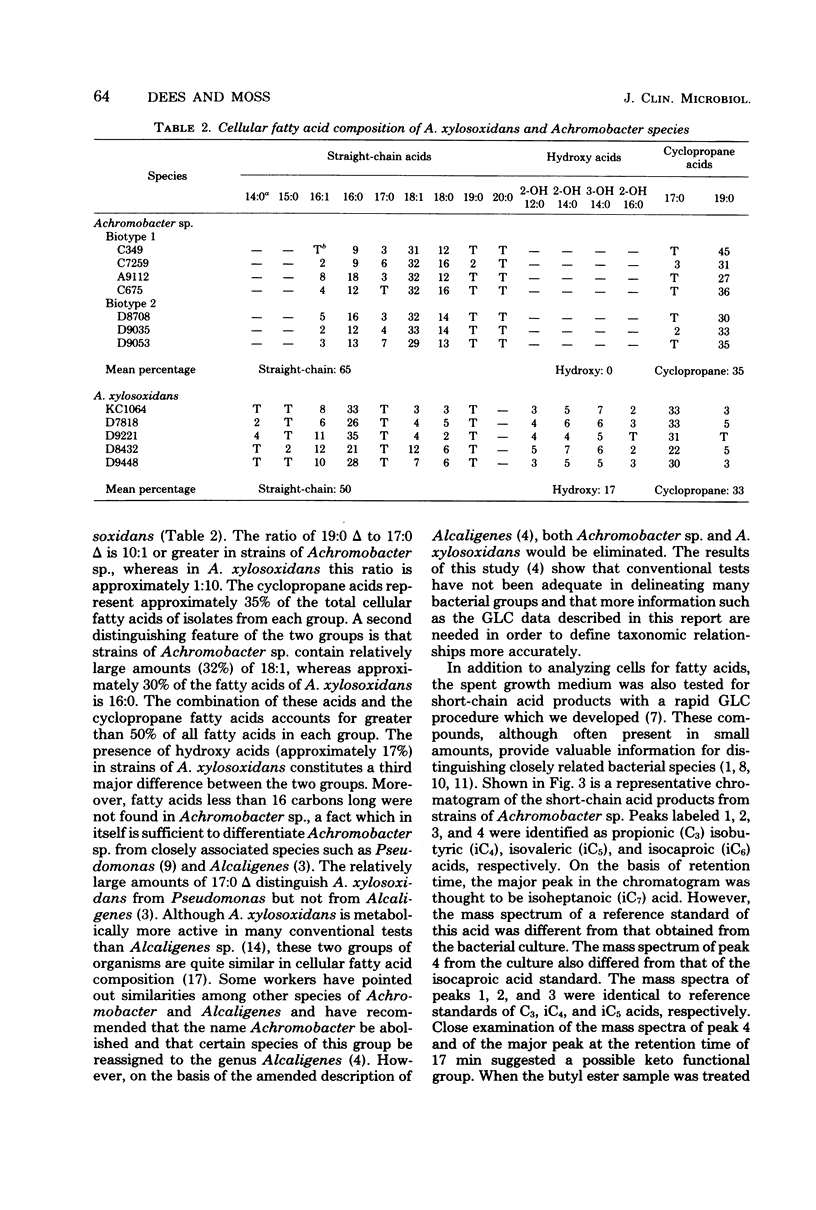
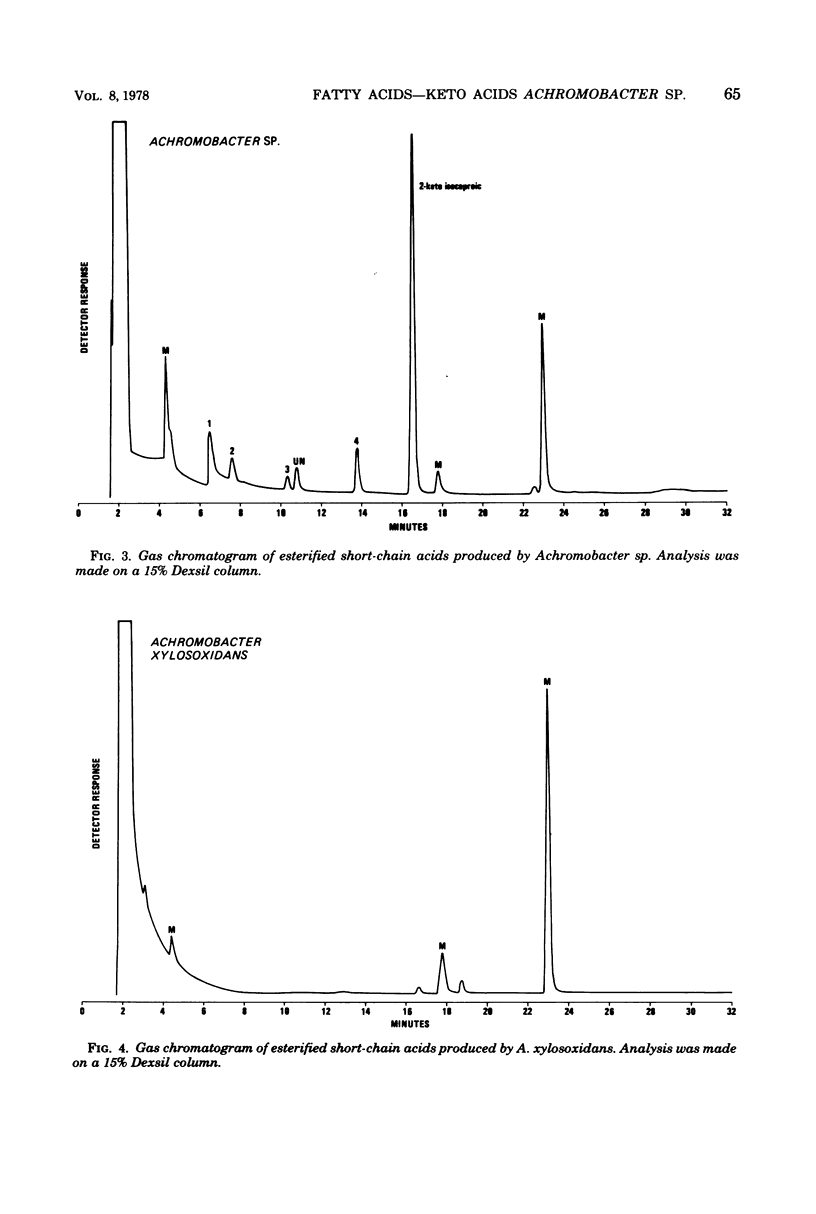
The cellular fatty acid composition and metabolic products of 12 reference strains of Achromobacter sp. and A. xylosoxidans were determined by gas-liquid chromatography (GLC). Results showed that the two Achromobacter groups are strikingly different and can be readily distinguished on the basis of cellular fatty acids and the short-chain acids produced by Achromobacter sp. The major cellular fatty acids of Achromobacter sp. were octadecenoic (18:1) and a 19-carbon cyclopropanoic (19:0 delta) acid, whereas hexadecanoic (16:0) and a 17-carbon cyclopropanoic (17:0 delta) acid were principal components of the lipids of A. xylosoxidans. Hydroxy acids were not found in strains of Achromobacter sp. but comprised approximately 20% of the cellular fatty acids of A. xylosoxidans. In addition, Achromobacter sp. produced relatively large amounts of 2-ketoisocaproic acid, which was detected in only trace amounts from strains of A. xylosoxidans. The data show that GLC tests provide additional criteria for differentiating groups which are very closely related when evaluated with conventional tests. The GLC tests can be readily adapted in the clinical laboratory because they are rapid, highly reproducible, relatively inexpensive, and simple to perform.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks J. B., Weaver R. E., Tatum H. W., Billingsley S. A. Differentiation between Pseudomonas testosteroni and P. acidovorans by gas chromatography. Can J Microbiol. 1972 Sep;18(9):1477–1482. doi: 10.1139/m72-226. [DOI] [PubMed] [Google Scholar]
- Choudhary G., Moss C. W. Gas chromatography-mass spectrometry of some biologically important short chain acid butyl esters. J Chromatogr. 1976 Dec 8;128(2):261–270. doi: 10.1016/s0021-9673(00)99257-4. [DOI] [PubMed] [Google Scholar]
- Dees S. B., Moss C. W. Cellular fatty acids of Alcaligenes and Pseudomonas species isolated from clinical specimens. J Clin Microbiol. 1975 May;1(5):414–419. doi: 10.1128/jcm.1.5.414-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Cellular fatty acids and metabolic products of Pseudomonas species obtained from clinical specimens. J Clin Microbiol. 1976 Dec;4(6):492–502. doi: 10.1128/jcm.4.6.492-502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Identification of microorganisms by gas chromatographic-mass spectrometric analysis of cellular fatty acids. J Chromatogr. 1975 Oct 29;112:594–604. doi: 10.1016/s0021-9673(00)99988-6. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Kaltenbach C. M. Production of glutaric acid: a useful criterion for differentiating Pseudomonas diminuta from Pseudomonas vesiculare. Appl Microbiol. 1974 Feb;27(2):437–439. doi: 10.1128/am.27.2.437-439.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Samuels S. B., Liddle J., McKinney R. M. Occurrence of branched-cahin hydroxy fatty acids in Pseudomonas maltophilia. J Bacteriol. 1973 Jun;114(3):1018–1024. doi: 10.1128/jb.114.3.1018-1024.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Samuels S. B. Short-chain acids of Pseudomonas species encountered in clinical specimens. Appl Microbiol. 1974 Mar;27(3):570–574. doi: 10.1128/am.27.3.570-574.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi E., Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971 Sep;15(5):477–481. doi: 10.1111/j.1348-0421.1971.tb00607.x. [DOI] [PubMed] [Google Scholar]


