Abstract
Human pharmacokinetic in vivo studies are often presumed to serve as the “gold standard” to assess product bioequivalence (BE) of immediate-release (IR) solid oral dosage forms. However, when this general assumption is re-visited, it appears that in vitro studies are sometimes better than in vivo studies in assessing BE of IR solid oral dosage forms. Reasons for in vitro studies to sometimes serve as the better method are that in vitro studies: (a) reduce costs, (b) more directly assess product performance, and (c) offer benefits in terms of ethical considerations. Reduced costs are achieved through avoiding in vivo studies where BE is self-evident, where biopharmaceutic data anticipates BE, and where in vivo BE study type II error is high. In vitro studies more directly assess product performance than do conventional human pharmacokinetic BE studies, since in vitro studies focus on comparative drug absorption from the two products, while in vivo BE testing can suffer from complications due to its indirect approach. Regarding ethical considerations, in vitro studies better embrace the principle “No unnecessary human testing should be performed” and can result in faster development. Situations when in vitro test should be viewed as preferred include Class I drugs with rapid dissolution, Class III drugs with very rapid dissolution, and highly variable drugs with rapid dissolution and that are not bio(equivalence)problem drugs. Sponsors of potential in vivo human pharmacokinetic BE testing should be required to justify why in vitro data is insufficient, similar to proposed animal testing requires justification to not employ an in vitro approach.
KEY WORDS: bioavailability, bioequivalence, biopharmaceutics, Biopharmaceutics Classification System, dissolution, in vitro, therapeutic equivalency
INTRODUCTION
On-Going Drug Product Quality
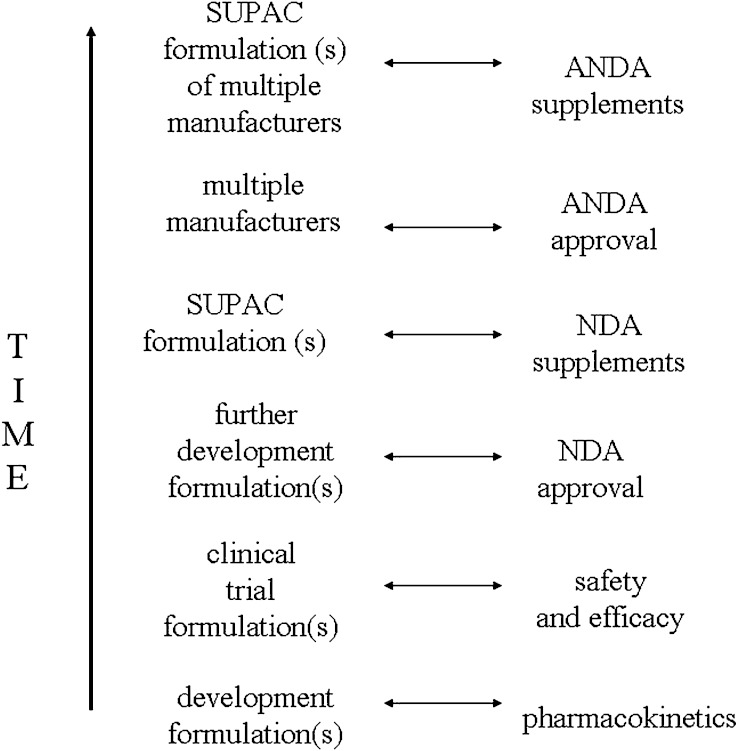
One major aim in the pharmaceutical sciences is the assurance of drug product quality throughout a drug’s life cycle. In the life cycle of any drug, formulation variants often include prototype formulations during early development, pivotal clinical trial formulations, further development formulations that are approved for marketing in a New Drug Application (NDA), innovator formulations that differ from their NDA formulations as a result of scale-up and post-approval changes (SUPAC changes), generic (i.e. multi-source) formulations that are approved as Abbreviated New Drug Applications (ANDAs), and generic formulations as a result of SUPAC changes. Figure 1 illustrates these formulation variants over time. These differing formulations are the result of (a) the normal course of drug product development where better formulation aspects are incorporated into the product during development, including during phase 3 clinical trials and after approval; (b) the normal course of needing to manufacture commercial product at larger scales of manufacture, as well as at different locations; (c) the normal course of generic competition; and (d) the desirable course of implementing improved manufacturing technologies.
Fig. 1.
Formulations in the normal course of a drug product’s life cycle. Even shortly after marketing of a new drug, the marketed formulation differ from the clinical trial formulation that demonstrated drug safety and efficacy, due to formulation changes in later development and SUPAC changes. In later stages of drug market life, formulations from several generic manufacturers are available, including generic formulations with SUPAC changes
Critical to these different formulations is the assurance that each product is bioequivalent (BE) to the clinical trial material that was shown to be safe and effective. In practice, a major contributor to this assurance is human pharmacokinetic in vivo bioequivalence (BE) testing, along with several other requirements (e.g. current good manufacturing practices, proper labeling, compendial requirements). A conventional human pharmacokinetic in vivo BE study employs a single dose, two period, two treatment, two sequence, open label, randomized crossover design comparing equal doses of the test and reference products in fasted, adult, healthy volunteers (e.g. n = 24); test and reference drug plasma profiles are compared. The comparison of test and reference drug plasma profiles to demonstrate BE is the most commonly used and successful biomarker. It would seem practically impossible to assure on-going drug product BE and quality of pharmaceuticals without methods that are substantially less resource-intensive than clinical safety and efficacy trials.
Clinical safety and efficacy trials are generally not required to demonstrate BE. 21 CFR 320.1(f) indicates the “[b]ioequivalence requirement means a requirement imposed by the Food and Drug Administration for in vitro and/or in vivo testing of individual drug products which must be satisfied as a condition of marketing” (1). The Food and Drug Administration (FDA) announced a web site to communicate recommendations concerning the design of BE studies of specific drug products to support ANDAs (2). A review of this web site describing 260 newly added and individual drug recommendations (3) indicates that clinical safety and efficacy trials generally are not required for IR solid oral dosage forms. In fact, clinical safety and efficacy trial is not recommended in any case for any drug in an IR solid oral dosage form on this web site. This preference against clinical studies reflects the FDA’s previous assessments in 21 CFR 320.24 and recent guidance that comparative clinical trials are generally insensitive in BE testing and should be avoided where possible (1,4). Comparative clinical studies are can be appropriate only when a pharmacokinetic approach or pharmacodynamic approach is infeasible (4).
Best Alternative to Comparative Clinical Trials
Given the need to assure drug product quality throughout the drug product life cycle and limitations of comparative clinical trials, what method is the best method to assess BE? Bioequivalence is defined in 21 CFR 320.1 as the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study (1).
Recent FDA guidance indicates that several in vivo and in vitro methods can be used to establish BE and include, in descending order of preference: pharmacokinetic studies, pharmacodynamic studies, clinical studies, and in vitro studies (4). FDA indicates that an in vivo study is generally recommended for all IR solid oral dosage forms approved after 1962 and for bioproblem drug products approved before 1962 (4). This viewpoint is well reflected in the FDA web site describing 260 individual drug recommendations (3), where a conventional human pharmacokinetic in vivo BE study in healthy volunteers is recommended for IR solid oral dosage forms in all but three of the following cases. Benzphetamine HCl tablets and benzonatate capsules may each be considered for in vivo biowaiver if test and reference in vitro dissolution profiles are comparable. A steady-state pharmacokinetic study in patients is recommended for the anti-cancer product mercaptopurine tablets. In no case is either a pharmacodynamic study or clinical study recommended. These observations are consistent with the prior FDA assessment that pharmacodynamic studies are not recommended for orally administered drug products when the drug is absorbed into the systemic circulation and a pharmacokinetic approach can be used to assess systemic exposure and establish BE (4).
It is accepted here that pharmacokinetic studies are generally preferred over pharmacodynamic studies as an alternative to comparative clinical studies (4). It is also accepted here that in vivo and in vitro methods can be used to establish BE (4). However, it is not clear as to why today that pharmacokinetic studies are generally preferred over in vitro studies. This commentary re-visits the frequent presumption that human pharmacokinetic in vivo studies should be the “gold standard” to assess BE for IR solid oral dosage forms. The objective of this paper is to describe reasons why in vitro studies are sometimes better than conventional human pharmacokinetic in vivo studies in assessing BE for IR solid oral dosage forms. Reasons are that in vitro studies: (a) reduce costs, (b) more directly assess product performance, and (c) offer benefits in terms of ethical considerations. Situations when in vitro test should be viewed as preferred include Class I drugs with rapid dissolution (i.e. 85% in 30 min or less in pH 1.2, 4.5 and 6.8 media), Class III drugs with very rapid dissolution dissolution (i.e. 85% in 15 min or less in pH 1.2, 4.5 and 6.8 media), and highly variable drugs with rapid dissolution and that are not bio(equivalence)problem drugs.
REASON 1: IN VITRO STUDIES REDUCE COSTS
In vitro studies achieve reduced costs through avoiding in vivo studies where BE is self-evident, where biopharmaceutic data anticipates BE, and where in vivo BE study type II error is high. Prior to discussing these three situations, motivation for reducing drug cost is briefly described.
Motivation to Reduce Drug Costs Through Cost-Effective Testing
While there is little doubt that medicines offer tremendous health benefits and are often cost-effective in comparison to other treatment alternatives, it is also true that the cost of specific medicines for specific patients is problematic. Research indicates that up to 32% of elder adults take fewer drugs than prescribed due to cost (5–7). The cost-related medication nonadherence results in some patients not achieving the full therapeutic benefits of therapy, suffering reduced health, exhibiting increased risks of adverse cardiac events, and requiring more emergency and institutional services (6,7). While prescription drugs compose only 10% of the US health costs (8), reduced costs through less expensive yet at least equi-effective product quality testing would seem desirable and achievable. With this assumed motivation, in vitro studies can sometimes serve as the better method than conventional human pharmacokinetic in vivo studies due to reduced costs.
Reduce the Cost Where BE is Self-Evident
In vitro studies achieve reduced costs by avoiding in vivo studies where BE is self-evident. In the context of the Biopharmaceutics Classification System (BCS) (9), Class I drugs are drugs with high solubility and high permeability. Rapidly dissolving IR formulations of solid dosage forms of Class I drugs represent scenarios where BE is self-evident. Cook and Bockbrader examine the potential cost savings of using BCS-based biowaviers for Class I drugs, in lieu of in vivo BE testing (10). They considered the number of BE studies performed by the pharmaceutical industry between January 1998 and May 2001 and assumed 25% of BE studies are for Class I drugs. They conservatively estimated in 2002 that “there is the potential to save one quarter the annual expenditures on bioequivalence studies, $22 to $38 million dollars/year.” The authors only considered direct costs of testing and indicate that additional indirect savings can occur if BE studies are rate limiting to drug regulatory submission (e.g. avoid lost sales of over one million dollars per day if product leads to sales of $400 million per year) and if opportunity costs are considered (e.g. resources not deployed to running in vivo studies can be redeployed to bring other drugs to market faster). While several tens of millions of dollars of saving each year can be viewed as minimal impact even if fully transferred to patients, it would appear that this level of direct savings is preferred over no level of direct saving, particularly since biowaivers of rapidly dissolving IR formulations of solid dosage forms of Class I drugs represent scenarios where BE is self-evident.
In Vitro Type I Risk
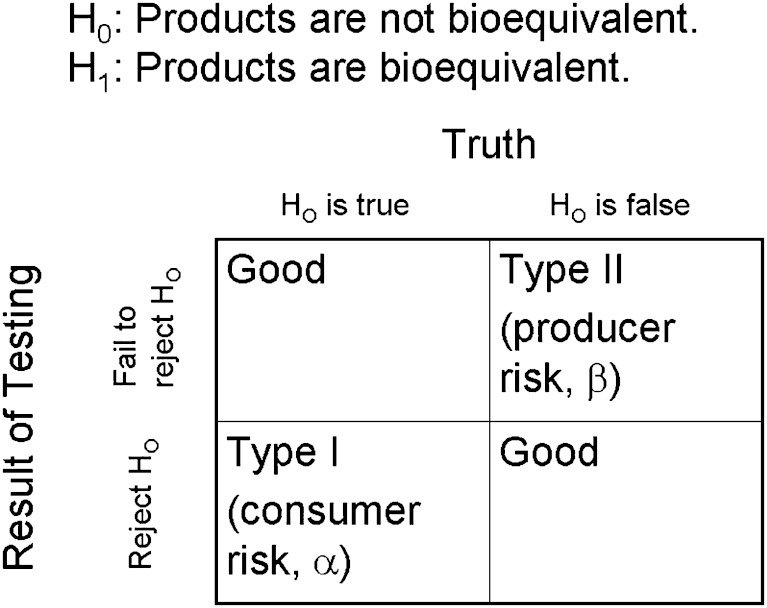
The above discussion assumes BE is self-evident for Class I drugs. This assumption is based upon the FDA’s and EMEA’s implementation of the BCS guidance about eight years ago (9,11), without either agency retracting or narrowing the guidances. Since the primary regulatory concern about BE is the protection of patients against approval of products that are not BE (12), a concern is the frequency that Class I drugs have passed with rapid dissolution but failed in vivo BE testing. Figure 2 illustrates Type I and Type II errors in the context of BE testing. Assuming conventional human pharmacokinetic in vivo BE testing is a perfect indication of whether products are BE, the extent that products pass Class I with rapid dissolution but fail in vivo BE testing is analogous to the Type I error rate of in vitro testing.
Fig. 2.
Bioequivalence, Hypothesis Testing, and Errors. In BE testing, the null hypothesis states that products are not BE, while the alternate hypothesis states that products are BE. Type I error occurs when products are erroneously concluded to be BE when they are not BE. Type I error represents a risk to the consumer (i.e. a health risk to the patient). Type II error occurs when products are erroneously concluded to be not BE when they are BE. Type II error represents a risk to the producer
Presentations report no documented BE failures for Class I drugs in the USA (13,14). A scientist at RIVM in the Netherlands has also indicated that there are no known BE failures for Class I drugs in the European Union [Personal communication from Dirk M. Barends (RijksInstituut voor Volksgezondheid en Milieu, Netherlands); March 15, 2007]. It appears that there have been no incidents of Type I errors in the use of in vitro testing to assess BE of Class I drugs in the USA or European Union (EU).
Reduce the Cost Where Biopharmaceutic Data Anticipates BE
In addition to scenarios where BE is self-evident, in vitro studies achieve reduced costs through avoiding in vivo studies where biopharmaceutic data anticipates BE. Specifically, in vitro studies avoid in vivo studies of rapidly dissolving IR formulations containing a BCS class III drug. Class III drugs exhibit high solubility and low permeability. While the FDA and EMEA BCS guidances, which remain unchanged since their implementations in 2000 and 2001 respectively, do not allow for Class III biowaivers, scientific consensus supports biowaivers for at least some Class III drugs whose formulations exhibit very rapid dissolution (15). Scientific support for such Class III biowaivers continues (16,17). As above, observations from FDA and EU scientists supports such biowaivers, as the risk for very rapidly dissolving Class III drugs to fail in in vivo BE testing is low.
Cook and Bockbrader conservatively calculated that $22 to $38 million dollars/year could be directly saved by employing BCS-based biowaivers for Class I drugs, assuming 25% of BE studies are for Class I drugs (10). Applying the same analysis to Class III drugs and assuming 25% of BE studies are for Class III drugs (18–20), another $22 to $38 million dollars/year could be directly saved by employing BCS-based biowaivers. Together, biowaivers for Class I and III drugs has the potential to directly save $44 to $76 million dollars/year in in vivo BE study expenditures.
The assumption that 50% of drugs are either Class I or III is reasonable, if not conservative. Takagi et al. (2006) provisionally BCS classified the orally administered, IR drug products in the top 200 drug product lists from the United States, Great Britain, Spain, and Japan. From these four lists, compounds were 30–36%, 30–34%, 19–28%, and 3–7% in BCS Class I, II, III, and IV, respectively (18). More than 50% on each list were determined to be high-solubility drugs (55–59%). This observation agrees with that of Benet and Wu, who, in proposing a Biopharmaceutics Drug Disposition Classification System (BDDCS), extensively examined 169 drugs in the WHO Essential Medicines List. These 169 compounds showed 39%, 30%, 26%, and 8% for BDDCS Class I, II, III, and IV, respectively (19). These distributions are further supported by Khandelwal et al., where drug disposition data for 56 previously unclassified drugs was obtained from an extensive literature search (20). These 56 compounds were distributed within BDDCS Class I, II, III, and IV as 47%, 20%, 25%, and 9%, respectively.
While there is scientific consensus and on-going support for such Class III biowaivers (15,16), there are a number of potential concerns (16). A comprehensive analysis of results of conventional human pharmacokinetic in vivo BE testing of Class III IR products, similar to what has previously been presented (13), would be beneficial. Such analysis has potential to measure the Type I error of in vitro BE testing for BCS class III drugs.
Reduce the Cost of Type II Errors
In vitro studies also achieve reduced costs through avoiding in vivo studies where in vivo BE study type II error is high. Highly variable drugs (HVDs) are drugs with high within-subject variabilities (ANOVA-CV ≥ 30%) in Cmax and/or AUC (21). HVDs typically have flat dose response curves and large therapeutic windows, such that clinically important adverse drug reactions (ADRs) occur at much higher doses than those required for efficacy. Currently in the USA, the same conventional BE statistical analysis [i.e. AUC and Cmax; log-transformed data; ANOVA model with period, sequence, subject(seq), and treatment; and 90% confidence intervals must fit between 80–125%] is applied to HVDs, as well as non-HVDs. It is well appreciated that HVDs often require a greater numbers of subjects than non-HVDs, in order to avoid type II error. Figure 2 illustrates Type II errors in the context of BE testing. Type II error occurs when products are erroneously concluded to be not BE when they are in fact BE. High variability is a frequent basis for low in vivo BE study power, necessitating larger number of subject to achieve sufficient power. Tanguay et al. examined over 1,200 BE studies performed between 1992 and 2002 (22) and observed “[d]rug formulations associated with an intra-individual variability of 35% or more failed to meet BE criteria at an astronomic rate of 85%.”
In spite of this pattern of high in vivo BE testing failure for HVDs, evidence indicates that high variability is frequently not due to poor product quality, even though the identification of products with poor quality is a central goal in BE testing. Davit et al. collected data from all in vivo BE studies reviewed at FDA’s Office of Generic Drugs from 2003–2005 (23). The review entailed over 1000 in vivo BE studies of 180 different drugs, of which 31% were highly variable. Of these HVDs, 51%, 10%, and 39% were either consistently, borderline, or inconsistently highly variable, respectively. Drug substance pharmacokinetic characteristics and drug product dissolution were considered to cause high variability. About 60% of the HVDs were highly variable due to drug substance pharmacokinetic characteristics. Formulation performance contributed to the high variability only about 20% of the time.
This perspective that conventional human pharmacokinetic in vivo BE testing is problematic and costly for HVDs has motivated the development of several novel in vivo BE methodologies and possible alternative acceptance criteria for HVDs. Buice et al. (24) state “[u]nreasonable bioequivalence costs, necessitating excess studies can only increase this [consumer] cost. ... Findings further suggest that the 90% confidence interval criteria should be adjusted for highly variable drugs.” Rather than loosening the BE criteria, it is suggested here that in vitro BE testing may be a better approach for HVDs, particularly if the drug’s biopharmaceutic properties are favorable and formulation performance is not suspect.
Estimating the potential direct cost savings by employing BCS-based biowaivers for HVDs is complicated by several factors. One factor is that in vivo BE testing of HVDs uses larger number of subjects than testing of non-HVDs. Another factor is that in vivo BE studies with increasingly larger numbers of subjects (i.e. drugs with increasing larger variability) suffer from the highest rates of failure, largely due to type II error. For example, the failure rate of studies using n = 49 to 60 subjects was three times larger than the failure rate of studies using n = 37 to 48 subjects (22). The potential direct cost savings for HVDs would seem at least as large as that for either Class I or Class III drugs. It should be noted that some HVDs are Class I and Class III drugs. Potential indirect savings (e.g. more rapid product development by reducing erroneous BE failures; freed resources now available for other projects) also seems very substantial.
Summary for In Vitro Studies Reduce Costs
The pharmaceutical science community should be motivated to reduce drug costs through cost-effective BE testing, as cost of medicines is problematic for some patients. About one-third of elder adults take fewer drugs than prescribed due to cost (5–7), causing reduced health that results from cost-related medication nonadherence. In vitro studies can sometimes serve as the better method than conventional human pharmacokinetic in vivo studies due to reduced costs. In vitro studies achieve reduced costs through avoiding in vivo studies where BE is self-evident, where biopharmaceutic data anticipates BE, and where in vivo BE study type II error is high.
REASON 2: IN VITRO STUDIES MORE DIRECTLY ASSESS DRUG PRODUCT PERFORMANCE
A second reason for in vitro studies to sometimes serve as the better BE method is that in vitro studies more directly assess product performance than do conventional human pharmacokinetic in vivo BE studies. In vitro studies focus on comparative drug absorption from the two products, while in vivo BE testing can suffer from complications due to its indirect approach.
In Vitro Studies Focus on Drug Absorption
Drug absorption is composed of the processes of drug release from the dosage form (i.e. dissolution) and drug permeation from the gastrointestinal lumen. While the pharmacokinetic metrics Cmax and AUC are by far the most common measures to assess BE in practice, neither the definition of bioequivalence nor the definition of the bioequivalence requirement (1) references Cmax or AUC, or even refer to pharmacokinetic plasma profiles. In fact, neither definition necessarily requires in vivo studies. Rather, Cmax and AUC are commonly used as metrics for the rate and extent of drug absorption. The definitions of bioavailability (1) and bioequivalent drug products (12), as well as the conditions under which products are considered bioequivalent (25), feature drug absorption rather than pharmacokinetic plasma profiles. Appendix 1 lists definitions of several terms, including bioequivalence, bioequivalence requirement, bioavailability, and bioequivalent drug products.
In vitro studies more directly assess drug absorption than do in vivo BE studies. In vitro dissolutions methods and in vitro (and in situ) permeation methods are broadly established. Compendial dissolution apparati are standardized. Dissolution specifications are routinely used to characterize product batch quality. In vitro (and in situ) permeation methods are used in many laboratories throughout the world at various stages of drug developing, from early discovery in screening for favorable permeability to regulatory applications in seeking BCS-based Class I biowaivers (16). The study of drug permeability and best permeability practice methods are a focus of academic drug delivery.
Limitations exist in in vitro dissolution testing and in vitro (and in situ) permeability testing. For example, there is no single universal dissolution media that a priori predicts in vivo drug dissolution. There is no single vitro (and in situ) permeability test condition that mimic the complex intestinal mucosa that drug can “see” over the course of it entire lifetime within the gastrointestinal lumen. In spite of the limitation that no single dissolution test condition or permeability test condition fully reflects in vivo conditions, multi-condition dissolution testing and multi-condition permeability testing address such limitations. Multi-condition dissolution testing and multi-condition permeation testing use a number of test conditions (e.g. multiple pH levels). In particular, the application of multi-condition in vitro dissolution and permeation testing within a drug absorption conceptual framework provides a focus on comparative drug absorption, where in vitro results have in vivo meaning in comparing products, including direct relevance to the term bioequivalent drug products and conditions under which products are considered bioequivalent (Appendix 1).
Complications of In Vivo BE Testing
Conventional human pharmacokinetic in vivo BE testing suffers from complications due to its indirect approach. Compared to the measurement of drug dissolution and drug permeability, pharmacokinetic plasma profiles represent an indirect approach to measure drug absorption. Post-absorption events such as metabolism and enterohepatic recycling can result in complex and variable pharmacokinetic profiles. These post-absorptive events can have little relevance to drug product quality or the rate and extent of drug absorption. For example, in one comprehensive survey, about 60% of HVDs were highly variable due to drug substance pharmacokinetic characteristics, rather than drug product characteristics (23). In particular, 83% of drugs that exhibit consistent or borderline high variability showed extensive first pass metabolism. Meanwhile, only 21% of drugs that are not highly variable show extensive first pass metabolism. This survey, in concert with the high rate of type II error for HVDs (22), indicates that extensive first pass metabolism is a basis for in vivo BE testing to function as an indirect approach to measure drug absorption, and at times a poor approach. It should be noted that while post-absorption metabolism can have little relevance to drug product quality or the rate and extent of drug absorption, the extent of first-pass metabolism can dependent on dissolution rate (e.g. clinically saturable first-pass metabolism).
Enterohepatic recirculation is also a post-absorption process that can modulate plasma profiles. It can cause drug to be secreted into bile after primary drug absorption, where drug is then exposed to the gut again, from which drug can be re-absorbed again. This secondary absorption can result in a second peak in the plasma profile and further plasma drug exposure. For drugs that are enterohepatically recycled, the hepatobiliary system impacts plasma profile kinetics, resulting in in vivo BE testing to function as an indirect approach to measure drug absorption. Within the context of BE, there appears to be no evidence that the enterohepatic recycling of drugs is formulation dependent. Rather, the hepatobiliary system is a post-absorptive system that is composed of several organs and tissues, and which is subject to several levels of physiologic control, including hormonal control. For drugs that are enterohepatically recycled, it is possible that even the subtlety of anticipating eating impacts the hepatobiliary system and drug plasma profile (26).
An additional scenario where in vivo BE testing suffers from its indirect approach is when the in vivo BE testing employs multiple dosing. Although infrequent, these situations occur when drug toxicity is high, such that in vivo studies cannot use healthy volunteers, but only patients. Since patients on maintenance therapy require multiple dosing regimens, in vivo BE studies are performed as multiple dose. It is well appreciated that pharmacokinetic profiles from multiple dosing typically reflect not only the most recent dose, but many of the most recent doses. Multiple dosing in vivo BE studies are viewed as less sensitive than single dose in vivo BE studies.
The In Vivo BE Standard is Not a Single Standard
These complications of conventional human pharmacokinetic in vivo BE testing manifest in the in vivo BE standard actually not being a single standard. The numerous BE criteria and proposals are indicative that in vivo BE testing is not a direct assessment of product performance, but an indirect assessment that can be confounded by non-product factors [e.g. within-subject variability in absorption, distribution, metabolism and excretion (ADME), enterohepatic recirculation]. For example, the Canadian agency does not require a confidence interval for Cmax, but corrects for drug content; FDA requirements differ. The CPMP/EMEA guideline allows broadening the BE limits (e.g. 75–133%) under certain situations. There are also proposals to broaden the BE limits according to the within-subject variability of the reference. Additionally, in vivo BE testing is subject to metric issues, where Cmax is not viewed as an ideal metric for rate, such that early exposure may sometime need to be measured. These limitations of in vivo BE testing have been frequently discussed, resulting in a range of different criteria to assess BE from pharmacokinetic data.
Summary for In Vitro Studies More Directly Assess Product Performance
A second reason for in vitro studies to sometimes serve as the better BE method is that they more directly assess product performance than do conventional human pharmacokinetic in vivo BE studies. For BE, product performance is intended to be aimed as comparative drug absorption, and not comparative pharmacokinetic profiles. In vitro studies more directly focus on comparative drug absorption from the two products. Multi-condition in vitro dissolution and permeation testing, along with a drug absorption conceptual framework, provides data that has focus toward, and in vivo meaning to, comparative drug absorption, including direct relevance to the term bioequivalent drug products and conditions under which products are considered bioequivalent. Additionally, because of its indirect approach, in vivo BE testing can suffer from complications such as post-absorptive metabolism, enterohepatic recirculation, and the uncommon situation of multiple dosing. The fact that the in vivo BE standard is not a single standard reflects limitations of in vivo BE testing in some circumstances and highlights when in vitro studies may be better.
REASON 3: IN VITRO STUDIES OFFER BENEFITS IN TERMS OF ETHICAL CONSIDERATIONS
A third reason for in vitro studies to sometimes serve as the better BE method is that in vivo studies better embrace the principle “No unnecessary human testing should be performed” and can result in faster development.
Better Embrace “No Unnecessary Human Testing”
For the US, 21 CFR 320.25(a) codifies the universal belief that “No unnecessary human testing should be performed” (1). Interestingly, 21 CFR 320.25(a) reads “The basic principle in an in vivo bioavailability study is that no unnecessary human research should be done.” This statement may at first appear oxymoronic by advocating minimal human research, while assuming an in vivo study is necessary. However, the scope of 21 CFR 320.25 is the guidelines for conducting an in vivo bioavailability study, so this statement is simply advocating aspects like using the fewest number of human subjects when human testing is conducted. However, it is interesting that 21 CFR 320 explicitly make no general preference against unnecessary human research or the preference for in vitro testing when in vitro testing is sufficient. Rather, recent FDA guidance indicates that in vitro studies are less preferable than pharmacokinetic studies, and even less preferable than pharmacodynamic studies and clinical studies (4). In spite of this recent guidance, FDA’s granting of BCS-based biowaivers for Class I drugs whose IR formulations exhibit rapid dissolution (16) suggests that in vitro studies are not less preferred in practice than pharmacodynamic studies and clinical studies.
In vivo BE testing is generally safe, where the majority of ADRs are mild (27). In particular, BE studies after drug has been approved as safe and effective can be expected to be generally safe. Adding to this safety is that conventional in vivo BE testing is single dose, limiting drug exposure. However, ADRs have occurred in BE testing. Aripiprazole treatments schizophrenia and bipolar I disorder. The reference listed drug (RLD) for aripiprazole is now the 5 mg tablet and not the 30 mg strength (12). The 30 mg strength caused ADRs in healthy volunteers, such that the lowest strength rather than highest strength is now used in BE testing of aripiprazole [Personal communication from Chris Hendy (Novum Pharmaceutical Research Services, Pittsburg, PA); March 7, 2007]. Clozapine also exemplifies that serious ARDs can occur in BE testing. The FDA guidance on clozapine BE testing (28) reads “In the 1996 guidance, the Agency recommended that doses of clozapine tablets be administered to healthy subjects ... Because a high number of healthy subjects experienced serious adverse effects such as hypotension, bradycardia, syncope, and asystole during clozapine bioequivalence studies, FDA is recommending that studies not be conducted using healthy subjects. In addition, a single-dose study using a 12.5 mg dose is no longer recommended. Instead, this guidance recommends a multiple-dose bioequivalence study conducted in patients using the highest dosage strengths (e.g., 100 mg tablets).”
As illustrated in Fig. 1, BE testing generally occurs during product development, prior to NDA filing. A typical NDA includes three to four BE studies (10,29). The question of what risk level is acceptable in research studies performed in healthy volunteers is persistent question (30). Peroxisome proliferator-activated receptor (PPAR) agonists are a drug class with significant potential. Over 50 INDs of PPAR agonists have commenced. However, numerous development programs of PPAR agonist have been terminated due to safety concerns (31). In 1997, troglitazone was approved and then removed three years later because of liver failure. While it is not evident that BE studies of experimental compounds have caused major ADRs, the philosophy that no unnecessary human testing should be performed would seem to favor in vitro BE testing over in vivo BE testing when in vitro BE testing is suitable, particularly if compound safety has not been established.
Several questions can be formulated around the issue of the ethics of conducting in vivo BE testing. Is it ethical to conduct an in vivo BE test for an IR solid oral dosage form containing a BCS Class I drug that would otherwise receive a BCS-based biowaiver? It would appear difficult to argue that the answer is “yes”. Is it ethically desirable to replace in vivo BE testing with in vitro BE testing? Animal testing may provide some insight into this most basic question. Institutional Animal Care and Use Committees (IACUCs) strongly promote the replacement of animal testing with non-animal alternatives. In proposing animal testing to IACUC, investigators typically must describe potential alternatives to animal testing, including why such alternatives are not preferred. Investigators must also describe how the proposed animal testing does not cause unnecessary duplication. Typically, investigators must cite literature searches using two different databases that support that in vitro and/or computer modeling alternatives are not preferred. A corollary to the question “Is it ethically desirable to replace in vivo BE testing with in vitro BE testing?” is “Should Institutional Review Boards (IRBs) strongly promote the replacement of in vivo BE testing with non-in vivo BE testing alternatives”. It would seem that the answer is “yes”.
In Vitro Studies Can Result in Faster Development
In Fig. 1, pre-approval BE studies are common within a development program. A typical NDA includes three to four BE studies (10,29) and can be rate limiting to drug development. One situation is when BE study results are needed before any further product development (10). Another situation is a final BE study is the last document needed for NDA filing (32). In vitro studies can be typically completed in less time (e.g. two months) than an in vivo BE study. In addition to having financial implications for the sponsor, these delays have implications for patients and the ethics of making therapies available to patients as soon as possible.
Summary for In Vitro Studies Offer Benefits in Terms of Ethical Considerations
A third reason for in vitro studies to sometimes serve as the better BE method is that in vitro studies offer benefits in terms of ethical considerations. Compared to conventional human pharmacokinetic BE in vivo studies, in vivo studies better embrace the principle “No unnecessary human testing should be performed” and can result in faster development.
SITUATIONS WHEN IN VITRO BE TESTING IS PREFERRED
Situations when in vitro BE testing should be viewed as preferred over conventional human in vivo BE testing include Class I drugs with rapid dissolution, Class III drugs with very rapid dissolution, and highly variable drugs with rapid dissolution and that are not bio(equivalence)problem drugs.
Class I and Class III Drugs
The scientific basis for BCS-based biowaivers of IR solid oral dosage forms containing a Class I drug is well accepted (9,11,15,16,33). Such biowaivers require test product to exhibit rapid dissolution (i.e. 85% in 30 min or less) in pH 1.2, 4.5 and 6.8 media, to dissolve similarly to reference, and to contain only certain types and quantities of excipients, along with other requirements (e.g. therapeutic index).
Scientific support continues for biowaivers for Class III compounds whose formulations exhibit very rapid dissolution (i.e. at least 85% in 15 min) (15,16,33). Rationale for such Class III biowaivers is that these products with very rapid dissolution perform like an oral solution in vivo, since intestinal permeability limits drug absorption. Formulation does not modulate BE results of these products when they meet this dissolution requirement and the other requirements for BCS Class I biowaivers (e.g. excipient limitation). This rationale for Class III biowaivers for very rapidly dissolving products is further supported by the regulatory practice of allowing biowaivers of oral solutions of Class III drugs.
Highly Variable Drugs
In vitro BE testing is preferred over in vivo BE testing for HVDs with rapid dissolution and that are not bio(equivalence)problem drugs. A review of over 1,000 BE studies from 2003–2005 suggests that about 31% of drugs are highly variable (23). However, it is difficult to estimate the annual frequency of in vivo BE studies of HVDs with rapid dissolution, since in vitro dissolution data in pH 1.2, 4.5 and 6.8 media is needed. Never the less, HVDs with rapid dissolution and that are not bio(equivalence)problem drugs appear to be excellent candidates for in vitro BE testing. HVDs typically have flat dose response curves and large therapeutic windows. Such products would benefit from in vitro testing since in vitro testing reduces costs, more directly assess product performance, and offer benefits in terms of ethical considerations.
REFUTING RATIONALE FOR ALWAYS PREFERRING IN VIVO BE TESTING
Four potential reasons for always favoring in vivo BE may be that conventional human in vivo BE testing is the “gold standard” and always has been, that in vivo BE is well accepted, that in vivo BE testing is perfectly designed to assess product equivalence, and that in vitro BE testing and in vivo BE testing can provide different results. Weaknesses in these rationales are discussed.
Conventional Human In Vivo BE Testing is the “Gold Standard” and Always Has Been
In recent decades, the single dose, two period, two treatment, two sequence, open label, randomized crossover design comparing equal doses of the test and reference products in fasted, adult, healthy volunteers has been the most common method to assess BE. However, this approach as the current “gold standard” reflects the historically wide utilization of this approach, rather than perhaps its relative merits against in vitro BE testing. It does not seem reasonable to discount in vitro BE testing as a better in some situation just because in vivo BE is most common and represents the “gold standard”. As described above, human pharmacokinetic in vivo BE testing does not follow a single standard anyway.
In vivo BE is not even recommended in all cases in current practice. Benzphetamine HCl tablets and benzonatate capsules may each be considered for waiver of in vivo BE testing provided test and reference in vitro dissolution profiles are comparable (3). Benzphetamine HCl tablets are Drug Efficacy Study Implementation-effective (DESI-effective) drug products without known BE problems, such that in vivo BE testing is not requested. Benzonatate capsules are soft gelatin capsules, where the 100 mg dose was approved prior to January 1, 1982. From FDA’s OGD web site (3), conventional human pharmacokinetic studies are by far the most recommended method to demonstrate BE, but pharmacokinetic studies are waived in many cases for lower doses, per 21 CFR 320.22(d) (2) based on (a) acceptable BE studies on the highest strength, (b) proportional similarity of the formulations across all strengths, and (c) acceptable in vitro dissolution testing of all strengths (1). Additionally, the FDA allows SUPAC changes in excipients, manufacturing site, manufacturing batch size, and manufacturing process/equipment to be allowed based upon in vitro tests, for both IR and modified release products (34–36).
In addition to in vivo BE not even being recommended in all cases, in vitro BE testing has a long history of use. 21 CFR 320.33 has provided criteria to assess actual or potential BE problems. In the latter 1970s, drug products that had met these criteria were deemed “bioproblem” drug products. In vitro studies were expected to correctly assess BE for products that were not “bioproblem” drug products. For IR products not containing a “bioproblem” drug, FDA allowed DESI-effective drugs to be assessed for BE through in vitro studies alone. Since 1979, such products that passed BE testing were assigned an AA rating in FDA’s “Approved Drug Products with Therapeutic Equivalence Ratings”. 21 CFR 320.24 also describes situations when in vitro studies can be used alone to document BE.
Like the USA, Germany has had a history of using in vitro BE testing. The German drug agency BfArM described situations when in vivo BE studies are not needed (37). A decision tree was based on pharmacodynamic, pharmacokinetic, and physicochemical criteria. No biowaiver was allowed if product was either oral controlled release or administered non-orally, if drug was for serious indications, if drug was a narrow therapeutic index drug, if sufficient bioavailability/pharmacokinetic data was not available, or if potential BE problems were known. In many cases, the decision tree indicated that in vivo BE studies were not required. In describing the use of this approach in Germany (37), Gleiter et al. indicate the names of 90 drugs for which in vivo BE studies were not generally required, as well as the names of 120 drugs for which in vivo BE studies would be requested. However, the decision tree allowing biowaivers for oral IR and solution dosage forms was withdrawn in 2003 after over 15 years of use, to facilitate European Union harmonization [Personal communication from Dirk M. Barends (RijksInstituut voor Volksgezondheid en Milieu, Netherlands); March 15, 2007].
In Vivo BE Testing is Well Accepted
In vivo BE testing is generally well accepted by prescribers. However, this level of acceptance should not preclude the use of an alternative method when the alternative method is better. In particular, in vitro BE testing has potentially to better gain prescriber confidence and understanding, since not all prescribers support conventional human in vivo BE testing. For example, the position of the American Academy of Neurology (38) is “[t]he FDA has allowed for significant differences between name-brand and generic drugs. This variation can be highly problematic for patients with epilepsy. Even minor differences in the composition of generic and name-brand anticonvulsant drugs for the treatment of epilepsy can result in breakthrough seizures....Anticonvulsant drugs for the treatment of epilepsy differ from other classes of drugs in several ways that make generic substitution problematic.”
Aspects of conventional human in vivo BE testing can also be difficult to understand, diminishing confidence at times in the BE standard. Conventional human in vivo BE testing employs average bioequivalence through the use of a 90% confidence internal approach. The confidence internal goalposts are typically 80–125%. This method can be confusing. Some prescribers can also be led to believe that a confidence internal goalpost of 80–125% can easily provide a situation where one generic is 80% of the reference and a second generic is 125% of the reference, such that the two genetics differ by over 50% (i.e. 125/80).
In Vivo BE Testing is Perfectly Designed to Assess Product Equivalence
A conventional human pharmacokinetic in vivo BE study employs a single dose, two period, two treatment, two sequence, open label, randomized crossover design comparing equal doses of the test and reference products in fasted, adult, healthy volunteers; test and reference drug plasma profiles are compared. The comparison of test and reference drug plasma profiles to demonstrate BE is the most commonly used and successful biomarker. However, in vivo BE testing is not perfect. Type I error can be expected to be up to 5%. Type II error is described above.
In addition to being subjected to type I and type II errors, conventional in vivo BE testing also is imperfectly designed. In traditional BE testing, the residual variance is composed of (a) analytical variability, (b) within-subject variability in ADME, (c) within-formulation variability, (d) subject-by-formulation interaction, and (e) unexplained variability. The conventional two period design cannot separate these variance components. Hence, passing the traditional BE test assumes that the two products have sufficient product quality in that within-formulation variability and subject-by-formulation interaction are small. Traditional BE testing does not consider differences in within-subject variability between test and reference. Replicate designs where each product is administered twice allows partitioning of the subject-by-formulation interaction from residual variance and estimation of within-subject variability of each the test and reference. Conventional in vivo BE testing is not sensitive to detecting a subject-by-formulation interaction effect or a reference that is a highly variable drug product (HVDP), except of course that such increases in variability will necessitate an increase in subject numbers to establish BE.
While a few theoretical scenarios provide a basis for a subject-by-formulation interaction effect, it perhaps is surprising that conventional in vivo BE testing is not sensitive to detecting a reference that is a HVDP. The term HVDP differs from HVD (21). HVDs are drugs with high within-subject variabilities (ANOVA-CV ≥ 30%) in Cmax and/or AUC. HVDs are typically associated with high first pass (21). A HVDP is a formulation of poor pharmaceutical quality where the drug itself is not highly variable, and where within-formulation variability [e.g. capsule to capsule variability] is large. Conventional in vivo BE testing is not sensitive to detecting a reference that is a HVDP.
In Vitro BE Testing and In Vivo BE Testing can Provide Different Results
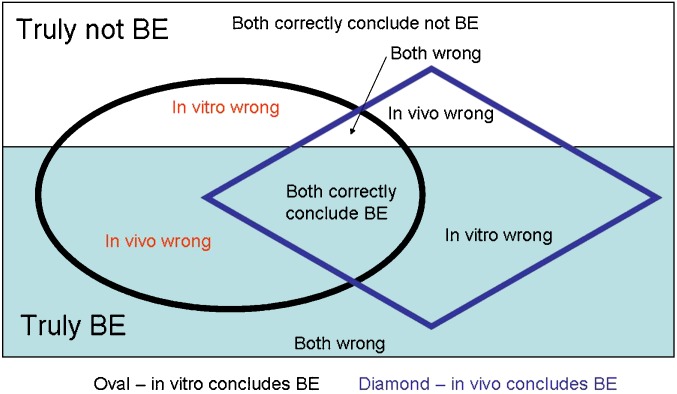
Perhaps the weakest reason to always favor in vivo BE over in vitro BE is that the two approaches can provide different results. Figure 3 illustrates situations of concordance and discordance between in vitro and in vivo testing. When products are truly BE (or truly not BE), both tests can be correct, both incorrect, or one correct and the other incorrect. Disconcordance between in vitro and in vivo results reflects type I and type II errors of each approach. Disconcordance does not imply in vitro is always incorrect, as limitation of in vivo BE testing are described above.
Fig. 3.
Concordance and Discordance between In Vitro and In Vivo Results. White area denotes products are not truly BE. Light blue area denotes products are truly BE. Oval denotes in vitro testing concludes BE. Diamond denotes in vivo testing concludes BE. Disconcordance between in vitro and in vivo results reflects type I and type II errors of each approach
Summary of Refuting Rationale for In Vivo BE Testing
It is limiting to favor in vivo BE with the view that it is the “gold standard” and always has been, it is well accepted, it is perfectly designed to assess product equivalence, and that it and in vitro BE testing can provide different results. In vivo BE testing is not always recommend, and in vitro BE testing has a long history. In vivo BE testing is generally well accepted by prescribers. However, this level of acceptance should not preclude the use of alternative method when the alternative method is better, particularly since in vitro BE testing has potentially to better gain prescriber confidence and understanding. In vivo BE testing is not perfect. It possesses Type I and Type II errors and is imperfectly designed. It assumes little within-formulation variability, little subject-by-formulation interaction, and equal within-subject variability for each test and reference. It is not sensitive to detecting a reference that is a HVDP. Perhaps the weakest reason to always favor in vivo BE over in vitro BE is that the two approaches can provide different results.
GOING FORWARD: HOW TO IDENTIFY BEST APPROACHES FOR BE TESTING
Simply always requiring or preferring in vivo demonstration of BE over in vitro methods is not rational and not scientific. For a rapidly dissolving IR solid oral dosage form containing a Class I drug, it is perhaps difficult to justify why in vitro BE test is not preferable over the conventional human pharmacokinetic in vivo BE testing. Difficulties in using the in vivo BE test for HVDs is well known. Tothfalusi et al. (21) indicate “An obvious remedy [of the HVD problem] is to increase the number of subjects participating in a study and thereby to narrow the CI. However, a BE study becomes, as a result, very expensive and cumbersome. ... The problem has been difficult and frustrating over many years and has often called for the use of unreasonably large numbers of subjects.”
Situations when in vitro test should be viewed as preferred include Class I drugs with rapid dissolution, Class III drugs with very rapid dissolution, and HVDs with rapid dissolution and that are not bio(equivalence)problem drugs. These situations represent a substantial majority of drugs. Class I and III drug make up about 50% of all marketed oral solid dosage forms (18,19). Upwards of 31% of drugs are HVDs (23). Since most HVDs show high first pass metabolism (23) and since many such drugs may be expected to be highly permeable (19), it can be estimated that a substantial majority of drugs are candidates for in vitro BE testing as the better BE test. Sponsors of potential in vivo human pharmacokinetic BE testing should be required to justify why in vitro data is insufficient, similar to proposed animal testing requires justification to not employ an in vitro approach.
Given the frequent use of the in vivo approach to evaluate BE, any effort to more broadly employ an in vitro approach would benefit from publicly available analysis of the relative performances of in vitro BE testing and in vivo BE testing. While there has been clear evidence of the regulatory impact of BCS with FDA providing regulatory relief via BCS-based biowaivers (16), there remains uncertainties among pharmaceutical companies and regulatory authorities about how to demonstrate the requirements for BCS-based biowaivers (39).
Type I errors of in vitro testing would be an obvious concern. Analyses, such as those previously performed and described (13), should be continuously updated and disclosed. In particular, written analyses would be most helpful, with due consideration to the fact that generic drug companies do not currently need to submit failed BE studies to the FDA. On-going open discussions about best practices in permeability classification (16,40) should be continuously encouraged. A better biopharmaceutic understanding of dosage form performance and kinetic role of in vivo dissolution in overall oral drug absorption is needed (41). More examples of detailed descriptions of how dosage forms achieve drug release in vivo are welcome. Better understanding of when and how in vitro dissolution methodologies do and do not reflect in vivo dissolution is needed. While Type I errors of in vitro testing is an obvious concern, a database for type II errors from in vitro dissolution would also be valuable. Ideally, quality-by-design (QbD) efforts during product development will help address some of these needs. Other topics needing better understanding are type II errors in current in vivo BE testing, which could be a major source in disconcordance between in vitro and in vivo BE results.
The path forward also requires a global effort. Most major products are registered worldwide. If one agency allows in vitro testing and another requires in vivo testing, in vivo testing will always be performed, even if in vitro testing is the better test. This lack of harmonized acceptance criteria is an obstacle that hinders wider utility of in vitro testing (42).
Acknowledgments
The author thanks Drs. Mehul Mehta, Jack Cook, Chris Hendy, Dirk Barends, and Kamal Midha for helpful discussions.
Appendix 1. Definitions
“Bioavailability means the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action.” (1).
“Bioequivalence means the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.” (1)
“Bioequivalence requirement means a requirement imposed by the Food and Drug Administration for in vitro and/or in vivo testing of specified drug products which must be satisfied as a condition of marketing.” (1)
“Bioequivalent Drug Products. This term describes pharmaceutical equivalentor pharmaceutical alternative products that display comparable bioavailability when studied under similar experimental conditions.” (12)
Conditions for bioequivalence. “A drug shall be considered to be bioequivalent to a listed drug if—(1) the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug when administered at the same molar dose of the therapeutic ingredient under similar experimental conditions in either a single dose or multiple doses; or (2) the extent of absorption of the drug does not show a significant difference from the extent of absorption of the listed drug when administered at the same molar dose of the therapeutic ingredient under similar experimental conditions in either a single dose or multiple doses and the difference from the listed drug in the rate of absorption of the drug is intentional, is reflected in its proposed labeling, is not essential to the attainment of effective body drug concentrations on chronic use, and is considered medically insignificant for the drug.” [Section 505 (j)[7](B) of the Federal Food, Drug, and Cosmetic Act] (25)
References
- 1.U.S. Government Printing Office. Code of Federal Regulations Title 21—Food and Drugs. Part 320—Bioavailability and Bioequivalence Requirements. http://www.access.gpo.gov/nara/cfr/waisidx_03/21cfr320_03.html (accessed February 9, 2008).
- 2.CDER/FDA. Draft Guidance for Industry, Bioequivalence Recommendations for Specific Products. May 2007. http://www.fda.gov/cder/guidance/6772dft.pdf (accessed February 9, 2008).
- 3.OGD/CDER/FDA. Individual Product Bioequivalence Recommendations. http://www.fda.gov/cder/guidance/bioequivalence/#Ind_Rec (accessed February 9, 2008).
- 4.CDER/FDA. Guidance for Industry, Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. March 2003. http://www.fda.gov/cder/guidance/5356fnl.pdf (accessed February 9, 2008).
- 5.D.G. Safran, P. Neuman, C. Schoen, M.S. Kitchman, I.B. Wilson, B. Cooper, A. Li, H. Chang, and W.H. Rogers. Prescription drug coverage and seniors: findings from a 2003 national survey. Health Aff. 10.1377/hlthaff.w5.152 (2005). [DOI] [PubMed]
- 6.Heisler M., Langa K. M., Eby E. L., Fendrick A. M., Kabeto M. U., Piette J. D. The health effects of restricting prescription medication use because of cost. Med. Care. 2004;42:626–634. doi: 10.1097/01.mlr.0000129352.36733.cc. [DOI] [PubMed] [Google Scholar]
- 7.Soumerai S. B., Pierre-Jacques M., Zhang F., Ross-Degnan D., Adams A. S., Gurwitz J., Adler G., Safran D. G. Cost-related medication nonadherence among elderly and disabled medicare beneficiaries: a national survey 1 year before the medicare drug benefit. Arch. Intern. Med. 2006;166:1829–1835. doi: 10.1001/archinte.166.17.1829. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. The Nation’s Health Dollar, Calendar Year 2005: Where It Went. http://www.cms.hhs.gov/NationalHealthExpendData/downloads/PieChartSourcesExpenditures2005.pdf (accessed February 9, 2008).
- 9.CDER/FDA. Guidance for Industry, Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. August 2000. http://www.fda.gov/cder/guidance/3618fnl.htm (accessed February 9, 2008).
- 10.J.A. Cook and H.N. Bockbrader. An industrial implementation of the Biopharmaceutics Classification System. Dissolution Technologies. May 2002. http://www.dissolutiontech.com/DTresour/0502art/DTMay02_art1.htm (accessed on February 9, 2008).
- 11.EMEA Committee for Proprietary Medicinal Products. Note for Guidance on the Investigation of Bioavailability and Bioequivalence. July 2001. http://www.emea.europa.eu/pdfs/human/ewp/140198en.pdf (accessed February 9, 2008).
- 12.CDER/FDA. Approved Drug Products with Therapeutic Equivalence Evaluations, 28th edition. 2008. http://www.fda.gov/cder/orange/obannual.pdf (accessed February 9, 2008).
- 13.M. U. Mehta. Presentation “Classification of New Drugs: NDA 1995–2001 Survey” at AAPS workshop Biopharmaceutics Classification System: Implementation Challenges and Extension Opportunities, Arlington, VA. September 25, 2002.
- 14.M. U. Mehta. Presentation “FDA Regulatory Use of BCS”. AAPS workshop on Bioequivalence, Biopharmaceutics Classification System, and Beyond, North Bethesda, MD. May 21, 2007.
- 15.Polli J. E., Yu L. X., Cook J. A., Amidon G. L., Borchardt R. T., Burnside B. A., Burton P. S., Chen M.-L., Conner D. P., Faustino J., Hawi A. A., Hussain A. S., Joshi H. N., Kwei G., Lee V. H. L., Lesko L. J., Lipper R. A., Loper A. E., Nerurkar S. G., Polli J. W., Sanvordeker D. R., Taneja R., Uppoor R. S., Vattikonda C. S., Wilding I., Zhang G. Summary workshop report: biopharmaceutics classification system—implementation challenges and extension opportunities. J. Pharm. Sci. 2004;93:1375–1381. doi: 10.1002/jps.20064. [DOI] [PubMed] [Google Scholar]
- 16.J. E. Polli, B. S. I. Abrahamsson, L. X. Yu, G. L. Amidon, J. M. Baldoni, J. A. Cook, P. Fackler, K. Hartauer, G. Johnston, S. L. Krill, R. A. Lipper, W. A. Malick, V. P. Shah, D. Sun, H. N. Winkle, Y. Wu, and H. Zhang. Summary Workshop Report: Bioequivalence, Biopharmaceutics Classification System, and Beyond. Submitted to AAPSJournal. [DOI] [PMC free article] [PubMed]
- 17.L. X. Yu, C. D. Ellison, D. P. Conner, L. J. Lesko, and A. S. Hussain. Influence of drug release properties of conventional solid dosage forms on the systemic exposure of highly soluble drugs. AAPS PharmSci. 3(3): article 24 (2001). DOI: 10.1208/ps030324 [DOI] [PMC free article] [PubMed]
- 18.Takagi T., Ramachandran C., Bermejo M., Yamashita S., Yu L. X., Amidon G. L. A Provisional Biopharmaceutical Classification of the Top 200 Oral Drug Products in the United States, Great Britain, Spain, and Japan. Mol. Pharmaceutics. 2006;3:631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 19.Wu C. Y., Benet L. Z. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 20.Khandelwal A., Bahadduri P. M., Chang C., Polli J. E., Swaan P. W., Ekins S. Computational models to assign biopharmaceutics drug disposition classification from molecular structure. Pharm. Res. 2007;24:2249–2262. doi: 10.1007/s11095-007-9435-9. [DOI] [PubMed] [Google Scholar]
- 21.Tothfalusi L., Endrenyi L., Midha K. K., Rawson M. J., Hubbard J. W. Evaluation of the bioequivalence of highly-variable drugs and drug products. Pharm. Res. 2001;18:728–733. doi: 10.1023/A:1011015924429. [DOI] [PubMed] [Google Scholar]
- 22.M. Tanguay, D. Potvin, J. Haddad, J. Lavigne, J. F. Marier, M. DiMarco, and M. P. Ducharme. When will a drug formulation pass or fail bioequivalence criteria? Experience From 1200 Studies. AAPS Pharm. Sci.4(4):Abstract R6193 (2002).
- 23.B. Davit, D. P. Conner, B. Fabian-Fritsch, S. H. Haidar, X. Jiang, D. T. Patel, P. R. Seo, K. Suh, C. L. Thompson, L. X. Yu. Highly variable drugs: observations from bioequivalence data submitted to the FDA for new generic drug applications. AAPS Journal (in press) (2008). [DOI] [PMC free article] [PubMed]
- 24.Buice R. G., Subramanian V. S., Duchin K. L., Uko-Nne S. Bioequivalence of a highly variable drug: an experience with nadolol. Pharm. Res. 1996;13:1109–1115. doi: 10.1023/A:1016031313065. [DOI] [PubMed] [Google Scholar]
- 25.Federal Food, Drug, and Cosmetic Act. http://www.fda.gov/opacom/laws/fdcact/fdcact5a.htm (accessed February 9, 2008).
- 26.Polli J. E., Bigora S., Piscitelli D. A., Straughn A. B., Young D. “Pavlovian” food effect on the enterohepatic recirculation of piroxicam. Biopharm. Drug Dispos. 1996;17:635–641. doi: 10.1002/(SICI)1099-081X(199610)17:7<635::AID-BDD981>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Huic M., Vrhovac B., Macolic-Sarinic V., Francetic I., Bakran I., Giljanovic S. How safe are bioequivalence studies in healthy volunteers? Therapie. 1996;51:410–413. [PubMed] [Google Scholar]
- 28.CDER/FDA. Guidance for Industry: Clozapine Tablets: In Vivo Bioequivalence and In Vitro Dissolution Testing. June 2005. http://www.fda.gov/cder/guidance/6077fnl.pdf (accessed February 9, 2008).
- 29.M. U. Mehta, L. J. Lesko, and M. L. Ching. Comparison of clinical pharmacology (CP) and biopharmaceutics (BP) studies submitted in NDAs during 1995 and 1997. 1998 ASCPT annual meeting abstract.
- 30.Stein C. M. Managing risk in healthy subjects participating in clinical research. J. Clin. Pharm. Ther. 2003;74:511–512. doi: 10.1016/j.clpt.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 31.J. El-Hage. Presentation “Peroxisome Proliferator-Activated Receptor (PPAR) Agonists: Preclinical and Clinical Cardiac Safety Considerations” at 42nd Annual Meeting of the Drug Information Association, Philadelphia, PA. June 18, 2006. www.fda.gov/Cder/present/DIA2006/El-Hage_CardiacSafety.ppt (accessed February 9, 2008).
- 32.A. Hussain, Presentation “An Update on the BCS Guidance”. Meeting of the FDA Advisory Committee for Pharmaceutical Science, Gaithersburg, MD. May 7, 1997.
- 33.Anonymous. “Annex 7: Multisource (Generic) Pharmaceutical Products: Guidelines on Registration Requirements to Establish Interchangeability”. In WHO Expert Committee on Specifications for Pharmaceutical Preparations: Fortieth Report; WHO: Geneva, Switzerland, 2006, pp. 347–390. http://whqlibdoc.who.int/trs/WHO_TRS_937_eng.pdf (accessed December 15, 2007).
- 34.CDER/FDA. Guidance for Industry: Immediate-Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation. November 1995. http://www.fda.gov/cder/guidance/cmc5.pdf (accessed February 9, 2008).
- 35.CDER/FDA. SUPAC-MR: Modified Release Solid Oral Dosage Forms Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation. September 1997. http://www.fda.gov/cder/guidance/1214fnl.pdf (accessed February 9, 2008).
- 36.CDER/FDA. Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. September 1997. http://www.fda.gov/cder/guidance/1306fnl.pdf (accessed February 9, 2008). [DOI] [PubMed]
- 37.Gleiter C. H., Klotz U., Kuhlmann J., Blume H., Stanislaus F., Harder S., Paulus H., Poethko-Muller C., Holz-Slomczyk M. When are bioavailability studies required? A German proposal. J. Clin. Pharmacol. 1998;38:904–911. doi: 10.1002/j.1552-4604.1998.tb04385.x. [DOI] [PubMed] [Google Scholar]
- 38.American Academy of Neurology. Position Statement on the Coverage of Anticonvulsant Drugs for the Treatment of Epilepsy. November 2006. http://www.aan.com/globals/axon/assets/2323.pdf (accessed February 9, 2008). [DOI] [PubMed]
- 39.EMEA Committee for Medicinal Products for Human Use. Concept Paper on BCS-based Biowaiver. May 2007. http://www.emea.europa.eu/pdfs/human/ewp/21303507en.pdf (accessed February 9, 2008).
- 40.Benet L. Z., Amidon G. L., Barends D. M., Lennernas H., Polli J. E., Shah V. P., Stavchansky S. A., Yu L. X. The Use of BDDCS in Classifying the Permeability of Marketed Drugs. Pharm. Res. 2008;25:483–488. doi: 10.1007/s11095-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polli J. E., Ginski M. J. Human drug absorption kinetics and comparison to Caco-2 monolayer permeabilities. Pharm. Res. 1998;15:47–52. doi: 10.1023/A:1011992518592. [DOI] [PubMed] [Google Scholar]
- 42.Gupta E., Barends D. M., Yamashita E., Lentz K. A., Harmsze A. M., Shah V. P., Dressman J. B., Lipper R. A. Review of global regulations concerning biowaivers for immediate release solid oral dosage forms. Eur. J. Pharm. Sci. 2006;29:315–324. doi: 10.1016/j.ejps.2006.05.001. [DOI] [PubMed] [Google Scholar]