Abstract
Phenethyl isothiocyanate (PEITC), an isothiocyanate abundantly found in cruciferous vegetables have been shown to induce apoptosis through MAPK pathway in prostate and colon cancer cells. In the present study, we investigate the effect of PEITC on cell cycle regulation of HT-29 colon cancer cells. Using flow cytometry and Western blot analyses, we found that PEITC significantly induced G1 cell cycle arrest in HT-29 cells. We showed that the cell cycle arrest was not related to beta-catenin translocation into the nucleus. Interestingly, inhibition of p38 attenuated the cell cycle arrest, suggesting that cell cycle arrest by PEITC was caused by the activation of MAPK pathway. Treatments of PEITC resulted in a dose-dependent down-regulation of cyclins A, D, E and pRb protein expression. The down-regulation can be attributed to the activation of the p38 pathway, since inhibition of its activities by specific inhibitor blocked PEITC’s ability to decrease the expression level of cyclins A and D and attenuate cell cycle arrest effect of PEITC. In conclusion, this study shows for the first time that PEITC can arrest HT-29 cells in G1 phase by down-regulation of cyclins through the activation of p38 MAPK signaling pathway.
Key words: cell cycle arrest, colon cancer, cyclin, PEITC, p38
INTRODUCTION
Colon cancer is one of the leading causes of cancer death in the US (1). Epidemiological studies have demonstrated an inverse association of colon cancer with intake of cruciferous vegetables (2). Dietary isothiocyanates are present in large quantities in cruciferous vegetables including watercress, broccoli and cabbage (3). Many isothiocyanates (ITCs) such as sulphoraphane (SFN), phenethyl isothiocyanate (PEITC) and ally isothiocyanate (AITC) are highly effective in chemoprevention and have anti-tumor activities in vitro and in vivo. Apart from the colorectum, isothiocyanates inhibit cancer formation in various tissues such as lung, esophagus, mammary gland, liver, small intestine and bladder (4).
While the exact mechanisms by which PEITC exerts its anti-tumor activity are still unclear, MAPK and AP-1 pathways are believed to be involved. Using PC-3 cells as a model, it was shown that PEITC can activate JNK and ERK signaling and mediate the transcriptional activity of AP-1, which in turn regulate the cell death (5). Apart from transcriptional control, PEITC may inhibit cap-dependent translation by regulating the level and phosphorylation of 4E-BP1 which may be an important mechanism in PEITC-induced apoptosis (6).
A lot of studies have been focused on PEITC induction of apoptosis in cancer cell lines. Previously, our lab demonstrated PEITC can induce apoptosis in HT-29 cells in a time- and dose-dependent manner via the mitochondria caspase cascade, and the activation of JNK is critical for the initiation of the apoptotic processes (7). In the present study, we demonstrated that in addition to activating apoptosis, MAPKs, p38 in particular have an important role in regulating cell cycle of HT-29 cells.
MATERIALS AND METHODS
Cell Culture and Reagents
Human colorectal cancer cell lines HT-29 was obtained from American Type Culture Collection (ATCC). The cells were maintained in minimum essential medium (MEM) with 10% fetal bovine serum (FBS), 2.2 g/l sodium bicarbonate, 100 U/ml penicillin and 100 μg streptomycin. Before treatment, the medium was removed and the cells were starved with 0.5% serum MEM overnight. Phenethyl isothiocyanate (PEITC) was obtained from Sigma (St Louis, MO, USA). Cyclin A, D and E were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). pRb, phospho-cdc2-Tyr15, p-JNK, p-ERK, (phosphorylated p38) were purchased from Cell Signalling Technology (Beverly, MA, USA). Specific MAPKs inhibitors SP600125, PD98059 and SB203580 were purchased from Calbiochem Technology (San Diego, CA, USA).
Flow Cytometry Analysis of Cell Cycle Distribution
HT-29 cells were seeded in 60-mm petri dishes in complete MEM overnight. The cells were then serum-starved for 24 h in serum free MEM medium. At treatment, the medium was replaced with complete MEM containing either 0.1% DMSO (as a negative control) or a varying dose of PEITC (5, 10 and 25 μM). Following PEITC treatment, the cells were collected by trypsinization. Cells were collected by centrifugation at 1,000 rpm for 5 min. The cell pellet was then re-suspended in 500 μl PBS, and the resulting cell suspension was passed through a 26.5 gauge needle three times. The cells were then fixed by adding 500 μl ethanol. The fixed cells were then stained by 100 μg/ml RNase A and 10 μg/ml propidium iodide (PI) at 4°C in the dark for 30 min. Cell cycle distribution was then analyzed by flow cytometry using FACS analysis core facility of University of Medicine and Dentistry of New Jersey.
Western Blotting Analysis
After treatment, HT-29 cells in six-well plates were washed with ice-cold PBS and lysed with 200 μl of whole cell lyses buffer (10 mM Tris–HCl, pH 7.9, 250 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 0.5% Triton X-100, 10% glycerol, 1 mM proteinase inhibitor mixture, 1 mM phenylmethylsulfonyl fluoride, 100 mM Na3VO4, 5 mM ZnCl2, 2 mM indole acetic acid). The cell lysates were centrifuged at 12,000×g for 10 min at 4°C. The protein concentrations of the whole cell lysate supernatants were determined using a Bio-Rad protein assay kit. An equal amount of protein (20 μg) was then resolved on a 10% SDS-polyacrylamide gel and transferred to PVDF membrane using semi-dry transfer system. The membrane was blocked in 5% non-fat milk for 1 h at room temperature, then incubated overnight at 4°C separately with a primary antibody specifically recognizing cyclin D1 (sc-718), cyclin A (sc-596), cyclin E (sc-247) or actin (sc-1616), p21CIP1 (sc-6246) (Santa Cruz Biotechnology) and p-JNK, pERK, phosphorylated p38, pRb, phospho-cdc2-Tyr15 (Cell Signalling Biotechnology). After incubation with the primary antibody, the membrane was washed with TBST (20 mM Tris–HCl, 8 g/l NaCl, 0.1% Tween 20, pH 7.6) three times, then incubated in horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution) for 45 min at room temperature followed by an additional three washes with TBST. Detection was performed using ECL reagents (Bio-Rad).
RESULTS
PEITC Induced G1 Cell Cycle Arrest in HT-29 Cells
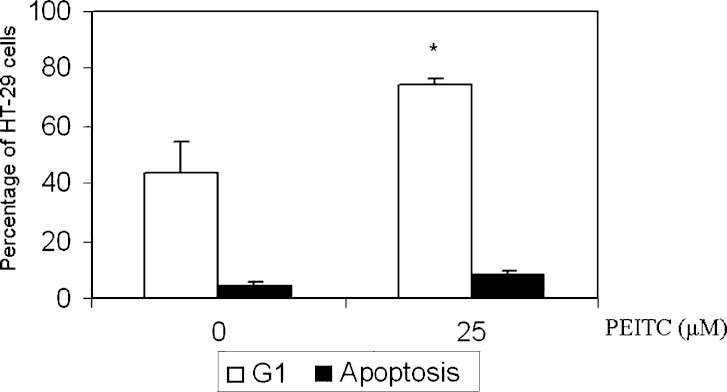
HT-29 cells were treated with various concentrations (0, 5, 10, 25 μM) of PEITC for 24 h. Flow cytometry was performed and the results showed a significant increase in the percentage of G1 phase cells at 25 μM (Fig 1). In agreement with our previous study, flow cytometry showed that apoptotic cells increase from 5% to about 11% at high concentrations after PEITC treatment. On the other hand, the percentage increase in G1 cells increased from 53% at concentration 0 μM to 71% at 25 μM. Together, these results suggest that apart from apoptosis, PEITC may exert its effect through cell cycle arrest.
Fig. 1.
The effect of PEITC on cell cycle of HT-29 cells. HT-29 cells were treated with PEITC (25 μM) for 24 h. PEITC significantly increased G1 cells from 51% at PEITC 0 μM to 73% at PEITC 25 μM. Student’s t-test was used to compare the means between treated (25 μM) groups vs control (0 μM) groups *p = 0.01
PEITC Decreased Expression of Cyclins
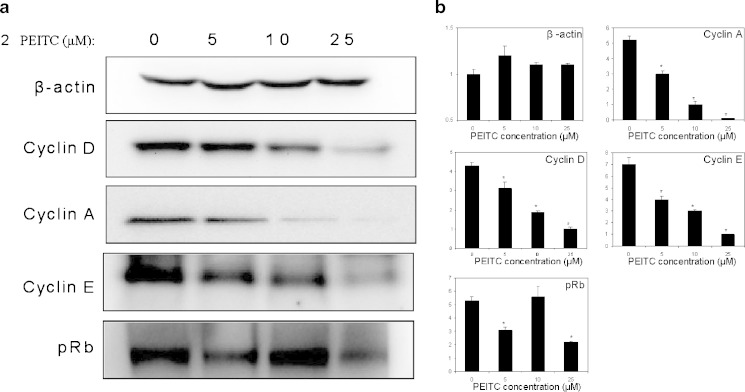
To determine the effect of PEITC on the expression level of various common proteins involved in cell cycle control, western blotting was performed. As shown in Fig. 2, PEITC decreased the expression of cyclin A, D and E in a dose-dependent manner. However, PEITC had no effect on p21, p27 and p53 (data not shown). Instead, pRb maybe the target where PEITC exerts it effect on HT-29, since increase in PEITC led to a reduction in pRb (Fig. 2).
Fig. 2.
a The effect of PEITC on cell cycle arrest markers in HT-29 cells. HT-29 cells were treated with an increasing dose of PEITC (0, 5, 10, 25 μM) for 24 h and cell cycle arrest markers were blotted. PEITC significantly suppressed the expression of cyclin A, D, E and pRb dose-dependently. b Densitometry data are representative of 3 independent experiments
PEITC Decrease Expression of Cyclins through a β-catenin Independent Pathway
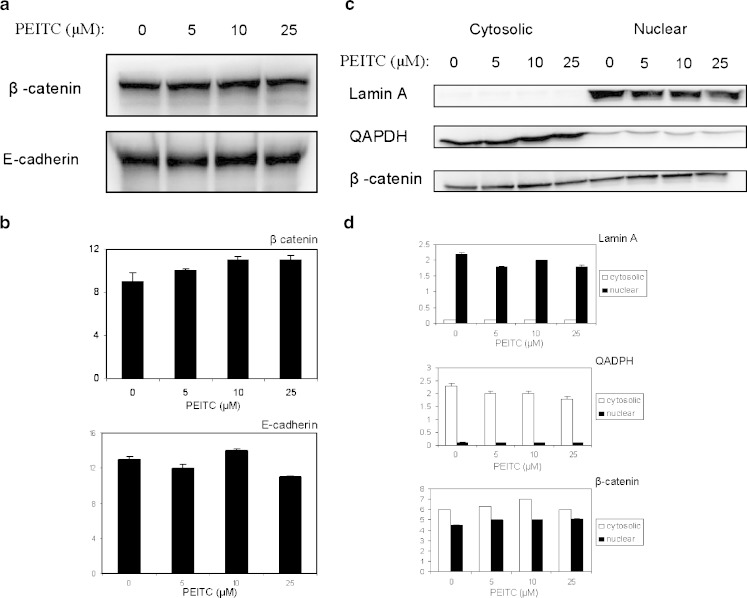
Aberrant activation of Wnt signaling pathway is known to be essential in colorectal carcinogenesis. To elucidate if β-catenin is involved in PEITC depression of cyclins, the expression of β-catenin and E-cadherin was examined. As shown in Fig. 3a, the total β-catenin and E-cadherin level remained unchanged after PEITC treatment. As shown in Fig. 3b, the nuclear β-catenin level did not change even PEITC increases, suggesting that β-catenin pathway may not be involved in the PEITC effects on HT-29 cells.
Fig. 3.
a The effect of PEITC on β-catenin expression. The expression level of β-catenin and E-cadherin in total cell lysate were not altered by administration of PEITC. b Densitometry data are representative of two independent experiments. c The effect of PEITC on β-catenin nuclear localization. The nuclear localization of β-catenin was not altered by administration of PEITC. d Densitometry data are representative of 2 independent experiments
PEITC Induced Phosphorylated p38 (pp38) Activity, Leading to G1 Cell Cycle Arrest
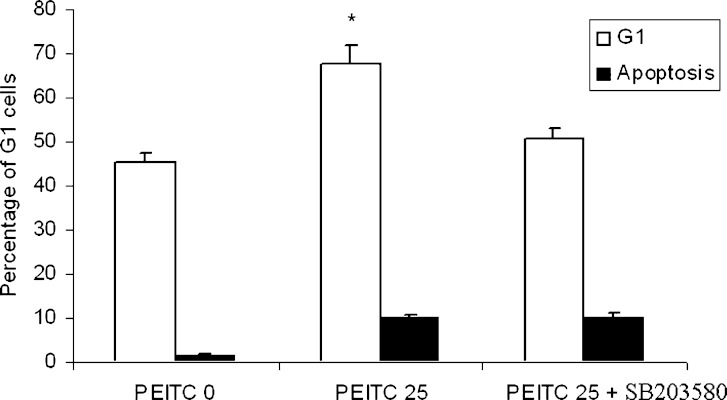
To investigate the role of different MAPKs on cell cycle arrest induced by PEITC, specific inhibitors of different MAPKs were used. JNK and ERK inhibitors did not significantly suppress G1 cell cycle arrest induced by PEITC (data not shown). However, by inhibiting the phosphorylated p38 (pp38) activity using specific inhibitor SB 203580 (10 μM and pretreatment of 1 h), we showed that the cell cycle arrest was attenuated. As shown in Fig. 4, the percentage of cells in G1 phase was 46.64% and 70.58% without and with treatment of 25 μM PEITC respectively. By inhibiting the action of p38, the percentage of cells in G1 phase after treatment of 25 μM PEITC dropped from originally 70.58% to 50.62% (p38 activity being inhibited). Therefore, we conclude that phosphorylated p38 (pp38) is involved in PEITC-mediated cell cycle arrest.
Fig. 4.
The effect of p38 inhibitor on G1 phase arrest of HT-29 cells induced by PEITC. PEITC (25 μM) significantly induced G1 cell cycle arrest while treatment of p38 inhibitor significantly attenuated this cell cycle arrest. Student’s t-test was used to compare the means between the groups *p = 0.01
PEITC Mediated Cyclin Level Through Phosphorylated p38 (pp38) Activity
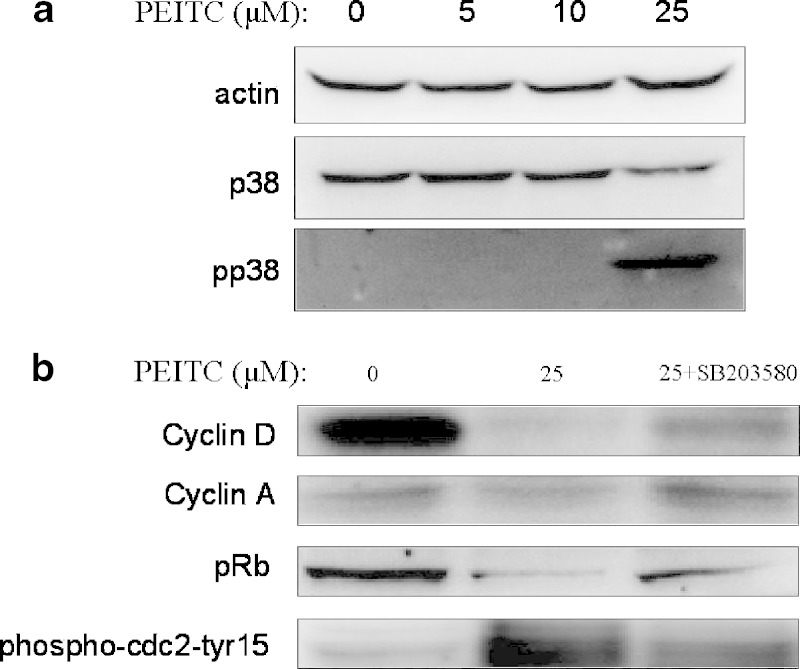
As shown in Fig. 5b, cyclin A and D expression, but not cyclin E (data not shown) decreased at the PEITC 25 μM while inhibitor of phosphorylated p38 partially restored the expression. This suggests phosphorylated p38 activity may play a role in regulating the expression of cyclins, probably regulation on cdc2 and pRb. pRb expression decreased upon PEITC treatment while inhibition of phosphorylated p38 partially inhibited the depression. In contrary, phospho-cdc2-Tyr15 expression increased upon PEITC treatment and was restored by inhibition of phosphorylated p38 (Fig. 5b).
Fig. 5.
a The effect of PEITC on expression of p38MAPK. PEITC induce the expression of phosphorylated p38 at PEITC 25 μM. The data are representative of three independent experiments. b The effect of p38 inhibitor on G1 phase arrest markers of HT-29 cells induced by PEITC. PEITC suppressed cyclin A, D and pRb, induced phosphor-cdc2 at tyr15 while treatment of p38 inhibitor significantly attenuated this. The data are representative of three independent experiments
DISCUSSION
Previous studies supported that isothiocyanates like PEITC and sulforaphane have anti-colon tumor effect. SFN regulates genes involved in apoptosis, cell growth and inflammation in the small intestinal polyps of ApcMin/ mice (8). It has been shown that the sulforaphane exert its anti-cancer effect through differential regulation of cell cycle and subsequent events lead to cell death (9). Little studies have been done on the anti-proliferative effect of PEITC on HT-29 cells. In this study, we used a concentration range of 0 to 25 μM (the range that does not produce toxic effect to HT-29 cells) and showed for the first time that in addition to apoptosis, PEITC inhibits cancer cell growth through cell cycle arrest.
Cyclin A, D and E were found to be down-regulated by PEITC in HT-29 cells. The results are consistent with flow cytometry results which show an increase in G1 cell cycle arrest. Several upstream signaling events have been suggested as responsible for up-regulations of cyclin A, D and E. For example, β-catenin can translocate into nucleus to up-regulate cyclin D through the Wnt signaling pathway (10). IKKα can stabilize β-catenin and induce the cyclins (11), leading to their proliferative effect. However, our results showed that PEITC do not suppress cyclin D through the (β)-catenin pathway.
At the heart of the cell-cycle control system is a family of protein kinases known as cyclin-dependent kinases (Cdks). Cdk activity is controlled by a complex array of enzymes and proteins, one of which is known as cyclins. Cylcin D belongs to the G1 cyclins which promote passage through restriction point in late G1, cyclin E belongs to the G1/S phase cyclins and cyclin A belongs to S phase cyclins. Our study showed that PEITC arrests HT-29 cells in G1 phase through suppression of cyclins in a dose dependent manner. It is known that DNA damage leads to the activation of p53, which in turn increase the expression of p21 that binds to G1/S Cdk and inhibits their activity, thus arresting the cells in G1 phase. However, our results showed that PEITC do not regulate the Cdk activity through p53 and p21.
We are then interested to know what is the upstream signaling for the increase in cyclin A, D and E levels. We found that β-catenin pathway is not involved since the basal level of β -catenin did not increase after PEITC treatment, nor did its nuclear localization. Specific inhibitor for JNK and ERK attenuates the G1 cell cycle arrest but is not significant (data not shown). However, we showed that abrogation of p38 attenuates the G1 cell cycle arrest induced by PEITC. This suggests that MAPK could be an upstream signaling controlling cyclins expression mediated by PEITC. Particularly, we provide a plausible mechanism explaining how phosphorylated p38 can mediate the effect of PEITC on HT-29 cells. Combining all the evidence, we suggest that activation of phosphorylated p38 is responsible for the G1 cell cycle arrest. Studies have shown that p38MAPK pathway is activated in response to the commonly used DNA-damaging agents cisplatin, doxorubicin, and camptothecin and that it has cell-cycle checkpoint function (12). It has been shown that phosphorylated p38 can phosphorylate cdc25A and phosphorylation of cdc2 at tyr15 leads to cell cycle arrest (13). Our results showed that PEITC induce phosphorylated p38 expression, leading to an increase in phospho-cdc2 at tyr15. The increase in inhibitory phosphorylation of this cyclin dependent kinase may results in a decrease in phosphorylation of Rb. And with more Rb binding to E2F, the cyclin expression is suppressed.
CONCLUSION
In conclusion, in addition to apoptotic effect, our study shows that PEITC also has a significant anti-proliferative effect, leading to a 20% increase in G1 arrest HT-29 cells. It is believed that p38 pathway plays an important role for the anti-cancer effect of PEITC. Activation of MAPK simultaneously stimulates the activation of caspases and depression of cyclins, leading to cell cycle arrest and apoptosis.
ACKNOWLEDGEMENT
We thank all the members in Dr. Tony Kong’s lab for their help in the discussion and preparation of this manuscript. This work was supported in part by National Institute of Health Grant R01 CA-073674-07.
REFERENCE
- 1.Wingo P. A., Tong T., Bolden S. Cancer statistics. CA Cancer J. Clin. 1995;45:8–30. doi: 10.3322/canjclin.45.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Potter J. D. Nutrition and colorectal cancer. Cancer Causes and Control. 1996;7:127–146. doi: 10.1007/BF00115644. [DOI] [PubMed] [Google Scholar]
- 3.Fahey J. W., Zalcmann A. T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54:7. [PubMed] [Google Scholar]
- 5.Xu C., Shen G., Yuan X., Kim J. H., Gopalkrishnan A., Keum Y. S., Nair S., Kong A. N. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;3:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 6.Hu J., Straub J., Xiao D., Singh S. V., Yang H. S., Sonenberg N., Vatsyayan J. Phenethyl isothiocyanate, a cancer chemopreventive constituent of cruciferous vegetables, inhibits cap-dependent translation by regulating the level and phosphorylation of 4E-BP1. Cancer Res. 2007;67:8. doi: 10.1158/0008-5472.CAN-07-0392. [DOI] [PubMed] [Google Scholar]
- 7.Hu R., Kim B. R., Chen C., Hebbar V., Kong A. N. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis. 2003;8:1361–1367. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 8.Khor T. O., Hu R., Shen G., Jeong W. S., Hebbar V., Chen C., Xu C., Nair S., Reddy B., Chada K., Kong A. N. Pharmacogenomics of cancer chemopreventive isothiocyanate compound sulforaphane in the intestinal polyps of ApcMin/ mice. Biopharm Drug Dispos. 2006;9:407–420. doi: 10.1002/bdd.522. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y. M., Jhanwar-Uniyal M., Schwartz J., Conaway C. C., Halicka H. D., Traganos F., Chung F. L. N-acetylcysteine conjugate of phenethyl isothiocyanate enhances apoptosis in growth-stimulated human lung cells. Cancer Res. 2005;18:8538–8547. doi: 10.1158/0008-5472.CAN-05-0236. [DOI] [PubMed] [Google Scholar]
- 10.Cong F., Zhang J., Pao W., Zhou P., Varmus H. A protein knockdown strategy to study the function of beta-catenin in tumorigenesis. BMC Mol Biol. 2003;4:10. doi: 10.1186/1471-2199-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins N. D. Integrating cell signaling pathways with NF-KB and IKK function. Nat Rev Mol Cell Biol. 2007;1:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 12.Reinhardt H. C., Aslanian A. S., Lees J. A., Yaffe M. B. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;2:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;11:3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]