Abstract
Chronic pseudomonal bronchopulmonary infections in cystic fibrosis patients are frequently controlled with inhaled antibiotics. Dry-powder inhalable antibiotics are an attractive alternative to nebulized medications. We produced and evaluated microparticles composed of dipalmitoylphosphatidylcholine, albumin, and lactose as a model system for intrapulmonary delivery of ceftazidime, ciprofloxacin, and several combinations of the two, none of which is presently available for inhalation. Microparticles containing one or both antibiotics were prepared by spray-drying. Their Anderson cascade impactor deposition profiles showed 10–30% fine particle fractions of the nominal dose. Microparticles containing varying amounts of each antibiotic showed statistically different deposition profiles. Aerodynamics and deposition of microparticles co-encapsulating both antibiotics were similar to those of single-drug microparticles with the same proportion of ciprofloxacin alone. The antipseudomonal activities of microparticles co-encapsulating half of the 50% effective concentration (EC50) of both ceftazidime and ciprofloxacin (5 mg of particles containing 5% ceftazidime and 10% ciprofloxacin) were at least additive compared to particles containing the EC50 of each antibiotic separately (5 mg of particles containing 10% ceftazidime or 5 mg of particles containing 20% ciprofloxacin). Co-encapsulation of the antibiotics in microparticles ensures co-deposition at desired ratios, improves the particles’ aerodynamics and fine particle fraction, as compared to microparticles with equivalent amounts of ceftazidime alone, and achieves additive antipseudomonal activity.
KEY WORDS: co-encapsulation, cystic fibrosis, dry-powder, inhalational delivery of antibiotics, microparticles
INTRODUCTION
Chronic bronchopulmonary infections with Pseudomonas aeruginosa account for significant morbidity and mortality in patients with cystic fibrosis (CF) (1,2) and frequently complicate other chronic pulmonary conditions. In such patients inhaled antibiotic therapy is an attractive alternative to oral or parenteral therapy, since it delivers the drugs directly to the desired site of action, which diminishes side effects and decreases the need for parenteral therapy. Inhaled antibiotics have shortened hospitalizations among patients with bacterial respiratory infections, decreased health-care expenditures, increased patient satisfaction, and ultimately improved clinical outcomes (3–5).
In clinical practice inhaled antibiotics are administered by nebulization, which has a number of limitations. The repertoire of the nebulized antibacterials is limited to several aminoglycosides, colistin (6), and vancomycin (7–9), prompting CF experts to recommend further pharmacologic testing of anti-pseudomonal agents (6). Moreover, the fine-particle fraction (FPF) of most commercially available nebulizers is around 10% (10). Hence, nebulized drugs must be highly potent; otherwise, the long administration time may impair patient compliance (11,12). Co-nebulizing several antibiotics is difficult because some combinations of antibiotic solutions can form precipitates (13). Finally, nebulization disperses antibiotics into the ambient air and generates antibiotic resistance among ubiquitous bacteria (14). The ideal vehicle for inhalational delivery of antibiotics should deliver the effective drug, provide a high FPF, allow for simultaneous delivery of multiple potentially chemically incompatible antibiotics, limit the contamination of ambient air, and be easy to use. Specially-formulated dry powders may be suitable for many of these goals (15,16).
In CF patients P. aeruginosa often develops resistance if treated with a single antibiotic (such as tobramycin, ceftazidime, or ciprofloxacin), and many clinicians use two or more agents of different classes simultaneously to prevent this (14,17–21). Co-encapsulating the two compounds in the same particles would assure their co-deposition in the airway at the intended doses. Furthermore, such combination particles may offer an attractive option for patients who harbor several types of microorganisms that may not be killed by a single antibiotic (22).
Here we have demonstrated an inhalable dry-powder formulation for the β-lactam ceftazidime and the quinolone ciprofloxacin. Each antibiotic is active and used clinically against P. aeruginosa, and their combination may be either synergistic or additive against the bacterium (22,23), but neither is available for inhalation. Presently, ceftazidime is only available for intravenous or intramuscular administration, while ciprofloxacin can be administered intravenously or orally. Inhalable ceftazidime may avoid the pain associated with parenteral administration and obviate the need for intravascular access as well as nursing care of and monitoring for indwelling vascular catheters. Inhalable ciprofloxacin may reduce the risk of tendon damage in the pediatric patient and prevent photosensitivity often seen with systemic administration of the drug to children or adults. We have also developed a microparticle system capable of co-encapsulating the two antibiotics. We used dipalmitoylphosphatidylcholine (DPPC), albumin, and lactose (referred to as “DAL”) particles (24) as a dry powder platform for inhalational delivery of ceftazidime and ciprofloxacin. Aerodynamic properties and antibacterial activities of the particles were evaluated in vitro.
MATERIALS AND METHODS
Materials
Ciprofloxacin (Cipro-IV, Bayer Pharmaceutical Corp., West Haven, CT) and ceftazidime (Fortaz, GlaxoSmithKline, Research Triangle Park, NC) were purchased from Pharmacy Department, Massachusetts General Hospital (Boston, MA). All cell lines were purchased from ATCC (Manassas, VA). All media and their components were purchased from Invitrogen (Carlsbad, CA). MTT viability assay kit was obtained from Promega (Fitchburg, WI). Dipalmitoyl phosphatidylcholine (DPPC) was purchased from Avanti Polar Lipids (Alabaster, AL). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of Microparticles
Microparticles were composed of 0% to 100% antibiotic, with the remainder composed of DPPC, albumin, and lactose (DPPC:albumin:lactose = 60:20:20 by weight). DPPC was dissolved in ethanol; the antibiotics, lactose, and albumin were dissolved in water. The solutions were mixed prior to spray-drying, creating a 70:30 ethanol/water cosolvent system (25). For formulations containing ciprofloxacin, a minimal amount of hydrochloric acid to adjust the pH to 3 was added to maintain the solubility of the drug. Spray-drying was performed using a Buchi-190 bench top spray drier (Buchi Co, Flawil, Switzerland). The operating parameters were: inlet temperature of 110–120°C; solution feed rate of 12–14 ml/min; drying airflow rate of 600 ml/min; and aspirator pressure of −18–20 bar. These conditions typically resulted in outlet temperatures of 50–55°C.
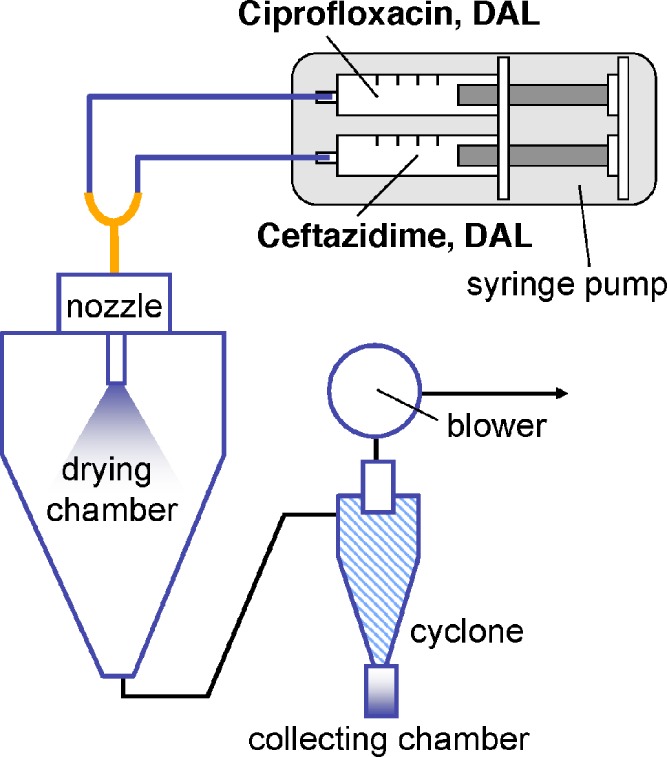
This procedure was modified for co-encapsulating ceftazidime and ciprofloxacin into the DAL particles (CTZ and CIP), since the two antibiotics were incompatible in aqueous solution (13). The solutions containing DAL and each antibiotic were prepared separately as above and dispensed into two respective 60-ml syringes. The two syringes were then mounted on a dual-syringe pump (KD Scientific, Holliston, MA) and connected to the spray nozzle via a Y-shaped adapter (Fig. 1). Both solutions were fed simultaneously into the spray-dryer at 7 ml/min per syringe (14 ml/min total). This minimized contact of the two drug solutions prior to formation of particles presumably preserving the homogeneity of the mixture and the consistency of the ceftazidime-to-ciprofloxacin ratio in the resulting microparticles. The other operating parameters remained the same. All spray-dried particles were stored in desiccated containers at 4°C until use.
Fig. 1.
Schematic of co-encapsulation of ciprofloxacin and ceftazidime by spray-drying. Individual antibiotics and the inert excipients (DPPC, albumin, and lactose; DAL) were dissolved in a co-solvent system. The final dry powder product accumulates in the collecting chamber
Drug loading was determined by dissolving the microparticles (5 mg/ml) in a 0.5% aqueous solution of sodium dodecyl sulfate. The concentration of antibiotics was measured by high performance liquid chromatography (HPLC).
High Performance Liquid Chromatography (HPLC) Analysis of Antibiotics
The ceftazidime and ciprofloxacin contents of the microparticles were analyzed by HPLC (1100 series, Agilent Technologies, Palo Alto, CA) with an Atlantis dC18 analytical column (dC18; 4.6 × 250 mm; particle size 5 μm). The mobile phase was a mixture of 10 mM phosphate buffer (pH 2.1) and acetonitrile (with an increasing ratio of acetonitrile from 20% to 70% over 8 min). The flow rate was 1 ml/min. Samples were filtered using a 0.45 μm syringe filter, and 5 μl was injected into the pre-equilibrated column followed by 10 min of wash with the mobile phase. The UV detector was set at 275 nm. A calibration curve was made by correlating the peak areas in the chromatograms with the concentrations of ceftazidime and ciprofloxacin standards.
Scanning Electron Microscopy
Spray-dried microparticles were attached to specimen stubs using double-coated tape and sputter-coated with gold in the presence of argon gas using a Desk II cold sputter/etch unit (Denton vacuum LLC, Moorestown, NJ). The specimens were imaged with a JEOL JSM 6060 scanning microscope (JEOL USA Inc., Peabody, MA) using 3 kV accelerating voltage at 6–9 mm working distance.
In-vitro Microparticle Aerodynamics
The aerodynamic properties of the microparticles were investigated in-vitro using an eight-stage Andersen cascade impactor (ACI) (1 ACFM Non-Viable Cascade Impactor, Andersen, Smyrna, GA). The environment was controlled to maintain the relative humidity at 50% and temperature at 20°C. The microparticle samples (nominal dose, 30 mg) were manually loaded into a custom-made passive inhaler. The ACI was operated according to manufacturer’s recommendations; the flow was maintained at 28.3 l/min. The effective cutoff aerodynamic diameter for each stage was as follows: Stage 0, 9 μm; Stage 1, 5.8 μm; Stage 2, 4.7 μm; Stage 3, 3.3 μm; Stage 4, 2.1 μm; Stage 5, 1.1 μm; Stage 6, 0.65 μm; Stage 7, 0.43 μm. The fine particle fraction (FPF) was defined as the amount of powder with an aerodynamic size <4.7 μm (particles deposited at stage 3 and lower) divided by the nominal dose and expressed as percentage. The cumulative mass of powder less than the effective cutoff diameter as percent of total mass recovered in the ACI was plotted against the effective cutoff diameter. The mass median aerodynamic diameter (MMAD) was defined on this graph as the particle size at which the line crossed the 50th percentile.
Antibacterial Activity of Microparticles
Five milligrams of microparticles containing various percentages of ceftazidime or ciprofloxacin were deposited into a pre-formed well in a Petri dish containing a reference strain of P. aeruginosa (ATCC 27853) on agar. The plates were incubated overnight to reveal the zone of inhibition. This zone was normalized to the total area of the Petri dish and expressed as a function of the logarithm of the percentage loading of ceftazidime or ciprofloxacin in the particles, yielding a dose–response curve for each antibiotic. Each microparticle type’s 50% effective concentration (EC50) was determined.
To study the interaction between ceftazidime and ciprofloxacin against the reference strain of P. aeruginosa, DAL microparticles co-encapsulating one-half of the amount corresponding to the EC50 of each antibiotic were produced. Their antipseudomonal activities were tested as described above.
Statistics
Data are expressed as mean ± standard deviation (SD) and analyzed using SigmaStat (SPSS Inc., Chicago, IL). Statistical differences were tested by using Student t-test or repeated-measures ANOVA. A value of p < 0.05 was considered statistically significant.
RESULTS
Microparticles
Microparticles with ceftazidime (CTZx, x indicating % of ceftazidime content) were fluffy and yellow; DAL microparticles without antibiotics (blanks) and particles containing ciprofloxacin (CIPy: y indicating % of ciprofloxacin content) formed a fluffy white powder. The particles containing both ceftazidime and ciprofloxacin (CTZx–CIPy) were a yellowish powder less fluffy than the blanks, CTZs, and CIPs. The particle yield ranged between 30% and 40% of total solute without significant differences among the particle types.
Scanning Electron Microscopy
Microparticle morphology was examined by scanning electron microscopy (Fig. 2). The presence of DPPC, albumin, and lactose (DAL) in the formulation resulted in irregularly shaped microparticles or rough spheroids. CTZ100s and CIP100s were spherical and smooth. The particle size of DAL and CTZ ranged from 2 to 20 μm, whereas that of CIP was slightly smaller (mostly less than 15 μm). On the other hand, both CTZ100s and CIP100s were in the range of 2–5 μm.
Fig. 2.
Scanning electron micrographs of spray-dried microparticles. CTZ and CIP are DAL microparticles containing ceftazidime and/or ciprofloxacin, respectively. Subscripts indicate the percentages of ceftazidime or ceftazidime in the microparticles by weight. The remainder was DPPC, albumin, and lactose (60:20:20, respectively, by weight %). All pictures were taken at ×5,000 magnification (scale bar = 5 μm)
In-vitro Microparticle Aerodynamics
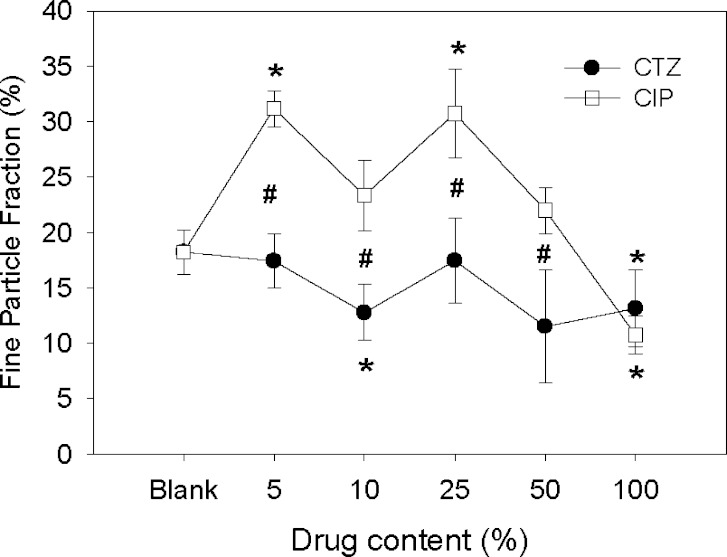
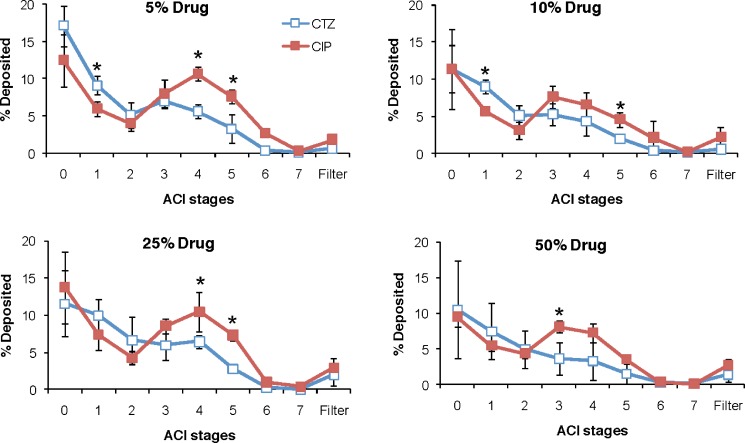
The aerodynamic properties of single-drug DAL-based microparticles were investigated using the Andersen Cascade Impactor (ACI; Fig. 3). Compared to blank particles, encapsulation of ceftazidime (CTZ10) decreased the FPF (expressed as percentage of the nominal dose), whereas encapsulation of ciprofloxacin (CIP5 and CIP25) increased it (p < 0.05). Compared to each other, the FPFs of CTZ and CIP particles were significantly different at drug contents of 5–50% (p < 0.05). CTZ100 or CIP100 had lower FPFs than blank DAL. CTZ and CIP particles showed different, albeit overlapping, ACI deposition profiles, with a significant proportion of CTZ particles depositing above the CIP particles at Stages 0 and 1 in the ACI (Fig. 4). The FPFs of CTZ-CIP particles were similar to those of microparticles with the same proportion of ciprofloxacin alone, but differed from those containing ceftazidime alone (Fig. 5). The mass of drug delivered to ACI stages 0–7 from the inhaler for most particles ranged from 40–55%, but were not significantly different from that of the blank particles, 45% (p > 0.05). The only exception were the microparticles composed entirely of drug (CIP100: 27% or CTZ100: 29%), which was expected since they did not contain DPPC, albumin, or lactose that contribute to favorable aerodynamics (26).
Fig. 3.
Fine particle fraction (relative to the nominal dose) of CTZ and CIP microparticles with varying drug contents. Data are means ± SD (n = 4). Asterisks p < 0.05 vs. blank microparticles; number sign p < 0.05 between CTZ and CIP microparticles
Fig. 4.
Deposition profiles of CTZ and CIP microparticles with varying drug contents in the Anderson cascade impactor (ACI). The fraction deposited in each stage is calculated by dividing the particle mass by the nominal dose. Data are expressed as means ± SD (n = 4). Asterisks p < 0.05 between CTZ and CIP microparticles
Fig. 5.
Fine particle fraction (relative to the nominal dose) of DAL microparticles containing ceftazidime and ciprofloxacin (CTZ–CIP), ceftazidime (CTZ), or ciprofloxacin (CIP). Subscripts indicate percentages of drugs in the microparticles. Data are mean ± SD (n = 4). Asterisks p < 0.05 between groups
Antibacterial Dose–Response Curves
The antibacterial activities of microparticles with varying drug contents were determined by measuring the extent to which they inhibited the growth of P. aeruginosa on agar. The EC50 for single-drug microparticles was defined as the % drug content (in 5 mg of microparticles) that achieved 50% of the inhibition zone that was obtained with microparticles made of pure drug. The EC50s were achieved with 5 mg of CTZ10 and 5 mg of CIP20. Based on these results, CTZ5–CIP10 particles (containing one-half of the EC50s of both ceftazidime and ciprofloxacin) were produced, and their zones of inhibition were compared with those of CTZ10 and CIP20. While the CTZ5–CIP10 microparticles and the CIP20 microparticles showed equal potency, the CTZ10 microparticles were approximately half as potent, showing that ceftazidime and ciprofloxacin co-delivered in microparticles have at least additive effects against Pseudomonas (Fig. 6).
Fig. 6.
Anti-bacterial activities of CTZ 5–CIP 10 (containing a mixture of half of the EC50s of ceftazidime and ciprofloxacin), CTZ 10 (containing the EC50 of ceftazidime), and CIP 20 (containing the EC50 of ciprofloxacin) microparticles. Data are mean ± SD (n = 3–6). Asterisks p < 0.05 between groups
DISCUSSION
Inhaled antibiotic therapy using dry-powder microparticles is an attractive means of drug delivery to the diseased airway. Compared with nebulization, dry-powder inhalation is faster, requires minimal equipment, and is less likely to disperse the drug into ambient air. Inhaled encapsulated drugs may avoid most of the toxicities associated with their systemically-administered counterparts. These factors are likely to improve compliance, overall microbiologic success, and patient outcomes.
In our study, ceftazidime and ciprofloxacin were co-encapsulated into the DAL-based microparticles using the simple method described here (Fig. 1). This dry powder system is advantageous as compared to a nebulizer for co-delivery of the two antibiotics, as the two tend to precipitate when they co-exist in solution (13).
The microparticles containing each antibiotic (CTZ and CIP microparticles) displayed different aerodynamic properties: adding ceftazidime decreased the microparticles’ FPF, whereas adding ciprofloxacin increased it. Moreover, ceftazidime and ciprofloxacin at all degrees of loading had different ACI deposition profiles, so separate administration of CTZ or CIP microparticles in vivo would not guarantee co-deposition, the lack of which may accelerate the development of antibiotic resistance. Although there were substantial areas of overlap between the ACI deposition profiles of the microparticles containing each drug alone, this could easily not be the case with other compounds. This potential problem would be obviated by co-encapsulation. Co-encapsulation also improved the microparticles’ aerodynamic properties and FPF as compared to the microparticles containing equivalent amount of ceftazidime alone; hence in vivo the particles co-encapsulating the two antibiotics will have a higher probability of deep airway deposition. These considerations suggest that co-encapsulation of antibiotics may be clinically warranted.
All the microparticles containing antibiotics showed antibacterial activity, allaying concerns about negative effects of spray-drying on the stability of the drugs. The co-encapsulation described here achieved additivity in antibiotic effectiveness; however, the potency of such particles could be vastly enhanced if the drugs were synergistic. This could reduce the mass of powder to be inhaled and/or require a lower proportion of active payload per particle, which could be beneficial if the drug adversely affected the aerodynamics. Co-encapsulation of compounds that enhance each other’s performance has been used effectively in other settings (27–29).
Although the ACI is a widely-accepted in-vitro model of pulmonary deposition of particulate matter, the results it provides may not be totally predictive of in-vivo behavior (30). In the airways of patients with CF and some other conditions, P. aeruginosa exists in a biofilm containing mucins and various products of neutrophil breakdown (2), which entrap it within tenacious mucus. Unless there is a mucolytic effect of a component of the formulation (which we have not demonstrated), microparticulate delivery systems are unlikely to enhance flux through the mucus.
Importantly, a potential impediment to the delivery of antibiotics by microparticulate systems is the low potency of the compounds, necessitating the delivery of large quantities of material. In preclinical studies, gentamicin doses of 160–180 mg (micronized into dry powder) (31,32) and tobramycin doses of 13 mg (as 25 mg of PulmoSphere® particles) (33) have been used in healthy volunteers and those affected with CF; 20 mg doses of particulate ciprofloxacin, doxycycline, or co-spray-dried ciprofloxacin–doxycycline were used in a recently-reported formulation study (34). Anti-P. aeruginosa MIC90 of gentamicin is 5 μg/ml (35), that of ciprofloxacin is less than 0.5 μg/ml (36), and that of ceftazidime is 2 μg/ml (37). One may therefore extrapolate that to achieve anti-P. aeruginosa effects approximately equal to those of 160 mg of gentamicin administered as DPI (32 times the MIC90 ) would require approximately 16 mg of ciprofloxacin or 64 mg of ceftazidime. Since from our data the ceftazidime and ciprofloxacin are at least additive against P. aeruginosa, their individual doses may be halved without loss of total activity if the drugs are co-administered in a single particle. Hence, only approximately 40 mg of drugs (8 mg of ciprofloxacin and 32 mg of ceftazidime) plus the requisite excipient mass would have to be inhaled to achieve the effect of 160 mg of gentamicin, while providing clinically advantageous double coverage. Preclinical studies will be necessary to determine whether sufficient doses of antibiotics can be delivered by an amount of particles that is practical.
CONCLUSION
Dry-powder inhalational delivery of antibiotics is a promising new development in therapy of CF. We produced and evaluated microparticles composed of dipalmitoylphosphatidylcholine, albumin, and lactose as a model system for intrapulmonary delivery of ceftazidime, ciprofloxacin, and combinations of the two, by spray-drying. Co-encapsulation of the antibiotics in microparticles ensured co-deposition at desired ratios, improved the particles’ aerodynamics and FPFs, as compared to microparticles with equivalent amount of ceftazidime alone, and achieved additive antipseudomonal activity. Co-encapsulation of two or more antibiotics is possible and may represent a useful design approach from the microbiological and pharmaceutical standpoint.
ACKNOWLEDGMENT
We would like to express our gratitude to Henry Dorkin, MD for helpful insights. This work was supported by Cystic Fibrosis Foundation Research and Clinical Fellowship grant (TSIFAN02B0) to MDT and NIH GM073626 to DSK.
Footnotes
Michael D. Tsifansky and Yoon Yeo contributed equally.
References
- 1.Penketh A. R., Wise A., Mearns M. B., Hodson M. E., Batten J. C. Cystic fibrosis in adolescents and adults. Thorax. 1987;42(7):526–532. doi: 10.1136/thx.42.7.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M. I. Gomez, and A. Prince. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol (2007). [DOI] [PubMed]
- 3.Hodson M. E., Gallagher C. G. New clinical evidence from the European tobramycin trial in cystic fibrosis. J. Cyst. Fibros. 2002;1(Suppl 2):199–202. doi: 10.1016/S1569-1993(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey B. W., Pepe M. S., Quan J. M., Otto K. L., Montgomery A. B., Williams-Warren J., Vasiljev K. M., Borowitz D., Bowman C. M., Marshall B. C., et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N. Engl. J. Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 5.Hodson M. E. Antibiotic treatment. Aerosol therapy. Chest. 1988;94(2 Suppl):156S–162S. doi: 10.1378/chest.94.2.156S. [DOI] [PubMed] [Google Scholar]
- 6.Campbell P. W., 3rd, Saiman L. Use of aerosolized antibiotics in patients with cystic fibrosis. Chest. 1999;116(3):775–788. doi: 10.1378/chest.116.3.775. [DOI] [PubMed] [Google Scholar]
- 7.Weathers L., Riggs D., Santeiro M., Weibley R. E. Aerosolized vancomycin for treatment of airway colonization by methicillin-resistant Staphylococcus aureus. Pediatr. Infect. Dis. J. 1990;9(3):220–221. doi: 10.1097/00006454-199003000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Máiz L., Cantón R., Mir N., Baquero F., Escobar H. Aerosolized vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. Pediatric. Pulmonology. 1998;26(4):287–289. doi: 10.1002/(SICI)1099-0496(199810)26:4<287::AID-PPUL9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Gradon J. D., Wu E. H., Lutwick L. I. Aerosolized vancomycin therapy facilitating nursing home placement. Ann. Pharmacother. 1992;26(2):209–210. doi: 10.1177/106002809202600214. [DOI] [PubMed] [Google Scholar]
- 10.Laube B. Aerosol delivery systems. In: Loughlin G., Eigen H., editors. Respiratory Disease in Children: Diagnosis and Management. Baltimore, MD: Williams and Wilkins; 1994. pp. 721–729. [Google Scholar]
- 11.Bernard R. S., Cohen L. L. Increasing adherence to cystic fibrosis treatment: a systematic review of behavioral techniques. Pediatr. Pulmonol. 2004;37(1):8–16. doi: 10.1002/ppul.10397. [DOI] [PubMed] [Google Scholar]
- 12.Wall M. A., Terry A. B., Eisenberg J., McNamara M., Cohen R. Inhaled antibiotics in cystic fibrosis. Lancet. 1983;1(8337):1325. doi: 10.1016/S0140-6736(83)92428-5. [DOI] [PubMed] [Google Scholar]
- 13.Elmore R. L., Contois M. E., Kelly J., Noe A., Poirier A. Stability and compatibility of admixtures of intravenous ciprofloxacin and selected drugs. Clin. Ther. 1996;18(2):246–255. doi: 10.1016/S0149-2918(96)80005-1. [DOI] [PubMed] [Google Scholar]
- 14.Prober C. G., Walson P. D., Jones J. Technical report: precautions regarding the use of aerosolized antibiotics. Committee on Infectious Diseases and Committee on Drugs. Pediatrics. 2000;106(6):E89. doi: 10.1542/peds.106.6.e89. [DOI] [PubMed] [Google Scholar]
- 15.Bosquillon C., Lombry C., Preat V., Vanbever R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release. 2001;70(3):329–339. doi: 10.1016/S0168-3659(00)00362-X. [DOI] [PubMed] [Google Scholar]
- 16.Bosquillon C., Rouxhet P. G., Ahimou F., Simon D., Culot C., Preat V., Vanbever R. Aerosolization properties, surface composition and physical state of spray-dried protein powders. J. Control. Release. 2004;99(3):357–367. doi: 10.1016/j.jconrel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Dostal R. E., Seale J. P., Yan B. J. Resistance to ciprofloxacin of respiratory pathogens in patients with cystic fibrosis. Med. J. Aust. 1992;156(1):20–24. doi: 10.5694/j.1326-5377.1992.tb121473.x. [DOI] [PubMed] [Google Scholar]
- 18.Watkins J., Francis J., Kuzemko J. A. Does monotherapy of pulmonary infections in cystic fibrosis lead to early development of resistant strains of Pseudomonas aeruginosa? Scand. J. Gastroenterol. Suppl. 1988;143:81–85. doi: 10.3109/00365528809090223. [DOI] [PubMed] [Google Scholar]
- 19.Cheer S. M., Waugh J., Noble S. Inhaled tobramycin (TOBI): a review of its use in the management of Pseudomonas aeruginosa infections in patients with cystic fibrosis. Drugs. 2003;63(22):2501–2520. doi: 10.2165/00003495-200363220-00015. [DOI] [PubMed] [Google Scholar]
- 20.Smith A. L., Ramsey B. W., Hedges D. L., Hack B., Williams-Warren J., Weber A., Gore E. J., Redding G. J. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr. Pulmonol. 1989;7(4):265–271. doi: 10.1002/ppul.1950070413. [DOI] [PubMed] [Google Scholar]
- 21.Burns J. L., Van Dalfsen J. M., Shawar R. M., Otto K. L., Garber R. L., Quan J. M., Montgomery A. B., Albers G. M., Ramsey B. W., Smith A. L. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J. Infect. Dis. 1999;179(5):1190–1196. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 22.Fish D. N., Choi M. K., Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J. Antimicrob. Chemother. 2002;50(6):1045–1049. doi: 10.1093/jac/dkf211. [DOI] [PubMed] [Google Scholar]
- 23.Pendland S. L., Messick C. R., Jung R. In vitro synergy testing of levofloxacin, ofloxacin, and ciprofloxacin in combination with aztreonam, ceftazidime, or piperacillin against Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2002;42(1):75–78. doi: 10.1016/S0732-8893(01)00312-1. [DOI] [PubMed] [Google Scholar]
- 24.Edwards D. A., Ben-Jebria A., Langer R. Recent advances in pulmonary drug delivery using large, porous inhaled particles. J. Appl. Physiol. 1998;85(2):379–385. doi: 10.1152/jappl.1998.85.2.379. [DOI] [PubMed] [Google Scholar]
- 25.Kohane D. S., Lipp M., Kinney R. C., Lotan N., Langer R. Sciatic nerve blockade with lipid–protein–sugar particles containing bupivacaine. Pharm. Res. 2000;17(10):1243–1249. doi: 10.1023/A:1026470831256. [DOI] [PubMed] [Google Scholar]
- 26.Vanbever R., Mintzes J. D., Wang J., Nice J., Chen D., Batycky R., Langer R., Edwards D. A. Formulation and physical characterization of large porous particles for inhalation. Pharm. Res. 1999;V16(11):1735. doi: 10.1023/A:1018910200420. [DOI] [PubMed] [Google Scholar]
- 27.Kohane D. S., Smith S. E., Louis D. N., Colombo G., Ghoroghchian P., Hunfeld N. G. M., Berde C. B., Langer R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 2003;104(1,2):415–421. doi: 10.1016/S0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 28.Castillo J., Curley J., Hotz J., Uezono M., Tigner J., Chasin M., Wilder R., Langer R., Berde C. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85(5):1157–1166. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Colombo G., Padera R., Langer R., Kohane D. Prolonged duration local anesthesia with lipid–protein–sugar particles containing bupivacaine and dexamethasone. J. Biomed. Mater. Res. A. 2005;75(2):458–464. doi: 10.1002/jbm.a.30443. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell J. P., Nagel M. W. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J. Aerosol. Med. 2003;16(4):341–377. doi: 10.1089/089426803772455622. [DOI] [PubMed] [Google Scholar]
- 31.Goldman J. M., Bayston S. M., O’Connor S., Meigh R. E. Inhaled micronised gentamicin powder: a new delivery system. Thorax. 1990;45(12):939–940. doi: 10.1136/thx.45.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowther Labiris N. R., Holbrook A. M., Chrystyn H., Macleod S. M., Newhouse M. T. Dry powder versus intravenous and nebulized gentamicin in cystic fibrosis and bronchiectasis. A pilot study. Am. J. Respir. Crit. Care Med. 1999;160(5 Pt 1):1711–1716. doi: 10.1164/ajrccm.160.5.9810080. [DOI] [PubMed] [Google Scholar]
- 33.Newhouse M. T., Hirst P. H., Duddu S. P., Walter Y. H., Tarara T. E., Clark A. R., Weers J. G. Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest. 2003;124(1):360–366. doi: 10.1378/chest.124.1.360. [DOI] [PubMed] [Google Scholar]
- 34.H. Adi, P. M. Young, H. K. Chan, P. Stewart, H. Agus, and D. Traini. Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci. (2007). [DOI] [PubMed]
- 35.Gentamicin. Micromedex® Healthcare Series. Thomson Micromedex
- 36.Ciprofloxacin. Micromedex® Healthcare Series. Thomson Micromedex
- 37.Ceftazidime. Micromedex® Healthcare Series. Thomson Micromedex