Abstract
The aim of the present investigation was to evaluate the potential use of mucoadhesive microspheres for gastroretentive delivery of acyclovir. Chitosan, thiolated chitosan, Carbopol 71G and Methocel K15M were used as mucoadhesive polymers. Microsphere formulations were prepared using emulsion-chemical crosslinking technique and evaluated in vitro, ex-vivo and in-vivo. Gelatin capsules containing drug powder showed complete dissolution (90.5 ± 3.6%) in 1 h. The release of drug was prolonged to 12 h (78.8 ± 3.9) when incorporated into mucoadhesive microspheres. The poor bioavailability of acyclovir is attributed to short retention of its dosage form at the absorption sites (in upper gastrointestinal tract to duodenum and jejunum). The results of mucoadhesion study showed better retention of thiolated chitosan microspheres (8.0 ± 0.8 h) in duodenal and jejunum regions of intestine. The results of qualitative and quantitative GI distribution study also showed significant higher retention of mucoadhesive microspheres in upper GI tract. Pharmacokinetic study revealed that administration of mucoadhesive microspheres could maintain measurable plasma concentration of acyclovir through 24 h, as compared to 5 h after its administration in solution form. Thiolated chitosan microsphere showed superiority over the other formulations as observed with nearly 4.0-fold higher AUC0–24 value (1,090 ± 51 ng h/ml) in comparison to drug solution (281 ± 28 ng h/ml). Overall, the result indicated prolonged delivery with significant improvement in oral bioavailability of acyclovir from mucoadhesive microspheres due to enhanced retention in the upper GI tract.
Key words: acyclovir, gastroretentive, in vivo evaluation, mucoadhesive microsphere, thiolated chitosan
INTRODUCTION
Acyclovir, the first agent to be licensed for the treatment of herpes simplex virus infections, is most widely used drug for infections such as cutaneous herpes, genital herpes, chicken pox, varicella zoster infections and herpes keratitis. Acyclovir is currently marketed as capsules (200 mg), tablets (200, 400 and 800 mg) and suspension for oral administration, intravenous injection and topical ointment (1). Oral acyclovir is mostly used as 200 mg tablets, five times a day. In addition, long term administration of acyclovir (6 month or longer) is required in immunocompetent patient with relapsing herpes simplex infection (2). The presently available conventional therapy is associated with a number of drawbacks such as highly variable absorption and low bioavailability (10–20%) after oral administration (6). Furthermore, with increase in dose, there was decrease in bioavailability. Moreover, because the mean plasma half life of the drug is 2.5 h, five times a day administration is required. In order to make oral therapy of acyclovir more patient compliant there is need to develop controlled drug delivery dosage form. Researchers have investigated formulating acyclovir in delivery systems using different approaches like matrix tablets (3), microspheres (4) and polymeric films (5).
The main problem with the therapeutic effectiveness of acyclovir is its absorption that is highly variable and dose dependent thus reducing the bioavailability to 10–20% (6). Acyclovir is soluble in acidic pH and is predominantly absorbed from upper gastro intestinal tract (GIT) to duodenum to jejunum regions (7). There are indications of its active absorption from the duodenum and jejunum regions of GIT (8). In commercially available dosages forms, the amount of drug absorbed is very low (10–20%) due to short residence time of the dosage forms at the absorption site. As a result, most of the drug is excreted in the faeces (50–60%) in unabsorbed form. Hence, it can be envisaged that increasing the residence time at the absorption site can enhance the absorption and bioavailability of acyclovir.
The present investigation, therefore aimed at formulating mucoadhesive microspheres of acyclovir. Chitosan and thiolated chitosan were selected as mucoadhesive polymers in present study and results were compared with widely used mucoadhesive polymers Carbopol 71G and Methocel K15M. Chitosan is a polysaccharide comprising copolymers of glucosamine and N-acetyl glucosamine (9). It is biodegradable, biocompatible, mucoadhesive polymer and has been used in the formulation of particulate drug delivery system. Embedding the drug in its micromatrix is expected to enhance the absorption of acyclovir from the site of absorption (10). Thiolated chitosan represents a new mucoadhesive polymer of promise. The higher mucoadhesive properties of thiomers are reported to intensify the contact with gastric mucosa, providing an increased epithelial permeability for many drugs (11,12). In addition, these polymers are reported to increase the intestinal permeability of drugs, and therefore besides their mucoadhesiveness, they may be beneficial for increasing the intestinal permeability of acyclovir.
MATERIALS AND METHODS
Materials
Acyclovir sodium and chitosan (degree of deacetylation 82% and molecular weight 650,000) were obtained as gift sample from Cipla Ltd, Mumbai and Central Fisheries Research Institute, Cochin, respectively. Methocel K15M and Carbopol 71G were obtained as gift sample from Colorcon Ltd., Mumbai and Degussa Ltd., Mumbai, respectively. 2-Iminothiolane-HCl and Traut's reagent were procured from Sigma-Aldrich Ltd., USA. Ethanol, acetonitrile, methanol and xylene were procured from E. Merck Ltd., Mumbai, India. Thiolated Chitosan was prepared in the lab according to the method reported by Bernkop-Schnurch et al. (13).
Preparation of Chitosan and Thiolated Chitosan Microspheres
Microspheres formulations using chitosan and thiolated chitosan as polymers were prepared using the method described by Wang et al. (14). A solution of chitosan or thiolated chitosan (2% w/v) was prepared in acetic acid (2% v/v) and aqueous solution of drug (0.3% w/w) was added to their respective solution. This was further added to a continuous phase (which consisted of light liquid paraffin and heavy liquid paraffin (1:1) containing Span 80 (0.5% w/v) as surfactant) under constant stirring (1,800 rpm) using a three blade propeller stirrer to form a w/o emulsion. This procedure was followed by addition of 0.25 ml of gluteraldehyde (25% v/v) dropwise at 15, 30, 45 and 60 min, respectively. The stirring was continued for 3.5 h. The microspheres so obtained were separated by centrifugation and washed with petroleum ether to remove liquid paraffin. The microspheres were suspended in 5% w/v sodium bisulfite solution and stirred for 15 min to remove residual gluteraldehyde. Final washing was done with distilled water, the microspheres were dried and stored in a vacuum desiccator.
Microspheres of Carbopol 71G and Methocel K15M were prepared by spray drying techniques as reported by Harikarnpakdee et al. (15). Methocel K15M or Carbopol 71G were dissolved in deionised water. Acyclovir was separately dissolved in distilled water. Colloidal silicon dioxide (Aerosil), maltodextrin and propylene glycol were then mixed with the polymer solution. The solution of each batch was spray dried employing inlet temperature of 120°C for Carbopol 71G and 130°C for Methocel K15M, with a pump setting of 5 ml/min; and a spray flow rate of 400 nl/h. Fluorescent-loaded microspheres were prepared similarly, except that the, drug solution was replaced with 0.3% w/v of 6-CF.
Characterization of Microspheres
Morphological Examination
The morphology of microspheres was examined by scanning electron microscopy (SEM, JSM-5310LV scanning microscope Tokyo, Japan). The microspheres were mounted on metal stubs using double-sided tape and coated with a 150 Å layer of gold under vacuum. Stubs were visualized under scanning electron microscope.
Particle Size Measurement
The particle size of the microspheres was measured using a stage micrometer scale. Dry microspheres (5 mg) were suspended in distilled water and ultrasonicated for 5 s. A drop of suspension was placed on a clean glass slide and microspheres were counted under stage ocular micrometer. A minimum of 200 microspheres was counted per batch.
Swelling Measurement
The swelling of microspheres was conducted in phosphate buffer pH 6.8. The sizes of dried microsphere and those after incubation in phosphate buffer (pH 6.8) for 0.3, 1.0, 3.0 and 5.0 h were measured by using microscopic method. The percentage of swelling at different time interval was determined by the difference between diameter of microspheres at time t (Dt) and initial time (t = 0 [D0]) as calculated from the following equation
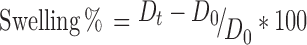 |
1 |
Production Yield
The production yield (percent w/w) was calculated from the ratio of average weight of dried microspheres (W1) recovered from each of three batches to the sum of initial dry weight of starting materials (W2).
Entrapment Efficiency
Acyclovir loaded microspheres (10 mg) of chitosan or thiolated chitosan were digested in HCl (0.01 M). Carbopol 71G and Methocel K15M microspheres were dispersed in 0.1 M NaOH and 0.05 M phosphate buffer (pH 6.8), respectively for overnight with intermittent shaking. The mixture was filtered and filtrate was assayed spectroflurometrically (Elico Spectrofluorometer, SL-174) at excitation wavelength of 256 nm and emission wavelength of 374 nm according to the method reported by Darwish et al. (16). The entrapment efficiency was calculated from the ratio of actual amount of the drug present in the formulation to the initial amount of the drug added.
Mucoadhesion Measurement Study
The mucoadhesion property of microsphere formulations was determined according to the method described by Vyas et al. (17). A 5 cm long piece of freshly cut pig intestine was obtained from a local abattoir within 1 h of killing of animal, and was cleaned by washing with isotonic saline solution. An accurate weight of microspheres was placed on the mucosal surface, which was attached to a polyethylene plate that was fixed at an angle of 40° relative to the horizontal plane. Phosphate buffer (pH 6.8) warmed at 37 ± 1°C was passed at a rate of 5 ml/min over the tissue. The time required for detaching all the microspheres from mucosal surface of the pig intestine was recorded by visual inspection (15).
In Vitro Drug Release Study
In vitro release of acyclovir from microspheres was determined by carrying out dissolution test using USP paddle method at a stirring rate of 50 ± 5 rpm at temperature 37 ± 0.5°C. Nine hundred milliliters of HCl buffer (pH 1.2) was used as dissolution medium for first hour and phosphate buffered saline (PBS, pH 6.8) was used for next 11 h. The dried microspheres were filled in hard gelatin capsules and were placed in dissolution vessels. A 5 ml sample was withdrawn at various time intervals and the volume of the media was replenished with an equal amount of dissolution media. The samples were then analyzed spectroflurometrically.
G.T.I. Distribution
Rats (Sprague Dawley strain), 6 to 8 months old, weighing 200–220 g were kept on fasting for 16–20 h before commencement of the experiment. Water was provided ad libitum. The protocols for these investigations were approved by the Institutional Animal ethics committee in accordance with the disciplinary principles and guidelines of CPCSEA (Committee for the purpose of control and supervision of experiments on animals). Five groups of animals were employed, with each group containing 15 rats. The first group received oral administration of aqueous solution of 6-CF (1 ml of 0.3% w/v). The second to fifth groups received microsphere of 6-CF prepared from chitosan (M-CH), thiolated chitosan (M-TCH), Carbopol 71G or Methocel K15M, respectively. Oral administration of microspheres was accomplished by suspending 20 mg sample of microspheres corresponding to 3.0 mg of 6-CF in 1.0 ml normal saline and administered via a rubber tube under non-anesthetic conditions. Three rats were sacrificed each after 2, 4, 6, 8 or 10 h of administration. Stomach (section 1) along with entire length of small intestine that was further subdivided into six sections (sections 2–7; length of each section 14 cm) were isolated (18). The stomach and intestinal sections were cut opened to expose the inner mucosal surface. All microspheres located in each part were collected by scratching the mucosa with a spatula. To the collected sample, 10 ml of 0.1 N HCl was added in case of chitosan and thiolated chitosan. The 0.1 N NaOH and phosphate buffer (0.05 M) were added in case of Carbopol 71G and Methocel K15M microsphere, respectively. The mixture was mashed using a homogenizer to extract 6-CF and kept for 24 h. After centrifugation at 805×g for 20 min, the supernatant was analyzed fluoremetrically at λexitation 489 nm and λemmission 515 nm for 6-CF. The extraction efficiency of 6-CF using this method was found to be approximately 95%. Spectrofluoremetric assay was selected in present study for estimation of 6-CF and acyclovir over the well known HPLC/LC-MS method because it was well reported in literature (16) and the assay have lower limit of detection (1.0 and 50 ng/ml for 6-CF and acyclovir, respectively), linearity, high extraction efficiency (>95%), and are less time consuming, low cost and easy to perform. In addition a 2 cm portion of section 2, 3 and 4 was taken out and further processed for fluorescence microscopy.
Fluorescence Microscopy
Fluorescence microscopy was performed to determine the extent of distribution and penetration of microsphere formulations. The excised tissue sections of GIT were blotted with tissue paper. The wiped tissue was fixed in fixative solution (3:1, absolute alcohol/chloroform) for 3 h. The pieces were first transferred to absolute alcohol for 0.5 h and then in absolute alcohol and xylene for 1 h. Wax scrapings were added in this solution till saturation and were kept for 24 h. Paraffin blocks were made by embedding the tissue in hard paraffin and matured at 62 ± 1.0°C. The sections (5 μm thickness) were cut using a microtome (Erma optical works, Japan) and examined under fluorescence microscope (Leica, DMRBE, Bensheim, Germany).
Hemolytic Toxicity Assay
The reported procedure was followed to perform the hemolytic toxicity studies (19). Blood from healthy donors was collected and anticogulated with 3% sodium citrate. Erythrocytes were separated from blood plasma by centrifugation (805×g, 5 min) and suspended in PBS (pH 7.4). The RBC suspension (1%) was mixed with distilled water, which was considered as producing 100% hemolysis and normal saline producing no hemolysis acted as a blank. A volume of 0.5 ml 2% dispersion of microspheres formulations in PBS (7.4) was added to 4.5 ml of normal saline and mixed with 1 ml RBC suspension. Similarly, 0.5 ml of 2% solution of acyclovir in PBS were mixed with 4.5 ml of normal saline and mixed with RBC suspension and kept in incubator for 1 h at 37 ± 1.0°C. After 1 h, mixture was centrifuged and supernatants were taken and diluted with an equal volume of normal saline and absorbance was measured at 540 nm against supernatant of normal saline diluted similarly as blank. The percentage hemolysis was thus determined for each sample by comparing to water as 100% hemolytic sample.
Pharmacokinetic Study
Rats (Sprague Dawley), 6 to 8 months old, weighing 200–220 g were divided into five groups, each consisting of five animals. Rats were kept on fasting 12 h before drug administration and until 24 h post dosing. Water ad libitum was given throughout the study. The dose selected of acyclovir was 5 mg/kg (20). The first group received oral administration of 0.3% w/v drug solution in PBS (pH 7.4). The second to fifth group received oral administration of chitosan, thiolated chitosan, Carbopol or Methocel microspheres, respectively. A 20 mg sample of microsphere corresponding to 3.0 mg of acyclovir were suspended in 1.0 ml saline and administered orally using a rubber tube under non-anesthetic condition. At 2.5, 5, 10, 15 and 24 h time intervals, blood was collected from jugular vein in ependorff tubes and centrifuged at 358×g for 10 min (REMI Equipment, Mumbai, India). Supernatant was collected and acetonitrile was added to precipitate the proteins. The precipitated proteins were settled by centrifugation at 358×g for 15 min. The supernatant was collected and filtered through a 0.45 μm filter into volumetric flask and drug concentration was determined by spectrofluoremetric assay.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD). Statistical analysis was carried out employing ANOVA followed by studentized range test using the Graph-pad PRISM software. A value of p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Preparation and In Vitro Characterization
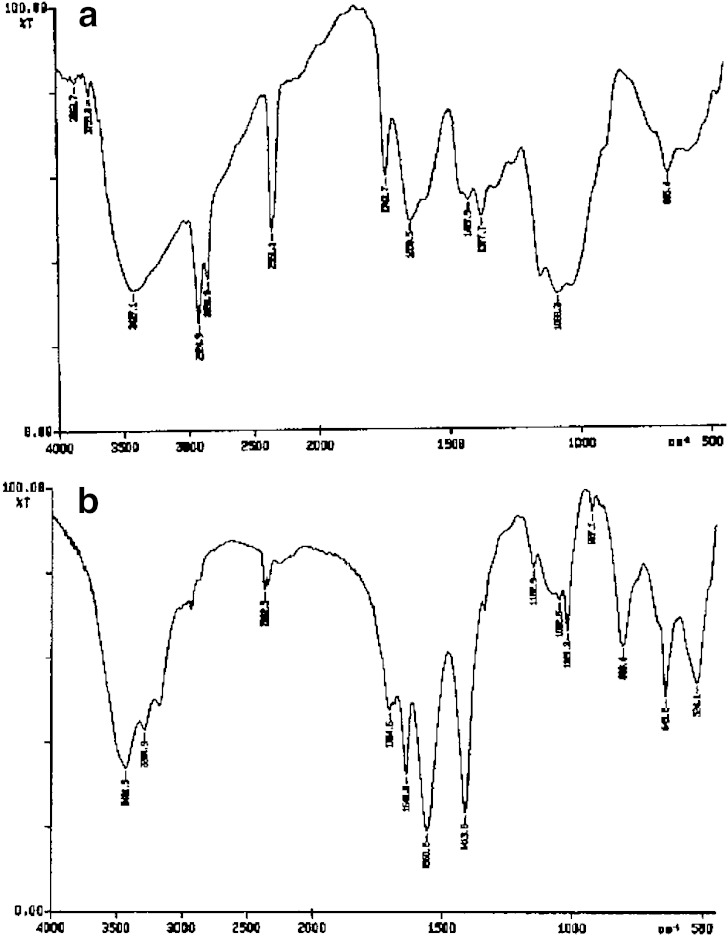
Table I shows the composition of different microsphere formulations. Synthesized polymer was characterized by IR spectroscopy. The FT-IR spectrum of chitosan (a) and synthesized thiolated chitosan (b) are shown in Fig. 1(a, b). In the spectra of thiolated chitosan –OH and –NH stretch were clearly seen at 3,431.5 and 3,284.9 cm−1, respectively in Fig. 1b. Additional presence of amidine I and amidine II bands are seen at 1,641.0 and 1,560.2 cm−1 corresponding to >=NH2+ stretch and NH>=NH2+, respectively, being two coupled vibrations. The presence of an additional band at 1,413.8 cm−1 can be assigned for N–H band of the salt NH2+Cl−. Other characteristic peaks of chitosan O–H stretch, C–H stretch and C–O stretch were present at 3,400–3,600, 2,800–2,900 and 1,020–1,180 cm−1, respectively (Fig. 1b). This confirmed the synthesis of thiolated chitosan. The spectrum of thiolated chitosan was well correlated with reports by Matsuda et al. (21). for thiolated chitosan. Thiol content of thiolated chitosan was found 214 ± 52 μmol/g (13).
Table I.
Composition of different mucoadhesive microsphere formulation
| Formulation code | Drug concentration (% w/w) | Polymer concentration (% w/v) | Cross linker (ml) | Maltodextrina (%) | Aerosilb (%) | Propylene glycolc (%) |
|---|---|---|---|---|---|---|
| M-CH | 0.3 | 2.0 | 1 | – | – | – |
| M-TCH | 0.3 | 2.0 | 1 | – | – | |
| M-CA | 0.3 | 2.0 | – | 80 | 10 | 30 |
| M-ME | 0.3 | 2.0 | – | 80 | 10 | 30 |
M-CH Chitosan microsphere, M-TCH thiolated chitosan microsphere; M-CA Carbopol 71G microsphere, M-ME Methocel K15M microsphere
aPercentage by weight of the total amount of drug and polymer
bPercentage by weight of formulation.
cPercentage by weight of amount of polymer
Fig. 1.
FT-IR spectra of chitosan a and thiolated chitosan b
The spray-dried mucoadhesive microspheres appeared as fine powder in white color (Methocel K15M) or pale yellow color (Carbopol 71G). The microspheres of chitosan or thiolated chitosan were brown in color.
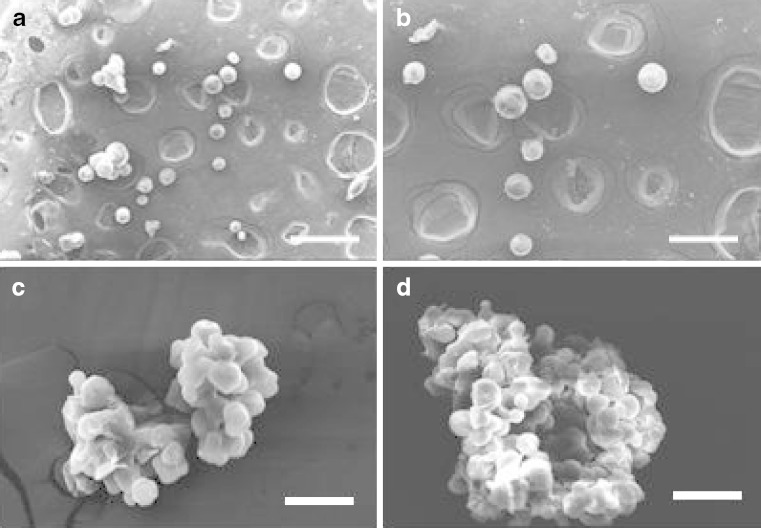
Figure 2(a–d) depict the photomicrographs of microspheres prepared using chitosan, thiolated chitosan, Carbopol 71G and Methocel K15M polymers. All microsphere formulations were spherical in shape and possessed smooth surface as visualized under SEM. The size of different microspheres formulation was found to range from 17.8 ± 1.5 to 21.3 ± 1.0 μm (Table II). The production yield of microsphere formulations was found to be 74.5 ± 3.5, 76.3 ± 3.8, 69.4 ± 4.1 and 54.1 ± 3.0, respectively, for chitosan, thiolated chitosan, Carbopol 71G and Methocel K15M polymers. The entrapment efficiency (percent w/w) of acyclovir in microspheres prepared from chitosan, thiolated chitosan, Carbopol 71G or Methocel K15M was found to be 88.0 ± 2.6, 86.8 ± 3.1, 91.4 ± 4.2 and 77.3 ± 4.2, respectively (Table II).
Fig. 2.
SEM photomicrograph of chitosan a, thiolated chitosan b, Carbopol 934 c and Methocel K15M d microsphere (×10,000). Scale bar = 50 μm
Table II.
In vitro characterizations of mucoadhesive microspheres
| Formulation code | Production yield (%) | Particle size (μm) | Entrapment efficiency (%) | Adhesion time (h) |
|---|---|---|---|---|
| M-CH | 74.5 ± 3.5 | 18.2 ± 0.8 | 88.0 ± 2.6 | 3.1 ± 0.4 |
| M-TCH | 76.3 ± 3.8 | 21.3 ± 1.0 | 86.8 ± 3.1 | 8.0 ± 0.8 |
| M-CA | 69.4 ± 4.1 | 20.6 ± 1.2 | 91.4 ± 4.2 | 0.2 ± 0.1 |
| M-ME | 54.1 ± 3.0 | 17.8 ± 1.5 | 77.3 ± 4.2 | 1.1 ± 0.2 |
Data are presented as mean ± SD (n = 3)
Mucoadhesive Measurement
Table II summarized the results of mucoadhesive measurement of different microspheres formulation in pig intestine. The adhesion time of microspheres followed the rank order of thiolated Chitosan (8.0 ± 0.8 h) > Chitosan (3.1 ± 0.4 h) > Carbopol 71G (1.1 ± 0.2) > Methocel K15M (0.2 ± 0.1 h). The comparatively poor mucoadhesion of Methocel microspheres could be attributed to its non-ionic property. On the contrary, strong electrostatic attraction seems to have contributed to good mucoadhesion between mucin and Carbopol 71G or Chitosan. Numerous hydrophilic functional groups such as carboxyl groups in chitosan molecules have an ability to form hydrogen bonds with the mucus molecules. This interaction is reported to be responsible for mucoadhesive property of this polymer (15). Carbopol microspheres possessed a negative charge which in the presence of the PBS buffer (pH 6.8) medium could have been repelled by the negatively charged mucus leading to poor mucoadhesion.
The excellent mucoadhesion observed in thiolated chitosan microspheres may be due to the presence of thiol groups, which are known to enhance the mucoadhesive property of chitosan due to formation of strong covalent bonds (disulfide bonds) with mucin. The formation of disulfide bonds between the thiomer and the mucus gel layer takes place either through thiol/disulfide exchange reaction or via a simple oxidation process of thiol groups (22). However, other polymers like chitosan or Carbopol form non-covalent bonds like hydrogen bonds, van der Waal's forces or ionic interactions, thereby resulting in weak mucoadhesion.
Swelling Study
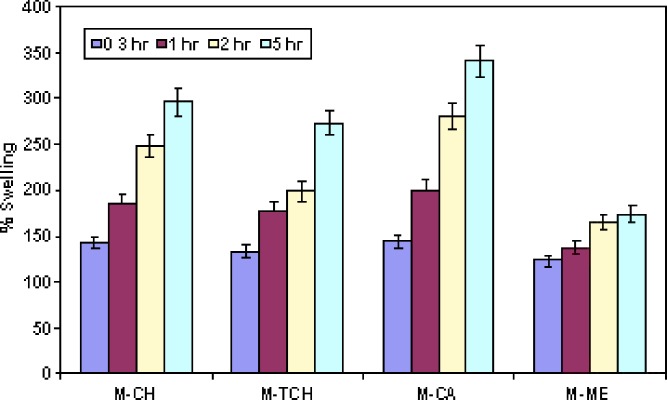
Figure 3 shows the percentage swelling of different microsphere formulations at different time intervals. The results revealed that all microsphere formulations swelled rapidly when immersed in phosphate buffer (pH 6.8). It is reported that adhesive properties and cohesiveness of mucoadhesive polymers are generally affected by their swelling behavior (23). Mucoadhesive microspheres are anticipated to take up water from the underlying mucosal tissue by absorbing, swelling, and capillary effects, leading to considerable stronger adhesion (24). The percent swelling of different microsphere formulation was found to follow the rank order 248 ± 18%, 198 ± 15%, 279 ± 26% and 164 ± 15%, respectively, after 2 h for microsphere prepared from chitosan, thiolated chitosan, Carbopol 71G and Methocel K15M. After 5 h of incubation percent swelling was observed to be 295 ± 28%, 273 ± 24%, 340 ± 30% or 173 ± 15%, respectively. Chitosan and Methocel K15M microspheres showed significantly less (p < 0.05) swelling as comparison to thiolated chitosan or Carbopol 71G microsphere. It was observed that thiolated chitosan microspheres swelled slowly and produced higher mucoadhesive strength. This is perhaps because slow swelling avoids the formation of over hydrated structure that loses its mucoadhesive properties before reaching the target (25). On the other hand, the highest swelling observed in microspheres of Carbopol 71G could be due to its high ionization at pH 6.8, which is capable of absorbing a high amount of water (26).
Fig. 3.
Percentage swelling measurement of microspheres formulations. Data are presented as mean ± SD (n = 3)
In Vitro Drug Release
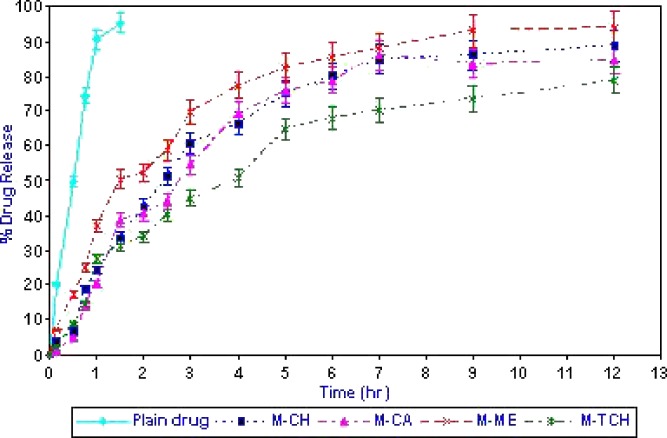
Figure 4 shows the release of acyclovir from various mucoadhesive microspheres. Drug powder enclosed in hard gelatin capsules was completely released (95.3 ± 4.1%) within 1 h. The time taken to release 75% of acyclovir (t75) from chitosan, thiolated chitosan, Methocel K15M or Carbopol 71G micospheres was 5.0 ± 0.4, 9.5 ± 0.7, 4.0 ± 0.3 and 5.5 ± 0.6 h, respectively. The significantly higher time required by the thiolated chitosan microspheres to release acyclovir was due to its better stability in acidic medium, which contributed significantly less amount of drug release during initial 1 h of dissolution (29.3 ± 1.1% and 20.5 ± 0.5% drug released from chitosan or thiolated chitosan microsphere during 1 h). The initial higher release of acyclovir from chitosan microspheres may be attributed to the higher solubility of chitosan in acidic medium. Chitosan is soluble in acidic medium but crosslinking with glutaraldehyde through its amino group stabilized the microspheres matrix and provides the sustained release (27). The significantly lesser drug release from thiolated chitosan microspheres is due to the presence of disulphide bonds in microsphere matrix further stabilized the structure along with glutaraldehyde as cross linking agent (12). The higher amount of drug released from Methocel K15M microspheres could be assigned to its linear structure and low viscosity at pH (10).
Fig. 4.
Percentage in vitro drug release of acyclovir from microspheres formulations. Data are presented as mean ± SD (n = 3)
Quantitative GIT Distribution
Table III shows the time course of distribution of mucoadhesive microspheres loaded with 6-Carboxyfluorescein in the GI tract, including the stomach (section 1) and small intestine (sections 2–7) after oral administration. 6-CF was selected as fluorescence marker because of its hydrophilic nature, higher extraction efficiency (>95%) and lower detection limit (1.0 ng). Following oral administration, more than 50% 6-CF solution was recovered from stomach after 2 h, but less than 10% was located after 4 h. The maximum amount of 6-CF was transferred to the lower part of intestine after 8 h of its administration in rats. The reason for the poor retention of 6-CF at absorption site may be due its soluble nature and very little affinity to GIT tissue. On the other hand oral administration of 6-CF loaded thiolated chitosan microspheres revealed a different GI distribution pattern. After 2 h, 22.3 ± 3.1% formulation was recovered from stomach (Section 1) and after 4 h, nearly 41.6 ± 2.9% was recovered from Section 2, 3 and 4 (duodenum and jejunum portion of intestine). Further, after 10 h of administration, 26 ± 2.1% formulation was recovered from Section 2, 3 and 4. The significantly higher quantity of acyclovir recovered (p < 0.05) from sections 2, 3 and 4 of GIT suggest gastroretentive characteristic of thiolated chitosan microsphere formulation. In comparison chitosan, Carbopol 71G and Methocel K15 microspheres showed 33.5 ± 4.2, 17 ± 2.8 and 9.6 ± 1.4% recovery from section 2, 3 and 4 of GIT after 4 h of oral administration and 12.9 ± 1.2, 2.5 ± 0.3 and 0% recovery after 10 h of administration. The 2-fold higher GI retention of thiolated microspheres in comparison to chitosan, Carbopol 71G and Methocel K15M microsphere formulation may be attributed to the better mucoadhesive properties of thiolated chitosan at pH 5–6, the pH of duodenum and jejunum region of intestine (28).
Table III.
GI distribution of 6-CF in rat gastrointestinal tract after administration as solution and mucoadhesive microspheres
| Section no. | % Dose recovered | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-CF solution (h) | M-TCH (h) | M-CH (h) | M-CA (h) | M-ME (h) | |||||||||||||||||||||
| 2 | 4 | 6 | 8 | 10 | 2 | 4 | 6 | 8 | 10 | 2 | 4 | 6 | 8 | 10 | 2 | 4 | 6 | 8 | 10 | 2 | 4 | 6 | 8 | 10 | |
| 1 Stomach | 52.2 ± 2.3 | 3.5 ± 0.2 | 0 | 0 | 0 | 22.3 ± 3.1 | 12.6 ± 1.4 | 6.8 ± 0.6 | 2.1 ± 0.3 | 0 | 23.8 ± 2.5 | 11.2 ± 0.8 | 4.3 ± 0.5 | 0 | 0 | 17.2 ± 1.5 | 5.4 ± 0.5 | 0 | 0 | 0 | 14.2 ± 1.2 | 0 | 0 | 0 | 0 |
| 2 Duodenum | 10.9 ± 1.4 | 19.5 ± 3.2 | 0 | 0 | 0 | 15.5 ± 1.6 | 16.3 ± 1.7 | 12.4 ± 1.1 | 7.8 ± 0.7 | 3.8 ± 0.2 | 14.2 ± 1.3 | 13.1 ± 1.2 | 10.2 ± 1.0 | 5.6 ± 0.5 | 1.2 ± 0.2 | 7.5 ± 0.9 | 7.5 ± 0.6 | 0 | 0 | 0 | 5.6 ± 0.5 | 2.1 ± 0.3 | 0 | 0 | 0 |
| 3 Jejunum | 0 | 10.6 ± 1.2 | 0 | 0 | 0 | 13.9 ± 1.4 | 14.1 ± 1.3 | 14.2 ± 1.4 | 10.1 ± 1.2 | 9.1 ± 0.7 | 10.8 ± 1.1 | 12.6 ± 1.2 | 10.6 ± 1.1 | 8.9 ± 0.7 | 4.2 ± 0.5 | 0 | 5.8 ± 0.5 | 6.8 ± 0.7 | 0 | 0 | 0 | 3.5 ± 0.5 | 0 | 0 | 0 |
| 4 Jejunum | 0 | 5.8 ± 0.6 | 6.9 ± 0.7 | 0 | 0 | 6.4 ± 0.5 | 11.2 ± 1.2 | 15.5 ± 1.6 | 14.8 ± 1.6 | 13.1 ± 1.5 | 3.6 ± 0.4 | 7.8 ± 0.6 | 15.4 ± 1.4 | 12.1 ± 1.0 | 7.5 ± 1.0 | 0 | 3.7 ± 0.4 | 5.8 ± 0.5 | 5.4 ± 0.4 | 2.5 ± 0.3 | 0 | 4.0 ± 0.5 | 8.0 ± 0.9 | 0 | 0 |
| 5 Jejunum | 0 | 0 | 3.9 ± 0.4 | 0 | 0 | 0 | 8.4 ± 0.9 | 15.1 ± 1.5 | 18.6 ± 2.0 | 17.5 ± 1.8 | 0 | 4.2 ± 0.5 | 9.6 ± 0.8 | 17.6 ± 1.8 | 13.5 ± 1.2 | 0 | 2.1 ± 0.2 | 4.4 ± 0.3 | 6.4 ± 0.5 | 0 | 0 | 3.2 ± 0.4 | 5.0 ± 0.5 | 0 | 0 |
| 6 Ileum | 0 | 0 | 0 | 5.9 ± 0.7 | 0 | 0 | 4.2 ± 0.4 | 10.8 ± 1.1 | 15.5 ± 1.6 | 11.7 ± 1.2 | 0 | 1.5 ± 0.2 | 7.3 ± 0.6 | 15.2 ± 1.4 | 10.7 ± 1.0 | 0 | 0 | 3.2 ± 0.3 | 4.0 ± 0.5 | 7.0 ± 0.6 | 0 | 3.0 ± 0.3 | 4.5 ± 0.3 | 5.2 ± 0.5 | 0 |
| 7 Remaining Intestine | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 ± 0.1 | 10.2 ± 1.0 | 12.8 ± 1.2 | 12.1 ± 1.4 | 0 | 0 | 6.4 ± 0.5 | 13.6 ± 1.2 | 8.7 ± 0.7 | 0 | 0 | 2.0 ± 0.2 | 8.8 ± 0.8 | 9.8 ± 1.0 | 0 | 0 | 2.1 ± 0.2 | 4.0 ± 0.3 | 0 |
Data are presented as mean ± SD (n = 3)
Qualitative GIT Distribution Study
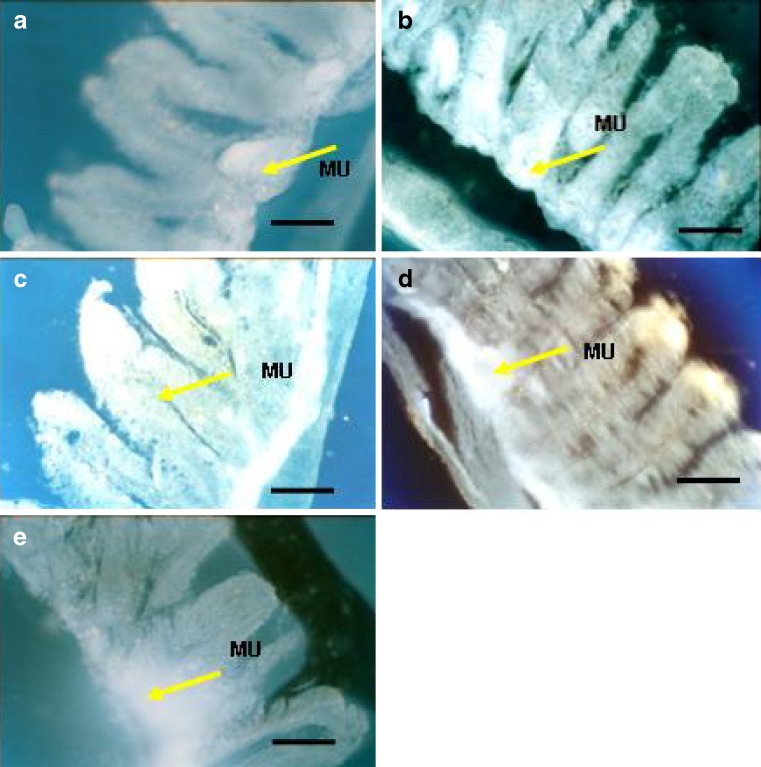
Distribution and extent of penetration of 6-CF as fluorescence marker from different formulation was characterized by fluorescence microscopy of duodenum and jejunum region (sections 2, 3 and 4). Figure 5(a–e) shows the photomicrograph of rat intestine after treatment with 6-CF solution (a), thiolated chitosan (b), chitosan (c), Carbopol 71G (d) or Methocel K15M (e) microspheres. The fluorescence photomicrographs revealed better qualitative uptake and localization of fluorescence marker loaded mucoadhesive microsphere in duodenum and jejunum as compared to its solution. Oral administration of 6-CF loaded thiolated chitosan microspheres showed higher fluorescence intensity accompanied with deep penetration of marker in intestinal tissue (Fig. 5b). This result indicates higher mucoadhesiveness and penetration enhancement effect of thiolated chitosan microsphere formulation. Thiolated chitosan is reported to open the tight junction of intestinal epithelium by interacting with intestinal protein, thus responsible for its penetration enhancing effect (28). Acyclovir is a Class III drug in the Biopharmaceutical Classification System. Its low permeability is the rate-limiting factor influencing its oral absorption (29). Hence, these results indicate that the penetration enhancement effect of thiolated chitosan microspheres could be beneficial in facilitating the oral absorption of acyclovir.
Fig. 5.
Penetration of 6-CF (1 ml of 0.3% w/v) as fluorescence probe across the duodenum section of intestinal mucosa after 3 h administration as solution a, thiolated chitosan b, chitosan c, Carbopol 934 d and Methocel K15M e microsphere formulation (×450). Scale bar = 250 μm. MU Mucosal surface, VI villi
Hemolytic Toxicity Assay
Hemolytic assay is a simple method widely used to study polymer–membrane interaction. It gives a quantitative measure of hemoglobin release. Table III compares the results of percent hemolysis of different microspheres formulations of acyclovir. Thiolated chitosan, chitosan, Carbopol 71 G and Methocel K15 M microspheres showed 13.1 ± 1.2%, 20.1 ± 2.0%, 26.2 ± 3.4% and 22.0 ± 2.8% hemolysis, respectively after 1 h of incubation. Thiolated chitosan microsphere displayed a lower membrane damaging effect by causing a significantly lower hemoglobin release than chitosan microsphere. This result from thiolated chitosan microspheres might be explained by the formation of intra- as well as inter-molecular disulfide bonds, thus leading to a higher rigidness of the microsphere matrix. Rigid molecules have more difficulties to attach to the cellular membrane than flexible molecules and showed lesser toxicity (30). These findings are in good agreement with previous studies of Guggi et al. (31). showing that chitosan-4-thio-butylamidine and glucosamine-4-thio-butylamidine conjugates have a significant less toxic effect on red blood cells in comparison to unmodified chitosan and glucosamine.
Pharmacokinetic Study
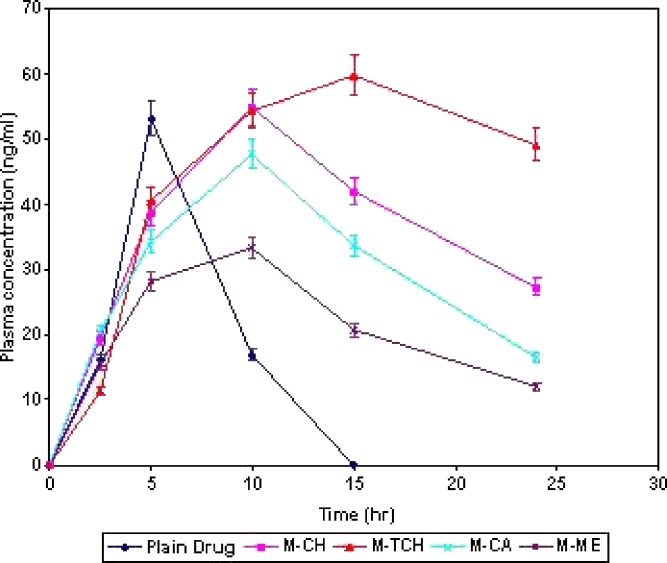
Figure 6 shows the plasma concentration profile of acyclovir after oral administration in the form of solution and microspheres. Thiolated chitosan microspheres showed superiority over the other formulations. Nearly four times higher AUC0–24 value of acyclovir for these microspheres (1091 ± 51 ng h/ml) as compared to drug solution (282 ± 28 ng h/ml) was observed. In addition, thiolated chitosan microspheres showed the ability to maintain the acyclovir plasma concentration through 24 h as compared to the drug solution that could maintain this level of drug only for 5 h. These results confirmed the sustained release potential of mucoadhesive microspheres of acyclovir prepared from thiolated chitosan. Hence, the overall better pharmacokinetic performance of thiolated chitosan microsphere in comparison to drug solution is due to (1) increased residence time within upper GI tract as evident by GI distribution study (2) an intensified contact between the intestinal mucosa and microspheres as evident by mucoadhesion study (3) increased drug concentration at site of absorption as evident by in vitro drug release study and (4) facilitated drug permeation through mucosa as evident by the fluorescence microscopy study (Table IV).
Fig. 6.
Plasma concentration of acyclovir after administration as drug solution and microsphere formulations. Data are presented as mean ± SD (n = 3)
Table IV.
Results of pharmacokinetic and hemolytic toxicity assay of different microsphere formulations of acyclovir
| Formulation code | AUC (ng h/ml)a | C max (ng)a | MRT (h)a | % Hemolysis |
|---|---|---|---|---|
| Drug solution | 282 ± 28 | 53.1 ± 6.8 | 5.4 ± 0.5 | 10.2 ± 1.4 |
| M-CH | 886 ± 72 | 54.9 ± 8.2 | 12.4 ± 1.2 | 20.1 ± 2.0 |
| M-TCH | 1,091 ± 51 | 59.7 ± 10.1 | 17.9 ± 1.8 | 13.1 ± 1.2 |
| M-CA | 728 ± 45 | 47.7 ± 6.8 | 11.6 ± 1.1 | 26.2 ± 3.4 |
| M-ME | 511 ± 35 | 33.3 ± 5.8 | 11.3 ± 1.0 | 22.0 ± 2.8 |
Data are presented as mean ±SD (n = 3)
aAnalyzed by WinNolin software
CONCLUSION
The results of present study revealed that the retention time of acyclovir at its absorption site, i.e. the upper GIT, could be increased by formulating it into microspheres using chitosan, thiolated chitosan, Carbopol 71G or Methocel K15M. The microspheres prepared from thiolated chitosan showed the highest mucoadhesiveness. Further, they were observed to penetrate through the intestinal mucosa qualitatively better than the microspheres prepared from chitosan, Carbopol 71G or Methocel K15M. These properties enabled sustained release of acyclovir from microspheres prepared from thiolated chitosan, and plasma drug concentration in rats was maintained for 24 h. Hence, microspheres of acyclovir prepared from thiolated chitosan may represent a useful approach for targeting its release at its site of absorption, sustaining its release and improving its oral availability.
Acknowledgements
Authors are grateful to East India Pharmaceutical, Ltd. India for providing as a gift sample of Acyclovir. The authors are also thankful to the Director, NIPER, Mohali, Punjab for providing the necessary facilities for lyophilization and spray drying.
References
- 1.Wagstaff A. G., Faulds D., Goa K. L. Aciclovir: a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ruhnese M., Sandstorm F., Andersson B. Treatment of recurrent genital herpes simplex infection with acyclovir. J. Antimicrob. Chemother. 1985;16:621–628. doi: 10.1093/jac/16.5.621. [DOI] [PubMed] [Google Scholar]
- 3.Fuertes I., Miranda A., Millan M., Caraballo I. Estimation of the percolation thersholds in acyclovir hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 2006;64:336–342. doi: 10.1016/j.ejpb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.De Jalon E. G., Blanco-Prieto M. J., Ygartua P., Santoyo S. Increased efficacy of acyclovir-loaded microparticles against herpes simplex virus type 1 in cell culture. Eur. J. Pharm. Biopharm. 2003;56:183–187. doi: 10.1016/S0939-6411(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 5.Rossi S., Sandri G., Ferrari F., Bonferoni M. C., Caramella C. Buccal delivery of acyclovir from films based on chitosan and polyacrylic acid. Pharm. Dev. Technol. 2003;8:199–208. doi: 10.1081/PDT-120018490. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien J. J., Campoli-Richards D. M. Acyclovir: an updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989;37:233–309. doi: 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Meadows K. C., Dressman J. B. Mechanism of acyclovir uptake in rat jejunum. Pharm. Res. 1990;7(3):299–303. doi: 10.1023/A:1015890516119. [DOI] [PubMed] [Google Scholar]
- 8.Park G. B., Shao Z., Mitra A. K. Acyclovir permeation enhancement across intestinal and nasal mucosae by bile salt–acylcarnitine mixed micelles. Pharm. Res. 1992;9:1262–1267. doi: 10.1023/A:1015845031488. [DOI] [PubMed] [Google Scholar]
- 9.Hirano S., Seino H., Akiyama Y. Chitosan: a biocompatible material for oral and intravenous administration. In: Gebelein C. G., Dunn R. L., editors. Progress in Biomedical Polymers. New York: Plenum; 1990. pp. 283–289. [Google Scholar]
- 10.Genta I., Conti B., Perugini P. F., Pavanetto F. A., Puglisi G. Bioadhesive microspheres for ophthalmic administration of acyclovir. J. Pharm. Pharmacol. 1997;49:737–742. doi: 10.1111/j.2042-7158.1997.tb06103.x. [DOI] [PubMed] [Google Scholar]
- 11.Aungst B. J. Intestinal permeation enhancers. J. Pharm. Sci. 2000;89:429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Maculotti K., Genta I., Perugini P., Imam M., Bernkop-Schnurch A., Pavanetto F. Preparation and in vitro evaluation of thiolated chitosan microparticles. J. Microencapsul. 2005;22:459–470. doi: 10.1080/02652040500162220. [DOI] [PubMed] [Google Scholar]
- 13.Bernkop-Schnurch A., Hornof M., Zoidl T. Thiolated polymers–thiomers: synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003;260:229–237. doi: 10.1016/S0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y. M., Sato H., Adachi I., Horikoshi I. Optimization of the formulation design of chitosan microspheres containing cisplatin. J. Pharm. Sci. 1996;85:1204–1210. doi: 10.1021/js960092j. [DOI] [PubMed] [Google Scholar]
- 15.S. Harikarnpakdee, V. Lipipun, N. Sutanthavibul, and G. C. Ritthidej. Spray dried mucoadhesive microspheres: preparation and transport through nasal cell monolayer. AAPS PharmSciTech.7(1):Article 12 (2006). [DOI] [PMC free article] [PubMed]
- 16.Darwish I. A., Khedr A. A., Askal H. F., Mahmoud R. M. Simple fluorimetric method for determination of certain antiviral drugs via their oxidation with cerium(IV) Farmaco. 2005;60:555–562. doi: 10.1016/j.farmac.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Vyas S.P., Talwar N., Karajgi J. S., Jain N. K. An erythrocyte based bioadhesive system for nasal delivery of propranolol. J. Control. Release. 1993;23:231–237. doi: 10.1016/0168-3659(93)90004-O. [DOI] [Google Scholar]
- 18.Morishita I., Morishita M., Takayama K., Machida Y., Nagai T. Internal insulin delivery by microspheres with three different formulations using Eudragit L100 55 and S 100. Int. J. Pharm. 1993;91:29–37. doi: 10.1016/0378-5173(93)90418-F. [DOI] [Google Scholar]
- 19.Singhai A. K., Jain S., Jain N. K. Evaluation of an aqueous injection of ketoprofen. Pharmazie. 1997;52:149–151. [PubMed] [Google Scholar]
- 20.Jain S. K., Jain R. K., Chaurasia M. K., Jain A. K., Chalasani K. B., Soni V., Jain A. Design and development of multivesicular liposomal depot delivery system for controlled systemic delivery of acyclovir sodium. AAPS PharmSciTech. 2005;6(1):E35–E41. doi: 10.1208/pt060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda A., Kobayashi H., Itoh S., Kataoka K., Tanaka J. Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding. Biomaterials. 2005;26:2273–2279. doi: 10.1016/j.biomaterials.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Leitner V. M., Walker G. F., Bernkop-Schnurch A. Thiolated polymers: evidence for formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003;56:207–214. doi: 10.1016/S0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- 23.Mortazavi S. A., Smart J. D. An investigation into the role of water movement and mucus gel dehydration in mucoadhesion. J. Control. Release. 1993;25:197–203. doi: 10.1016/0168-3659(93)90078-J. [DOI] [Google Scholar]
- 24.Duchene D., Ponchel G. Principle and investigation of bioadhesion mechanism of solid dosage forms. Biomaterials. 1992;13:709–714. doi: 10.1016/0142-9612(92)90132-8. [DOI] [PubMed] [Google Scholar]
- 25.Lehr C. M. From sticky stuff to sweet receptors—achievement, limits and novel approaches to bioadhesion. Eur. J. Drug Metab. Pharmacokinet. 1996;21:139–148. doi: 10.1007/BF03190262. [DOI] [PubMed] [Google Scholar]
- 26.Chng H. S., Park H., Kely P., Robinson J. R. Bioadhesive polymers as platforms for oral controlled drug delivery: I. Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers. J. Pharm. Sci. 1985;74:399–405. doi: 10.1002/jps.2600740407. [DOI] [PubMed] [Google Scholar]
- 27.Thanoo B. C., Sunny M. C., Jayakrishnan A. Crosslinked chitosan microspheres: preparation and evaluation as a matrix for the controlled release of pharmaceuticals. J. Pharm. Pharmacol. 1992;44:283–286. doi: 10.1111/j.2042-7158.1992.tb03607.x. [DOI] [PubMed] [Google Scholar]
- 28.Bernkop-Schnurch A., Guggi D., Pinter Y. Thiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release. 2004;94:177–186. doi: 10.1016/j.jconrel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Loftsson T., Brewster M. E., Masson M. Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Delivery. 2004;2(4):261–275. doi: 10.2165/00137696-200402040-00006. [DOI] [Google Scholar]
- 30.Fischer D., Li Y., Ahlemeyer B., Krieglstein J., Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/S0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 31.Guggi D., Langoth N., Hoffer M. H., Wirth M., Bernkop-Schnurch A. Comparative evaluation of cytotoxicity of glucosamine–TBA conjugate and a chitosan–TBA conjugate. Int. J. Pharm. 2004;278:353–360. doi: 10.1016/j.ijpharm.2004.03.016. [DOI] [PubMed] [Google Scholar]