Abstract
Nucleolytic processing of DNA double-strand breaks (DSBs) generates 3′ ssDNA tails that are essential for the assembly of DNA damage checkpoint signaling and DNA repair protein complexes. Genetic studies have provided evidence that multiple nuclease activities are involved in DSB end resection. Three recent studies, including work by Jackson and colleagues (pp. 2767–2772) in the October 15, 2008, issue of Genes & Development, have begun to shed some light on the intricacy of this process.
Keywords: RecQ helicases, DNA resection, DNA repair, DNA damage checkpoint, Exonuclease 1, ATR
Homologous recombination (HR) and DNA double-strand break (DSB) repair
DNA DSBs are induced by agents such as ionizing radiation (IR), and also arise during DNA replication when the DNA polymerase ensemble encounters obstacles such as DNA lesions or unusual DNA structures. Persistent or incorrectly repaired DSBs can result in chromosome loss, deletion, translocation, or fusion, which can lead to carcinogenesis through activation of oncogenes or inactivation of tumor-suppressor genes. Cells possess two main mechanisms for eliminating DSBs: nonhomologous end-joining (NHEJ) and HR. In NHEJ, DNA ends are simply religated, in a manner that requires limited or no sequence homology. Repair by NHEJ often leads to small deletions at the site of the DSB and is considered to be error-prone (Daley et al. 2005). In contrast, HR is directed by extensive homology in a partner DNA molecule and is predominantly faithful, particularly if the sister chromatid is utilized as the information donor. In mitotic cells, NHEJ occurs throughout all phases of the cell cycle, whereas HR is largely restricted to the S and G2 phases when the sister chromatid is available to mediate the repair process (Krogh and Symington 2004; Daley et al. 2005).
Not all DSBs are pathological in nature. For instance, DSBs are essential for V(D)J recombination in the immune system (Lewis 1994). Programmed DSBs that occur early in meiosis trigger genome-wide recombination that serves to tie chromosome homolog pairs together, so as to allow for their orderly segregation in the first meiotic division (Petronczki et al. 2003). Likewise, mating-type switching in budding yeast is accomplished by recombination initiated from a site-specific DSB at the MAT locus on chromosome 3 (Haber 1998). A great deal of valuable information regarding HR mechanism has emanated from studies on meiotic recombination and mating-type switching in the budding yeast (Haber 1998; Neale and Keeney 2006).
Recombinases and the presynaptic filament
The HR reaction is mediated by recombinases that catalyze the formation of hybrid joints of homologous DNA molecules. Two recombinases, Rad51 and Dmc1, exist in eukaryotes. Rad51 is crucial for both mitotic and meiotic HR events, whereas Dmc1 is expressed only in meiosis and its function is restricted therein. The catalytically active form of Rad51 or Dmc1 consists of a right-handed helical protein filament assembled on ssDNA; this recombinase-ssDNA nucleoprotein filament is often referred to as the presynaptic filament (San Filippo et al. 2008). Once assembled, the presynaptic filament conducts a search for a homologous chromatid and catalyzes the invasion of the donor chromatid to form a DNA joint molecule called a displacement (D)-loop. Subsequent steps include DNA synthesis, resolution of DNA intermediates through one of several pathways, and DNA ligation to complete the repair process (Krogh and Symington 2004; San Filippo et al. 2008).
DSB end resection in checkpoint signaling and presynaptic filament assembly
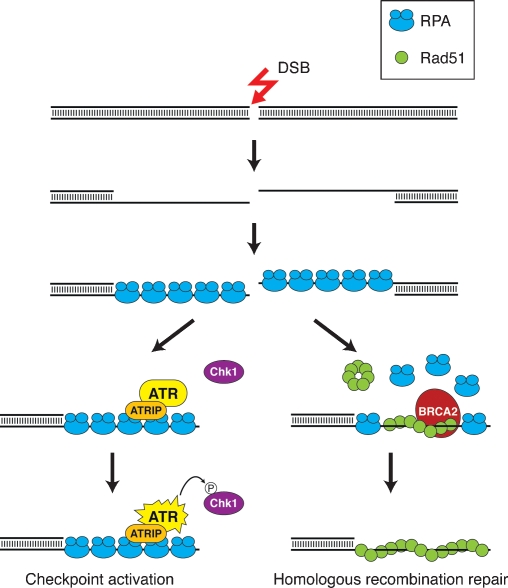
To provide the substrate for presynaptic filament assembly, DSB ends must first undergo extensive 5′-to-3′ end resection to generate 3′ ssDNA tails (Fig. 1). Studies in yeast and other organisms have provided evidence that in mitotic cells, such tails are first engaged by the ssDNA-binding protein replication protein A (RPA), and that the RPA-coated ssDNA plays a key role in DNA damage checkpoint activation by recruiting the PI3 kinase ATR (Mec1 in yeast) through its associated protein ATRIP (Ddc2 in yeast) (Cortez et al. 2001; Zou and Elledge 2003). The ATR/Mec1-mediated phosphorylation of downstream effector proteins, such as Chk1 (Liu et al. 2000), leads to the induction of cell cycle arrest and thus facilitates DNA damage repair. For homologous repair to occur, RPA must be displaced to allow for the loading and polymerization of Rad51. Accessory proteins, collectively known as recombination mediators, help dislodge RPA from the ssDNA tails. Several recombination mediators, including the breast tumor suppressor BRCA2 and Saccharomyces cerevisiae Rad52, have been identified (San Filippo et al. 2008).
Figure 1.
DSB end resection in checkpoint signaling and presynaptic filament assembly. DSBs are processed nucleolytically to expose 3′ ssDNA tails that are immediately bound by RPA. The RPA-coated ssDNA recruits the ATR–ATRIP complex, leading to the activation of ATR and downstream effectors such as Chk1 to initiate the checkpoint signaling cascade. For HR repair to occur, RPA is dislodged from the ssDNA and replaced by Rad51 with the assistance of recombination mediator proteins, such as BRCA2, to form the presynaptic filament.
Lessons from bacterial studies
In Escherichia coli, there are two known pathways that contribute to DNA end resection. The RecBCD complex functions in the major resection pathway, with the minor pathway being dependent on the RecQ–RecJ pair (Spies and Kowalczykowski 2005). The RecB subunit of the RecBCD complex harbors both helicase and endonuclease activities, and the RecD subunit also possesses a helicase activity. RecC recognizes a specific sequence in DNA (5′-GCTGGTGG-3′) called χ, and serves a scaffolding function in protein complex assembly. RecBCD engages a DNA end and, through the combined action of the RecB and RecD helicase activities, rapidly separates the two DNA strands in preparation for strand cleavage by RecB. The DNA unwinding and strand scission properties of the RecBCD complex are modulated by the χ sequence so as to favor the generation of a 3′ ssDNA tail for the nucleation of the bacterial recombinase, RecA (Anderson and Kowalczykowski 1997). In the RecQ–RecJ pathway of DNA end resection, the RecQ helicase separates DNA strands from an end, and the ensuing digestion of the 5′ DNA strand by the 5′-to-3′ exonuclease activity of RecJ leads to the accumulation of 3′ ssDNA (Shereda et al. 2007).
Role of the Mre11–Rad50–Xrs2 (NBS1) complex in DSB processing
Genetic studies in yeast have long implicated the highly conserved Mre11–Rad50–Xrs2 (MRX) complex in the sensing, processing, and repair of DSBs. In humans, hypomorphic mutations in MRE11 and NBS1 (the human Xrs2 equivalent) are associated with the cancer-prone diseases ataxia–telangiectasia-like disorder (ATLD) and Nijmegen breakage syndrome (NBS), respectively. The Mre11 protein has a 3′-to-5′ exonuclease activity and a DNA structure-specific endonuclease activity (Paull and Gellert 1998; Trujillo et al. 1998; Trujillo and Sung 2001). A role for the MRX complex in DSB end processing was first suggested by the finding that unresected meiotic DSBs accumulate in yeast strains expressing a RAD50 separation of function mutant, called rad50S (Cao et al. 1990). In vegetative cells, null mutants of any of the three MRX subunits are hypersensitive to DNA damaging agents, but the resection of DSBs, albeit delayed, still occurs (Ivanov et al. 1994; Bressan et al. 1999; Moreau et al. 1999; Llorente and Symington 2004). The function of the MRX(N) complex is dependent on the yeast Sae2 protein and its human ortholog CtIP (Lengsfeld et al. 2007; Sartori et al. 2007). Two new studies, by Mimitou and Symington (2008) and Zhu et al. (2008), have helped elucidate the role of the MRX–Sae2 ensemble in DNA end resection in yeast cells. With the use of strand-specific probes, these investigators have furnished evidence for a role of MRX–Sae2 in the initial resection of DSBs to yield 3′ ssDNA, and that it does so in increments of 100 or so nucleotides (Mimitou and Symington 2008; Zhu et al. 2008). Complementary evidence that MRX(N)-mediated DSB processing generates short ssDNA fragments has come from studies done in Xenopus extracts (Jazayeri et al. 2008). By chromatin immunoprecipitation (ChIP) and cytological analyses, it has been concluded that MRX associates only in a transient fashion with regions proximal to DSBs (Lisby et al. 2004; Shroff et al. 2004; Zhu et al. 2008), which could explain why the involvement of MRX in end resection is apparently limited to the immediate vicinity of the breaks (Mimitou and Symington 2008; Zhu et al. 2008).
Involvement of Exo1 in end resection
Exo1, an evolutionarily conserved protein that possesses 5′-to-3′ dsDNA exonuclease and 5′ flap-endonuclease activities (Fiorentini et al. 1997; Tishkoff et al. 1997; Tran et al. 2002), participates in DNA repair, HR, DNA damage signaling, and telomere maintenance (Nakada et al. 2004; Tran et al. 2004). The exo1Δ mutation alone causes a subtle defect in end resection of DSBs, but it does not prevent mating-type switching, nor does it engender any sensitivity to IR (Moreau et al. 2001; Llorente and Symington 2004). Interestingly, in a mre11-null mutant background, exo1Δ has a synergistic effect for IR sensitivity, and the double mutant cells exhibit slow growth, reduced HR capacity, impaired DNA damage signaling, and delayed mating-type switching (Tsubouchi and Ogawa 2000; Moreau et al. 2001; Nakada et al. 2004; Llorente and Symington 2004). Moreover, overexpression of EXO1 can partially suppress the IR sensitivity of mre11-, rad50-, and xrs2-null mutants, and this Exo1 attribute is dependent on its nuclease activity (Tsubouchi and Ogawa 2000; Moreau et al. 2001; Lewis et al. 2002). In two of the newly published studies (Mimitou and Symington 2008; Zhu et al. 2008), the physical analysis of DSB end resection at the budding yeast mating-type locus and examination of a specialized type of direct repeat recombination that is indicative of the extent of end resection have provided direct evidence that Exo1 resects DSB ends that have been processed initially via MRX–Sae2. Taken together, it is clear from these results that Exo1 contributes actively to DSB processing. However, because end resection still occurs in the mre11Δ exo1Δ double mutant, albeit at a slow rate, there must be another resection mechanism that works in redundant fashion to Exo1.
Sgs1/Dna2-dependent pathway of end resection
As summarized above, genetic evidence indicates the existence of an Mre11- and Exo1-independent means of DSB end resection in yeast. The identity of this novel mechanism of end resection is revealed in a study published in the October 15, 2008, issue of Genes & Development by Gravel et al. (2008) and in related works by Mimitou and Symington (2008) and Zhu et al. (2008). Given that DNA end resection in bacteria proceeds through DNA unwinding mediated by a DNA helicase (either RecBD or RecQ) before nucleolytic scission, the authors of these new studies asked whether Sgs1, the RecQ ortholog in S. cerevisiae, plays a role in this regard. Importantly, they found that in an exo1Δ background, deletion of the Sgs1 helicase leads to a pronounced hypersensitivity to DNA damaging agents, an inability to mount the Mec1-dependent DNA damage checkpoint, a substantial increase in genomic instability, and an impairment of HR. Results from the physical analysis of DSB end resection and examination of recombination between direct repeats revealed that little end resection beyond the initial processing mediated by the MRX–Sae2 ensemble occurs in the exo1Δ sgs1Δ double mutant, and the use of catalytically null mutants helps establish the requirement for the Exo1 nuclease and Sgs1 helicase activities.
In their further attempt to identify the nuclease that works with Sgs1, Zhu et al. (2008) have tested a possible involvement of Dna2, a conserved protein that possesses both 5′ flap-endonuclease and DNA helicase activities and that is recruited to DSBs in yeast cells. Dna2 is involved in Okazaki fragment processing (Budd and Campbell 1995; Bae et al. 2001), and several hypomorphic dna2 mutants are sensitive to DNA damage induced by IR and by the DNA methylating agent methylmethane sulfonate (Formosa and Nittis 1999; Budd et al. 2000). The latest results of Zhu et al. (2008) show convincingly that cells simultaneously lacking Exo1 and Dna2 are strongly impaired for DNA end resection and, importantly, Dna2 appears to work in conjunction with Sgs1 in this regard.
Evolutionary conservation
In order to examine whether the pathways of DSB end resection are conserved in humans, Gravel et al. (2008) used siRNA to deplete the EXO1 protein and the Sgs1 ortholog BLM, which is mutated in the cancer-prone Bloom syndrome. The investigators then treated the cells with camptothecin to induce DSBs during S phase and monitored RPA focus formation as an indicator of ssDNA accumulation. While depletion of either BLM or EXO1 alone is accompanied by a modest reduction in RPA foci, simultaneous depletion of both proteins leads to a further drop in the frequency of these foci. Moreover, using phospho-specific antibodies against specific ATR phosphorylation sites of RPA and Chk1, Gravel et al. (2008) found that activation of the ATR signaling pathway is impaired in BLM/EXO1-depleted cells, with a concomitant increase in the cellular sensitivity to camptothecin. These results thus provide good evidence for the conservation of DSB processing pathways in human cells.
Model for DSB processing
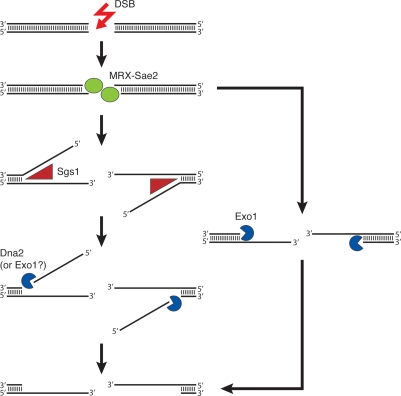
The results from the three new studies (Gravel et al. 2008; Mimitou and Symington 2008; Zhu et al. 2008) provide valuable insights from which we can begin to formulate a mechanistic model for understanding the intricacy of DSB end processing in yeast cells. In this model (Fig. 2), DSB ends are first engaged by the MRX–Sae2 ensemble, which catalyzes a limited amount of DSB end resection. The 3′-tailed DNA ends are then rapidly processed by either the Sgs1–Dna2 complex or Exo1, to yield long 3′ ssDNA tails. Importantly, these pathways of DNA end resection are likely conserved in human cells (Gravel et al. 2008).
Figure 2.
Model for 5′ DSB end resection pathways in yeast. The MRX–Sae2 ensemble first engages the DSB ends and catalyzes limited end resection. The 3′-tailed intermediate is rapidly processed through the coordinated helicase and nuclease activities of Sgs1 and Exo1 or Dna2, or by the 5′-to-3′ exonucleolytic activity of Exo1, to expose long 3′ ssDNA tracts.
Some future questions
The identification and characterization of the genetic pathways that promote DSB processing set the stage for dissecting the biochemical mechanisms of these pathways. For instance, one would like to know whether Sgs1 and Dna2 function together as a complex in end resection and, if so, whether it requires post-translational modifications of these proteins or other bridging factors. It also seems important to ask whether there is functional cross-link among the three end resection pathways, and if these pathways cooperate to process meiotic DSBs made by the conserved topoisomerase-like protein Spo11 (Neale and Keeney 2006). Sae2 also harbors a nuclease function (Lengsfeld et al. 2007), but the relevance of this Sae2 attribute in the initiation of DSB processing remains to be elucidated.
Acknowledgments
The research in our laboratory has been supported by grants from the U.S. National Institutes of Health, the U.S. Department of Defense, and the Susan G. Komen For the Cure Foundation.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1742408.
References
- Anderson D.G., Kowalczykowski S.C. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a χ-regulated manner. Cell. 1997;90:77–86. doi: 10.1016/s0092-8674(00)80315-3. [DOI] [PubMed] [Google Scholar]
- Bae S.H., Bae K.H., Kim J.A., Seo Y.S. RPA governs endonucleases switching during processing of Okazaki fragments in eukaryotes. Nature. 2001;412:456–461. doi: 10.1038/35086609. [DOI] [PubMed] [Google Scholar]
- Bressan D.A., Baxter B.K., Petrini J.H. The Mre11–Rad50–Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd M.E., Campbell J.L. A yeast gene required for DNA replication encodes a protein with homology to DNA helicases. Proc. Natl. Acad. Sci. 1995;92:7642–7646. doi: 10.1073/pnas.92.17.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd M.E., Choe W., Campbell J.L. The nuclease activity of the yeast DNA2 protein, which is related to the RecB-like nucleases, is essential in vivo. J. Biol. Chem. 2000;275:16518–16529. doi: 10.1074/jbc.M909511199. [DOI] [PubMed] [Google Scholar]
- Cao L., Alani E., Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Cortez D., Guntuku S., Qin J., Elledge S.J. ATR and ATRIP: Partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- Daley J.M., Palmbos P.L., Wu D., Wilson T.E. Nonhomologous end joining in yeast. Annu. Rev. Genet. 2005;39:431–451. doi: 10.1146/annurev.genet.39.073003.113340. [DOI] [PubMed] [Google Scholar]
- Fiorentini P., Huang K.N., Tishkoff D.X., Kolodner R.D., Symington L.S. Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol. Cell. Biol. 1997;17:2764–2773. doi: 10.1128/mcb.17.5.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T., Nittis T. Dna2 mutants reveal interactions with DNA polymerase α and Ctf4, a Pol α accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics. 1999;151:1459–1470. doi: 10.1093/genetics/151.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel S., Chapman J.R., Magill C., Jackson S.P. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes & Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J.E. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 1998;32:561–599. doi: 10.1146/annurev.genet.32.1.561. [DOI] [PubMed] [Google Scholar]
- Ivanov E.L., Sugawara N., White C.I., Fabre F., Haber J.E. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A., Balestrini A., Garner E., Haber J.E., Costanzo V. Mre11–Rad50–Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 2008;27:1953–1962. doi: 10.1038/emboj.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh B.O., Symington L.S. Recombination proteins in yeast. Annu. Rev. Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- Lengsfeld B.M., Rattray A.J., Bhaskara V., Ghirlando R., Paull T.T. Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol. Cell. 2007;28:638–651. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.M. The mechanism of V(D)J joining: Lessons from molecular, immunological, and comparative analyses. Adv. Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- Lewis L.K., Karthikeyan G., Westmoreland J.W., Resnick M.A. Differential suppression of DNA repair deficiencies of yeast rad50, mre11 and xrs2 mutants by EXO1 and TLC1 (the RNA component of telomerase) Genetics. 2002;160:49–62. doi: 10.1093/genetics/160.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M., Barlow J.H., Burgess R.C., Rothstein R. Choreography of the DNA damage response: Spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Liu Q., Guntuku S., Cui X.S., Matsuoka S., Cortez D., Tamai K., Luo G., Carattini-Rivera S., DeMayo F., Bradley A., et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes & Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Llorente B., Symington L.S. The Mre11 nuclease is not required for 5′ to 3′ resection at multiple HO-induced double-strand breaks. Mol. Cell. Biol. 2004;24:9682–9694. doi: 10.1128/MCB.24.21.9682-9694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou E.P., Symington L.S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008 doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S., Ferguson J.R., Symington L.S. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S., Morgan E.A., Symington L.S. Overlapping functions of the Saccharomyces cerevisiae Mre11, Exo1 and Rad27 nucleases in DNA metabolism. Genetics. 2001;159:1423–1433. doi: 10.1093/genetics/159.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D., Hirano Y., Sugimoto K. Requirement of the Mre11 complex and exonuclease 1 for activation of the Mec1 signaling pathway. Mol. Cell. Biol. 2004;24:10016–10025. doi: 10.1128/MCB.24.22.10016-10025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M.J., Keeney S. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature. 2006;442:153–158. doi: 10.1038/nature04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull T.T., Gellert M. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Siomos M.F., Nasmyth K. Un menage a quatre: The molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- San Filippo J., Sung P., Klein H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- Sartori A.A., Lukas C., Coates J., Mistrik M., Fu S., Bartek J., Baer R., Lukas J., Jackson S.P. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereda R.D., Bernstein D.A., Keck J.L. A central role for SSB in Escherichia coli RecQ DNA helicase function. J. Biol. Chem. 2007;282:19247–19258. doi: 10.1074/jbc.M608011200. [DOI] [PubMed] [Google Scholar]
- Shroff R., Arbel-Eden A., Pilch D., Ira G., Bonner W.M., Petrini J.H., Haber J.E., Lichten M. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr. Biol. 2004;14:1703–1711. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M., Kowalczykowski S.C. Homologous recombination by RecBCD and RecF pathways. In: Higgins N.P., editor. The bacterial chromosome. ASM Press; Washington, DC: 2005. pp. 389–403. [Google Scholar]
- Tishkoff D.X., Boerger A.L., Bertrand P., Filosi N., Gaida G.M., Kane M.F., Kolodner R.D. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc. Natl. Acad. Sci. 1997;94:7487–7492. doi: 10.1073/pnas.94.14.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P.T., Erdeniz N., Dudley S., Liskay R.M. Characterization of nuclease-dependent functions of Exo1p in Saccharomyces cerevisiae. DNA Repair (Amst.) 2002;1:895–912. doi: 10.1016/s1568-7864(02)00114-3. [DOI] [PubMed] [Google Scholar]
- Tran P.T., Erdeniz N., Symington L.S., Liskay R.M. EXO1—A multi-tasking eukaryotic nuclease. DNA Repair (Amst.) 2004;3:1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Trujillo K.M., Sung P. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50-Mre11 complex. J. Biol. Chem. 2001;276:35458–35464. doi: 10.1074/jbc.M105482200. [DOI] [PubMed] [Google Scholar]
- Trujillo K.M., Yuan S.S., Lee E.Y., Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J. Biol. Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H., Ogawa H. Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:2221–2233. doi: 10.1091/mbc.11.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chung W., Shim E.Y., Lee S.E., Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Elledge S.J. Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]