Abstract
Central nervous system (CNS) drug delivery remains a major challenge, despite extensive efforts that have been made to develop novel strategies to overcome obstacles. Prodrugs are bioreversible derivatives of drug molecules that must undergo an enzymatic and/or chemical transformation in vivo to release the active parent drug, which subsequently exerts the desired pharmacological effect. In both drug discovery and drug development, prodrugs have become an established tool for improving physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active agents that overcome barriers to a drug’s usefulness. This review provides insight into various prodrug strategies explored to date for CNS drug delivery, including lipophilic prodrugs, carrier- and receptor-mediated prodrug delivery systems, and gene-directed enzyme prodrug therapy.
Key words: BBB, CNS, delivery drug, prodrug
INTRODUCTION
The blood–brain barrier (BBB) presents an efficient structural and functional barrier for the delivery of therapeutic agents to the central nervous system (CNS). Due to its unique properties, passage across the BBB often becomes the main limiting factor for the delivery of potential CNS drugs into the brain parenchyma. In fact, it is estimated that more than 98% of small-molecular weight drugs and practically 100% of large-molecular weight drugs developed for the CNS diseases do not readily cross the BBB (1,2). Many of the pharmacologically active drugs tend to fail early in their development phase because these molecules lack the structural features that are essential for passing the BBB (3).
The BBB segregates the CNS from the systemic circulation, and its main physiological functions include maintaining homeostasis at the brain parenchyma and protecting the brain from potentially harmful chemicals. The BBB is primarily formed from capillary endothelial cells, which differ from the other tissues (4). The brain capillary endothelial cells are very closely joined together by tight intercellular junctions that efficiently restrict the paracellular diffusion of hydrophilic drugs. In addition, perivascular elements such as pericytes, which partially encircle the endothelium, astrocytic end-foot processes and neuronal cells, are important in the function of the BBB (5–7).
In addition to being a selective structural diffusion barrier, the BBB constitutes an efficient functional barrier for solutes crossing the cell membrane. The high metabolic activity of brain capillary endothelial cells (8), as well as effective efflux systems that actively remove solutes from brain tissue and return them back to the blood stream (9–11), create a great challenge for potential neuro-therapeutics. Furthermore, the BBB expresses a number of specific carrier-mediated inward transport mechanisms that ensure an adequate nutrient supply for the brain (12).
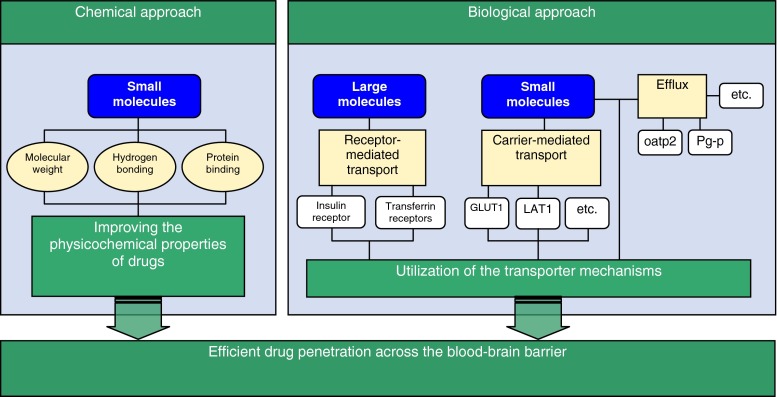
Traditionally, various medicinal chemistry- (e.g., lipophilic drug analogs and prodrugs, or disruption of BBB) and physical neurosurgery-based invasive approaches (e.g., interstitial drug delivery) have been attempted to increase brain delivery of therapeutic agents. Increased information and understanding of BBB physiology has led to rational chemistry- and biology-based drug delivery strategies that are presented in Fig. 1 (13). Novel CNS-targeted neuro-therapeutics should possess either the optimal physicochemical characteristics that allow for passive diffusion through the BBB via the transcellular route, or have the structural features necessary to serve as a substrate for one of the endogenous influx transport systems of the BBB (14,15). To be able to readily cross the BBB by passive diffusion in pharmacologically significant amounts, a drug should be relatively small (have a molecular weight of less than 500 Da), lipid soluble and, be either neutral or significantly uncharged at physiologically pH, and be capable of forming less than eight H-bonds with water (16). On the other hand, new knowledge of endogenous BBB transporters can be used in the rational reformulation of drug molecules for active transport. However, it is important to recognize that the degree of BBB drug penetration and resultant CNS concentrations are relative to the potency of the drug. Although a small amount of the drug may penetrate into the CNS, if it is potent, very small concentrations binding to the receptor will result in the desired effect.
Fig. 1.
Chemistry- and biology-based approaches to increase brain delivery of CNS targeted therapeutic agents (modified from Pardridge 2003 (13))
The human brain microvasculature consists of approximately 640 km of capillaries, with a surface area of about 20 m2. Every neuron is essentially perfused by its own blood vessels, and these vessels are typically 40 μm apart from each other. A small molecule may diffuse through this 40 μm space in about 1 s, which indicates that after passage across the BBB the drug is almost instantly distributed within the whole cerebral tissue (13). These physiological facts indicate that the vascular route would be very promising in drug delivery for targeting the brain, if the CNS transport challenge could be solved.
An attractive and rewarding chemistry-based strategy that has been successfully employed to increase the CNS transport of poorly penetrating therapeutic agents is their transient chemical modification by using the prodrug approach.
PRODRUG CONCEPT
The term “prodrug” or “pro-agent” was first introduced in 1958 to describe compounds that undergo biotransformation prior to their therapeutic activity (17). Prodrugs are described as bioreversible derivatives of drug molecules that must undergo a chemical or enzymatic biotransformation to the active forms within the body, prior to exerting a pharmacological action (Fig. 2). Release of the active drug is controlled and can occur before, during or after absorption, or at the specific site of action within the body, depending upon the purpose for which prodrug is designed (18,19). The major goal in prodrug design is to overcome the various physicochemical, pharmaceutical, biopharmaceutical, and/or pharmacokinetic limitations of parent drug, which otherwise would hinder its clinical use (20–25). For example, the prodrug approach may provide an effective tool in solving drug formulation and delivery problems, such as poor aqueous solubility, chemical instability, insufficient oral absorption, rapid presystemic metabolism, inadequate brain penetration, toxicity and local irritation. Prodrug technologies can also be used to improve targeting of drug action. Finally, the development of a prodrug of an existing drug with improved properties may represent a life-cycle management opportunity.
Fig. 2.
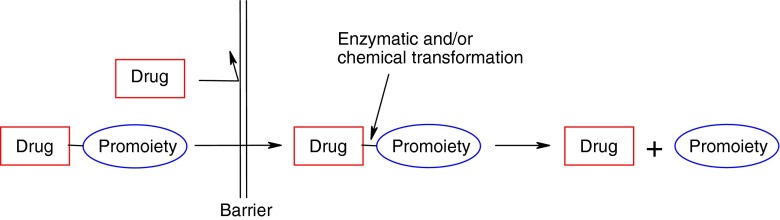
A representative illustration of the prodrug concept. The Drug-Promoiety indicates that part of the prodrug is pharmacologically inactive. The Barrier can be any limitation of a parent drug that prevents optimal (bio)pharmaceutical or pharmacokinetic performance, which must be overcome for the development of a marketable drug (adapted from Rautio et al. 2007 (25))
By applying prodrug technology, the clinical usefulness of a drug molecule may be enhanced without modifying the pharmacological activity of a parent drug. However, the design of an appropriate prodrug structure should ideally be considered at the early stages of preclinical development, bearing in mind that prodrugs, while not common, may alter the tissue distribution, efficacy and the toxicity of the parent drug. Moreover, promoieties used should ideally be safe and rapidly excreted from the body. The choice of promoiety should be considered with respect to the disease state, dose, and the duration of therapy. The prodrug approach can be exploited for almost all administration routes and dosage forms, and it can be applied to a wide variety of existing medicines on the market, as well as to novel drug molecules in the lead optimization step early in the drug discovery process (20,26,27). Prodrug approaches are used to improve drug delivery and targeting in the CNS, and utilize passive drug uptake processes into CNS by chemically modifying a drug to become more lipophilic. Such chemical derivatives include, for example, “traditional” lipophilic esters and other lipophilic compounds that release brain-trapped intermediates, which are also referred to as chemical delivery systems (CDSs). More sophisticated prodrug approaches comprising endogenous transporters (e.g., carrier-mediated prodrug transport), macromolecular delivery mechanisms (e.g., receptor-mediated prodrug transport) as well as gene-directed enzyme prodrug therapy have also been utilized and will be discussed in this review.
THE ROLE OF INCREASED LIPOPHILICITY IN CNS DELIVERY
A frequent challenge with new drug candidates, in regard to CNS delivery, is the candidates’ high polarity. The endothelial cells that line the BBB microvasculature are joined together by highly resistant tight junctions (28), thus preventing the paracellular passage of polar solutes. In addition, the BBB capillaries allow minimum pinocytosis, thus making transcellular diffusion through the cell membranes the only feasible passive route for entering the CNS. This makes adequate lipophilicity one of the key elements in passive CNS entry. A very simple approach to increase the CNS entry of polar molecules would be the masking of polar functionalities within such compounds. This is sometimes referred to as a lipidization of molecules (16). In practice, lipidization through lipophilic drug analogues often results in diminished therapeutic effect, due to decreased activity of the analogues or increased toxicity. Lipidization through prodrugs offers a possibility for a more efficient CNS delivery of polar drugs. Prodrugs, being more lipophilic than the parent drug, enter the CNS more readily, and are then converted back to the parent drug within the CNS.
Lipidization Approach
With increased lipophilicity, for example through simple ester prodrugs, one would predict increased CNS access due to the more lipophilic nature of the prodrugs. Indeed, one can usually accomplish better CNS access by using these lipophilic derivatives. However, this highly one-dimensional approach does not usually lead to more feasible therapeutic results (29). There are only a limited number of really successful examples of improved CNS therapy through plain lipidization of polar molecules. The best known, and from a technological point of view a highly successful example, is the diacetylated form of morphine, heroin (30). Heroin, being more lipophilic, crosses the BBB about 100-fold better than morphine.
The challenge with CNS delivery is that if one wants to achieve a truly site-specific CNS delivery of a drug, various parameters need to be considered and optimized. The general criteria for site-specific drug delivery through prodrugs can be summarized by the following three criteria; (1) the prodrug must have ready access to the appropriate tissue within the CNS, (2) bioconversion back to the parent drug should be highly site-selective, and (3) the parent drug should exhibit prolonged retention within the target tissue (31). In the case of chlorambucil, an anticancer agent, more lipophilic prodrugs have been developed in order to gain enhanced anticancer activity through increased brain entry (32,33). For example, chlorambucil’s tertiary butyl ester enters and remains within the brain with peak concentrations at 15 min and a half-life of 37 min. Further, after chlorambucil and chlorambucil-tertiary butyl ester administration, the brain-to-plasma ratios of the active chlorambucil were recorded to be 0.018 and 0.85, respectively. However, despite increased brain-to-plasma ratios, the chlorambucil prodrugs did not demonstrate superior anticancer activity in disease models when compared to equimolar parent chlorambucil administration.
Lipophilic chlorambucil prodrugs nicely demonstrate that increased lipophilicity through prodrugs does not alone ensure more efficient CNS therapy. Instead, increased lipophilicity, while improving CNS access, also tends to increase uptake in other tissues as well, which can lead to concerns of toxicity. Increased lipophilicity may also increase the plasma protein binding, and invariably increased molecular weight may also hamper the passive transcellular diffusion of a prodrug.
When considering increased lipophilicity through prodrugs as means to increase CNS delivery, the parent molecular properties dictate whether or not the prodrug approach has the basic elements for success. When the parent molecule’s CNS entry and exit are similar, it can be estimated that lipidization may improve the CNS delivery of poorly permeable highly polar drugs exhibiting negligible CNS uptake. In such a case, the lipophilic prodrug has improved CNS access and after bioconversion the more hydrophilic parent molecule gets “trapped” in the brain tissue. Here lipophilic ester prodrugs may provide modest advantage in CNS delivery. While many ester prodrugs suffer from unfavorable bioconversion selectivity, since they can be prematurely hydrolyzed both during absorption process and in systemic circulation, any additional parent drug generated from lipophilic ester prodrug, which has entered the brain, may ensure the applicability of the approach in improved CNS drug delivery. In general, the peripheral bioconversion should to be slow enough to avoid excess premature bioconversion, and the CNS bioconversion fast enough to enable therapeutic drug levels within the CNS before prodrug clearance.
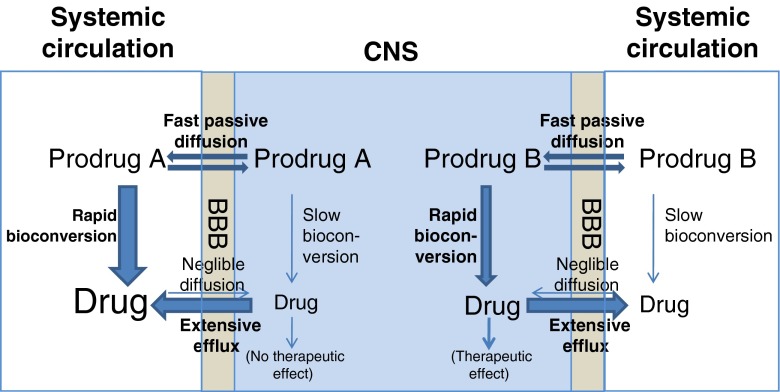
Unfortunately, there are only a limited number of optimal candidates for a pure lipidization approach. Various compounds have built-in limitations to begin with; i.e., one major limitation being a substrate for an active efflux mechanism at the BBB. Figure 3 illustrates the challenge when the parent drug is a substrate for an efflux transporter at the BBB. In the case of Prodrug A, an active efflux component combined with a relatively slow bioconversion within the CNS gives only minimum parent drug levels in the CNS. In the case of Prodrug B, however, the relatively fast bioconversion within the CNS enables substantial levels of parent drug within the CNS, despite the active efflux component. Prodrugs may also offer a way to overcome the efflux challenges that are faced by drugs. This is discussed in the chapter “Overcoming efflux transport”.
Fig. 3.
The role of BBB efflux transporters in lipophilic prodrug CNS entry (modified from Anderson 2007 (29))
Prodrugs can surely be considered one of the most potentially useful technologies when considering strategies of overcoming the BBB. One just has to appreciate the complexity of the BBB anatomy and physiology, and to be ready for more sophisticated approaches than simple lipidization through prodrugs. At the same time, although not a short-cut to success, increased lipophilicity still remains one of the key aspects that must be taken into an account when developing prodrugs for CNS delivery. With CNS prodrugs, the target tissue bioconversion needs to be both rapid and selective enough to compete with elimination from the target tissue, and also to ensure that the premature bioconversion of the prodrug is low enough. Together with the passive efflux component, one also has to acknowledge the role of active efflux. Therefore, a successful CNS delivery strategy via prodrugs has to be tailor-made for the parent molecule. There are two excellent reviews that discuss the lipophilicity, bioconversion and related issues in more detail (29,31).
Chemical Drug Delivery Systems
A successful prodrug approach that utilizes improved lipophilicity and also requires a sequential bioactivation steps for conversion to an active drug and a brain tissue trapped intermediate is often referred in the literature as a chemical drug delivery system (CDS; 34–37). The CDS term was originally coined by Bodor and coworkers to distinguish this approach from prodrugs that typically require only a single activation step. However, many sophisticated prodrugs are nowadays activated in multiple steps.
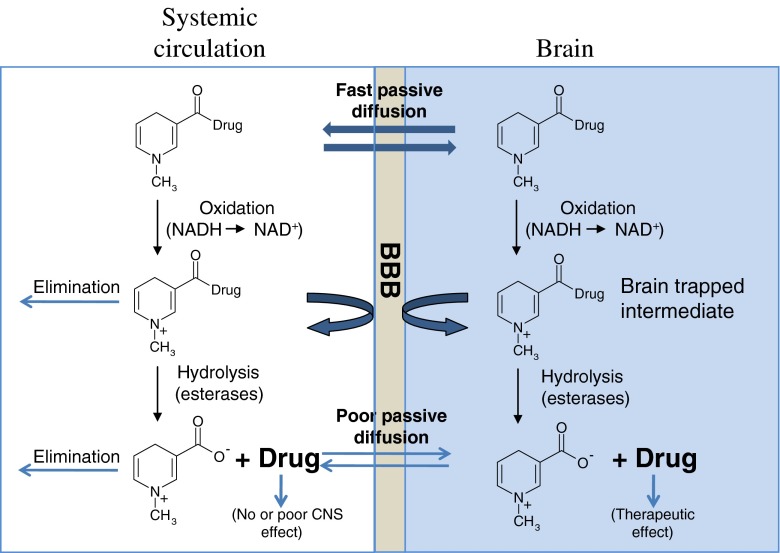
The principle of CDS, in addition to providing access to the brain by increasing the lipophilicity of a drug, exploits specific properties of the BBB to lock drugs in the brain on arrival and prevent them from re-crossing the BBB. The most studied CDS exploits the linking of an active drug molecule to a bio-removable lipophilic targetor moiety, 1,4-dihydro-N-methylnicotinic acid (dihydrotrigonelline), which results in a derivative that readily distributes throughout the body and brain after administration due to its lipophilic character. Once inside the brain parenchyma, and also everywhere in the body, the lipophilic dihydrotrigonelline is oxidized to form a cationic intermediate (Fig. 4). The acquisition of charge both accelerates the rate of systemic elimination of this hydrophilic intermediate and captures the ionic drug-targetor inside the brain. Subsequently, slow release of the drug from the targetor can result in a sustained and brain-specific release of free active drug. Furthermore, the targetor is readily removed from the brain by active processes. The CDS has been explored with a wide variety of hydroxy- and amino-containing drugs (35,36), and considerably increased brain targeting has been achieved, for example, for zidovudine (AZT) (38,39), ganciclovir (40), benzylbenicillin (41, 42) and estradiol (43).
Fig. 4.
An illustration of the chemical drug delivery system (CDS)
Among all CDSs, estradiol-CDS (Estredox) is in the most advanced stages of investigation (36). Estradiol is a lipophilic drug with an octanol/water partition coefficient (log P) of 3.3, and derivatization with the targetor, 1,4-dihydrotrogonelline, further increases lipophilicity (log P = 4.5), thus enabling better transport across the BBB. Oxidation of the targetor moiety leads to a more ionic and less lipophilic form, with a log P value of only −0.14, which is retained in the brain. After the slow and sustained hydrolysis of estradiol-CDS, the concentration of estradiol in rat brain was elevated four to five times longer than after estradiol treatment (44). Moreover, clinical evaluations suggested a potent central effect with only slight elevations in systemic estrogen levels (45). Estredox is currently undergoing Phase II clinical testing for the treatment of postmenopausal symptoms.
Alternative methods of generating brain-trapped intermediates have also been developed. For example, a cationic thiazolium intermediate formed after disulfide reduction-mediated ring-closure (46), and CNS targeted prodrugs utilizing phosphates (47), phosphonates (48–50), and phosphinates (51) as anionic trapped intermediates have been explored in preclinical studies with varying degrees of success. Psilocin and its phosphate ester psilocybin, both from the Psilocybe species of fungi, provided the first example of this mechanism of oral drug delivery to the brain, once it was identified in the mid-1950s by Sandoz.
ENDOGENOUS TRANSPORTERS IN CNS PRODRUG DELIVERY
Recent progress in molecular cloning and functional analysis of transporter genes has greatly contributed to our understanding of membrane transport phenomena (52). The BBB expresses several different transport mechanisms that enable the CNS delivery of compounds that are fundamental to the normal functions of the brain, but are restricted from CNS entry by passive diffusion due to their polar nature. Moreover, these transporters have become a target to drug/prodrug design in an attempt to ferry drug molecules across the BBB via carrier-mediated transport (15,53,54). Some of the transporters can be expressed on both sides of the barrier, and some of them act only as influx or as efflux transporters. The endogenous BBB transporters can therefore be classified into three categories: carrier-mediated transporters and active efflux transporters, which are responsible for small molecule transport across the BBB, and receptor-mediated transport systems, which are responsible for the brain uptake of large endogenous molecules (55).
Carrier-mediated Transport
Several specific endogenous influx transporters have been identified at the brain capillary endothelium that forms the BBB. These include transporters for nutrients, such as amino acids, glucose and vitamins (12). As many drug molecules have similar structural properties to endogenous substrates, it is clear that some membrane transporters can take part in drug transport as well (52). Chemical drug modification in a way that the drug can be recognized by specific transporters, but still maintaining therapeutic efficacy, has proven to be very challenging. One attractive approach is to conjugate an endogenous transporter substrate to the active drug molecule in a bioreversible manner; i.e., to utilize the prodrug approach. The prodrug should be designed in such a way that it is recognized by the specific transporter mechanism at the BBB, and more importantly transported across the BBB to brain tissues, where the release of an active drug from the prodrug should predominantly take place. Here again, the CNS drug delivery via prodrugs can be compromised because of premature systemic bioconversion of the prodrug, although structural requirements for transporter recognition are fulfilled. By using the prodrug approach, BBB penetration properties of a drug molecule can be enhanced without modifying its pharmacological properties (19,31).
Large neutral amino acid transporter (LAT1)
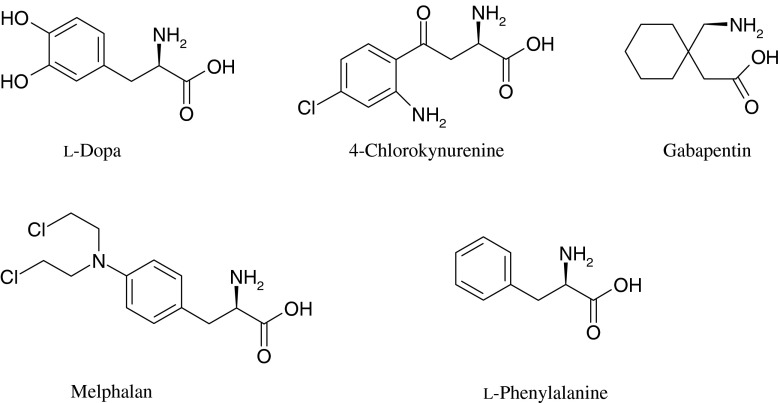
This transport system (LAT1) is expressed on the luminal and abluminal membranes of capillary endothelial cells, and efficiently transports neutral L-amino acids (e.g., phenylalanine and leucine) into the brain (56–58). Several clinically useful amino acid mimicking drugs, such as gabapentin and melphalan, have been shown to be delivered into the brain predominantly via cerebrovascular LAT1-mediated transport, thus demonstrating the ability of LAT1 to be utilized in drug delivery (59,60). It may be no surprise that all of these drugs bear a very close structural resemblance to endogenous LAT1-substrates (Fig. 5).
Fig. 5.
Structures of exo- and endogenous LAT1 substrates. All LAT1 substrates have a positively charged amino group, a negatively charged carboxyl group and a hydrophobic side chain
The only prodrug that is used clinically for entering the brain predominantly through LAT1-mediated transport is L-dopa. The neurotransmitter dopamine is not able to cross the BBB due to its hydrophilic nature (61). However, the conversion of dopamine into its α-amino acid, L-dopa, enables the brain to uptake dopamine via LAT1 (62). L-Dopa is decarboxylated into dopamine by L-amino acid decarboxylase in the brain tissue, and also in the peripheral circulation (63). Although approximately 95% of L-dopa is metabolized to dopamine in the peripheral tissues, the percentage of remaining L-dopa has been therapeutically enough to apply this approach in clinic practice for more than 30 years (64). Another example of LAT1 utilizing prodrugs is 4-chlorokynurenine, a prodrug of 7-chlorokynurenic acid (65). 7-Chlorokynurenic acid is an N-methyl-d-aspartate antagonist that crosses the BBB poorly because of its high hydrophilicity. In these examples, the parent drug has a structure that closely resembles that of an amino acid. However, this prodrug approach does not generalize to structurally different molecules.
Another approach to utilize LAT1 for BBB transport is to conjugate a small molecular drug with a LAT1 substrate, typically an amino acid. Killian et al. (66) conjugated L-cysteine with the anticancer agent 6-mercaptopurine and a model compound 2-methyl-1-propanethiol. The prodrugs were able to inhibit LAT1 mediated brain uptake of [14C]L-leucine using an in situ rat brain perfusion technique, which indicated that the prodrugs are able to bind to LAT1. The amino acid l-tyrosine is a LAT1-substrate that has a phenolic hydroxyl group suitable for the conjugation of various structurally different drug molecules with a biodegradable linkage (67). In a study by Walker et al. (68), a phosphoformate l-tyrosine conjugate was able to inhibit the transport of [3H]l-tyrosine in porcine brain microvessel endothelial cells. In another study, p-nitro and p-chlorobenzyl ether conjugates of l-tyrosine inhibited the transport of [3H]l-tyrosine in rabbit corneal cell line (69). These results indicate that l-tyrosine conjugates are able to bind to the LAT1-transporter. However, the ability of these conjugates to cross the cell membrane has not yet been studied. In contrast, an l-tyrosine prodrug of ketoprofen demonstrated significant reversible inhibition in brain uptake of the radiotracer [14C]l-leucine in the in situ rat brain perfusion model, indicating that the prodrug binds to the LAT1 (104). More importantly, the prodrug entered the brain with both concentration-dependent and saturable uptake. In addition, the LAT1 inhibitor 2-aminobicyclo-(2, 2, 1)-heptane-2-carboxylic acid (BCH) significantly decreased the brain uptake of the prodrug, further confirming that the drug-substrate conjugate was not only recognized but also transported across the rat BBB by LAT1.
Glucose transporter (GLUT1)
The glucose transporter (GLUT1) is present both on the luminal and the abluminal membrane of the endothelial cells forming the BBB (70). GLUT1 transports glucose and other hexoses, and has the highest transport capacity of the carrier-mediated transporters present at the BBB being therefore an attractive transporter for prodrug delivery (31).
Glycosylation strategy has been utilized to increase the uptake of 7-chlorokynurenic acid (71). The concentration of 7-chlorokynurenic acid and kynurenic acid in the rat brain was determined with microdialysis after systemic injection of 7-chlorokynurenic acid with two glucose conjugates of 7-chlorokynurenic acid. The glycosylation increased the brain uptake of the parent drug, but the mechanism that caused the increased uptake was unfortunately not conclusively demonstrated in that study.
Several studies have been performed with different drug molecules, in order to determine the ability of their glycosyl derivatives to bind to GLUT1 (61,72). A glucose–chlorambucil derivative was able to inhibit the uptake of [14C]D-glucose into human erythrocytes (72). However, in these in vitro uptake studies the prodrug was found to be an inhibitor rather than a substrate of GLUT1. Fernandez et al. (61) synthesized several glycosyl derivatives of dopamine and tested the affinity of the prodrugs to GLUT1 in human erythrocytes. Dopamine was linked to glucose with different linkers at the C-1, C-3 and C-6 positions of glucose (Fig. 6). The results of glucose uptake inhibition showed that the glucose derivatives that were conjugated at position C-6 had the best affinity for GLUT1. There was also a difference in the affinity between carbamate and succinamate prodrugs, with the carbamate prodrug having a better affinity for the carrier. In an earlier study by Fernandez et al. (73) glycosyl derivatives of dopamine that were conjugated with succinyl linker did not exhibit an ability to induce a recovery of motor activity in mice pretreated with reserpine. This was attributed to slow bioconversion of the prodrugs into dopamine.
Fig. 6.
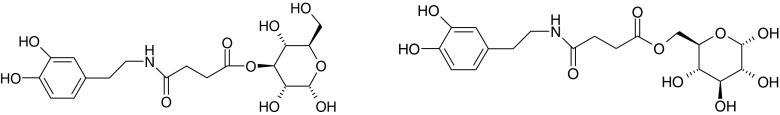
Structures of a C-3-glucose dopamine succinyl ester and a C-6-glucose dopamine succinyl ester
Bonina et al. (74) synthesized L-dopa and dopamine glycoside prodrugs by conjugating the parent drugs with glucose at the C-3 position and galactose at the C-6 position by a succinyl linker with the aim of overcoming the problem of the low BBB permeability of dopamine. The prodrugs were tested with classic dopaminergic models, morphine induced locomotion in mice and reserpine-induced hypolocomotion in rats. Both of the dopamine glycosidic prodrugs were more active in reversing the reserpine-induced hypolocomotion in rats than L-dopa or the L-dopa prodrugs. In reducing morphine-induced locomotion in mice, the galactose–dopamine conjugate was the most effective and glucose–dopamine had the least efficacy of all the tested prodrugs. However, the glucose-L-dopa prodrug was more effective than the galactose-L-dopa prodrug. By conjugating dopamine with glycosides, the pharmacological efficacy was increased but the mechanism of brain uptake remains unclear. In the study by Fernandez et al. (61), the dopamine-galactose prodrug, conjugated with a succinyl linker, had poor affinity for GLUT1 in human erythrocytes.
Overcoming Efflux Transport
While much attention has been given to the transport of compounds into the brain, transport out of the brain also plays a critical role in efficient drug delivery. There are several different efflux transporters that are present in the BBB and functioning as clearance systems for both metabolic and catabolic compounds produced in the brain (75–77). Moreover, these efflux transporters have broad substrate recognition for xenobiotics, which contributes to the restricted BBB permeability and the subsequent distribution of structurally diverse molecules. For example, quinolone antibiotics are effluxed 10- to 100-fold faster from the brain when compared to their influx rate, thus resulting in low brain interstitial concentration (78). Some examples of substrates and inhibitors of efflux transporters are presented in Table 1 (79).
Table 1.
Drug efflux transporters present at the BBB and examples of their substrates as well as inhibitors (79)
| Transporter | Substrate | Inhibitor |
|---|---|---|
| P-glycoprotein | Saquinavir, Vincristine | Verapamil, OC144–093 |
| MRP1-6 | Melphalan, AZT | Probenecid |
| BCRP | Topotecan | CF 120918 |
| Oatp1-3 | Rosuvastatin, Enalapril | Rifampicin, Probenecid |
| OATP-A | Bile acids | Rifampicin |
| OAT3 | Cephalosporin antibiotics | Acyclovir, Probenecid |
The prodrug approach is indeed interesting, but still a very challenging means of overcoming efflux transport of neuro-pharmaceuticals. The important functional groups of drug molecules that afford recognition of the efflux transporter could be, at least in theory, masked with promoieties, and the resulting prodrug, which might not be an efflux candidate, would cross the BBB. However, as indicated above, the efflux transporters have wide substrate specificity, and therefore chemical modification of a drug with the hope of preventing its efflux transporter recognition is very challenging, and more like hit and miss at this time. Thorough SAR studies on structurally related efflux substrates, as in case of P-glycoprotein (Pgp) substrates (BACE inhibitors), may be useful and afford molecules with similar potency but reduced efflux liabilities. (80,81)
The prodrug approach could also enable the efficient brain uptake of drugs while inhibiting the function of efflux transporters. Co-administration of efflux substrate drugs with efflux inhibitors is a well-known strategy for enhanced CNS drug delivery (82,83). Several potent and specific Pgp modulators such as elacridar, tariquidar and laniquidar have been tested in the preclinical studies to assess whether concomitant administration of Pgp inhibitors can enhance the brain penetration into the brain. For example, Polli et al. demonstrated a 13-fold increase in the brain concentrations of amprenavir in mice pretreated with elacridar (82). Moreover, the brain concentration of ketoprofen after administering its lipophilic prodrug was maintained for a significantly longer period following co-administration of the nonspecific efflux inhibitor probenecid (84), compared to ketoprofen alone. An efflux inhibitor could also be conjugated with therapeutic drugs to form a codrug. A codrug consists of two pharmacologically active drugs that are coupled together in a single molecule, so that each drug acts as a promoiety for the other (85,86). After degradation of the codrug in the systemic circulation, the efflux inhibitor would enable better brain uptake of the therapeutic drug. The codrug approach has been explored in an effort to improve L-dopa brain delivery with a potent catechol-O-methyltransferase inhibitor, entacapone, as a form of L-dopa-entacapone codrug (86). However, a drug-efflux inhibitor codrug approach has not yet been pursued to our knowledge. This approach would be applicable to lipophilic drugs that were able to cross the BBB, due to their lipophilicity, but that are also restricted from efficient brain entry by efflux transporters. However, this approach might not be the best option for drugs that are for the treatment of chronic disorders of the brain, as the brain produces compounds that may cause neurodegenerative diseases (87). These metabolites are cleared from the CNS by efflux systems and the chronic inhibition of these efflux systems might lead to an accumulation of neurotoxins in the brain. In addition, the chronic dosing of efflux inhibitors may possibly upregulate other efflux systems at the BBB as a compensatory mechanism raising further issues associated with clinical use of these inhibitors.
Receptor-mediated Prodrug Delivery
Receptor-mediated drug delivery also takes advantage of the endogenous BBB-transport system, and aims to improve brain uptake by coupling non-transportable therapeutic molecules to a drug-transport vector (88,89). The brain capillary endothelium expresses specific transcytosis systems for important circulating nutrients and signalling molecules that ordinarily cannot diffuse through the BBB. For example, these include systems for the transport of insulin, insulin-like growth factors, transferrin, and leptin. Thus, a drug-transport vector may include endogenous peptides, such as insulin or transferrin, a modified protein, or it may include anti-receptor specific monoclonal antibodies (MAb) that undergo transcytosis through the BBB via the endogenous receptor system within the brain capillary endothelium. Conjugation of a drug to a transport vector can be facilitated either by chemical linkers, avidin-biotin technology, polyethylene glycol linkers, or liposomes.
One of the most common model vectors for receptor-mediated transcytosis has been the anti-rat transferrin-receptor antibody OX26 (90). Since the transferrin receptor is highly expressed on brain capillaries, the binding of the OX26 MAb to this receptor enables an ordinarily non-transportable molecule to penetrate the BBB when coupled to OX26 MAb. To allow different peptide ligands to be released following transcytosis, these peptides were coupled with the vector by a bioreversible disulfide linkage. A biotinylated peptide (i.e., a peptide conjugated with biotin) linked covalently with an OX26-avidin conjugate binds to the BBB transferrin receptor and the peptide-carrier conjugate is transported across the BBB. In brain tissue the disulfide bond between the drug molecule and biotin is cleaved by disulfide reductases and the peptide is released (88). Applicability of this strategy was demonstrated for the first time in vivo, when a vasoactive intestinal peptide analog produced a significant increase in cerebral blood flow (91).
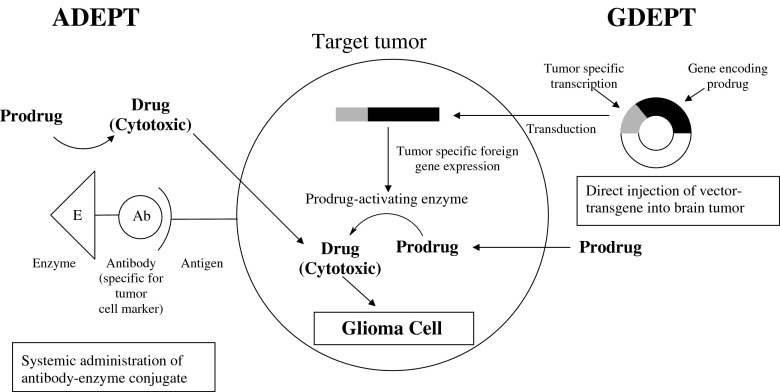
ANTIBODY- AND GENE-DIRECTED PRODRUG THERAPIES
Most endogenous enzymes have a ubiquitous distribution, which diminishes the possibilities for selective activation and, consequently, the targeting for potential prodrugs in the CNS. More selective prodrug activation in targeted tissues can be achieved by exogenous prodrug bioconverting enzymes that are delivered via monoclonal antibodies or generated from genes encoding an exogenous enzyme. These approaches are referred to as ADEPT (antibody-directed enzyme prodrug therapy) and GDEPT (gene-directed enzyme prodrug therapy; Fig. 7), and also known as “suicide gene therapy”, because these enzymes are not regenerated for further use (92,93). Both are useful means of targeting prodrugs, especially towards various tumors. To our knowledge no examples of ADEPT for the treatment of human brain tumors exist, however numerous GDEPT clinical trials have been conducted in treating for CNS malignancies.
Fig. 7.
An outline of antibody-directed enzyme prodrug therapy (ADEPT) and gene-directed enzyme prodrug therapy (GDEPT) for site-specific activation of cytotoxic drugs in brain tumor cells
From a number of GDEPT approaches have been described in the past decade (93–96), several have been for the treatment of CNS malignancies, such as gliomas (96). A widely studied example is the bacterial gene cytosine deaminase (CD), which sensitizes tumor cells to the anti-fungal drug 5-fluorocytosine (5-FC; 94,97). As a result, 5-FC is bioconverted to the anti-cancer drug, 5-fluorouracil (5-FU). The most commonly explored system of this approach in clinical trials is a pioneering effort that centers on inserting a thymine kinase gene into the herpes simplex virus (HSV-TK), which is delivered to tumor cells and followed by combination treatment with the prodrug ganciclovir (GCV). The expressed thymidine kinase enzyme selectively converts GCV to its monophosphate, which is subsequently converted to its active triphosphate form by a series of intracellular reactions. A large 248 patient multicenter phase III clinical trial has demonstrated the ability of this approach of using a retrovirus-mediated gene delivery system for the treatment of glioblastoma multiforme. However, no significant benefit was found when compared to standard therapy (i.e., surgical resection and radiotherapy; 98), which was attributed to low transduction efficiency of the retroviral vector-producing cells (99). During the past few years, adenoviruses have been considered as viable gene transfer vectors, and some recent clinical trials have demonstrated significant efficacy (96,100, 101). Further optimization of gene transfer vectors is essential for improving the clinical effectiveness of GDEPT.
CONCLUSIONS
The worldwide market for prescription neuro-therapeutic drugs will expand at a double-digit pace over the next years to surpass the €28 billion mark by the year 2009. Recent advances in biotechnology and pharmaceutical sciences have greatly expanded the number of drugs that are being developed for the treatment of CNS disorders. However, the BBB presents a major structural and functional barrier for drugs that have pharmacological targets within the brain. A potential option to improve CNS drug delivery is in the utilization of prodrug technology. Prodrugs have already become an integral part of the drug design and delivery processes. Moreover, it has been estimated that 5–6% of all marketed drugs worldwide are prodrugs; in 2001 and 2002, approximately 15% of all new approved drugs were prodrugs (23,102,103). Since the introduction of L-dopa, over 30 years ago, only a few prodrugs for the treatment of CNS disorders have even reached clinical trials. Those few include estradiol prodrug (Estredox) and prodrugs used in gene-directed enzyme prodrug therapy. However, there is a strong belief to justify the idea that prodrugs hold great potential for CNS drug delivery. A better understanding of molecular biology will certainly provide more insight in the mechanisms of enzymes and endogenous transporters that exist at the BBB. In addition, a better understanding of the multi-dimensional nature of prodrug design, when targeting prodrugs into the CNS, is of crucial importance when developing CNS prodrugs.
Acknowledgements
Authors thank Dr. Jace Callaway for his valuable comments and the Academy of Finland (KL,108569) for financial support.
References
- 1.Pardridge W. M. Why is the global CNS pharmaceutical market so under-penetrated. Drug Discov. Today. 2002;7(1):5–7. doi: 10.1016/s1359-6446(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge W. M. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begley D. J. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury M. W. The structure and function of the blood–brain barrier. Fed. Proc. 1984;43(2):186–190. [PubMed] [Google Scholar]
- 5.Janzer R. C., Raff M. C. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 6.Kim J. H., Kim J. H., Park J. A., et al. Blood–neural barrier: intercellular communication at glio–vascular interface. J. Biochem. Mol. Biol. 2006;39(4):339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- 7.Lai C. H., Kuo K. H. The critical component to establish in vitro BBB model: Pericyte. Brain Res. Brain Res. Rev. 2005;50(2):258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Begley D. J. The blood–brain barrier: principles for targeting peptides and drugs to the central nervous system. J. Pharm. Pharmacol. 1996;48(2):136–146. doi: 10.1111/j.2042-7158.1996.tb07112.x. [DOI] [PubMed] [Google Scholar]
- 9.Loscher W., Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schinkel A. H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug. Deliv. Rev. 1999;36(2–3):179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 11.Begley D. J. ABC transporters and the blood–brain barrier. Curr. Pharm. Des. 2004;10(12):1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge W. M., Oldendorf W. H. Transport of metabolic substrates through the blood–brain barrier. J. Neurochem. 1977;28(1):5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- 13.Pardridge W. M. Blood–brain barrier genomics and the use of endogenous transporters to cause drug penetration into the brain. Curr. Opin. Drug. Discov. Devel. 2003;6(5):683–691. [PubMed] [Google Scholar]
- 14.Halmos T., Santarromana M., Herscovici J., Scherman D. Brain drug delivery through the blood–brain barrier transport systems. Attempted strategies and issues. STP Pharma. Sci. 1997;7:37–42. [Google Scholar]
- 15.Yang C., Tirucherai G. S., Mitra A. K. Prodrug based optimal drug delivery via membrane transporter/receptor. Expert Opin. Biol. Ther. 2001;1(2):159–175. doi: 10.1517/14712598.1.2.159. [DOI] [PubMed] [Google Scholar]
- 16.Pardridge W. M. Blood–brain barrier delivery. Drug Discov. Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Albert A. Chemical aspects of selective toxicity. Nature. 1958;182:421–422. doi: 10.1038/182421a0. [DOI] [PubMed] [Google Scholar]
- 18.Sinkula A. A., Yalkowsky S. H. Rationale for design of biologically reversible drug derivatives: prodrugs. J. Pharm. Sci. 1975;64(2):181–210. doi: 10.1002/jps.2600640203. [DOI] [PubMed] [Google Scholar]
- 19.Stella V. J., Charman W. N., Naringrekar V. H. Prodrugs. Do they have advantages in clinical practice? Drugs. 1985;29(5):455–473. doi: 10.2165/00003495-198529050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Stella V. J., Borchardt R. T., Hageman M. J., Oliyai R., Maag H., Tilley J. W. Prodrugs: Challenges and Rewards. Vol. 1–2. New York: Published by AAPS Press and Springer; 2007. [Google Scholar]
- 21.Sherwood R. F. Advanced drug delivery reviews: Enzyme prodrug therapy. Adv. Drug Del. Rev. 1996;22:269–288. [Google Scholar]
- 22.Stella V. Prodrug strategies for improving drug-like properties. In: Borchardt R., Hageman M., Stevens J., Kerns E., Thakker D., editors. Optimizing the “drug-like” properties of leads in drug discovery. New York: Springer; 2006. pp. 221–242. [Google Scholar]
- 23.Stella V. J. Prodrugs as therapeutics. Expert Opin. Ther. Patents. 2004;14(3):277–280. [Google Scholar]
- 24.Stella V. J., Nti-Addae K. W. Prodrug strategies to overcome poor water solubility. Adv. Drug Deliv. Rev. 2007;59:677–694. doi: 10.1016/j.addr.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Rautio J., Kumpulainen H., Heimbach T., et al. Prodrugs: design and clinical applications. Nat. Rev. Drug Discovery. 2008;7:1–16. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 26.Järvinen T., Rautio J., Masson M., Loftsson T. Design and pharmaceutical applications of prodrugs. In: Gad S., editor. Drug discovery handbook. Hoboken: John Wiley & Sons, Inc.; 2005. pp. 733–796. [Google Scholar]
- 27.Beaumont K., Webster R., Gardner I., Dack K. Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: Challenges to the discovery scientist. Curr. Drug Metab. 2003;4(6):461–485. doi: 10.2174/1389200033489253. [DOI] [PubMed] [Google Scholar]
- 28.Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell. Biol. 1969;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson B. D. Prodrug approaches for drug delivery to the brain. In: Stella V. J., Borchardt R. T., Hageman M. J., Oliyai R., Maag H., Tilley J. W., editors. Prodrugs: Challenges and Rewards. Part 1. New York: AAPS Press/Springer; 2007. pp. 573–651. [Google Scholar]
- 30.Oldendorf W. H., Hyman S., Braun L., Oldendorf S. Z. Blood–brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178(64):984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- 31.Anderson B. D. Prodrugs for improved CNS delivery. Adv Drug Deliv Rev. 1996;19:171–202. [Google Scholar]
- 32.Greig N. H., Genka S., Daly E. M., Sweeney D. J., Rapoport S. I. Physicochemical and pharmacokinetic parameters of seven lipophilic chlorambucil esters designed for brain penetration. Cancer Chemother Pharmacol. 1990;25(5):311–319. doi: 10.1007/BF00686229. [DOI] [PubMed] [Google Scholar]
- 33.Genka S., Deutsch J., Shetty U. H., et al. Development of lipophilic anticancer agents for the treatment of brain tumors by the esterification of water-soluble chlorambucil. Clin Exp Metastasis. 1993;11(2):131–140. doi: 10.1007/BF00114971. [DOI] [PubMed] [Google Scholar]
- 34.Bodor N., Buchwald P. Drug targeting via retrometabolic approaches. Pharmacol. Ther. 1997;76(1–3):1–27. [PubMed] [Google Scholar]
- 35.Bodor N., Buchwald P. Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems. Adv. Drug Deliv. Rev. 1999;36(2–3):229–254. doi: 10.1016/s0169-409x(98)00090-8. [DOI] [PubMed] [Google Scholar]
- 36.Bodor N., Buchwald P. Barriers to remember: brain-targeting chemical delivery systems and Alzheimer's disease. Drug. Discov. Today. 2002;7(14):766–774. doi: 10.1016/s1359-6446(02)02332-2. [DOI] [PubMed] [Google Scholar]
- 37.Prokai L., Prokai-Tatrai K., Bodor N. Targeting drugs to the brain by redox chemical delivery systems. Med. Res. Rev. 2000;20(5):367–416. doi: 10.1002/1098-1128(200009)20:5<367::aid-med3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Brewster M. E., Anderson W. R., Helton D. O., Bodor N., Pop E. Dose-dependent brain delivery of zidovudine through the use of a zidovudine chemical delivery system. Pharm. Res. 1995;12(5):796–798. doi: 10.1023/a:1016240432455. [DOI] [PubMed] [Google Scholar]
- 39.Brewster M. E., Anderson W. R., Webb A. I., et al. Evaluation of a brain-targeting zidovudine chemical delivery system in dogs. Antimicrob Agents Chemother. 1997;41(1):122–128. doi: 10.1128/aac.41.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewster M. E., Raghavan K., Pop E., Bodor N. Enhanced delivery of ganciclovir to the brain through the use of redox targeting. Antimicrob. Agents. Chemother. 1994;38(4):817–823. doi: 10.1128/aac.38.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu W. M., Pop E., Shek E., Bodor N. Brain-specific chemical delivery systems for beta-lactam antibiotics. In vitro and in vivo studies of some dihydropyridine and dihydroisoquinoline derivatives of benzylpenicillin in rats. J. Med. Chem. 1989;32(8):1782–1788. doi: 10.1021/jm00128a020. [DOI] [PubMed] [Google Scholar]
- 42.Wu W. M., Pop E., Shek E., Clemmons R., Bodor N. Brain and CSF specific chemical delivery systems for beta-lactam antibiotics. Study of two dihydropyridine derivatives of benzylpenicillin in rabbits and dogs. Drug Des. Deliv. 1990;7(1):33–43. [PubMed] [Google Scholar]
- 43.Estes K. S., Brewster M. E., Simpkins J. W., Bodor N. A novel redox system for CNS-directed delivery of estradiol causes sustained LH suppression in castrate rats. Life Sci. 1987;40(13):1327–1334. doi: 10.1016/0024-3205(87)90590-x. [DOI] [PubMed] [Google Scholar]
- 44.Mullersman G., Derendorf H., Brewster M. E., Estes K. S., Bodor N. High-performance liquid chromatographic assay of a central nervous system (CNS)-directed estradiol chemical delivery system and its application after intravenous administration to rats. Pharm. Res. Mar. 1988;5(3):172–177. doi: 10.1023/a:1015964907110. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar D. K., Friedman S. J., Yen S. S., Frautschy S. A. Chronic inhibition of hypothalamic-pituitary-ovarian axis and body weight gain by brain-directed delivery of estradiol-17 beta in female rats. Neuroendocrinology. 1989;50(2):204–210. doi: 10.1159/000125223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikura T., Senou T., Ishihara H., Kato T., Ito T. Drug delivery to the brain. DOPA prodrugs based on a ring-closure reaction to quaternary thiazolium compounds. Int. J. Pharm. 1995;116(1):51. [Google Scholar]
- 47.Tan X., Boudinot F. D., Chu C. K., et al. Pharmacokinetics of bis(t-butyl-SATE)-AZTMP, a bispivaloylthioethyl prodrug for intracellular delivery of zidovudine monophosphate, in mice. Antivir. Chem. Chemother. 2000;11(3):203–211. doi: 10.1177/095632020001100303. [DOI] [PubMed] [Google Scholar]
- 48.Somogyi G., Buchwald P., Bodor N. Targeted drug delivery to the central nervous system via phosphonate derivatives (anionic delivery system for testosterone) Pharmazie. 2002;57(2):135–137. [PubMed] [Google Scholar]
- 49.Somogyi G., Nishitani S., Nomi D., Buchwald P., Prokai L., Bodor N. Targeted drug delivery to the brain via phosphonate derivatives: I. Design, synthesis and evaluation of an anionic chemical delivery system for testosterone. Int. J. Pharm. 1998;166(1):15. [Google Scholar]
- 50.Somogyi G., Buchwald P., Nomi D., Prokai L., Bodor N. Targeted drug delivery to the brain via phosphonate derivatives II. Anionic chemical delivery system for zidovudine (AZT) Int. J. Pharm. 1998;166(1):27. [Google Scholar]
- 51.Chen H., Noble F., Roques B. P., Fournie-Zaluski M. C. Long lasting antinociceptive properties of enkephalin degrading enzyme (NEP and APN) inhibitor prodrugs. J. Med. Chem. 2001;44(21):3523–3530. doi: 10.1021/jm0102248. [DOI] [PubMed] [Google Scholar]
- 52.Tamai I., Tsuji A. Transporter-mediated permeation of drugs across the blood–brain barrier. J. Pharm. Sci. 2000;89(11):1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 53.Anand B. S., Dey S., Mitra A. K. Current prodrug strategies via membrane transporters/receptors. Expert. Opin. Biol. Ther. 2002;2(6):607–620. doi: 10.1517/14712598.2.6.607. [DOI] [PubMed] [Google Scholar]
- 54.Majumdar S., Duvvuri S., Mitra A. K. Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Adv. Drug Deliv. Rev. 2004;56(10):1437–1452. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Pardridge W. M. Drug targeting to the brain. Pharm. Res. 2007;24(9):1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 56.Boado R. J., Li J. Y., Nagaya M., Zhang C., Pardridge W. M. Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc. Natl. Acad. Sci. U S A. 1999;96(21):12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duelli R., Enerson B. E., Gerhart D. Z., Drewes L. R. Expression of large amino acid transporter LAT1 in rat brain endothelium. J. Cereb. Blood Flow Metab. 2000;20(11):1557–1562. doi: 10.1097/00004647-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Smith Q. R. Carrier-mediated transport to enhance drug delivery to brain. International Congress Series. 2005;1277:63–74. [Google Scholar]
- 59.Cundy K. C., Branch R., Chernov-Rogan T., et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J. Pharmacol. Exp. Ther. 2004;311(1):315–323. doi: 10.1124/jpet.104.067934. [DOI] [PubMed] [Google Scholar]
- 60.Goldenberg G. J., Lam H. Y., Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J. Biol. Chem. 1979;254(4):1057–1064. [PubMed] [Google Scholar]
- 61.Fernandez C., Nieto O., Fontenla J. A., Rivas E., de Ceballos M. L., Fernandez-Mayoralas A. Synthesis of glycosyl derivatives as dopamine prodrugs: interaction with glucose carrier GLUT-1. Org. Biomol. Chem. 2003;1(5):767–771. doi: 10.1039/b212066f. [DOI] [PubMed] [Google Scholar]
- 62.Gomes P., Soares-da-Silva P. L-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res. 1999;829(1–2):143–150. doi: 10.1016/s0006-8993(99)01387-6. [DOI] [PubMed] [Google Scholar]
- 63.Dairman W., Christenson J. G., Udenfriend S. Decrease in liver aromatic L-amino-acid decarboxylase produced by chronic administration of L-dopa. Proc. Natl. Acad. Sci. U S A. 1971;68(9):2117–2120. doi: 10.1073/pnas.68.9.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mena I., Cotzias G. C. Protein intake and treatment of Parkinson’s disease with levodopa. N. Engl. J. Med. 1975;292(4):181–184. doi: 10.1056/NEJM197501232920404. [DOI] [PubMed] [Google Scholar]
- 65.Hokari M., Wu H. Q., Schwarcz R., Smith Q. R. Facilitated brain uptake of 4-chlorokynurenine and conversion to 7-chlorokynurenic acid. Neuroreport. 1996;8(1):15–18. doi: 10.1097/00001756-199612200-00004. [DOI] [PubMed] [Google Scholar]
- 66.Killian D. M., Hermeling S., Chikhale P. J. Targeting the cerebrovascular large neutral amino acid transporter (LAT1) isoform using a novel disulfide-based brain drug delivery system. Drug Deliv. 2007;14(1):25–31. doi: 10.1080/10717540600559510. [DOI] [PubMed] [Google Scholar]
- 67.Smith Q. R., Cooper A. J. L. Mammalian amino acid transport. New York: Plenum Press; 1992. pp. 165–193. [Google Scholar]
- 68.Walker I., Nicholls D., Irwin W. J., Freeman S. Drug delivery via active transport at the blood–brain barrier: affinity of a prodrug of phosphonoformate for the large amino acid transporter. Int. J. Pharm. 1994;104(2):157. [Google Scholar]
- 69.Balakrishnan A., Jain-Vakkalagadda B., Yang C., Pal D., Mitra A. K. Carrier mediated uptake of -tyrosine and its competitive inhibition by model tyrosine linked compounds in a rabbit corneal cell line (SIRC)—strategy for the design of transporter/receptor targeted prodrugs. Int. J. Pharm. 2002;247(1–2):115. doi: 10.1016/s0378-5173(02)00405-2. [DOI] [PubMed] [Google Scholar]
- 70.Farrell C. L., Pardridge W. M. Blood–brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc. Natl. Acad. Sci. U. S. A. 1991;88(13):5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Battaglia G., La Russa M., Bruno V., et al. Systemically administered D-glucose conjugates of 7-chlorokynurenic acid are centrally available and exert anticonvulsant activity in rodents. Brain Res. 2000;860(1–2):149–156. doi: 10.1016/s0006-8993(00)01962-4. [DOI] [PubMed] [Google Scholar]
- 72.Halmos T., Santarromana M., Antonakis K., Scherman D. Synthesis of glucose–chlorambucil derivatives and their recognition by the human GLUT1 glucose transporter. Eur. J. Pharmacol. 1996;318(2–3):477–484. doi: 10.1016/s0014-2999(96)00796-0. [DOI] [PubMed] [Google Scholar]
- 73.Fernandez C., Nieto O., Rivas E., Montenegro G., Fontenla J. A., Fernandez-Mayoralas A. Synthesis and biological studies of glycosyl dopamine derivatives as potential antiparkinsonian agents. Carbohydr. Res. 2000;327(4):353–365. doi: 10.1016/s0008-6215(00)00073-2. [DOI] [PubMed] [Google Scholar]
- 74.Bonina F., Puglia C., Rimoli M. G., et al. Glycosyl derivatives of dopamine and L-dopa as anti-Parkinson prodrugs: synthesis, pharmacological activity and in vitro stability studies. J. Drug Target. 2003;11(1):25–36. doi: 10.1080/1061186031000086090. [DOI] [PubMed] [Google Scholar]
- 75.Ohtsuki S., Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm. Res. 2007;24(9):1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 76.Lee G., Dallas S., Hong M., Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol. Rev. 2001;53(4):569–596. [PubMed] [Google Scholar]
- 77.Tsuji A., Tamai I. I. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv. Drug. Deliv. Rev. 1999;36(2–3):277–290. doi: 10.1016/s0169-409x(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 78.Ooie T., Terasaki T., Suzuki H., Sugiyama Y. Kinetic evidence for active efflux transport across the blood–brain barrier of quinolone antibiotics. J. Pharmacol. Exp. Ther. 1997;283(1):293–304. [PubMed] [Google Scholar]
- 79.Loscher W., Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Moore K. P., Zhu H., Rajapakse H. A., et al. Strategies toward improving the brain penetration of macrocyclic tertiary carbinamine BACE-1 inhibitors. Bioorg. Med. Chem. Lett. 2007;17(21):5831–5835. doi: 10.1016/j.bmcl.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 81.Stauffer S. R., Stanton M. G., Gregro A. R., et al. Discovery and SAR of isonicotinamide BACE-1 inhibitors that bind beta-secretase in a N-terminal 10s-loop down conformation. Bioorg. Med. Chem. Lett. 2007;17(6):1788–1792. doi: 10.1016/j.bmcl.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 82.Polli J. W., Jarrett J. L., Studenberg S. D., et al. Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm. Res. 1999;16(8):1206–1212. doi: 10.1023/a:1018941328702. [DOI] [PubMed] [Google Scholar]
- 83.Breedveld P., Beijnen J. H., Schellens J. H. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol. Sci. 2006;27(1):17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Deguchi Y., Hayashi H., Fujii S., et al. Improved brain delivery of a nonsteroidal anti-inflammatory drug with a synthetic glyceride ester: a preliminary attempt at a CNS drug delivery system for the therapy of Alzheimer’s disease. Eur J Pharm. 2000;8:371–378. doi: 10.3109/10611860008997913. [DOI] [PubMed] [Google Scholar]
- 85.Kiptoo P. K., Hamad M. O., Crooks P. A., Stinchcomb A. L. Enhancement of transdermal delivery of 6-beta-naltrexol via a codrug linked to hydroxybupropion. J. Control. Release. 2006;113(2):137–145. doi: 10.1016/j.jconrel.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 86.Leppanen J., Huuskonen J., Nevalainen T., Gynther J., Taipale H., Jarvinen T. Design and synthesis of a novel L-dopa-entacapone codrug. J. Med. Chem. 2002;45(6):1379–1382. doi: 10.1021/jm010980d. [DOI] [PubMed] [Google Scholar]
- 87.Taylor E. M. The impact of efflux transporters in the brain on the development of drugs for CNS disorders. Clin. Pharmacokinet. 2002;41(2):81–92. doi: 10.2165/00003088-200241020-00001. [DOI] [PubMed] [Google Scholar]
- 88.Bickel U., Yoshikawa T., Pardridge W. M. Delivery of peptides and proteins through the blood–brain barrier. Adv. Drug. Deliv. Rev. 2001;46(1–3):247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 89.Pardridge W. M. Vector-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 1999;36(2–3):299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 90.Saito Y., Buciak J., Yang J., Pardridge W. M. Vector-mediated delivery of 125I-labeled beta-amyloid peptide A beta 1–40 through the blood–brain barrier and binding to Alzheimer disease amyloid of the A beta 1–40/vector complex. Proc. Natl. Acad. Sci. U S A. 1995;92(22):10227–10231. doi: 10.1073/pnas.92.22.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bickel U., Yoshikawa T., Landaw E. M., Faull K. F., Pardridge W. M. Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. Proc. Natl. Acad. Sci. U S A. 1993;90(7):2618–2622. doi: 10.1073/pnas.90.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greco O., Dachs G. U. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J. Cell. Physiol. 2001;187(1):22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 93.Dachs G. U., Tupper J., Tozer G. M. From bench to bedside for gene-directed enzyme prodrug therapy of cancer. Anticancer Drugs. 2005;16(4):349–359. doi: 10.1097/00001813-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Aghi M., Hochberg F., Breakefield X. O. Prodrug activation enzymes in cancer gene therapy. J Gene Med. 2000;2(3):148–164. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 95.Denny W. A. Tumor-activated prodrugs—a new approach to cancer therapy. Cancer Invest. 2004;22(4):604–619. doi: 10.1081/cnv-200027148. [DOI] [PubMed] [Google Scholar]
- 96.Pulkkanen K. J., Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 2005;12(4):585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z. H., Samuels S., Gama Sosa M. A., Kolodny E. H. 5-Fluorocytosine-mediated apoptosis and DNA damage in glioma cells engineered to express cytosine deaminase and their enhancement with interferon. J Neurooncol. 1998;36(3):219–229. doi: 10.1023/a:1005883128175. [DOI] [PubMed] [Google Scholar]
- 98.Rainov N. G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene. Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 99.Rainov N. G., Ren H. Clinical trials with retrovirus mediated gene therapy–what have we learned? J. Neurooncol. 2003;65(3):227–236. doi: 10.1023/b:neon.0000003652.71665.f2. [DOI] [PubMed] [Google Scholar]
- 100.Immonen A., Vapalahti M., Tyynela K., et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther. 2004;10(5):967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Sandmair A. M., Loimas S., Puranen P., et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene. Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 102.Ettmayer P., Amidon G. L., Clement B., Testa B. Lessons learned from marketed and investigational prodrugs. J Med Chem. 2004;47(10):2393–2404. doi: 10.1021/jm0303812. [DOI] [PubMed] [Google Scholar]
- 103.Stella V. J. Prodrugs: Challenges and Rewards. Part 1. New York: AAPS Press/Springer; 2007. A Case for Prodrugs; pp. 3–33. [Google Scholar]
- 104.M. Gynther, K. Laine, J. Ropponen, et al. Large neutral amino acid transporter enables brain drug delivery via prodrug. J. Med. Chem. (2008), In press. [DOI] [PubMed]