Abstract
A subset of integrins function as cell surface receptors for the profibrotic cytokine transforming growth factor-β (TGF-β). TGF-β is expressed in an inactive or latent form, and activation of TGF-β is a major mechanism that regulates TGF-β function. Indeed, important TGF-β activation mechanisms involve several of the TGF-β binding integrins. Knockout mice suggest essential roles for integrin-mediated TGF-β activation in vessel and craniofacial morphogenesis during development and in immune homeostasis and the fibrotic wound healing response in the adult. Amplification of integrin-mediated TGF-β activation in fibrotic disorders and data from preclinical models suggest that integrins may therefore represent novel targets for antifibrotic therapies.
The multifunctional cytokine transforming growth factor-β (TGF-β) plays major roles in the biology of immune, endothelial, epithelial, and mesenchymal cells during development and adult life in invertebrate and vertebrate species.1,2 In mammals, these functions are mediated by three isoforms, TGF-β1, 2, and 3, which are each widely expressed.3 All three isoforms interact with the same cell surface receptors (TGFBR2 and ALK5) and signal through the same intracellular signaling pathways, which involve either canonical (ie, SMADs) or noncanonical (ie, MAPK, JUN, PI3K, PP2A, Rho, PAR6) signaling effectors.4,5 The canonical TGF-β signaling pathway, whereby TGF-β signaling is propagated from the TGF-β receptor apparatus through phosphorylation of cytoplasmic SMAD-2/3, complex formation with SMAD-4, nuclear translocation of the SMAD-2/3/4 complex, and binding to SMAD response elements located in the promoter regions of many genes involved in the fibrogenic response, has been the most intensively studied.6 However, despite having similar signaling partners, each isoform serves individual biological functions, perhaps due to differences in binding affinity to TGF-β receptors, activation mechanism, signaling intensity or duration, or spatial and/or temporal distribution.7
Knockout and conditional deletion models of TGF-β isoforms, receptors, and signaling mediators, as well as function-blocking reagents targeting all TGF-β isoforms, have revealed essential roles for TGF-β in T-cell, cardiac, lung, vascular, and palate development.8,9,10,11,12,13,14,15 For instance, mice deficient in TGF-β1 either die in utero owing to defects in yolk sac vasculogenesis or are born and survive into adult life but develop severe multiorgan autoimmunity.12 Genetic deletion of TGF-β signaling mediators has shown an essential role for Smad2 in early patterning and mesodermal formation,16,17 and mice lacking Smad3 are viable and fertile, but exhibit limb malformations,18 immune dysregulation, colitis,19 colon carcinomas,20 and alveolar enlargement.21
In adult tissues, the TGF-β pathway is thought to regulate the dynamic interactions among immune, mesenchymal, and epithelial cells to maintain homeostasis in response to environmental stress.22 The normal homeostatic pathways mediated by TGF-β are perturbed in response in chronic repetitive injury. In cases of injury, TGF-β becomes a major profibrogenic cytokine, delaying epithelial wound healing by inhibiting epithelial proliferation and migration and promoting apoptosis and expanding the mesenchymal compartment by inducing fibroblast recruitment, fibroblast contractility, and extracellular matrix deposition.23 Indeed, intratracheal transfer of adenoviral recombinant TGF-β1 to the rodent lung dramatically increases fibroblast accumulation and expression of type I and type III collagen around airways and in the pulmonary interstitium,24,25 and neutralizing anti-TGF-β antibodies can block experimental bleomycin or radiation-induced pulmonary fibrosis.26,27 Increased activity of the TGF-β pathway has also been implicated in fibrotic lung disease, glomerulosclerosis, and restenosis of cardiac vessels.23,28,29,30 Most TGF-β-mediated pathological changes appear to be attributed to the TGF-β1 isoform.31
The complexity of TGF-β1 function in humans is illuminated by hereditary disorders with generalized or cell type-specific enhancement or deficiency in either TGF-β1 itself or its signaling effectors. Mutations that increase the activity of the TGF-β pathway lead to defects in bone metabolism (ie, Camurati-Engelmann disease) and in connective tissue (ie, Marfan syndrome), and in aortic aneurysms (ie, Loeys-Dietz syndrome), whereas mutations that lead to decreased activity of the TGF-β pathway correlate with cancer occurrence and prognosis.32 The role of TGF-β as a tumor suppressor in cancer is not straightforward, however, because TGF-β can also enhance tumor growth and metastasis, perhaps through its roles in immune suppression, cell invasion, epithelial-mesenchymal transition, or angiogenesis.19,33,34,35
Despite the multiple essential functions of TGF-β, a single dose or short-term administration of a pan-TGF-β neutralizing antibody is reportedly well tolerated at doses that inhibit organ fibrosis or experimental carcinoma cell growth and metastasis, with no reported side effects in adult mice and rats. This treatment has shown therapeutic efficacy in inhibiting experimental fibrosis.27,28,36,37,38,39,40 Because of these promising results, single-dose phase I/II clinical trials using neutralizing pan-TGF-β antibodies have been performed or are ongoing for metastatic renal cell carcinoma, melanoma, focal segmental glomerulosclerosis, and idiopathic pulmonary fibrosis (Genzyme Corporation, http://www.genzymeclinicalresearch.com, last accessed August 27, 2009). However, it is likely that long-term global inhibition of TGF-β will have undesirable side effects, because targeted deletion of TGF-β signaling in various cell types may lead to accelerated atherosclerosis, autoimmunity, or carcinoma development.9,12,41 Clearly, careful targeting of the TGF-β pathway to minimize systemic effects is a highly desirable goal.
TGF-β Is Ubiquitously Expressed as a Latent Complex
The function of TGF-β can be modulated in disease states through changes in expression of either TGF-β itself or of proteins that interact directly or indirectly with TGF-β. These expression changes may affect the activity or accessibility of TGF-β as well as the activity of the TGF-β receptor apparatus or of the downstream TGF-β signaling pathways. Increased TGF-β expression is seen in both fibrotic and neoplastic disorders, and this increase often correlates with clinical disease severity.31 However, increased expression of TGF-β itself is not sufficient to increase TGF-β function, because all three TGF-β isoforms are expressed in tissues as inactive latent precursors. These latent precursors are known as small latent complexes (SLCs) and are sequestered in the extracellular matrix (ECM) through covalent interactions to form multimeric complexes with latent TGF-β binding proteins and other ECM proteins, such as fibrillin and fibronectin (Figure 1).3
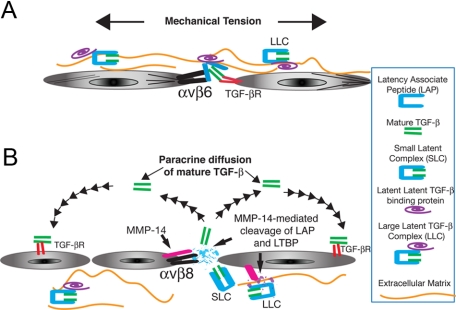
Figure 1.
Mechanisms TGF-β activation by αvβ6 and αvβ8. A: Integrin αvβ6 mediates TGF-β activation through a mechanism requiring mechanical tension, facilitated by covalent linkage of the SLC to the latent TGF-β binding protein (LTBP) and to ECM proteins. This tension opens LAP, rendering the mature TGF-β peptide accessible to the TGF-β receptor (TGF-βR) expressed by an adjacent epithelial cell. No active TGF-β is released from the cell surface, preventing αvβ6/TGF-β-dependent signaling to cells not in direct contact.50 B: The αvβ8 metalloproteolytic (MMP-14) mechanism of activation results in release of the mature TGF-β peptide from the SLC, which can diffuse as a paracrine signal to adjacent cell types.52 This mechanism of activation of TGF-β may be facilitated by the MMP-14-dependent cleavage of LTBP, causing release of the SLC from the large latent complex (LLC).71
The SLC is formed from the noncovalent association of the homodimeric amino-terminal propeptide, known as the latency-associated peptide (LAP), with the homodimeric C-terminal peptide, which is the bioactive TGF-β peptide known as mature or active TGF-β.3 The association of LAP with active TGF-β is conferred through association with the thrombospondin-1-binding domain, LSKL, of LAP, which binds in proximity to the ligand-binding domain of active TGF-β1 and prevents TGF-β from interacting with its receptors (Figure 1)3,42 Therefore, the activation of the SLC is a highly regulated process that serves as a gatekeeper of TGF-β function.
Activation of the TGF-β SLC may involve either proteolytic or nonproteolytic mechanisms that, respectively, result in either cleavage or conformational alteration of the LAP, both of which can allow the active TGF-β peptide to interact with cell surface TGF-β receptors, with varying consequences.3 Thus, proteolytic liberation of the active TGF-β peptide from LAP allows for paracrine interactions with other cells over distances constrained by the concentration, diffusion, and half-life of active TGF-β. In contrast, conformational activation involving interactions with cell surface proteins requires cells in direct contact with the cell presenting the active TGF-β complex.
In vitro, TGF-β conformational activation can result from treatment of latent TGF-β with heat, denaturants, reactive oxygen species,43 acidification,44 interaction with the extracellular-matrix molecule thrombospondin-1, or interactions with the integrins αvβ3, αvβ5, and αvβ645,46,47,48,49,50 Proteolytic activation occurs through interactions with matrix metalloprotein (MMP)-2 or -9, plasmin or the integrin αvβ850,51,52 However, in mouse models only thrombospondin (tsp1) or the integrins αvβ6 (itgb6) and αvβ8 (itgb8) have been conclusively shown to have a role in TGF-β activation.45,50,53
Multiple mechanisms of activation of TGF-β are likely to operate simultaneously in vivo. For example, tsp1/itgb6 double knockout mice display a more severe functional phenotype than mice with either knockout alone.54 In this regard, the integrins αvβ6 and αvβ8 are of particular interest because the combined loss of these integrins reproduces the combined phenotypes of TGF-β1- and TGF-β3-null mice.55 The integrin αvβ6 is mainly expressed in epithelial cell types, and the integrin αvβ8 is expressed in subsets of epithelial, neural, immune, and mesenchymal cell types.56,57,58,59
Mechanisms of Integrin-Mediated TGF-β Activation
Integrins are a large family of heterodimeric cell surface receptors, consisting of a single α and β subunit, which bind to ECM proteins. They act as mechanotransducers by relaying information from the ECM to the cell interior or vice versa and have diverse roles in mediating cell adhesion, shape, migration, proliferation, survival, differentiation, and invasion.60
The interactions of TGF-β1 and TGF-β3 with integrins include interactions that may or may not result in TGF-β activation. In general, high-affinity binding interactions (ie, αvβ6 and αvβ8) seem to lead to efficient activation of TGF-β, and lower-affinity interactions (αvβ1, αvβ3, and αvβ5) are either much less efficient or do not activate TGF-β at all.61 These data suggest that high-affinity ligand binding is more efficient in initiating the global conformational rearrangements that integrin extracellular domains are known to undergo, which in some way would enhance the activation of the latent TGF-β complex62,63,64 Much remains to be learned about how the dynamics of integrin conformation affects the interaction with and ability to activate TGF-β. Certainly, the determination of the crystal structure of the SLC of TGF-β alone and in association with integrins will be a significant advance.
Current mechanistic understanding of integrin-mediated activation of TGF-β relies on bioassays of TGF-β function using purified integrins, cofactors, or integrin-expressing cells cocultured with TGF-β reporter cells to determine what pathways are involved in integrin activation.49,52,61 With this approach, it has been determined that integrins require association with the cell membrane to support TGF-β activation, because purified integrins bind to the latent TGF-β complex but do not support TGF-β activation.52,61 Certain integrins can act as transmembrane tethers that link the cell cytoskeleton to matrix-bound latent TGF-β. Thus, agents or culture conditions that increase the contractile forces on integrin-bound TGF-β-ECM complexes can increase the efficiency of TGF-β activation.49,65 The role of mechanical tension in TGF-β activation has recently been the subject of an excellent review.66
The efficiency of αvβ6-mediated TGF-β activation is increased by the binding of the SLC to latent TGF-β binding protein-1.65 Furthermore, αvβ6 integrin-mediated TGF-β activation is increased by thrombin via PAR-1 and by lipophosphatidic acid via the LPA2 receptor, both of which use G-protein-coupled receptors (RhoA and Gaq) to increase actin stress fiber formation.67,68
The αvβ3 and αvβ5 integrins, which bind with low affinity to the SLC, have recently been shown to support activation of TGF-β in the presence of cell contraction-promoting agents in rat lung fibroblasts in vitro.49 However, this activation is dispensable in vivo during development because the combined β3/β5-deficient mice develop normally.69 In humans, expression of the β3 and β5 integrin subunits has been shown to be up-regulated in dermal fibroblasts of skin samples from scleroderma patients.46,48 Furthermore, increased expression of these integrins has been shown to increase autocrine activation of TGF-β and support conversion of dermal fibroblasts to a profibrotic phenotype.46,47,48 Whether αvβ3, αvβ5, or other integrins that bind with low affinity to TGF-β account for a component of TGF-β activation in pathological tissues remains to be determined using in vivo models.
As opposed to the mechanical tension model of integrin-mediated TGF-β activation, the integrin αvβ8 appears to have evolved to use a mechanism independent of matrix tension. Thus, the integrin β8 subunit can support TGF-β activation in the absence of its cytoplasmic domain, which notably is completely divergent in sequence from the highly conserved, actin cytoskeleton-linked cytoplasmic domains of the other αv-associating β subunits (β1, 3, 5, and 6).52 In addition, integrin αvβ8-mediated TGF-β activation is inhibited by metalloprotease inhibitors.52 Cell lines deficient in the transmembrane metalloprotease MT1-MMP (MMP-14) do not support αvβ8-mediated activation of TGF-β, and reconstitution of MT1-MMP can rescue αvβ8-mediated TGF-β activation.52 However, it is likely that additional metalloproteases are involved in αvβ8-mediated activation of TGF-β, because MT1-MMP-deficient mice do not recapitulate the phenotype of β8 deficiency.70
Indeed, there are six membrane-associated matrix metalloproteases expressed in mammals that are likely to share functional redundancy with MT1-MMP.71 In addition, αvβ8 does not efficiently support TGF-β activation in all expressing cell types (ie, airway epithelial cells) but requires stimulation with phorbol esters, suggesting a regulatory role for protein kinase in integrin αvβ8-mediated activation of TGF-β.57 Interestingly, protein kinase C has been shown to enhance the metalloproteolytic activity of MT1-MMP and its ability to proteolytically liberate the SLC from latent TGF-β binding protein-1.72 The increased liberation of SLC would be predicted to have the effect of increasing the efficiency of αvβ8-mediated activation of TGF-β, because αvβ8 does not require the incorporation of the SLC into the ECM.52 In contrast, liberation of the SLC would decrease the matrix tension exerted by binding to αvβ3, αvβ5, and αvβ6, which would decrease TGF-β activation mediated by these integrins (Figure 1).
Integrins as Activators of TGF-β during Organogenesis and in Development of the Immune System
Five integrins share the αv subunit (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8), and this subfamily recognizes ligands containing the Arg-Gly-Asp (RGD) tripeptide motif. The αv subfamily of integrins is of interest because individual αv integrins have distinctive spatiotemporal distribution and varied roles in diverse pathological processes such as angiogenesis, pulmonary fibrosis, acute lung injury, and neoplasia, making them potentially attractive therapeutic targets.60 The αv integrin subunit has been genetically deleted in mice and surprisingly almost exactly recapitulates the embryonic and perinatal lethal phenotype of β8 integrin subunit-deficient mice.73,74 Although the integrin β3, β5, or β6 subunit knockouts are not lethal and even the combined β3/β5-null mice survive and develop normally,69,75 integrin β1 subunit knockout mice are early embryonic lethal. This lethality is expected because the integrin β1 subunit forms heterodimers not only with αv but also with 11 other α-subunits (α1-11), many of which (eg, α5β1) are essential for development.76 However, it can be inferred that αvβ1 alone is not essential for development, because the αv- and β8-null phenotypes almost completely overlap.
The LAPs of the TGF-β1 and 3 SLCs contain an RGD motif that mediates binding to integrins, with high-affinity binding to integrins αvβ6 and αvβ8 and lower-affinity binding to integrins αvβ1, αvβ3, αvβ5, and α8β1.61,77 In contrast, there is no evidence to suggest that the TGF-β2 isoform, which lacks an RGD site, interacts with integrins. Mice engineered for a single amino acid mutation in the integrin-binding motif in the LAP of TGF-β1 (RGD to RGE), which disrupts integrin binding but incorporates normally into the ECM, recapitulate the partial embryonic lethality and the multiorgan autoimmune inflammatory phenotype of TGF-β1-null mice, suggesting a general role for integrins in regulating the function of TGF-β1.35 Moreover, when the TGF-β1 (RGE) mice were crossed to a TGF-β3 null background, they almost completely recapitulated the early yolk sac vascular defects and the perinatal hemorrhagic brain vascular phenotype of the αv- or β8 subunit-deficient mice.13 These experiments suggest that the αvβ8 integrin is a crucial regulator of the vasculogenic TGF-β1 and 3 function during development via interaction with the LAP RGD integrin-binding domain.
TGF-β3-null mice develop cleft palate, which is more severe when mice are crossed to TGF-β1 (RGE) mutant mice, suggesting a role for integrin-mediated modulation of TGF-β3 function in palate fusion.13 A small percentage of integrin αv- or β8-subunit null mice develop cleft palate as well, although cleft palate is not observed in integrin β3, β5, and β3/5 double knockout or β6 knock-out mice.69,73,74,75 However, work from Munger’s laboratory has shown that when activities of both integrin αvβ8 and αvβ6 subunits are reduced, cleft palate develops in 100% of mice surviving to birth.55 These experiments suggest that αvβ8- and αvβ6-mediated activation of TGF-β1 and 3 are functionally redundant during craniofacial development.
The TGF-β pathway has been widely implicated in vasculogenesis, as many TGF-β1-null mice die early in embryogenesis owing to failure of proper vascular development of the yolk sac.12 Mice with a D to E mutation in the RGD integrin-binding site of TGF-β1 develop a hemorrhagic brain vascular disorder with an underlying vascular “dysplasia” when crossed to TGF-β3-null mice, which phenocopies the brain vascular phenotype of mice individually deficient in either the αv or the β8 integrin subunit.13 αvβ8 integrin is expressed in the brain not by endothelial cells but by neurons, mature astrocytes, and radial glial cells, the later of which are thought to act as a scaffold for neurovascular development.59,74 Conditional deletion of αv or β8 in immature neuroglial cells, of which radial glia are a major subset, recapitulates much of the phenotype of complete αv or β8 integrin subunit deletion.73,78 A mechanism underlying this phenotype has been proposed using in vitro evidence whereby human astrocytes support αvβ8-mediated activation of TGF-β, which inhibits proliferation and promotes differentiation of adjacent brain microvascular endothelial cells.79
The specific contribution of integrin-mediated TGF-β activation to the functions of TGF-β in the development of the immune system has been explored in conditional and traditional integrin knockout models. Integrin β6-null mice survive and breed normally, yet display mild lung and skin inflammation, increased alveolar macrophage expression of MMP-12 with subsequent MMP-12-induced airspace enlargement, and alteration in surfactant homeostasis, all functions attributable to the role of αvβ6 in activation of TGF-β.75,80,81 However, the inflammatory phenotype of the β6-null mice is much milder than that seen in TGF-β1-null or TGF-β1 (RGE) mice, suggesting the involvement of another RGD-binding integrin or other molecule (ie, tsp1) in the regulation of TGF-β1 function.54
In addition to its role in TGF-β activation during organogenesis, the integrin αvβ8 probably also contributes to TGF-β activation in immune regulation. To study the role of the integrin αvβ8 in adult mice, the embryonic and perinatal lethality of the β8-null phenotype had to be overcome. The Sheppard laboratory has taken the approach of conditionally deleting αvβ8 integrin in T-cell and dendritic cell lineages that normally express integrin αvβ8. Dendritic cell deletion of β8 resulted in reduced dendritic TGF-β1 activation, which resulted in diffuse T-cell activation, reduced colonic regulatory T-cell development, autoimmunity, and colitis.53 This phenotype was not as severe as the multiorgan inflammatory phenotype seen in TGF-β1-null or TGF-β1 (RGE) mice.12,15 However, these differences may occur because activation of TGFβ by αvβ8 on other cells contributes to immune regulation, αvβ6 and αvβ8 integrins share functional redundancy in TGF-β activation in sites where they are co-expressed, or other RGD-binding integrins or other nonintegrin mechanisms are involved in TGF-β activation.82
To answer these questions, the Munger laboratory capitalized on the finding that integrin β8-null mice survived postnatally in an outbred mouse strain. Thus, the integrin β8-null mice in the ICR background survived an average of 2 months without histological evidence of organ inflammation.55 Integrin β8-null mice in the outbred ICR background, like their inbred counterparts, developed perinatal brain vasculogenic defects; however, the phenotype was less severe and allowed survival of many mice to adulthood. Surviving mice developed a progressive neurological syndrome accompanied by wasting.55 When the integrin β8-null mice were treated at birth with high doses of a neutralizing murine anti-β6 antibody, they developed a multiorgan inflammatory disease, associated with a defect in dendritic cell development that was similar to but more severe than that seen in the TGF-β1-null and TGF-β1 RGE mice.55 One possible explanation for this result is that the combined loss of αvβ6 and αvβ8-mediated activation of TGF-β1 and TGF-β3 results in a more severe inflammatory phenotype than loss of TGF-β1 alone. Of note, the contribution of both integrins to the immune phenotype may be dependent on the age of the mice, as only a mild inflammatory phenotype was seen when treatment of integrin β8-null mice with the neutralizing anti-β6 antibody was begun at postnatal day 3.55 This later result may indicate that there is a narrow window at birth during which integrin-mediated TGF-β activation is required for murine dendritic cell development.
Integrin-Mediated TGF-β Activation in Fibrogenic Reactions
Lung
The lung is divided into two functional components: the alveoli where gas exchange occurs and the conducting airways that deliver, humidify, and filter the air. Studies using human tissues have shown increased epithelial expression of the αvβ6 integrin in type I and II epithelial cells in interstitial fibrosis (ie, idiopathic pulmonary fibrosis and lung fibrosis due to scleroderma).83 There is no evidence as yet that expression of the integrin αvβ8 or that of other TGF-β-activating integrins is altered in interstitial fibrosis.84 Several excellent recent reviews have addressed integrin-mediated activation of TGF-β in the lung.85,86
In mouse models of lung injury using β6 integrin subunit-null mice, αvβ6 integrin-mediated TGF-β activation has been implicated in lung permeability changes (high tidal volume,68 bleomycin, LPS,87 and interleukin [IL]-1β-induced88), surfactant homeostasis,80and fibrosis (bleomycin- and radiation-induced27). Mechanisms involving alveolar/endothelial cell junction formation in lung permeability or epithelial mesenchymal transition in fibrosis have been proposed (Figure 2).88,89
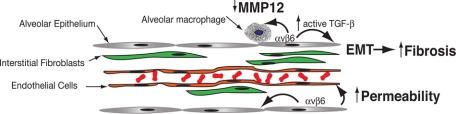
Figure 2.
Integrin αvβ6-mediated TGF-β activation in interstitial lung pathological conditions . Integrin αvβ6 is expressed by alveolar epithelial cells, where it mediates activation of TGF-β. Complete loss of β6 in knockout mice results in increased expression of MMP-12 by alveolar macrophages, which causes age-dependent airspace enlargement81 and also increases susceptibility to pulmonary edema by increasing alveolar and endothelial permeability.88 Increased expression of αvβ6 is associated with pulmonary inflammation and fibrotic lung disorders, and deficiency of β6 or antibody neutralization of αvβ6 in bleomycin or radiation-treated mice can decrease pulmonary fibrosis,27,50,83 an effect that may in part be due to a role for αvβ6-mediated TGF-β activation in epithelial mesenchymal transition (EMT).89
Less is know of the role of the integrin αvβ6 in the airway. In vitro, integrin αvβ6-mediated TGF-β activation may play a role in promoting squamous metaplasia of airway epithelial cells.56 The degree of squamous metaplasia is associated with the severity of airway obstruction and, in turn, squamous metaplasia is associated with increased secretion of the profibrogenic cytokine IL-1β.56 Thus, αvβ6 may play an indirect role in promoting airway remodeling through its role in squamous metaplasia and IL-1β expression.
There is more evidence for the role that αvβ8-mediated TGF-β activation plays in airway remodeling. Increased expression of the integrin αvβ8 has been found in airway fibroblasts in patients with chronic obstructive pulmonary disease, and its expression correlates with the severity of airway obstruction, suggesting that fibroblast αvβ8-mediated activation of TGF-β leads to airway fibrosis.56 In addition, β8 is normally highly expressed in the human airway epithelium and its expression is lost in lung adenocarcinomas, suggesting that αvβ8-mediated activation of TGF-β inhibits airway epithelial proliferation and therefore delays wound healing.52,84
The in vivo role of αvβ8 in the airways has been difficult to model because basal cells, a major cell type in which αvβ8 is expressed in the human airways, are not present in mouse bronchioles but rather are mostly confined to the mouse trachea.90 Thus far, studies of airway αvβ8-mediated TGF-β activation have been confined to complex in vitro models of the human airway. These models have mainly used intact fragments of human bronchi or cocultures of airway epithelial cells and fibroblasts and have verified that αvβ8 integrin-mediated activation of TGF-β plays a role in inhibiting airway epithelial migration and proliferation. In addition, αvβ8 integrin-mediated activation of TGF-β has been shown to promote the transition of airway fibroblasts to a profibrogenic phenotype characterized by increased smooth muscle actin and collagen type I and decreased human growth factor expression.56,57,91,92
The increased expression of αvβ8 integrin found in the airway fibroblasts of patients with chronic obstructive pulmonary disease may be driven by the inflammatory response seen in smokers, because β8 is a transcriptional target of IL-1β56 and IL-1β is induced by cigarette smoke.56 Transgenic or adenoviral delivered IL-1β to the airways of mice causes airway and interstitial fibrosis, which is associated with marked increases in TGF-β expression.25 Furthermore, the absence of Smad3 abrogates adenoviral IL-1β-induced fibrosis, indicating that IL-1β increases TGF-β function.93
It is clear from the above that the αvβ6 and αvβ8 integrins play nonredundant but highly interactive roles in pathological airway remodeling based on their patterns of expression and respective functions in autocrine and paracrine TGF-β signaling. The pathological reciprocal interactions that take place between the airway epithelial cells and fibroblasts in the so-called epithelial-mesenchymal trophic unit during airway remodeling involve increased αvβ6-mediated activation of TGF-β, which leads to increased squamous metaplasia accompanied by increased expression of IL-1β. Increased levels of IL-1β then drive increased αvβ8 expression on adjacent fibroblasts, leading to increased TGF-β activation and autocrine suppression of human growth factor secretion (Figure 3).56,57
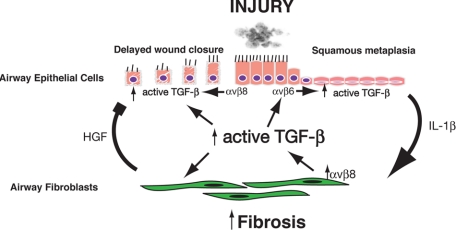
Figure 3.
Possible roles for αvβ6 and αvβ8-mediated activation of TGF-β in airway remodeling. Airway epithelium injury due to noxious stimuli causes increased αvβ6-mediated TGF-β activation. Increased αvβ6-mediated activation of TGF-β contributes to squamous metaplasia, a TGF-β driven process.56 Squamous metaplastic epithelial cells secrete increased levels of IL-1β, which acts as a paracrine factor on adjacent airway fibroblasts.56 Airway fibroblasts respond to IL-1β by up-regulating β8 expression and αvβ8-mediated TGF-β activation.56 Increased TGF-β activation by airway fibroblasts causes autocrine effects on the fibrogenic fibroblast phenotype by up-regulating type I collagen and α-smooth muscle actin (α-SMA) and paracrine effects on adjacent airway epithelium by decreasing fibroblast secretion of human growth factor, which inhibits epithelial proliferation.57 Airway epithelial wounding also causes increased αvβ8-mediated activation of TGF-β, which inhibits airway epithelial migration.92 Finally, increased levels of active TGF-β contribute to the increased expression of β6 by airway epithelial cells because the β6 integrin is a TGF-β-responsive gene, thus forming a self-amplifying loop of TGF-β activation.94
In airway remodeling, integrin-mediated activation of TGF-β may form a self-amplifying loop, because β6 is a TGF-β-responsive gene and β6 expression is increased in areas of inflammation.58,94 Indeed, there is evidence that during in vitro squamous metaplasia, airway epithelial β6 expression increases as does αvβ6-mediated TGF-β activation, an effect that can be blocked with neutralizing anti-β6 antibodies.56 Thus, squamous metaplasia ultimately ties together IL-1β and αvβ6- and αvβ8-mediated activation of TGF-β in pathological airway remodeling (Figure 3).
Skin
The expression of integrin αvβ6 is increased in the migrating epidermal cells adjacent to wounds.58 TGF-β is thought to play a crucial role in regulating the epithelial-mesenchymal interactions that guide a proper wound healing response.95 Indeed, either overexpression or deficiency of αvβ6 leads to skin pathological changes.75,96 Thus, transgenic overexpression of the integrin β6 subunit in the epidermis results in spontaneous skin ulcers with hypertrophic scars, and αvβ6 deficiency in aged mice leads to delays in wound healing.96,97
There have been several reports that dermal fibroblasts in biopsy samples from patients with scleroderma express increased αvβ3 and αvβ5 integrins, and both of these integrins have been shown to mediate TGF-β activation in primary cultures isolated from scleroderma fibroblasts.47,48
Kidney
The integrin αvβ6 is expressed at low levels in the normal kidney, but its expression increases in the renal tubular epithelium in a variety of renal diseases including membranous and lupus glomerulonephritis, diabetic and IgA nephropathy, and Goodpasture’s and Alport syndrome.28 Mice deficient in the collagen 4A3 isoform (COL4A3) develop a progressive fibrosing glomerulonephritis similar to Alport syndrome in humans.28 COL4A3-deficient mice that are also deficient in β6 or treated with anti-β6 integrin-blocking antibodies (or a soluble TGF-β type 2 receptor) are protected from renal fibrosis.28
Liver
The expression of integrin αvβ6 is increased in humans in acute, but not chronic, biliary injury, where it is localized to cholangiocytes.98 In rodent models, increased expression of αvβ6 can be markedly induced by acute bile duct obstruction and a specific αvβ6 small molecule antagonist (EMD527040) can reduce periductal collagen deposition by ∼50%.99
Integrins as Therapeutic Targets
Increased expression of the TGF-β-activating integrins αvβ3, αvβ5, αvβ6, and αvβ8 have been described in scleroderma, fibrotic kidney disease, idiopathic pulmonary fibrosis, and chronic obstructive lung disease.28,46,47,48,56,83 Data from preclinical models of lung,27,83 kidney,28,100 and liver fibrosis98,99 suggest that inhibition of integrin-mediated TGF-β activation (specifically αvβ6) may be useful to treat a range of fibrotic disorders in humans. Most of these preclinical data have been obtained using a monoclonal antibody that was developed by immunizing β6 subunit knockout mice with human αvβ6, which resulted in several high-affinity specific αvβ6 monoclonal antibodies, one of which, clone 6.3G9, was broadly cross-species reactive and used in preclinical models.101 Furthermore, partial blockade of αvβ6-mediated TGF-β activation with submaximal doses of clone 6.3G9 bypasses the autoinflammatory and emphysema phenotype seen in itgb6 knockout mice while maintaining antifibrotic efficacy.83 Clone 6.3G9 has been humanized and is licensed by Stromedix (Cambridge, MA) as STX-100.
Small molecule inhibitors to αvβ6 have also been developed by Merck KGaA (Darmstadt, Germany) and one, EMD527040, is a peptidomimetic compound that specifically inhibits αvβ6 function in vitro and in vivo.102 Small molecules or therapeutic antibodies have relative merits based on bioavailability, route of administration, half-life, specificity, and toxicity, and there are currently Food and Drug Administration-approved examples of each being used as anti-integrin αIIbβIIIa antiplatelet agents (tirofiban103 and abciximab,104 respectively).
Other therapeutic monoclonal anti-integrin antibodies have been developed or are in various phases of preclinical testing. For instance, an antibody to α4β1 (natalizumab) has shown benefit in reducing multiple sclerosis disease activity105,106 but has had rare serious side effects (ie, progressive multifocal leukoencephalopathy) due to immunosuppression.106 Thus, the use of anti-integrin therapies to target TGF-β-driven pathological conditions is likely to continue to expand and evolve but their potential efficacy will have to be closely measured against off-target effects.
Summary
Teleologically it makes sense that integrins mediate activation of TGF-β because integrins are cell-associated sensors of the extracellular environment and TGF-β1 and TGF-β3 are secreted molecules that serve as central regulators of cell movement, proliferation, and differentiation. Activation of TGF-β by individual integrins serves highly specialized functions to meet homeostatic needs in specific cellular microenvironments. It appears that amplification of integrin-mediated TGF-β might be a general theme for a number of fibrotic disorders. Although the mechanism of this amplification remains to be determined, it is possible that genetic variation in TGF-β-activating integrins or their cofactors may play a role. Together, these features make integrin-mediated TGF-β activation a very interesting therapeutic target in specific fibrosing disorders.
Acknowledgments
I thank Dean Sheppard for his careful reading and suggestions for this manuscript and Stephanie Cambier for her editorial assistance.
Footnotes
Address reprint requests to Stephen L. Nishimura, M.D., Department of Anatomic Pathology, University of California San Francisco, San Francisco, CA 94143. E-mail: stephen.nishimura@ucsf.edu.
Supported by the National Institutes of Health (grants HL63993 and NS04415) and by the Sandler Foundation.
References
- Goumans MJ, Liu Z, ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-β activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Massagué J, Gomis RR. The logic of TGFβ signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGF-β-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim SJ, Fu XY, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor-β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGF-β1 and TGF-β3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor-β-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-β-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor-β in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Transforming growth facto-β: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon NJ, Ward RW, McGrew G, Last JA. TGF-β1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 2003;58:772–777. doi: 10.1136/thorax.58.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, 3rd, Sheppard D, Gardner HA, Violette SM. αvβ6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-β signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Wang W, Koka V, Lan HY. Transforming growth factor-β and Smad signalling in kidney diseases. Nephrology (Carlton) 2005;10:48–56. doi: 10.1111/j.1440-1797.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- Gordon KJ, Blobe GC. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Levy L, Hill CS. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EY, Moses HL. Transforming growth factor-β1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol. 1990;111:731–741. doi: 10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Moses HL. Transforming growth factor-β: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–9111. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-β antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006;65:876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Li X, Jha S, Wang W, Karetskaya L, Pratt B, Ledbetter S. Therapeutic role of TGF-β-neutralizing antibody in mouse cyclosporin A nephropathy: morphologic improvement associated with functional preservation. J Am Soc Nephrol. 2003;14:377–388. doi: 10.1097/01.asn.0000042168.43665.9b. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol. 2002;22:975–982. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-β. J Biol Chem. 2004;279:38032–38039. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-β1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- Jullien P, Berg TM, Lawrence DA. Acidic cellular environments: activation of latent TGF-β and sensitization of cellular responses to TGF-β and EGF. Int J Cancer. 1989;43:886–891. doi: 10.1002/ijc.2910430525. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of αvβ5 integrin in the establishment of autocrine TGF-β signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126:1761–1769. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of αvβ5 integrin-mediated activation of latent transforming growth factor-β1 in autocrine transforming growth factor-β signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52:2897–2905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, Sheppard D, Lawler J. Characterization of integrin β6 and thrombospondin-1 double-null mice. J Cell Mol Med. 2005;9:421–437. doi: 10.1111/j.1582-4934.2005.tb00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122:227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J, Cambier S, Morris A, Finkbeiner W, Nishimura SL. Integrin mediated TGF-β activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol. 2006;169:405–415. doi: 10.2353/ajpath.2006.060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin β6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- Nishimura SL, Sheppard D, Pytela R. Integrin αvβ8. Interaction with vitronectin and functional divergence of the β8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-β are ligands for the integrin αvβ1. Mol Biol Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αvβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin αvβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor-β1—an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Jenkins G. The role of proteases in transforming growth factor-β activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances αvβ6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Bauvois B. Transmembrane proteases in focus: diversity and redundancy? J Leukoc Biol. 2001;70:11–17. [PubMed] [Google Scholar]
- Tatti O, Vehvilainen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-β1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res. 2008;314:2501–2514. doi: 10.1016/j.yexcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin α8β1 mediates adhesion to LAP-TGF-β1. J Cell Sci. 2002;115:4641–4648. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires β8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koth LL, Alex B, Hawgood S, Nead MA, Sheppard D, Erle DJ, Morris DG. Integrin β6 mediates phospholipid and collectin homeostasis by activation of latent TGF-β1. Am J Respir Cell Mol Biol. 2007;37:651–659. doi: 10.1165/rcmb.2006-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin αvβ6-mediated TGF-β activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- Derks RA, Jankowska-Gan E, Xu Q, Burlingham WJ. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J Immunol. 2007;179:3443–3451. doi: 10.4049/jimmunol.179.6.3443. [DOI] [PubMed] [Google Scholar]
- Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O'Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- Cambier S, Mu DZ, O'Connell D, Boylen K, Travis W, Liu WH, Broaddus VC, Nishimura SL. A role for the integrin αvβ8 in the negative regulation of epithelial cell growth. Cancer Res. 2000;60:7084–7093. [PubMed] [Google Scholar]
- Aluwihare P, Munger JS. What the lung has taught us about latent TGF-beta activation. Am J Respir Cell Mol Biol. 2008;39:499–502. doi: 10.1165/rcmb.2008-0003ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. The role of integrins in pulmonary fibrosis. Eur Respir Rev. 2008;17:157–162. [Google Scholar]
- Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-β is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, Matthay MA, Pittet JF. Interleukin-1β causes acute lung injury via αvβ5 and αvβ6 integrin-dependent mechanisms. Circ Res. 2008;102:804–812. doi: 10.1161/CIRCRESAHA.107.161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper CG, Hyde DM. The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther. 2008;21:755–766. doi: 10.1016/j.pupt.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol. 2003;163:533–542. doi: 10.1016/s0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurohr C, Nishimura SL, Sheppard D. Activation of transforming growth factor-β by the integrin αvβ8 delays epithelial wound closure. Am J Respir Cell Mol Biol. 2006;35:252–259. doi: 10.1165/rcmb.2006-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-β and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175:5390–5395. doi: 10.4049/jimmunol.175.8.5390. [DOI] [PubMed] [Google Scholar]
- Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Respir Cell Mol Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Shull JH, Smith JM, Ward JM, Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983;219:1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Häkkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava H. Increased expression of β6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlDahlawi S, Eslami A, Hakkinen L, Larjava HS. The αvβ6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen. 2006;14:289–297. doi: 10.1111/j.1743-6109.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of αvβ6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin αvβ6 on cholangiocytes blocks transforming growth factor-β activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevillian P, Paul H, Millar E, Hibberd A, Agrez MV. αvβ6 integrin expression in diseased and transplanted kidneys. Kidney Int. 2004;66:1423–1433. doi: 10.1111/j.1523-1755.2004.00904.x. [DOI] [PubMed] [Google Scholar]
- Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, Sheppard D, Violette SM. Function-blocking integrin αvβ6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- Goodman SL, Holzemann G, Sulyok GA, Kessler H. Nanomolar small molecule inhibitors for αvβ6, αvβ5, and αvβ3 integrins. J Med Chem. 2002;45:1045–1051. doi: 10.1021/jm0102598. [DOI] [PubMed] [Google Scholar]
- Hartman GD, Egbertson MS, Halczenko W, Laswell WL, Duggan ME, Smith RL, Naylor AM, Manno PD, Lynch RJ, Zhang G, Change CT-C, Gould RJ. Non-peptide fibrinogen receptor antagonists. 1. Discovery and design of exosite inhibitors. J Med Chem. 1992;35:4640–4642. doi: 10.1021/jm00102a020. [DOI] [PubMed] [Google Scholar]
- Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med. 1994;330:956–961. doi: 10.1056/NEJM199404073301402. [DOI] [PubMed] [Google Scholar]
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, Sandrock AW. Natalizumab plus interferon β-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]