Abstract
Bioactive sphingosine 1-phosphate (S1P) and S1P receptors (S1PRs) have been implicated in many critical cellular events, including inflammation, cancer, and angiogenesis. However, the role of S1P/S1PR signaling in the pathogenesis of liver fibrosis has not been well documented. In this study, we found that S1P levels and S1P3 receptor expression in liver tissue were markedly up-regulated in a mouse model of cholestasis-induced liver fibrosis. In addition, the S1P3 receptor was also expressed in green fluorescent protein transgenic bone marrow (BM)-derived cells found in the damaged liver of transplanted chimeric mice that underwent bile duct ligation. Silencing of S1P3 expression significantly inhibited S1P-induced BM cell migration in vitro. Furthermore, a selective S1P3 receptor antagonist, suramin, markedly reduced the number of BM-derived cells during cholestasis. Interestingly, suramin administration clearly ameliorated bile duct ligation-induced hepatic fibrosis, as demonstrated by attenuated deposition of collagen type I and III, reduced smooth muscle α-actin expression, and decreased total hydroxyproline content. In conclusion, our data suggest that S1P/S1P3 signaling plays an important role in cholestasis-induced liver fibrosis through mediating the homing of BM cells. Modulation of S1PR activity may therefore represent a new antifibrotic strategy.
Fibrosis is a wound-healing response that engages a range of cell types and mediators to encapsulate injury from multiple causes ranging from viral infection, alcohol abuse, and drug or chemical toxicity to autoimmune and metabolic disorders. Cirrhosis is the advanced stage of fibrosis, typically characterized by nodule formation and organ contraction.1 It is worth noting that cholestatic liver disorders are a serious clinical problem and often require liver transplantation due to cirrhosis.2 The most common cholestatic liver diseases affecting adults are primary biliary cirrhosis and primary sclerosing cholangitis, which may present diagnostic and therapeutic difficulties.3 Generally, the pathogenesis of fibrosis/cirrhosis is characterized by the excessive deposition and histological redistribution of extracellular matrix components in the tissue as consequences of chronic liver injury. Cytokines regulating the scarring responses to injury modulate hepatic fibrogenesis in vivo and in vitro. Strategies with the aim of disrupting the cytokine synthesis and/or signaling pathways markedly decreased hepatic fibrosis in experimental models.4
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid generated by sphingomyelin metabolism that acts almost ubiquitously, influencing many key biological parameters including cell proliferation, differentiation, motility, and survival and the regulation of immune function.5,6 S1P is generated by phosphorylation of sphingosine catalyzed by sphingosine kinases (SphKs). Two isoforms of mammalian SphK have been cloned and characterized: SphK types 1 and 2 (SphK1 and SphK2).7 SphK1 is slightly more efficient than SphK2 in phosphorylating their primary intracellular substrate, sphingosine, whereas SphK2 is significantly more efficient toward unnatural substrates such as the immunomodulatory drug FTY720.8 The concentration of S1P in cells is normally low and is regulated tightly by the equilibrium between its formation, catalyzed by SphK, and its degradation, catalyzed by S1P lyase and S1P phosphatase. SphK is stimulated by numerous external stimuli resulting in increased intracellular S1P concentration and increased release from certain cell types.7
Of note, most of the characterized actions of S1P are mediated through a family of five G protein-coupled receptors named S1P1, S1P2, S1P3, S1P4, and S1P5, originally referred to as endothelial differentiation gene-1, 5, 3, 6, and 8, respectively.7 The different activities triggered by S1P depend on the pattern of expression of S1P receptors (S1PRs) in each cell type. Among the S1PRs, S1P1-3 are widely expressed in various tissues, whereas the expression of S1P4 is confined to lymphoid and hematopoietic tissue and that of S1P5 to the central nervous system.9 From this pattern of expression, it is possible to understand the pathophysiological role of each S1PR. Indeed, the role of S1P and its receptors has been reported in various disease-related models including lung fibrosis10,11,12; in addition, our previous study unraveled a major role of S1P2 receptor in the wound healing response to acute liver injury induced by carbon tetrachloride.13 However, the exact functional role of S1P as well as the therapeutic potential of strategies aimed at S1P/S1PRs signalings in liver fibrogenesis is still unknown.
It is well established that myofibroblasts are the principal effector cells that are responsible for the overproduction of extracellular matrix in fibrotic liver.1,4 Myofibroblasts can be produced by the activation of hepatic stellate cells, portal fibroblasts, and fibrocytes, as well as cells derived from epithelial mesenchymal transition.1,4,14 Recently, several lines of evidence have indicated that a significant proportion of myofibroblasts are of bone marrow (BM) origin in liver fibrosis.15,16,17,18,19 However, much less is known about the mechanism that governs the mobilization of BM cells after chronic liver injury. Importantly, several reports have demonstrated that S1P strongly stimulated BM stem cells migration in vitro.20,21,22 Along this line, we hypothesized that S1P is involved in liver fibrogenesis through mediation of the migration of BM cells to the damaged liver. In an effort to establish what factors govern BM cells engraftment after chronic liver injury, we have sought to investigate the pathogenesis of liver fibrosis in experimental animals induced by bile duct ligation (BDL). This pattern is rather unique, being sustained by compensatory proliferation of biliary epithelial cells. Chronic obstruction of the bile duct causes massive activation of periductal myofibroblasts and ultimately results in biliary fibrosis/cirrhosis.23,24 In this study, we evaluated the importance of S1P/S1P3 signaling in liver fibrogenesis through mediation of the homing of BM cells during cholestasis, which may represent a novel antifibrotic target.
Materials and Methods
Isolation of BM Cells
Enhanced green fluorescent protein (EGFP)-transgenic ICR mice (3 weeks old) were sacrificed by cervical dislocation at the time of BM harvest. EGFP-positive BM cells were extracted from the tibias and femurs by flushing with culture medium (Invitrogen, Grand Island, NY) using a 25-gauge needle. The cells were then passed through a 70-mm nylon mesh and were washed three times with PBS containing 2% fetal bovine serum (Biochrom, Berlin, Germany). All animal work was performed under the guidelines of the Ethics Committee of Capital Medical University.
BM Transplantation
ICR mice aged 6 weeks received lethal irradiation (8 Gy) and immediately received transplantation by a tail vein injection of 1.5 × 107 whole BM cells, which were obtained from 3-week-old EGFP transgenic mice. Four weeks later, mice were subjected to the BDL-induced liver fibrosis as follows. After 2 weeks, mice were sacrificed, and tissue was harvested.
To further demonstrate that homing of BM cells mediated by S1P is via the S1P3 receptor, we performed another series of experiments. After lethal irradiation and transplantation of EGFP-positive BM cells, mice underwent BDL in the absence or presence of suramin (Sigma-Aldrich, St. Louis, MO) administration. Suramin (20 mg/kg body weight, twice per week) in saline was injected i.p. All mice received 2 weeks of suramin or saline alone treatment, and liver tissue was harvested at next day after last injection.
Mouse Model of Cholestasis-Induced Liver Fibrosis
ICR mice were allocated randomly to two experimental groups, and either BDL or sham operations were performed as described previously by Uchinami et al25 In brief, mice were anesthetized to receive a midline laparotomy, and then the common bile duct was exposed and ligated three times. Two ligatures were placed in the proximal portion of the bile duct, and one ligature was located in the distal portion of the bile duct. The bile duct was then cut between the ligatures. The abdomen was closed in layers, and mice were allowed to recover on a heat pad. Two weeks later, mice were anesthetized to collect blood and liver samples.
Immunofluorescence and Immunohistochemistry
Liver samples were fixed in 4% paraformaldehyde and embedded in Tissue Tek OCT compound (Electron Microscopy Sciences, Hatfield, PA); 5-μm frozen sections were used for immunofluorescence. They were blocked with 3% bovine serum albumin for 1 hour and then incubated with anti-S1P3 receptor polyclonal antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) and Cy3-conjugated AffiniPure goat anti-rabbit IgG antibody (1:1000, Jackson ImmunoResearch Laboratories Inc., West Grove, PA) as a secondary antibody. The sections were covered with Vectashield mounting medium containing 4,6-diamidino-2-phenylindole and observed under a confocal microscope (LSM510; Carl Zeiss MicroImaging GmbH, Jena, Germany).
Immunohistochemical analysis was performed using anti-smooth muscle α-actin (α-SMA) (1:80, mouse monoclonal clone 1A4; Sigma-Aldrich) with a Mouse on Mouse kit (Vector Laboratories, Burlingame, CA). Detection of the primary antibody was performed by using a biotinylated antibody and DAB Peroxidase kit (Vector Laboratories). For negative controls, sections were processed as described earlier, except that incubation with the primary antibody was omitted.
Measurement of S1P by High-Performance Liquid Chromatography Analysis
S1P concentrations in serum, liver tissue, and BM were determined as described previously by Min et al26 with minor modifications. Tissue samples were homogenized in 25 mmol/L HCl/1 mmol/L NaCl. BM was obtained by flushing the femur and tibia of donor mice with 25 mmol/L HCl/1 mol/L NaCl. The samples were ultrasonicated for 10 minutes in ice-cold water. Serum was used in measurements without any pretreatment. After transferring a 20-μl aliquot of the sample to a fresh tube for protein assay, 250 μl of methanol containing 0.6 μl of concentrated HCl were added and the samples were ultrasonicated for 10 minutes in ice-cold water. Lipids were extracted by addition of 500 μl of CHCl3/1 mol/L NaCl (1:1, v/v) and 25 μl of 3 N NaOH. The basic aqueous phase containing S1P was transferred to a siliconized glass tube. The residual S1P in the organic phase was extracted twice with 250 μl of methanol/1 mol/L NaCl (1:1, v/v) plus 15 μl of 3 N NaOH, and all of the aqueous fractions were then combined. The aqueous phase containing S1P was mixed with 150 μl of buffer (200 mmol/L Tris-HCl and 75 mmol/L MgCl2 in 2 mol/L glycine buffer, pH 9.0) and 50 units of alkaline phosphatase (bovine intestinal mucosa, type VII-T; Sigma-Aldrich). The mixture was incubated at 37°C for 45 minutes. To terminate the reaction, 15 μl of concentrated HCl was added and the dephosphorylated sphingosine was extracted twice with 500 μl of CHCl3. The pooled CHCl3 phase was washed three times with alkaline water and then dried under nitrogen in siliconized glass tubes. The dried lipid residue was resuspended in 200 μl of ethanol at 67°C for 30 minutes. The dissolved lipid solution was incubated with 30 μl of o-phthalaldehyde (Sigma Chemical) reagent for 1 hour at room temperature. Sample analysis was performed by using Agilent 1100 high-performance liquid chromatography equipment. The isocratic mobile phase was acetonitrile-deionized distilled water (90:10, v/v), and the flow rate was 1 ml/min. The derivatives were detected using a spectrofluorometer, with an excitation wavelength of 340 nm and an emission wavelength of 455 nm.
Migration Assays
Cell migration was determined in Boyden chambers as described previously.21 In brief, serum-starved BM cells (4 × 104 cells) were seeded to the upper chamber. Cell migration was allowed to proceed for 6 hours at 37°C in 5% CO2 by adding S1P (1 nmol/L) to the lower chamber. Cells migrating to the lower surface of the filter were stained and quantified by cell counting. The migration index was defined as the number of cells in the lower chamber under the tested condition divided by the number of cells under control.
RNA Interference
The siRNA sequence targeting mouse S1P3 specifically was synthesized (L-040957-00; Dharmacon, Lafayette, CO). Forty to 50% confluent BM cells were prepared in six-well dishes. Transient transfection of siRNA (40 nmol/L) was performed by using Lipofectamine RNAiMAX as recommended by the manufacturer. Control cells were treated with 40 nmol/L RNAi negative control duplexes (scramble siRNA). After 48 hours cells were used to perform the migration assay.
Real-Time RT-PCR
Total RNA was extracted from liver frozen specimens using an RNeasy kit (Qiagen, Hilden, Germany). Real-time RT-PCR was performed in an ABI Prism 7300 sequence detecting system (Applied Biosystems, Foster City, CA), as described previously.13 Primers (MWG Biotech, Ebersberg, Germany) used for real-time RT-PCR were as follows: mouse S1P1 receptor: sense, 5′-ACTTTGCGAGTGAGCTG-3′ and antisense, 5′-AGTGAGCCTTCAGTTACAGC-3′; S1P2 receptor: sense, 5′-TTCTGGAGGGTAACACAGTGGT-3′ and antisense, 5′-ACACCCTTTGTATCAAGTGGCA-3′; S1P3 receptor: sense, 5′-TGGTGTGCGGCTGTCTAGTCAA-3′ and antisense, 5′-CACAGCAAGCAGACCTCCAGA-3′; and 18S rRNA: sense, 5′-GTAACCCGTTGAACCCCATT-3′ and antisense, 5′-CCATCCAATCGGTAGTAGCG-3′. Probes (Applied Biosystems) used for real time RT-PCR were as follows: procollagen α1(I): MA00801666; procollagen α1(III): MA00802331; α-SMA: MA00725412; SphK1: MA00448841; S1P phosphatase: MA00473016; and S1P lyase: MA00486079.
Quantitative Analysis of Liver Fibrosis and Necrosis
Liver tissues were fixed in PBS containing 4% paraformaldehyde for 24 hours and embedded in paraffin. Sections (5 μm) were stained with Sirius red for collagen visualization and H&E for analysis of necrotic area. The fibrotic area and necrotic area were assessed by computer-assisted image analysis with MetaMorph software (Universal Imaging Corporation, Downingtown, PA). The mean value of 15 randomly selected areas per sample was used as the expressed percentage of fibrosis or necrosis area.
Hydroxyproline Content Assay
Hydroxyproline content of the liver was measured as described previously27 with minor modifications. In brief, three small fragments of each liver were pooled, homogenized in distilled water, and lyophilized, and 20 mg of the freeze-dried sample was hydrolyzed at 95°C for 20 minutes. After hydrolysis, the samples were neutralized at pH 6.0 to 6.8. The hydrolysates were then treated with activated charcoal. After centrifugation at 1000 × g for 10 minutes, aliquots of the hydrolysates were used to measure hydroxyproline content spectrophotometrically by reaction with Ehrlich’s reagent. Absorbance was measured at 560 nm. The hydroxyproline content of the liver was expressed as micrograms per gram of dry weight.
Serum Biochemistry
Serum total bilirubin and serum aminotransferase were measured using commercial assay kits (Stanbio, Boerne, TX).
Analysis of Bile Flow
ICR mice aged 6 weeks were given one-time suramin or saline alone administration (20 mg/kg b.wt. i.p.). After 24 hours, mice were anesthetized to receive midline laparotomy, and the common bile duct and duodenum were exposed. The bile duct was then cut in the proximal portion of the duodenal papilla. Bile flow was collected by drainage tube for 30 minutes.
Western Blot Analysis
Western blot analysis of S1P3 receptor was performed with 50 μg of protein extract, obtained as described previously28 using rabbit polyclonal antibody to S1P3 (1:500; Santa Cruz Biotechnology) and peroxidase-conjugated goat anti-rabbit IgG antibody (1:10,000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) as a secondary antibody. Protein expression was visualized by using an enhanced chemiluminescence (ECL Plus) assay kit according to the manufacturer’s instructions (Amersham Biosciences, Arlington Heights, IL). Signals were normalized to the glyceraldehyde-3-phosphate dehydrogenase signals (rabbit monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase antibody, 1:1000; Sigma).
Statistics
Results are expressed as mean ± SEM. Statistical significance was assessed by using Student’s t-test or one-way analysis of variance for analysis of variance when appropriate. A value of P < 0.05 was considered to be statistically significant.
Results
Cholestasis-Induced Liver Injury Promotes Activation of the Hepatic S1P System
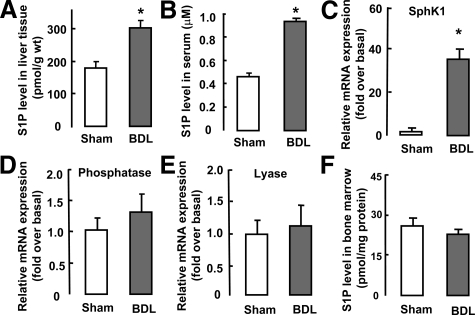
We first examined S1P levels in cholestasis-induced liver fibrosis. High-performance liquid chromatography analysis of liver extracts from BDL mice revealed that S1P levels rose markedly compared with those in sham-operated liver (Figure 1A). Likewise we found that the serum S1P concentration in BDL mice was increased by approximate twofold higher than that for sham-operated mice (Figure 1B).
Figure 1.
Activation of the S1P system after BDL-induced liver injury. Mice were subjected to sham or BDL operation and were sacrificed after 2 weeks (n = 10 per group). S1P levels in liver tissue (A), serum (B), and BM (F) from sham- and BDL-operated mice, were measured by high-performance liquid chromatography. Relative hepatic levels of SphK1 (C), S1P phosphatase (D), and S1P lyase mRNA (E) were measured by real-time RT-PCR. Data are presented as the mean ± SEM. *P < 0.05, compared with sham-operated mice.
We next examined whether the enzymes involved in determining S1P abundance (SphK, S1P phosphatase, and S1P lyase) in liver tissue were affected by BDL. As expected, 2 weeks after BDL, expression of SphK1 mRNA was significantly increased compared with that in sham-operated mice (Figure 1C), although expression of SphK2 mRNA was unchanged (data not shown). Moreover, expression of S1P phosphatase and lyase mRNAs was unchanged after BDL (Figure 1, D and E). These findings indicated that the up-regulation of SphK1 expression resulted in increased S1P concentration in the liver during cholestasis.
In addition, we tested S1P levels in BM and found that there was no difference in S1P concentration of BM between the BDL-operated and sham-operated mice (Figure 1F). Likewise, these enzymes (SphK, S1P phosphatase, and S1P lyase) in BM were unaffected after BDL (data not shown), indicating that the hepatic S1P system was exclusively activated after BDL-induced liver injury.
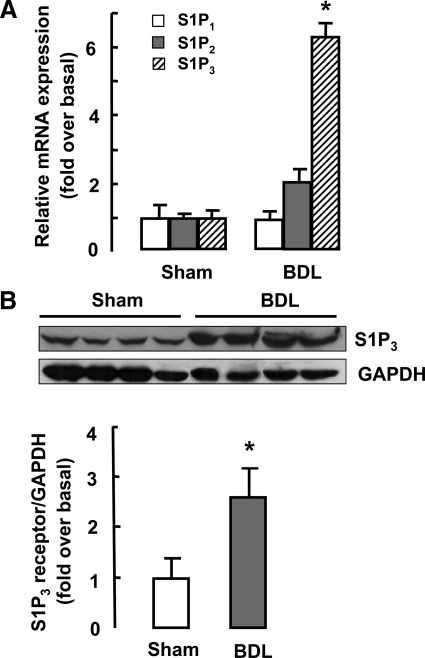
We further determined expression of S1PRs in sham- and BDL-operated mice liver. Real time RT-PCR analysis showed that expression of S1P3 receptor, but not that of S1P1 or S1P2, was markedly up-regulated in BDL-operated liver (Figure 2A). Meanwhile, we evaluated the protein levels of S1P3 receptor in the BDL liver tissue. As shown in Figure 2B, Western blot analysis also revealed a pronounced increase in S1P3 receptor expression after BDL.
Figure 2.
Expression of S1P receptors in liver tissue after BDL. Two weeks after BDL, liver tissue was collected. Real-time RT-PCR and Western blot analysis for expression of S1P receptors in sham- or BDL-operated livers were performed (n = 10 per group). A: S1P1-3 receptor mRNA expression. B: Western blot analysis of S1P3 receptor protein level. Typical autoradiograms are shown. After quantification of the signals, results were normalized relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Data are presented as the mean ± SEM. *P < 0.05, compared with sham-operated mice.
Taken together, these data reveal that SphK1/S1P and related receptors (here, in particular, S1P3) are significantly increased in BDL mice and may play a role in the pathogenesis of cholestatic liver injury.
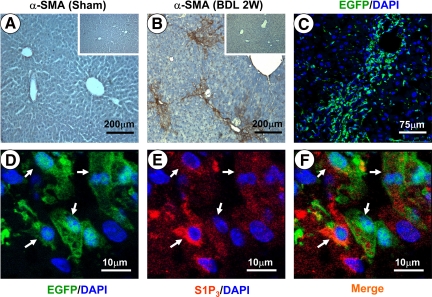
In addition, immunohistochemical staining for α-SMA was performed to evaluate the BDL-induced fibrosis model (Figure 3, A and B). Collagen deposition around the portal tracts with the formation of bridging fibrosis was seen in the livers of the BDL mice.
Figure 3.
Expression of S1P3 receptor in BM-derived cells. A and B: Two weeks (2W) after BDL, immunostaining for α-SMA in sham- or BDL-operated livers representative of 10 independent samples. Insets: Respective negative control staining. C: Confocal microscopy image of livers from mice that had received BM transplants (n = 5). D–F: Representative images of immunofluorescence analysis by confocal microscopy to track the expression of S1P3 receptor (red) in BM-derived cells (green). Arrows indicate the cells positive for both S1P3 receptor and EGFP. DAPI, 4,6-diamidino-2-phenylindole.
S1P3 Receptor Is Expressed in BM-Derived Cells in the Damaged Liver
S1P has been shown to serve as a very good candidate for the induction of cell mobilization.29 There is, in fact, a growing body of evidence to indicate that S1P strongly stimulates BM stem cells migration in vitro.20,21,22 Recently, several studies have shown that the BM contributes significantly to liver fibrosis. Thus, it was of interest to examine whether S1P mediated migration of BM cells to the damaged liver during cholestasis.
We first transplanted BM cells from EGFP transgenic mice into recipients. Four weeks later, hematological reconstitution was complete.16 We then sought to examine BM-derived cells in mouse fibrotic liver induced by BDL. Two weeks after BDL, a confocal microscopy image of livers showed that significant numbers of EGFP-positive cells (BM origin) were found in the fibrotic foci (Figure 3C). Thus, we confirm in our BM transplant model that BM cells can migrate to the damaged liver.
Next, we examined the expression of S1P3 in those BM-derived cells in the damaged liver by immunofluorescence analysis. As shown in Figure 3, D–F, many BM-derived EGFP-positive cells in fibrotic areas were found to express S1P3 receptor, suggesting that the S1P3 receptor was involved in the homing of BM cells probably mediated by S1P after BDL.
S1P Stimulates Migration of BM Cells via S1P3 Receptor
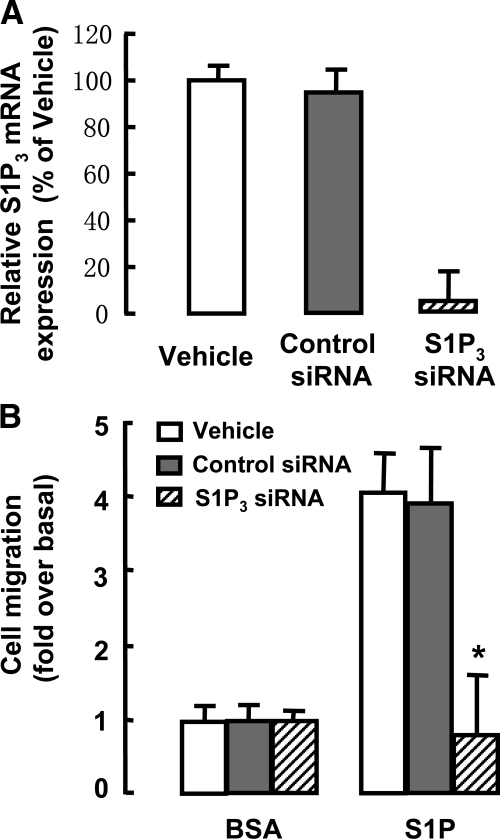
To determine whether S1P induced migration of BM cells via S1P3 receptor, a transwell migration assay was performed with or without silencing of S1P3 expression. As shown in Figure 4A, S1P3 mRNA was down-regulated by 97% in BM cells by siRNA transfection. Moreover, silencing S1P3 expression markedly inhibited S1P-induced BM cell migration (Figure 4B). These results demonstrate that S1P3 receptor is required in S1P-induced BM cell migration.
Figure 4.
S1P-induced migration of BM cells via the S1P3 receptor. A: Knockdown of S1P3 mRNA in BM cells by S1P3-siRNA transfection, measured by real-time RT-PCR. B: Effect of silencing S1P3 expression on BM cell migration in response to S1P. The migration index was defined as the number of cells in the lower chamber under the tested condition divided by the number of cells in the control. Data are presented as the mean ± SEM *P < 0.05 compared with control siRNA. BSA, bovine serum albumin.
Homing of BM Cells Is Prevented by Suramin during Cholestasis
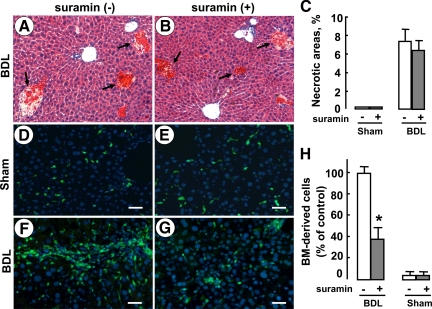
We used a selective S1P3 receptor antagonist, suramin, to further determine whether S1P/S1P3 signaling mediated homing of BM cells in BDL-induced liver injury. At first, we analyzed the effects of suramin on the underlying liver injury process. H&E-stained sections showed that after 3 days of BDL and/or sham operations, necrotic areas were unchanged following suramin administration (Figure 5, A–C). Analysis of bile flow demonstrated that suramin administration did not reduce bile flow in mice (Table 1). Moreover, no significant differences in biochemical parameters (serum total bilirubin, aspartate aminotransferase, and alanine aminotransferase) were found between suramin-treated and untreated mice (Table 1). These results indicated that in the sham/BDL model mice suramin did not affect the extent of inflammation and necrosis in the liver.
Figure 5.
The effect of suramin on the homing of BM cells in mice during cholestasis. Mice (n = 6 per group) received sham or BDL operation with or without one-time suramin administration (20 mg/kg b.wt. i.p.). Three days later, mice were sacrificed and liver tissue was harvested. A and B: Representative H&E-stained liver sections (original magnification, ×200). Necrotic areas are indicated by arrows. C: Quantification of necrotic areas. At least 10 view fields per animal were included. Mice (n = 5 per group) were lethally irradiated and received transplants of EGFP-positive whole BM cells. Then liver fibrosis was induced by BDL over 2 weeks, simultaneously with or without suramin administration (20 mg/kg, twice per week, i.p.). Shown are representative fluorescent images of liver sections with (E and G) or without suramin administration (D and F). Scale bars = 40 μm. H: The average number of BM-derived cells was quantified by analyzing at least 10 random fields per animal, with Image-Pro Plus software. The quantitative result is the percentage of the number of BM-derived cells in BDL mice without suramin administration. Data are presented as the mean ± SEM. *P < 0.05, compared with control.
Table 1.
Biochemical Parameters and Bile Flow in Suramin-treated and Untreated Mice
| ALT (U/L)
|
AST (U/L)
|
Total bilirubin (μmol/L)
|
Bile flow (μl/g b.wt.)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Suramin (−) | Suramin (+) | Suramin (−) | Suramin (+) | Suramin (−) | Suramin (+) | Suramin (−) | Suramin (+) | |
| Sham | 27.7 ± 2.3 | 19.7 ± 3.4 | 62.3 ± 7.7 | 61.7 ± 2.6 | 4.5 ± 1.1 | 3.2 ± 0.6 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| BDL | 923.8 ± 151.3 | 813.2 ± 147.7 | 1467.8 ± 308.6 | 1719.0 ± 485.4 | 170.7 ± 14.2 | 229.1 ± 13.3 | ||
Mice (n = 6 per group) received sham or BDL operation with or without one time suramin administration (20 mg/kg b.wt. i.p.). Three days later, mice were sacrificed, and serum was harvested. Serum aminotransferase (ALT and AST) and total bilirubin were measured using commercial assay kits. Mice (n = 8 per group) received one time suramin administration or saline alone (20 mg/kg b.wt. i.p.). After 24 hours, mice were anesthetized to measure bile flow for 30 minutes. Data are presented as the mean ± SEM.
ALT, alanine aminotransferase; LST, aspartate aminotransferase.
Next, referring to the previous reports,30,31 we performed an EGFP-positive BM cell transplantation experiment followed by BDL-induced liver injury with or without suramin administration. As expected, over the 2 weeks of suramin administration, the proportion of BM-derived cells in the damaged liver decreased markedly compared with that in the liver without suramin treatment (Figure 5, D–H). These results demonstrated that homing of BM cells was mediated by S1P/S1P3 signaling during cholestasis and that the decrease in BM-derived cells in the liver by suramin administration was probably not a consequence of a reduced infiltration.
Suramin Administration Attenuates BDL-Induced Liver Fibrosis
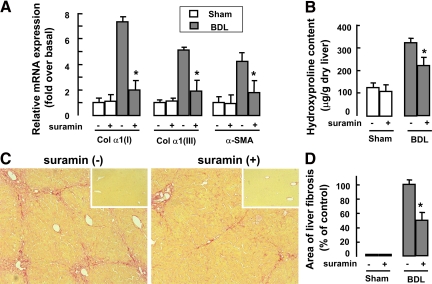
Next, we assessed the potential effect of suramin on liver fibrosis induced by BDL. Two weeks after BDL and suramin administration, mRNA expressions of procollagen α1(I), procollagen α1(III), and α-SMA in liver tissue were markedly down-regulated after suramin administration (Figure 6A). Moreover, hepatic collagen deposition was evaluated by morphometric analysis of Sirius red staining (Figure 6C) and quantified by digital image analysis (Figure 6D). The result showed that collagen deposition was markedly attenuated after suramin administration. We also measured total liver hydroxyproline content (Figure 6B). After suramin administration, there was a significant decrease in hydroxyproline content compared with that in untreated mice.
Figure 6.
Antifibrotic effect of suramin on BDL-induced liver fibrosis in mice. After lethal irradiation and transplantation of EGFP-positive BM cells, mice (n = 5 per group) underwent sham or BDL operation with or without suramin administration. A: Expression of procollagen α1(I) (Col α1(I)), procollagen α1(III) (Col α1(III)), and α-SMA mRNA levels in liver, measured by real-time RT-PCR. B: Hydroxyproline content in the liver. C: Representative pictures of Sirius red staining (original magnification, ×100) in the BDL- or sham-operated livers. Inset: Sirius red staining for sham-operated livers. D: Quantitative analysis of liver fibrosis. Ten randomly selected fields were quantitated for each mouse using the Leica QWin V3 software. Data are presented as the mean ± SEM. *P < 0.05. compared with BDL-operated mice without suramin administration.
Discussion
S1P is a pleiotropic lipid mediator capable of influencing many cell types. However, in mammalian systems S1P is found mainly in the blood and lymph. Furthermore, its receptors are expressed in the vasculature and immune organs. Thus, the functions of S1P in these two organ systems have been characterized extensively.32 S1P/S1PRs are thought to regulate important physiological actions, such as immune cell trafficking, vascular development, vascular tone control, cardiac function, and vascular permeability. In addition, S1P may participate in various pathological conditions. For instance, S1P has been implicated as an important mediator in autoimmunity, transplant rejection, cancer, angiogenesis, vascular permeability, female infertility, and myocardial infarction.33 However, the important role of S1P/S1PRs in liver fibrosis is still poorly understood. In the present study, we hypothesized that S1P production by SphK1 may play a role in liver fibrogenesis involving homing of BM cells. Using the S1P3 receptor antagonist, suramin, we demonstrated that S1P/S1P3 signaling was involved in cholestasis-induced liver fibrosis.
We observed a significant activation of the S1P system in the damaged liver but no change in BM after BDL. These results demonstrate that the S1P/S1PR signaling pathway plays a role in the process of liver fibrogenesis induced by BDL. Moreover, previous studies have demonstrated that S1P acts as an extracellular ligand for a family of G protein-coupled receptors that are crucial in cell migration, including BM stem cells.20,21,22,29 Thus, our data suggest that after cholestatic liver injury, the homeostasis of systemic S1P levels is destroyed, which in turn facilitates the recruitment of BM cells from the BM into the circulation and then into the liver. A similar phenomenon was reported in a previous study about lymphocyte egress from the thymus and peripheral lymphoid organs.11
S1P can function as an extracellular first messenger and intracellular second messenger. Before the discovery of the S1PRs, it was believed that S1P acted as an intracellular mediator.9 However, the intracellular molecular targets of S1P remain to be identified and, thus, much less is known of the intracellular actions of S1P. To date, five S1PRs, S1P1-5, have been identified. They have overlapping and distinct patterns of expression in different tissues. In addition, the coupling of these receptors to different G proteins explains their differential signal transduction properties and also the varied cellular effects of S1P.9 In particular, S1P1, S1P2, and S1P3 were shown to be involved in S1P-induced chemotaxis.29 Moreover, contrary to the stimulatory effects of S1P1 and S1P3, S1P2 inhibits cell migration in most cell types.34 That S1P can both stimulate and inhibit cell migration at first seems contradictory, but the net effect of S1P on cell migration may depend on the relative levels of S1PRs expression. Indeed, in this report, up-regulation of S1P3 expression in liver tissue after chronic liver injury and its expression in the BM-derived cells, as well as S1P-induced BM cell migration via the S1P3 receptor, strongly suggest that S1P/S1P3 may play a role in the migration of BM cells to the damaged liver. This notion is further supported by the observation that administration of the selective S1P3 receptor antagonist, suramin, markedly reduced the number of BM-derived cells during cholestasis. In fact, suramin is a polycyclic anionic compound impermeable to plasma membrane and blocks many ligand-receptor interactions including those of the S1P3 receptor. Specifically, more recent studies have demonstrated that, as an antagonist of S1P3 receptor but not the other S1PRs, suramin almost completely inhibited S1P-induced biological effects via the S1P3 receptor.30,31,35,36,37 In agreement with these studies, our data demonstrate that suramin inhibits S1P/S1P3-induced migration of BM cells in BDL mice. It is noteworthy that in in vitro migration assays, BM stem cells can respond to many growth factors and chemokines such as platelet-derived growth factor and stromal-derived factor-1.38,39 Here, we evaluate an important role of S1P in the homing of BM cells. Nonetheless, further studies are necessary to elucidate the exact mechanism by which S1P mediates migration of BM cells via the S1P3 receptor.
Interestingly, using Sirius red staining, hydroxyproline quantification, and real time RT-PCR analysis of collagen and α-SMA, we observed that suramin administration clearly attenuated BDL-induced hepatic fibrosis, involving blockade of homing of BM-derived cells. However, suramin administration did not affect the extent of inflammation and necrosis in the BDL liver. One possible explanation for these seemingly contradictory results is that suramin mainly lessens the number of BM-derived fibrogenic cells during tissue repair but not the extent of inflammation and necrosis. It is well known that myofibroblasts are the main cellular type involved in extracellular matrix deposition. Recent studies have revealed that extracellular matrix-producing myofibroblasts may also come from BM-derived cells,15,16,17,18,19 in addition to the portal fibroblasts and resident stellate cells. In keeping with these reports, our results strongly suggest that these BM-derived cells in the fibrotic regions, have, at least in part, a myofibroblast phenotype. Therefore, suramin administration attenuated BDL-induced fibrosis most likely because of the inhibition of homing of BM cells and subsequent decrease in fibrogenic cells. In addition, the biological effects of S1P overlap with those of a wide array of cytokines and growth factors, in particular, transforming growth factor-β and platelet-derived growth factor,40 the key mediators during liver fibrogenesis. There is increasing evidence that this overlap in functions relates to both the ability of growth factors to transactivate S1P signaling cascades as well as the ability of S1P binding to the S1PRs to transactivate growth factor-signaling cascades.40 Therefore, another possibility is that inactivation of S1PR by suramin attenuates the interaction between S1P and growth factors. It is also possible other actions of S1P/S1PRs involved in fibrogenesis besides the ability to regulate cell motility were eliminated. In this regard, modulation of S1PR activity may represent a new antifibrotic strategy.
There is growing realization that BM stem cells are a potential therapy for many diseases including chronic liver diseases.27,41,42,43 On the other hand, the fact that BM cells may also contribute to fibrosis in many solid organs suggests that BM cells are also involved in disease progression.15,16,17,18,19 BDL is a unique experimental animal model of hepatic fibrosis/cirrhosis. This model of fibrosis is characterized by moderate inflammation, and the main factors responsible for inducing portal lesions are an increase in biliary pressure and probable modifications in bile composition.44 There is little doubt that myofibroblast activation is one of the key steps that initiate fibrotic lesions in this model. However, the exact origin of these periductal myofibroblasts is yet to be explored, although it is often presumed that they are from local activation of residential fibroblasts or hepatic stellate cells.14,44 Consistent with the previous reports, we confirm that BM-derived cells contribute to liver fibrosis during cholestasis. Therefore, the profibrotic potential of BM cells should not be underevaluated, in particular when BM stem cells are used as therapy for fibrosis/cirrhosis.
In conclusion, our findings suggest that S1P/S1P3 signaling plays an important role in cholestasis-induced liver fibrosis through mediation of the migration of BM cells to the injured liver, which will help to provide a possible therapeutic strategy to control chronic liver diseases.
Acknowledgments
We thank Hong Wang for technical assistance.
Footnotes
Address reprint requests to Dr. Liying Li, Ph.D., No.10 Xitoutiao, You An Men, Beijing 100069 P. R. China. E-mail: liliying@ccmu.edu.cn.
Supported by the Natural Science Foundation of Beijing (grant 7072008), the Basic Science and Technology Research Program Foundation of Ministry of Education (grants 106003 and 208001), the Basic Science and Technology Research Program Foundation of Ministry of Personnel, the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, a Science and Technology Grant from Education Department of Beijing (KM200710025001), the National Basic Research Program (grant 2008CB517401), and the Beijing Municipal Science and Technology Commission (grant Z09050200890906).
C.L. and X.J. contributed equally to this work.
References
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeffe EB. Liver transplantation: current status and novel approaches to liver replacement. Gastroenterology. 2001;120:749–762. doi: 10.1053/gast.2001.22583. [DOI] [PubMed] [Google Scholar]
- Kim WR, Ludwig J, Lindor KD. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol. 2000;95:1130–1138. doi: 10.1111/j.1572-0241.2000.01999.x. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-β1. Am J Respir Cell Mol Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Iwasaki T, Kitano M, Kuno H, Hashimoto N, Kawahito Y, Azuma M, Hla T, Sano H. Role of sphingosine 1-phosphate in the pathogenesis of Sjögren’s syndrome. J Immunol. 2008;180:1921–1928. doi: 10.4049/jimmunol.180.3.1921. [DOI] [PubMed] [Google Scholar]
- Serriere-Lanneau V, Teixeira-Clerc F, Li LY, Schippers M, de Wries W, Julien B, Tran-Van-Nhieu J, Manin S, Poelstra K, Chun J, Carpentier S, Levade T, Mallat A, Lotersztajn S. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med. 2008;233:109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M, Davit A, Francica S, Novelli F, Colombatto S, Fagioli F, Parola M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223–231. doi: 10.1136/gut.2006.111617. [DOI] [PubMed] [Google Scholar]
- Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56:716–724. doi: 10.1136/gut.2006.098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi B, Thibeault S, Lee YT, Bousquet-Gagnon N, Eliopoulos N, Barrette S, Galipeau J, Beliveau R. Matrix metalloproteinase regulation of sphingosine-1-phosphate-induced angiogenic properties of bone marrow stromal cells. Exp Hematol. 2003;31:640–649. doi: 10.1016/s0301-472x(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Meriane M, Duhamel S, Lejeune L, Galipeau J, Annabi B. Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells. 2006;24:2557–2565. doi: 10.1634/stemcells.2006-0209. [DOI] [PubMed] [Google Scholar]
- Jaganathan BG, Ruester B, Dressel L, Stein S, Grez M, Seifried E, Henschler R. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells. 2007;25:1966–1974. doi: 10.1634/stemcells.2007-0167. [DOI] [PubMed] [Google Scholar]
- Xia JL, Dai CS, Michalopoulos GK, Liu YH. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500–1512. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure T, Sakamoto T, Tsuji H, Lunz JG, Murase N, Fung JJ, Demetris AJ. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol. 2000;156:1627–1639. doi: 10.1016/s0002-9440(10)65034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–429. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase l. Anal Biochem. 2002;303:167–175. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- Li LY, Grenard P, Van Nhieu JT, Julien B, Mallat A, Habib A, Lotersztajn S. Heme oxygenase-1 is an antifibrogenic protein in human hepatic myofibroblasts. Gastroenterology. 2003;125:460–468. doi: 10.1016/s0016-5085(03)00906-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S, English D, Milstien S. Sphingosine 1-phosphate signaling: providing cells with a direction. Trends Cell Biol. 2002;12:236–242. doi: 10.1016/s0962-8924(02)02277-8. [DOI] [PubMed] [Google Scholar]
- Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1. EDG-3, and EDG-5. J Biol Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, Waeber C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur J Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- Hla T, Venkataraman K, Michaud J. The vascular S1P gradient—cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun DJ, Lee JH, Choi BH, Koh TK, Ha DC, Jeong MW, Kim KT. Sphingosine-1-phosphate modulates both lipolysis and leptin production in differentiated rat white adipocytes. Endocrinology. 2006;147:5835–5844. doi: 10.1210/en.2006-0579. [DOI] [PubMed] [Google Scholar]
- Nodai A, Machida T, Izumi S, Hamaya Y, Kohno T, Igarashi Y, Iizuka K, Minami M, Hirafuji M. Sphingosine 1-phosphate induces cyclooxygeriase-2 via Ca2+-dependent, but MAPK-independent mechanism in rat vascular smooth muscle cells. Life Sci. 2007;80:1768–1776. doi: 10.1016/j.lfs.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Wang XQ, Mao LJ, Kobayashi T, Kawasaki S, Mori N, Toews ML, Kim HJ, Cerutis DR, Liu XD, Rennard SI. Sphingosine 1-phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptor. Am J Resp Cell Mol Biol. 2008;39:356–363. doi: 10.1165/rcmb.2006-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Lebman DA, Spiegel S. Thematic review series: sphingolipids—cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J Lipid Res. 2008;49:1388–1394. doi: 10.1194/jlr.R800008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Fang BJ, Shi MX, Liao LM, Yang SG, Liu YH, Zhao RC. Systemic infusion of FLK1+ mesenchymal stem cells ameliorate carbon tetrachloride- induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y, Okita K, Sakaida I. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmouliere A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]