Abstract
Fetal liver progenitor cell suspensions (FLPC) and hepatic precursor cells derived from embryonic stem cells (ES-HPC) represent a potential source for liver cell therapy. However, the relative capacity of these cell types to engraft and repopulate a recipient liver compared with adult hepatocytes (HC) has not been comprehensively assessed. We transplanted mouse and human HC, FLPC, and ES-HPC into a new immunodeficient mouse strain (Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice) and estimated the percentages of HC after 3 months. Adult mouse HC repopulated approximately half of the liver mass (46.6 ± 8.0%, 1 × 106 transplanted cells), whereas mouse FLPC derived from day 13.5 and 11.5 post conception embryos generated only 12.1 ± 3.0% and 5.1 ± 1.1%, respectively, of the recipient liver and smaller cell clusters. Adult human HC and FLPC generated overall less liver tissue than mouse cells and repopulated 10.0 ± 3.9% and 2.7 ± 1.1% of the recipient livers, respectively. Mouse and human ES-HPC did not generate HC clusters in our animal model. We conclude that, in contrast to expectations, adult HC of human and mouse origin generate liver tissue more efficiently than cells derived from fetal tissue or embryonic stem cells in a highly immunodeficient Alb-uPA transgenic mouse model system. These results have important implications in the context of selecting the optimal strategy for human liver cell therapies.
Transplanted adult hepatocytes (HC) engraft in a recipient liver and morphologically as well as functionally connect with the surrounding cells.1,2 In animal models with liver injury and/or selective growth advantage engrafted cells respond to growth stimuli and repopulate recipient livers.3,4 Elucidation of the molecular pathways of liver regeneration and extensive preclinical cell transplantation experiments in animals have led to the application of HC transplantation in a limited number of patients with hereditary metabolic liver disease and acute liver failure.5,6,7,8 However, the shortage of donor organs and the difficulties of cryopreservation and long-term culturing of mature HC have limited the clinical application of cell-based therapies.
Stem cells have attracted considerable interest for cell replacement therapy, because they expand in cell culture or can be easily harvested from patients.9,10 Adult, fetal, and embryonic stem cell (ESC) sources have been studied as a potential substitute for primary adult HC in liver cell therapy. The generation of HC has been reported in recipient livers of animals, which have been transplanted with adult hematopoietic and mesenchymal stem cells.11,12,13,14 More recent studies, however, have not convincingly shown formation of HC in therapeutically relevant numbers in mouse liver repopulation or toxic injury models.15,16,17 In one study in fumarylacetoacetate hydrolase (Fah)(−/−) deficient mice, liver tissue formation from transplanted bone marrow cells was found to be the result of monocyte fusion with recipient liver cells.18 In contrast to adult stem cells, fetal liver progenitor cells (FLPC) already express an induced immature hepatic phenotype and can be isolated, cultured, and expanded in vitro. Transplantation experiments in several laboratories have demonstrated engraftment of FLPC and subsequent liver tissue formation.19,20 Transplanted FLPC, which were isolated from murine fetal liver tissue, were shown to acquire the adult HC phenotype over a period of 6 to 8 weeks after transplantation.21 Although FLPC can be expanded in cell culture, the availability of donated fetal tissues restricts the clinical application of this cell source.
With their unlimited potential to grow in vitro and to develop into virtually any cell type, ESCs, and more recently, induced pluripotent stem cells, might be the ideal source of donor liver cells for cell therapies in the future.22,23,24 We and others have generated hepatic precursor cells from human and mouse ESC lines.25,26,27,28 With the existing differentiation protocols a primitive hepatic phenotype with fetal gene expression patterns can be induced in the majority of the ESCs.28,29 Transplantation of these cells, however, have so far resulted only in scattered formation of HC or were reported to form small HC clusters in major urinary protein promoter driven urokinase-type plasminogen activator (uPA) mice30 and Fah(−/−) mice.31
Multiple progenitor cell types have been studied extensively in transplantation experiments in animals with normal liver, in toxic liver injury models, and in liver repopulation models such as the albumin promoter/enhancer (Alb) directed uPA transgenic or Fah(−/−) mice. Although the potential of transplanted stem cell derived hepatic precursor and progenitor cells to generate HC has been clearly demonstrated, a comparative analysis of the individual capacity to form liver tissue is not available. In our present study we aimed to establish and validate an animal model, which would allow us to compare side-by-side the degree of liver repopulation of various human and murine cell types in a recipient liver. To this end, we performed standardized transplantation experiments in immunodeficient heterozygous Alb-uPA mice. In this animal model the transgene is expressed under transcriptional control of the albumin promoter/enhancer sequence exclusively in HC, which causes postnatal toxic liver injury.32 Homozygous mice die from liver failure, but can be rescued by the transplantation of HC. In heterozygous mice, endogenous HC delete the transgene and regenerate the liver. Transplanted cells thus compete with endogenous HC to regenerate the liver. The capacity of a given cell type to repopulate a recipient liver organ after transplantation in this animal model is determined by its engraftment properties, the in vivo differentiation potential, and the proliferation capacity in a recipient liver. We generated a new immunodeficient xenograft mouse model by crossing Alb-uPA transgenic (tg) mice onto the Rag2(−/−)γc(−/−) background (Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice). This new model was then transplanted with various primary human and mouse cells with hepatic phenotype and liver tissues of the transplanted animals were harvested 3 months after transplantation and analyzed for the presence of HC derived from transplanted cells. Our data indicate that immature hepatic cell types of both human and mouse origin are unexpectedly less competitive compared with adult HC in repopulation of the Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mouse liver. Additionally, the overall repopulation rates observed after transplantation of human fetal and adult cells were significantly lower compared with similar transplantations performed with respective mouse cells.
Materials and Methods
Animals
C57BL/6 and enhanced green fluorescent protein (EGFP)-transgenic mice (C57BL/6-TgN(ACTbEGFP)1Osb) were purchased from the Jackson Laboratory (Bar Harbor, ME). Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice were generated by breeding of Alb-uPA transgenic mice32,33 on the severe combined immunodeficiency background34 with Rag2(−/−)γc(−/−) mice35 on the nonobese diabetic background (J.P. Di Santo, unpublished). All animals were maintained and handled in accordance with institutional guidelines of the Hannover Medical School and the Helmholtz Center for Infection Research.
Isolation of Cells from Human Adult and Fetal Liver Tissue
Human adult HC were isolated as described previously by a modified three-step collagenase perfusion from surgical resectates, which have been obtained from patients with informed consent.36 Perfusion solutions were introduced into the tissue through catheters placed into the portal or hepatic vein branches. After the digestion phase, the liver tissue was manually disrupted with sterile scissors and scalpels. To separate undigested tissue pieces, the suspended HC were passed through 750 and 500 μm filters into 50 ml Falcon tubes. The cell suspensions were centrifuged at 50 g for 10 minutes and the cell pellet was resuspended in an ice cold buffer. An aliquot of the cell preparation was separated for cell count and viability analysis (light microscopy and trypan blue exclusion test). In all transplantation experiments, suspensions with >85% of viable HC were used. The non-HC fraction (leukocytes, nonparenchymal liver cells) in the cell suspension was less than 5% based on microscopic analysis. Plating efficacy of the HC on collagen-coated cell culture surfaces exceeded 80% in all preparations.
Human fetal livers (14 to 17 weeks of gestation) were obtained after termination of pregnancy with the informed consent of the mothers. The procedure and the transplantation protocols were approved by the ethical committee of the Hannover Medical School. The tissues were mechanically disrupted and the resulting fragments were treated with 0.1 to 1% collagenase D (Roche, Mannheim, Germany) at 37°C. The resulting FLPC suspensions were washed with William’s E media containing fetal calf serum (FCS) and centrifuged at 50 g. The cell pellet was resuspended with red blood cell lysis buffer (Sigma-Aldrich, München, Germany), centrifuged and resuspended in William’s E medium. Viability (as evaluated by trypan blue exclusion) always exceeded 85% in transplanted samples.
Human ESCs
Human ESC line H1 was obtained from WiCell research institute (Madison, WI). The passage number of the H1 cells used in the transplantation experiment was 43. The culture and hepatic differentiation of human ESCs were performed as previously described in the lab of Hongkui Deng.28 After 18 days of differentiation, the cells were dissociated with Accutase (Sigma) and then transplanted.
Isolation of Cells from Mouse Adult and Fetal Liver Tissue
Adult HC from C57BL/6 and EGFP-transgenic C57BL/6 mice were isolated by a two-step collagenase perfusion method originally described by Seglen et al37 with minor modifications. For the generation of mouse FLPC suspensions, the livers from mouse embryos (embryonic day [ED] 11.5 and 13.5 post conception [p.c.]) were removed under the binocular microscope. Cells were isolated by collagenase/dispase (Roche, Mannheim, Germany) digestion for 20 minutes at 37°C. The cells were treated with red blood cell lysis buffer (Sigma), washed twice in cold Dulbecco’s modified Eagle’s medium with 10% FCS and resuspended in PBS and stored on ice until transplantation.
Mouse ESCs
ESCs derived from Rosa26 mice were cultured on mouse embryonic fibroblasts on gelatinized dishes (Falcon BD) in culture medium I (Knock-out Dulbecco’s modified Eagle’s medium, Invitrogen, Karlsruhe, Germany) supplemented with 15% FCS (selected batches) and the following additives: β-mercaptoethanol (5 × 10−5 M); l-glutamine (2 mmol/L); nonessential amino acids; penicillin-streptomycin (all Invitrogen); and 10 ng/ml recombinant human leukemia inhibitory factor (Chemicon, Millipore, Billerica, MA), as previously described.38 For hepatic differentiation, 600 ESCs were cultivated in hanging drops (20 μl), as described previously to form embryoid bodies. On day 5, embryoid bodies were plated onto gelatinized dishes in culture medium II (IMDM; Invitrogen) supplemented with 20% FCS, 450 μmol/L α-monothioglycerol (Sigma) and all additives except leukemia inhibitory factor. On days 5 and 9, embryoid bodies outgrowths were trypsinized, replated onto collagen type I-coated dishes in differentiation medium (HC culture medium, Cambrex, East Rutherford, NJ), and cultivated until days 5, 9, and 20. For hepatic phenotype selection, hepatic precursor cells derived from embryonic stem cells (ES-HPC) were transduced on day 31 (day 5 + 9 + 17) of differentiation with the lentivirus vector RRL.PPT. Alb.GFPpre (MOI ∼10) and analyzed for EGFP fluorescence after 3 days with a fluorescence microscope. After visual analysis, the cells were trypsinized and sorted for EGFP in a MoFlow cytometer.
Flow Cytometry of Murine and Human FLPC
Cells expressing Dlk-1, a member of the δ-like family of cell surface transmembrane proteins, have been shown in rats to contain the full liver repopulation potential present in fetal liver cell suspensions.39 The surface marker was analyzed by flow cytometry by using 2 μg of a rabbit polyclonal antibody (Abcam, Cambridge, MA) in our cell preparations. Stainings with a rabbit antibody against green fluorescent protein (Invitrogen) at identical concentrations served as isotype controls. Cells were washed once with PBS before applying the fluorescein isothiocyanate-conjugated goat anti-rabbit antibody in a 1:200 dilution. All stainings were performed on ice for 30 minutes in a total volume of 100 μl PBS. Cells were washed again with PBS and analyzed with the LSR II flow cytometer (BD Biosciences, Heidelberg, Germany) and the FlowJo software (Tree Star, Ashland, Oregon).
Cell Transplantation
Intrasplenic transplantations of the various cell types were performed under sterile conditions. Briefly, recipient mice were anesthetized via isoflurane inhalation with an appropriate vaporator. A lateral abdominal incision was performed, the spleen was localized, and cells in a total volume of 50 μl of medium containing no FCS were injected intrasplenically. Sutured spleens were returned carefully, and the skin was closed.
Analysis of Engraftment and Distribution
ES-HPC, FLPC suspensions (ED 11.5 and 13.5), and primary adult HC were incubated with the PKH26 fluorescent dye (Sigma), as previously described.38 Cells, 5 × 105, (viability >85% by trypan blue exclusion) were transplanted via the intrasplenic route into the liver of C57BL/6 mice. After 24 hours, 48 hours, 14 days, and 28 days, the livers (n = 4 for each time point) were harvested and native fluorescence microscopy was performed on cryosections (10 μm) to detect and count PKH26 positive cells. Autofluorescence was excluded by parallel examination of the red (617 nm) and green emission (528 nm). The numbers of PKH-fluorescent cells were assessed in 20 high power fields (×200) of 10 representative tissue sections.
Histology and Immunohistochemistry
Liver tissues were harvested and fixed in 4% paraformaldehyde (Merck, Darmstadt, Germany) and embedded in paraffin. For H&E staining, sections were immersed in hematoxylin solution for 3 minutes and in 0.5% eosin solution for an additional 3 minutes. Immunofluorescence staining of the EGFP antigen was performed in paraformaldehyde-fixed 5-μm permeabilized (in 0.25% Triton-X PBS) sections by using an anti-green fluorescent protein-Alexa 594 antibody (Molecular Probes, Invitrogen) in a 1:200 dilution. Human albumin and human cytokeratin (CK) 18 staining was performed in 6 μm sections. After dewaxing and rehydration endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 10 minutes, followed by antigen retrieval in target retrieval solution (Dako, Glostrup, Denmark) for 20 minutes in a water bath at 98°C. Primary monoclonal anti-human albumin (1:100) (Bethyl Laboratories, Montgomery, TX) and monoclonal anti-human CK 18 (1:200) (Dako) antibodies were incubated overnight. Biotinylated secondary antibody was incubated for 1 hour, followed by incubation with avidin-coupled peroxidase for 1 hour (Vector Laboratories, Peterborough, UK). Three-amino-9-ethylcarbazole was used as a chromogen (Dako) and Gill’s No. 3 hematoxylin for the counter stain.
Biometric Analysis of Liver Tissue
Ten formalin fixed and paraffin embedded tissue slides from all liver lobes of recipient animals were chosen for analysis. The number of HC derived from transplanted cells was counted in ten view fields/section (original magnification, ×400) in individual mice 3 months after transplantation and expressed as percentage of the total number of HC.
Statistical Analyses
Data were compared for all individual groups of animals with the Student’s t-test. A P value of <0.05 was considered as statistically significant.
Results
Transplantation of Murine Cells
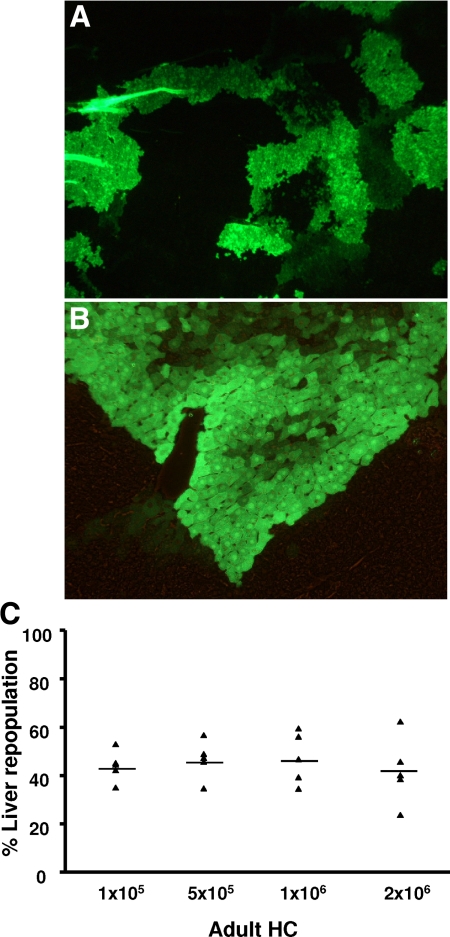
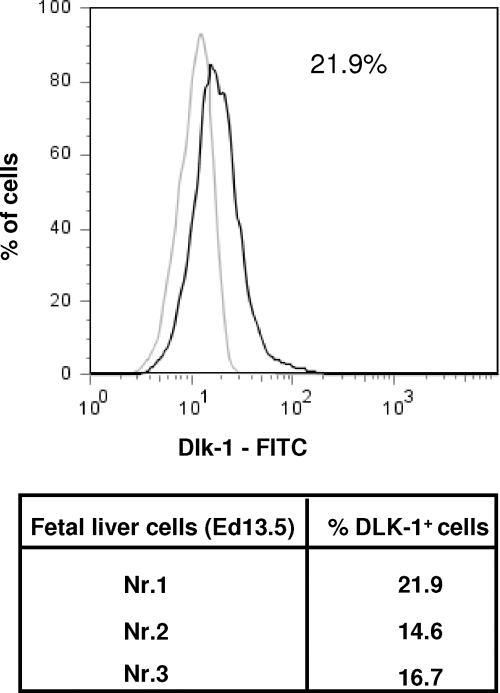
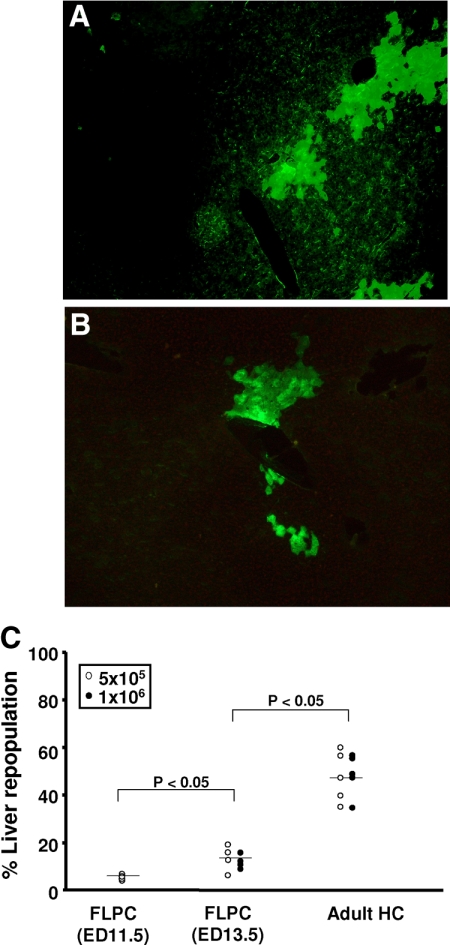
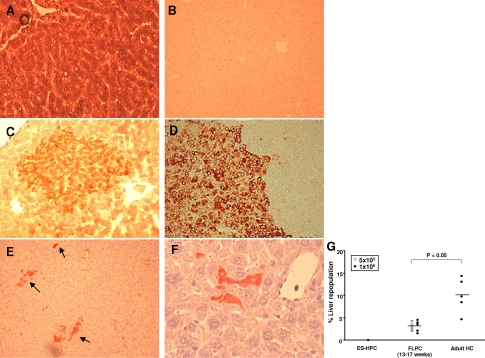
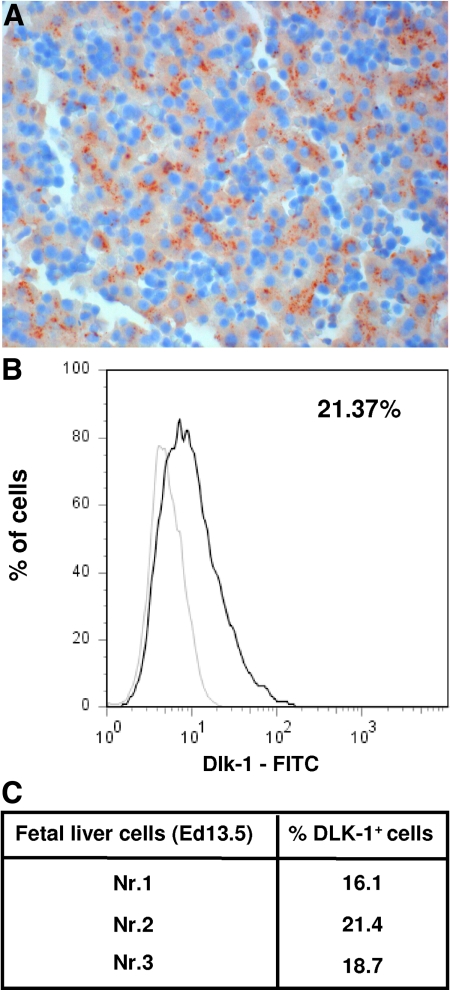
In the first experiment, groups of animals (n = 5) were transplanted with 1 × 105, 5 × 105, 1 × 106, and 2 × 106 EGFP–mouse HC and analyzed for the presence of fluorescent HC at 3 months after transplantation (Figure 1, A and B). Mice from group 1, which were transplanted with 1 × 105 cells, repopulated the recipient liver with 44.0 ± 6.5% of the HC expressing the EGFP. In groups 2, 3, and 4 (receiving 5 × 105, 1 × 106, and 2 × 106 cells), 46.6 ± 8.0%, 47.5 ± 10.7%, and 42.0 ± 14.0% of recipient livers were repopulated by the transplanted cells, respectively (Figure 1C). These results, which did not show statistically significant differences, indicate a saturation effect of intrasplenic liver cell transplantation and a limited effect on the overall repopulation in this particular animal model at numbers exceeding 1 × 105 cells. In contrast to the homogenous adult HC suspensions (< 5% of nonhepatic cells) murine FLPC suspensions derived from ED 11.5 and 13.5 fetal livers contain hepatoblasts and cells of hematopoietic origin. In previous studies the Dlk-1 surface marker has been shown to be exclusively expressed by liver repopulating hepatoblasts.38 After red blood cell lysis, an average of 17.8 ± 4% (n = 3) of the ED 13.5 p.c. liver cell suspensions expressed the Dlk-1 protein as determined by flow cytometric analysis (Figure 2). In transplantation, experiments freshly isolated those murine FLPC suspensions and were less efficient for subsequent repopulation of recipient livers compared with the respective adult HC transplantations (Figure 3, A and B). EGFP–transgenic cells, 5 × 105 and 1 × 106, (containing ∼1 × 105 and 2 × 105 Dlk-1+ hepatoblasts) isolated from ED 13.5 p.c. fetal liver resulted in reduced repopulation rates of the recipient liver organ (12.1 ± 3.0% of the total number of HC) compared with the adult mouse HC transplantations. A further decrease in repopulation efficacy was noted after transplantation of cells isolated from ED 11.5 p.c. fetal liver (5.1 ± 1.1%, P < 0.05) (Figure 3C). The reduced repopulation rate in animals transplanted with mouse FLPC was the result of smaller numbers of cell clusters and smaller sizes of individual groups of cells. Nonsorted mouse ES-HPC did not result in liver tissue formation, but formed teratoma tissue in the liver and spleen of most of the transplanted animals. To facilitate normal liver tissue and HC formation from ES-HPC, a selection strategy based on albumin driven EGFP expression for cells with a hepatic phenotype was evaluated. Transplantation of sorted murine ES-HPC in Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice did not generate teratomas but also did not result in HC cluster formation, a result that confirms earlier data obtained in Fah(−/−) mice.38 Only a few EGFP expressing cells in groups of fewer than six cells with the morphological appearance of HC were detectable in the recipient livers (not shown).
Figure 1.
Microscopic analysis of histological sections (5 μm) from a formalin-fixed liver of a recipient mouse transplanted with 5 × 105 adult EGFP transgenic mouse HC (original magnification, ×200, A; ×400, B). Native EGFP fluorescence, as shown here, was confirmed in selective sections by anti-EGFP immunofluorescence (not shown). To test the relevance of transplanted cell numbers, 1 × 105, 5 × 105, 1 × 106, and 2 × 106 EGFP–transgenic mouse HC were transplanted into Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice via intrasplenic injection. C: Three months after transplantation the number of EGFP transgenic HC detected in the recipient liver and expressed as percentage of the total number of HC was not significantly different in the four analyzed groups of animals (n = 5; p = not significant).
Figure 2.
Murine FLPC suspensions were stained with Dlk-1 antibody and an appropriate isotype. Three representative samples were analyzed and the number of Dlk-1 positive cells were calculated.
Figure 3.
Fluorescence microscopy of a representative formalin-fixed liver section with EGFP transgenic cell clusters from a mouse transplanted with ED 13.5 FLPC (original magnification ×200, A) and ED11.5 FLPC (original magnification ×400, B). Significantly different results were obtained between animals of groups 1 and 2, which were injected with ED 11.5 and 13.5 FLPC, respectively, as compared with animals in group 3, which were transplanted with adult HC. Results of groups 1 and 2 were not statistically different (C). The average size of the cell clusters was smaller than those observed in animals transplanted with adult mouse HC. Sorted ES-HPC did not generate sizable cell clusters in the recipient livers.
Homing, Engraftment, and Proliferation Properties
To rule out that differences in homing and engraftment properties of the various transplanted cell types could have influenced the repopulation capacity in recipient livers, we performed short-term transplantation experiments with PKH26 labeled cells into C57BL/6 mice. After intrasplenic injection of fluorescent labeled cells (1 × 105) most of the cells were detected in the sinusoids or attached to the sinusoidal endothelium in all groups 24 and 48 hours after transplantation. At later time points (14 and 28 days), only a fraction of the labeled murine cells were detectable in the liver plate. The numbers of detectable FLPC and ES-HPC at 14 and 28 days after transplantation were lower, but not statistically different, as compared with adult HC (see Supplemental Figure S1 at http://ajp.amjpathol.org). The transplanted and engrafted adult HC and FLPC were organized as single cells or in groups of maximally three cells at day 28 and morphologically appeared as integrated HC. In contrast, ES-HPC appeared as rounded cells within the liver plate and were negative by immunostaining with hepatocyte specific markers as CK18.
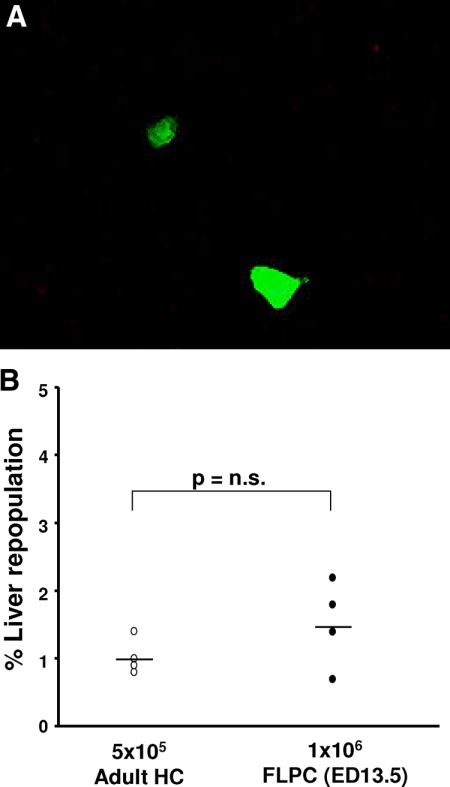
An autonomous growth and repopulation potential has been reported for rat FLPC, which were transplanted into dipeptidylpeptidase (DPPIV−) IV mutant rats with normal liver.20 We therefore tested whether mouse FLPC can grow in a normal recipient liver environment. Mouse HC (5 × 105) and FLPC were transplanted into wild-type litter mate Alb-uPAtg(−/−)Rag2(−/−)γc(−/−) mice not expressing the uPA transgene and analyzed for the presence of fluorescent HC after 3 months. Only cell clusters of up to three cells were dectable with an overall contribution of 1.3 ± 0.9% (FLPC) and 0.9 ± 0.5% of the HC derived from transplanted cells (Figure 4, A and B). Similar observations were also made for human FLPC suspensions (not shown). Some animals were followed for up to 8 months, but no further expansion of the transplanted cells was observed.
Figure 4.
Mouse ED 13.5 FLPC and adult mouse HC were transplanted into Alb-uPAtg(−/−)Rag2(−/−)γc(−/−) mice, which do not show a liver pathology. Mostly single doublet cells were identified in the recipient livers after 3 months (original magnification ×400, A). The percentage of cells derived from FLPC and adult HC was low (<2%) and statistically not different in the two groups of animals (B).
Transplantation of Human Cells
Adult human HC (5 × 105 and 1 × 106) were transplanted via intrasplenic injection and identified in recipient liver tissue sections by immunohistochemistry for human albumin and CK 18 antigens after 3 months. Positive and negative controls (see Figure 5, A and B, respectively) for human albumin immunostaining and a representative section obtained from a liver of a mouse transplanted with human HC and stained for human albumin and CK 18 are shown in Figure 5, C and D, respectively. Human HC formed integrated cell clusters and repopulated 10.0 ± 3.9% of the recipient mouse liver 3 months after transplantation (Figure 5G). The degree of repopulation correlated with the expected serum levels (932 ± 321 μg/ml) of human albumin in these animals. Transplantation of more HC (2 × 106 cells) did not significantly increase the repopulation efficacy in recipient animals (not shown).
Figure 5.
Microscopic analysis of representative histological sections derived from human liver tissue (A), liver tissue from nontransplanted Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) (B), and from livers of Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice transplanted with human cells (C–E). Human liver tissue stained uniformly positive for human albumin antigen (A). Staining was absent in nontransplanted mouse liver (B). Transplanted adult human HC formed mostly medium-sized human albumin (C, dark red colored cells) and human cytokeratin (CK) 18 (D, brown colored cells) antigen positive cell clusters surrounded by mouse liver tissue. Transplanted human FLPC (stained for human albumin, dark red color) formed single cells, which were scattered throughout the liver, and small clusters of HC (arrows) (E and F). The percentage of liver tissue repopulation in Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice transplanted with 1 × 106 human ES-HPC, 5 × 105 to 1 × 106 human FLPC and 1 × 106 adult human HC is shown (G). The number of human albumin positive HC expressed as the average percentage of the total number of HC per microscopic field in liver sections from recipient mice was significantly different for FLPC and adult HC. Nonsorted ES-HPC did not form albumin/CK 18 positive cells in the recipient liver (not shown).
As observed for the mouse FLPC, the transplantation of human FLPC resulted in lower repopulation rates of the recipient liver organ compared with adult HC transplantations. In the group of animals injected with human FLPC (5 × 105 and 1 × 106 cells) suspensions only 2.7 ± 1.1% were identified as human HC (Figure 5G) in the recipient liver. The percentages of cells in the animals transplanted with 5 × 105 and 1 × 106 cells were statistically not different. The human cells were distributed as scattered single cells or as small clusters of cells throughout the liver (Figure 5, E and F). In contrast to mouse FLPC the human cells mostly retained the morphology of hepatoblasts. Nonsorted human ES-HPC did not result in liver tissue formation, but formed teratoma tissue in the liver and spleen of most of the transplanted animals (see Supplemental Figure S2 at http://ajp.amjpathol.org).
Human fetal liver tissues and isolated FLPC suspensions were extensively characterized before application in the transplantation experiments. Immunostaining of fetal tissue sections with an antibody recognizing α-fetoprotein as a characteristic marker of hepatoblasts (brown color) is shown in Figure 6A. The percentage of Dlk-1+ repopulating hepatoblasts in the FLPC suspensions (18.7 ± 3, n = 3) was similar to what was observed for mouse ED 13.5 p.c. FLPC (Figure 6, B and C).
Figure 6.
Detection of α–fetoprotein (AFP) expressing cells in human fetal liver tissue (week 17) by immunohistology. Fetal liver tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Six micron thick sections were analyzed for the expression of human AFP by using a monoclonal mouse anti-human AFP (1:200) (AbD Serotec) antibody. The AFP expressing hepatoblasts (brown color) represent ∼20% of the cells in representative sections (A). Human FLPC suspensions were stained with Dlk-1 antibody and an appropriate isotype control (B). Three representative samples were analyzed and the number of Dlk-1 positive cells calculated (C).
Discussion
In this study we established a novel mouse model for efficiency analyses of various hepatic cell transplant sources: the immunodeficient heterozygous Alb-uPA mice, ie, Alb-uPAtg(+/−)Rag2(−/−)γc(−/−), which combines two important features. First, due to the heterozygous expression of Albumin-promoter-driven urokinase-type plasminogen activator [Alb-uPAtg(+/−)] transplanted cells compete with endogenous cells that spontaneously had inactivated or lost the transgene and, therefore, allows quantitative analyses of engraftment and repopulation efficiency of transplanted cells. Second, the combination of the Rag2- and the γc-knockout results in a complete lack of T-, B- and NK-cells and, therefore, is highly permissive for the transplantation of human cells. Taken together, this particular mouse model allows the direct comparisons of specific murine and human hepatic cell derivatives in the same animal model. Furthermore, we standardized the time period of transplantation (ie, 4 to 14 days after birth) to account for comparability of the transplantation results obtained with the various cell types, because the expression of the urokinase-type plasminogen protein in HC has its peak expression around birth and subsequently induces a subacute liver failure. Regeneration in heterozygous animals is completed between 8 to 12 weeks after birth. At our chosen endpoint of tissue analysis (3 months after transplantation), complete regeneration of the liver tissue was observed with only minor areas of remnant necrosis in some animals. To further reduce variability in transplantation outcome, the viability of various cell preparations always exceeded 85%, as determined by the trypan blue dye exclusion test.
In the first set of experiments we analyzed the effect of the transplanted cell number on the repopulation efficiency in the heterozygous uPA-RAG2-γc mice and confirmed the hypothesis that once we transplanted a threshold cell number, a further increase in transplanted cell numbers does not produce higher repopulation efficiency. In our hands intrasplenic transplantation of 1 × 105 primary murine adult HC results in a repopulation of 44%, which is not further increased after transplantation of higher cell numbers, such as 5 × 105, 1 × 106, or 2 × 106.
In earlier studies, adult human HC were shown to engraft after transplantation and to extensively regenerate a recipient homozygous Alb-uPA mouse liver in various immunodeficient backgrounds.40,41 In our experiments, transplantation of 5 × 105 and 1 × 106 human cells repopulated approximately 10% of the recipient liver mass after 3 months. This result confirms previous data, which showed up to 15% of liver repopulation by transplanted human HC in immunodeficient heterozygous uPA mice.42 The proliferation rate and tissue forming capacity of human adult HC, however, were significantly lower than those observed for transplanted autologous murine HC (>40% of the HC), although similar numbers of cells were transplanted. Incompatibilities of cell-to-cell and cell-to-matrix contacts, as well as differences in response to growth stimuli may have been responsible for reduced repopulation capacity.
FLPC, which have the ability to differentiate into mature HC or biliary epithelial cells, are considered as a potential alternative to adult HC for liver cell therapy. The cells extensively proliferate in vitro and differentiate into adult parenchymal phenotypes after transplantation into a host liver. Nierhoff et al43 reported up to 80% liver repopulation in a retrorsine treated DPPIV(−) mouse model by transplanted mouse fetal hepatoblast preparations. In another report, Oertel et al40 showed that rat FLPC not only proliferated in vitro but also extensively expanded after transplantation in a normal liver and induced apoptotic cell death of surrounding endogenous HC without evidence for tumor formation. Accordingly, transplantations of small numbers of FLPC into the liver of patients with metabolic liver disease could result in complete correction of the disease phenotype without preconditioning of the recipient organ.
To test the capacity to generate liver tissue in our experimental animal model, 5 × 105 and 1 × 106 human as well as murine FLPC (ED 13.5) were transplanted into the spleen of recipient mice according to our standardized protocol. Although HC were frequently detected in the recipient livers after 3 months, the degree of liver repopulation was significantly lower compared with the results obtained from the transplantation experiments with either human or murine adult HC. The average size of HC clusters derived from transplanted FLPC was smaller compared with the clusters derived from transplanted adult HC. Furthermore, transplantation of mouse FLPC from ED 11.5 mouse fetal liver repopulated the recipient liver even less than the more mature FLPC from ED 13.5 fetal livers. Although the cells in the recipient liver 3 months after FLPC transplantation were detectable by CK 18 and albumin immunohistochemistry, the morphology only occasionally resembled mature HC. In contrast, murine FLPC have been shown in previous experiments to mature into the adult hepatic phenotype over a period of 5 to 8 weeks.
Several other groups reported transplantations of human fetal liver cell suspensions and studied engraftment as well as repopulation rates in recipient mouse livers. However, only one study showed a repopulation of up to 10% of the liver (ranging from 1 to 10%) after transplantation of primary human fetal hepatoblasts (11 to 13 weeks of gestation) in a nonconditioned athymic mouse model indicating autonomous in vivo proliferation potential.44 More recent studies reported either a maximum of 4% of human HC in D-galactosamine preconditioned mouse livers after transplantation of primary human fetal hepatoblast suspensions (6 to 10 weeks of gestation) or showed only marginal capacities for repopulation after transplantation of either freshly isolated epithelial cell adhesion molecule, a surface marker present on fetal hepatoblasts, sorted hepatic progenitor cells or cultured multipotent progenitor cells.45,46,47
The observed degree of repopulation after transplantation of mouse FLPC was in the same range observed for the ED 14 derived bipotential mouse embryonic liver cell line transplanted into heterozygous Alb-uPA(+/−)/severe combined immunodeficiency(−/−) mice.19 Strick-Marchand et al19 also elegantly demonstrated the contribution of the transplanted bipotential mouse embryonic liver cell to the bile duct epithelial compartment of the recipient liver. Although we have seen occasional generation of biliary epithelial cells from transplanted cells, it was beyond the scope of our study to quantify the contribution to bile duct formation.
In the already mentioned studies from Sandhu et al20 and Oertel et al, 40 embryonic ED14 FLPC derived from DPPIV+ rats proliferated for up to 6 months after transplantation into a DPPIV− host liver after partial hepatectomy. In contrast, in our model we could not observe a further increase in the size of regeneration nodules derived from liver progenitor cells beyond the 12 weeks period in animals that have been observed for up to 8 months. Most probably, this is due to the fact that the liver has already been fully repopulated by endogenous adult HC that have deleted the transgene. Furthermore, transplantation of FLPC into wild-type Alb-uPAtg(−/−)Rag2(−/−)γc(−/−) mice, which do not provide a proliferation advantage to transplanted cells, resulted into mostly single engrafted cells or clusters of not more than three cells.
Fetal liver is the site of hematopoiesis and contains among other cell types considerable amounts of hematopoietic stem and precursor cells. Immunohistochemistry (albumin, α-fetoprotein), quantitation of Dlk-1 expressing hepatoblasts by flow cytometry and quantitative gene expression analysis in reporter transgene selected human fetal liver cells, identified ∼20% of the cells as hepatoblasts. Similar numbers of Dlk-1 expressing cells were identified in FLPC suspensions derived from ED 13.5 mouse livers. The reduced repopulation capacity of human and mouse FLPC could thus have been the result of lower numbers of transplanted FLPC compared with respective adult HC. From the maximal number of transplanted FLPC (1 × 106) in our experiments ∼2 × 105 cells expressed the hepatoblast phenotype with the potential to form HC. The calculated numbers of transplanted murine FLPC (ED 13.5) in our experiments, however, exceeded (∼twofold) the lowest number of transplanted murine adult HC (1 × 105), which resulted in repopulation of 44% of the recipient liver. Interestingly, further increase of transplanted adult murine HC numbers did not significantly increase the repopulation in this particular animal model. These results are in line with earlier observations by other laboratories that only a very small fraction of transplanted HC enters the liver plates permanently, whereas most of the initially transplanted cells are lost within the first days after transplantation.2
Our data suggest that human FLPC show repopulation capacities in recipient Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice, which are similar to mouse FLPC and lack autonomic growth characteristics in vivo after transplantation, as suggested by others.20,40 Our results obtained by using xenogeneic and allogeneic mouse-based HC transplantation models suggest that we should be cautious when extrapolating either data from different animal species to humans or results generated by using different experimental models. Nevertheless, the results presented here in the mouse-to-mouse and human-to-mouse settings are fully consistent.
Interestingly, transplantation of human and mouse ES-HPC did not result in significant cell cluster formation derived from transplanted cells. In our particular animal model a crude suspension of mouse ES-HPC generated teratoma tissue and caused death within 5 weeks in 100% of the transplanted animals. Selection of cells with a hepatic phenotype by transduction with a lentivirus encoding an Alb-EGFP cassette and subsequent cell sorting avoided teratoma formation. However, only a few single scattered cells with the phenotype of HC were detected in transplanted Alb-uPAtg(+/−)Rag2(−/−)γc(−/−) mice. Our data confirm previous studies from our laboratory38 and others,31 demonstrating that most, if not all, protocols for ES-HPC differentiation not yet provide the full capacity of repopulation. Duan et al48 were recently the first to transplant selected (α1-antitrypsin driven EGFP expression) and isolated hepatic precursor cells from human ESCs into nonobese diabetic-severe combined immunodeficiency mice. By whole mouse bioluminescence imaging, the intrahepatically transplanted cells were visible for 1 week after transplantation and, by PCR and albumin levels in the serum, for more than 3 weeks.48 Long-term survival of these cells in mouse liver repopulation models, however, was not yet reported.
The reduced capacity of FLPC and ES-HPC to form cell clusters after transplantation into the Alb-uPAtg(−/−)Rag2(−/−)γc(−/−) mouse may also—at least partially—result from differences in engraftment efficacies. Although transplanted PKH26 stained murine FLPC and ES-HPC were detected throughout the observation period of 28 days in the recipient liver, the numbers were considerably, although not significantly, lower compared with adult HC.
In conclusion our experimental in vivo model provide conclusive data on side-by-side comparison of potential cell sources for cellular liver therapies for liver. The obtained results have important implications in the context of establishing the optimal strategy for implementing human cell therapies. In our hands, the applied protocols for differentiation of ESCs into ES-HPC are not yet sufficient to generate cell phenotypes, which can generate significant numbers of HC or form regular liver tissue after an intrahepatic injection in vivo. However, considering recent advances in the field, it is expected that ES-HPC with an adequate level of differentiation will be available for clinical applications in the field in the upcoming years.
Supplementary Material
Footnotes
Address reprint requests to Michael Ott, Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School and Twincore Centre for Experimental and Clinical Infection Research, Feodor-Lynen-Strasse 7, 30625 Hannover, Germany. E-mail: ott-mhh@gmx.de.
Supported by a grant from the Bill and Melinda Gates Foundation. Authors from the Hannover Medical School, Helmholtz Center for Infection Research, Institut Pasteur and Academic Medical Center are part of the Human Vaccine Consortium “Grand Challenges in Global Health: Devise Reliable Testing Systems for New Vaccines.”
D.H. and Q.Y. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Gupta S, Rajvanshi P, Lee CD. Integration of transplanted hepatocytes into host liver plates demonstrated with dipeptidyl peptidase IV-deficient rats. Proc Natl Acad Sci USA. 1995;92:5860–5864. doi: 10.1073/pnas.92.13.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, Novikoff PM. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29:509–519. doi: 10.1002/hep.510290213. [DOI] [PubMed] [Google Scholar]
- Weglarz TC, Degen JL, Sandgren EP. Hepatocyte transplantation into diseased mouse liver: kinetics of parenchymal repopulation and identification of the proliferative capacity of tetraploid and octaploid hepatocytes. Am J Pathol. 2000;157:1963–1974. doi: 10.1016/S0002-9440(10)64835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grompe M. Principles of therapeutic liver repopulation. J Inherit Metab Dis. 2009;29:421–425. doi: 10.1007/s10545-006-0311-2. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- Schneider A, Attaran M, Meier PN, Strassburg C, Manns MP, Ott M, Barthold M, Arseniev L, Becker T, Panning B. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation. 2006;82:1115–1116. doi: 10.1097/01.tp.0000232451.93703.ab. [DOI] [PubMed] [Google Scholar]
- Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, Schmidt J, Ott M, Pettenazzo A, Luecke T, Bertram H, Hoffmann GF, Burlina A. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009;87:636–641. doi: 10.1097/TP.0b013e318199936a. [DOI] [PubMed] [Google Scholar]
- Cantz T, Manns MP, Ott M. Stem cells in liver regeneration and therapy. Cell Tissue Res. 2008;331:271–282. doi: 10.1007/s00441-007-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa Y, Verma IM. Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11850–11853. doi: 10.1073/pnas.1834198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Cantz T, Sharma AD, Jochheim-Richter A, Arseniev L, Klein C, Manns MP, Ott M. Reevaluation of bone marrow-derived cells as a source for hepatocyte regeneration. Cell Transplant. 2004;13:659–666. doi: 10.3727/000000004783983521. [DOI] [PubMed] [Google Scholar]
- Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, Weiss MC. Bipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytes. Proc Natl Acad Sci USA. 2004;101:8360–8365. doi: 10.1073/pnas.0401092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am J Pathol. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantz T, Zuckerman DM, Burda MR, Dandri M, Göricke B, Thalhammer S, Heckl WM, Manns MP, Petersen J, Ott M. Quantitative gene expression analysis reveals transition of fetal liver progenitor cells to mature hepatocytes after transplantation in uPA/RAG-2 mice. Am J Pathol. 2003;162:37–45. doi: 10.1016/S0002-9440(10)63796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen J, Rathjen PD. Mouse ES cells: experimental exploitation of pluripotent differentiation potential. Curr Opin Genet Dev. 2001;11:587–594. doi: 10.1016/s0959-437x(00)00237-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Kania G, Blyszczuk P, Jochheim A, Ott M, Wobus AM. Generation of glycogen- and albumin-producing hepatocyte-like cells from embryonic stem cells. Biol Chem. 2004;385:943–953. doi: 10.1515/BC.2004.123. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Jochheim A, Hillemann T, Kania G, Scharf J, Attaran M, Manns MP, Wobus AM, Ott M. Quantitative gene expression profiling reveals a fetal hepatic phenotype of murine ES-derived hepatocytes. Int J Dev Biol. 2004;48:23–29. doi: 10.1387/ijdb.15005571. [DOI] [PubMed] [Google Scholar]
- Heo J, Factor VM, Uren T, Takahama Y, Lee JS, Major M, Feinstone SM, Thorgeirsson SS. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology. 2006;44:1478–1486. doi: 10.1002/hep.21441. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nature Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Heckel JL, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell. 1990;62:447–456. doi: 10.1016/0092-8674(90)90010-c. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- Morosan S, Hez-Deroubaix S, Lunel F, Renia L, Giannini C, Van Rooijen N, Battaglia S, Blanc C, Eling W, Sauerwein R, Hannoun L, Belghiti J, Brechot C, Kremsdorf D, Druilhe P. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J Infect Dis. 2006;193:996–1004. doi: 10.1086/500840. [DOI] [PubMed] [Google Scholar]
- Silva-Barbosa SD, Butler-Browne GS, Di Santo JP, Mouly V. Comparative analysis of genetically engineered immunodeficient mouse strains as recipients for human myoblast transplantation. Cell Transplant. 2005;14:457–467. doi: 10.3727/000000005783982837. [DOI] [PubMed] [Google Scholar]
- Alexandrova K, Griesel C, Barthold M, Heuft HG, Ott M, Winkler M, Schrem H, Manns MP, Bredehorn T, Net M, Vidal MM, Kafert-Kasting S, Arseniev L. Large-scale isolation of human hepatocytes for therapeutic application. Cell Transplant. 2005;14:845–853. doi: 10.3727/000000005783982530. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Hepatocyte suspensions and cultures as tools in experimental carcinogenesis. J Toxicol Environ Health. 1979;5:551–560. doi: 10.1080/15287397909529766. [DOI] [PubMed] [Google Scholar]
- Sharma AD, Cantz T, Vogel A, Schambach A, Haridass D, Iken M, Bleidissel M, Manns MP, Schöler HR, Ott M. Murine embryonic stem cell-derived hepatic progenitor cells engraft in recipient livers with limited capacity of liver tissue formation. Cell Transplant. 2008;17:313–323. doi: 10.3727/096368908784153896. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Chen YQ, Teisner B, Jensen CH, Shafritz DA. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Gupta S, Rogler CE. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1998;95:310–315. doi: 10.1073/pnas.95.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- Nierhoff D, Ogawa A, Oertel M, Chen YQ, Shafritz DA. Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology. 2005;42:130–139. doi: 10.1002/hep.20735. [DOI] [PubMed] [Google Scholar]
- Mahieu-Caputo D, Allain JE, Branger J, Coulomb A, Delgado JP, Andreoletti M, Mainot S, Frydman R, Leboulch P, Di Santo JP, Capron F, Weber A. Repopulation of athymic mouse liver by cryopreserved early human fetal hepatoblasts. Hum Gene Ther. 2004;15:1219–1228. doi: 10.1089/hum.2004.15.1219. [DOI] [PubMed] [Google Scholar]
- Nowak G, Ericzon BG, Nava S, Jaksch M, Westgren M, Sumitran-Holgersson S. Identification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivo. Gut. 2005;54:972–979. doi: 10.1136/gut.2005.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci USA. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M, Follenzi A, Cheng K, Surana M, Joseph B, Benten D, Bandi S, Qian H, Gupta S. Phenotype reversion in fetal human liver epithelial cells identifies the role of an intermediate meso-endodermal stage before hepatic maturation. J Cell Sci. 2008;121:1002–1013. doi: 10.1242/jcs.019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS, Zern MA. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.