Abstract
Autosomal dominant polycystic kidney disease (ADPKD) results from mutations in either PKD1 or PKD2 and accounts for 10% of all patients on renal replacement therapy. The kidney disease phenotype is primarily characterized by cyst formation, but there are also prominent interstitial changes (inflammation, apoptosis, proliferation, and fibrosis). Using a model of unilateral ischemia-reperfusion injury, we tested the hypothesis that Pkd2 heterozygous kidneys are more sensitive to injury and that this could lead to interstitial inflammation and fibrosis. Baseline tubular proliferation in heterozygous kidneys was twofold higher than in wild-type kidneys. The magnitude and duration of tubular and interstitial proliferative responses was consistently greater in injured heterozygous compared with wild-type kidneys at all time points. Conversely, tubular p21 expression in heterozygotes was lower at baseline and following injury at all time points. Significantly more neutrophils and macrophages were detected in injured Pkd2 heterozygous kidneys at 2 days, correlating with increased expression of the cytokines interleukin (IL)-1β and keratinocyte-derived chemokine and resulting in interstitial fibrosis at 28 days. We conclude that Pkd2 dosage influences both susceptibility and nature of the repair responses following injury. Polycystin-2 is therefore likely to play multiple roles in regulating tubular cell viability, repair, and remodeling in the mature kidney.
Autosomal dominant polycystic kidney disease (ADPKD) results in kidney failure in 10% of all patients on renal replacement therapy and is caused by mutations in PKD1 or PKD2.1,2 The ADPKD proteins, polycystin-1 (PC1) and polycystin-2 (PC2), function as a heterodimeric complex and have been shown to regulate multiple signaling pathways. These include intracellular Ca2+, heterotrimeric and monomeric G-proteins, protein kinase C, Janus kinase and signal transducers and activators of transcription, which in turn maintain normal tubular structure and function (reviewed in Ref. 3). Although a weak phenotype-genotype correlation for renal survival has been reported for PKD1, it is clear that nonallelic factors such as genetic modifying loci and/or environmental factors exert a greater influence on the cystic phenotype and probably account for the marked intrafamilial phenotypic variability often observed in ADPKD pedigrees.4,5,6
Cyst formation is a focal process and it has been estimated that cysts arise from <1% of all nephrons carrying a germline mutation of PKD1 or PKD2. The mutational mechanism underlying cyst formation is thought to require somatic mutation (“two hit” model); evidence of somatic mutations in cystic cells isolated from PKD1 or PKD2 kidneys has been shown.7 Nevertheless, there is accumulating evidence that somatic mutation may not be essential for cyst formation and that gene dosage could be equally important. Thus, mice transgenic for PKD1 or Pkd1 as well as Pkd1 hypomorphs have been shown to develop renal cysts.8,9,10,11 Equally, other modifying genes (eg, TSC2) and gender play key roles in determining phenotypic severity probably by pathways independent of the rate of somatic mutation.12,13,14 The cellular triggers for cyst formation could differ between the developing and mature kidney since recent reports of Pkd1 inactivation using Cre-Lox mice have revealed marked differences in phenotype depending on the timing of Pkd1 deletion in the kidney.15,16,17
The presence of prominent interstitial changes in the cystic kidney has been largely attributed to inflammatory cell infiltration or matrix deposition secondary to cyst growth. However in a recent study, we noted that Pkd2 heterozygous mice (with minor cystic disease) developed more prominent interstitial fibrosis than age-matched wild-type mice.18 In this study, the degree of interstitial fibrosis was significantly correlated not only to cyst number but also to the tubular proliferative index (PI) (detected in noncystic tubules). These results suggested that interstitial fibrosis could occur independently of cyst formation, possibly secondary to the increase in proliferating tubular cells or as the result of an increase in interstitial inflammation. To test this hypothesis, we used a model of transient unilateral ischemia-reperfusion injury (IRI) to investigate if Pkd2 haploinsufficiency could render the kidney more susceptible to the development of interstitial scarring following a single-shot injury.
Materials and Methods
Experimental Animals
Pkd2+/− founder mice were obtained from Prof. S. Somlo (Yale University). These mice carry a null allele (ws183) for pkd2 resulting from homologous recombination at exon-1 of the Pkd2 locus.19 They have been backcrossed onto a C57BL/6 strain background for over 20 generations.
Experimental Groups
Female Pkd2 heterozygous or wild-type mice of 4 to 6 months of age (n = 5–7/group) were housed at constant temperature (21°C), humidity (45%) on a 12-hour light/dark cycle and had free access to drinking water and standard laboratory chow. Under Fluothane (ZENECA, UK) anesthesia, the left renal artery and vein were exposed using a flank incision and occluded using a nontraumatic clamp for 25 minutes. Following ischemia, the clamped kidney was reperfused for 2, 7, and 28 days before harvest. After the stated time of reperfusion, the ischemic (left) and nonischemic (right) kidney were removed. Half of each kidney was fixed in 10% neutral-buffered formalin, and the other half was snap frozen in liquid nitrogen and stored at −80°C until further analysis. All experimental procedures were performed according to the rules and regulations as laid down by the Home Office (Animal Scientific Procedures Act 1986, UK).
Human Nephrectomy Tissue
We analyzed stored archival kidney tissue (Department of Histopathology, Northern General Hospital) that had been formalin fixed and paraffin embedded. ADPKD kidney sections (n = 5–7) were derived from patients who had undergone nephrectomy for various reasons, including intractable pain, huge abdominal masses, pretransplantation, recurrent hematuria, or cyst infection. As normal controls, tissue sections from the uninvolved pole of kidneys removed because of a localized renal tumor were used (n = 4). All of the archived material (stored before the year 2000) was dissociated, and therefore, retrospective permission to use the tissue was not appropriate.
Histomorphometric Analysis
H&E sections were used for analysis of cyst number and mean cyst area. To differentiate between cysts and normal tubular profiles, only epithelial lined cavities of the size of a glomerulus (∼6000 μm2) or larger were counted as cysts.13,18 The fibrosis score was estimated on Masson Trichrome-stained sections by multiphase image analysis as described previously.18,20 For each section, 10 randomized selected areas of cortex and medulla were analyzed at ×200 magnification.
A tubular injury score was evaluated on periodic acid-Schiff-stained sections. The average internal cross-sectional diameter of a normal cortical tubule was evaluated on eight control kidney sections (left and right) from four wild-type mice and estimated as 10 μm. Necrotic tubules were defined as those with loss of brush border and a minimum dilatation of 1.5 times the average luminal diameter of a normal tubule. All evaluation was performed using touch count and arbitrary distance measurement using AnalySIS5 software (Soft Imaging System], Germany). A minimum of 100 tubules were counted per section. The number of necrotic tubules was expressed as a percentage of the total number of tubules counted. Both kidneys were included in all histological analysis and analyzed in a single-blinded manner.
Immunohistochemistry
Immunodetection for proliferating cell nuclear antigen (PCNA), Ki-67, α-SMA, F4/80, CD45, CD68, myeloperoxidase, and p21 was performed on formalin-fixed paraffin-embedded sections using previously published protocols.18,20 The number of positive cells in each section was counted in 10 consecutive nonoverlapping fields of renal cortex and medulla at ×200 magnification, and the results expressed as mean ± SE per mm2.20 The PI was defined as the number of PCNA or Ki-67-positive cells per section in 10 sequentially selected nonoverlapping fields (×200 magnification) of renal cortex and medulla, expressed per mm2.
Costaining of F4/80 and PCNA was conducted using primary antibodies raised in different species (rabbit, mouse) as described previously.18 Bound antibody was detected using a horseradish peroxidase-conjugated goat anti-rabbit IgG for F4/80 staining and an alkaline phosphatase-conjugated goat anti-mouse IgG for PCNA staining. Diaminobenzidine and Vector Red (Vector Laboratories, Peterborough, UK) were used as chromogens, respectively. Only clearly definable nuclei with heavy/or granular staining were counted as PCNA positive (red) and expressed as a percentage of F4/80 positive (brown) cells.
Assessment of Apoptosis
Apoptotic cells in kidney sections were detected by the terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine (dUTP) end labeling (TUNEL) method (Chemicon International, Southampton, UK) following the manufacturer’s protocol.20 The apoptotic index was defined as the number of TUNEL-positive cells per section in 10 sequentially selected nonoverlapping fields (×200 magnification) of renal cortex and medulla, expressed per mm2.
Kidney Lysates
Snap-frozen kidneys were ground under liquid nitrogen and homogenized in lysis buffer (2 M Tris-HCl and 1 mmol/L EDTA supplemented with complete protease inhibitor tablet (Roche, Welwyn Garden City, UK), 5 μmol/L sodium vanadate, and 5 μmol/L sodium phosphate) using 10 strokes of a Dounce homogenizer (Wheaton, Millville, NJ) followed by sonication. Crude debris was sedimented using a low-speed (1000 × g) centrifugation and discarded. Lysates were finally centrifuged in Beckman TL-100 ultracentrifuge at (100,000 × g) for 2 hours to give a cytosolic fraction (supernatant) and a membrane pellet. Membrane pellets were resuspended in 1% SDS to prepare the membrane fraction. Total protein concentration was quantified using the Bio-Rad DC Assay (Bio-Rad Laboratories UK, Hemel Hempstead, UK).
Immunoblotting
The generation and detailed characterization of the polycystin-1 (7e12) and polycystin-2 (p30) antibodies raised to human sequence have been described in previous papers.13,21 Immunoblotting was performed on protein lysates as described previously.22 In brief, membranes were incubated overnight at 4°C with primary antibodies: mouse mAb to PCNA (Dako UK, Ely, UK), polycystin-1 (7e12), and polycystin-2 (p30). These were incubated with an appropriate horseradish peroxidase-linked secondary antibody: goat anti-mouse IgG2a or IgG1 (Southern Biotechnology Associates, Cambridge, UK) or goat anti-rabbit (Dako UK). Membranes were stripped and reprobed using an antibody against the endoplasmic reticulum (ER) resident protein, calnexin (BD Transduction Laboratories, Dorset, UK), to control for protein loading and integrity. Band intensities of PC1 and PC2 were quantified using a Bio-Rad GS-690 scanning densitometer using Molecular Analyst version 4 software and adjusted to that of calnexin as described previously.22 Since PC2 and calnexin were sometimes detectable as doublet bands, we quantified the upper band of each protein because this was consistently present and likely to represent the mature protein in each case.
Real-Time Quantitative PCR Assays
Total RNA was extracted from snap-frozen kidney tissue using TRIzol, DNAase treated, and quantified using a fluorescent nuclei acid assay (Quant-iT RiboGreen reagent; Invitrogen). DNase-treated RNA (1.5 μg) was reverse transcribed using the Superscript II RT system (Invitrogen). All samples were analyzed in triplicate and reverse transcribed using the same master mix (SYBR Green; Stratagene) in a single batch to avoid differences in the efficiency of the Reverse Transcriptase (RT) reaction between samples. The cDNA concentration was measured to ensure equal loading of template. Primer specificity was first validated by standard PCR to check that a single product of the right size was obtained on agarose gel electrophoresis. For quantitative PCR, a MX3000P Thermal Cycler (Stratagene) was used with a cycle profile of 40 cycles at 95°C for 30 seconds and 60°C for 1 minute. The number of template cDNA molecules in each sample was determined by recording the amplification cycle number during the exponential phase (cycle threshold or CT). On the basis of this principle, the starting number of template cDNA is inversely proportional to CT. Standard curves were generated using a range of 1:3 serial dilutions by plotting CT against log of known standard RNA concentrations. The reaction was optimized to obtain an efficiency between 95 and 110%. The relative mRNA concentration in each sample was calculated by extrapolation from the standard curve and expressed in arbitrary units.
Cytokine Measurements
Cytokines were assayed using a Bio-Plex murine 23-plex microsphere assay (Bio-Rad) according to the manufacturer’s instructions. The assay was run on a Luminex 200 system using high Photomultiplier Tube (PMT) settings for narrow range standard curve (system calibrated with CAL2 high RP1 target value). Lysates were diluted one in two with Bio-Plex assay buffer before the assay. Results were expressed as pg/100 μg protein.
Statistical Analysis
Values are expressed as means ± SEM. Comparison between groups was performed using the Mann-Whitney U-test for nonparametric data. Correlation analyses used the Spearman correlation test. A value of P < 0.05 was considered statistically significant.
Results
Cell Proliferation, Apoptosis, and Inflammation in Human ADPKD Kidney
We first examined human ADPKD and normal kidney tissue to assess changes in proliferation, apoptosis and inflammation. Cell proliferation in human ADPKD kidney was markedly increased in both tubular and interstitial compartments using the marker PCNA, which labels cycling cells (Supplemental Fig. S1, C and D, see http://ajp.amjpathol.org). Few PCNA-positive cells were visualized in the cyst lining, the majority of positive cells being found in undilated or dilated (but noncystic) tubular segments (Supplemental Fig S1B, see http://ajp.amjpathol.org). Similarly, the number of apoptotic cells in tubular segments was two to three times higher that that in cyst lining (Supplemental Fig S1, F and G, see http://ajp.amjpathol.org). There was a significant increase in the interstitial apoptotic index compared with normal kidneys (Supplemental Fig S1H, see http://ajp.amjpathol.org). The ADPKD interstitium was characterized by a noticeable increase in surface area, inflammatory cells, and α-smooth muscle actin (α-SMA)-positive fibroblasts (data not shown). Approximately 20% of interstitial cells were CD68 positive, suggesting a prominent macrophage infiltrate in these diseased kidneys (Supplemental Fig S1, J and K, see http://ajp.amjpathol.org).
Baseline Tubular and Interstitial Proliferation in Pkd2 Heterozygous and Wild-Type Kidneys
The baseline mean tubular PI was twofold higher in heterozygous mice (n = 6) compared with age-matched female wild-type (n = 7) mice (left kidney (LK): 6.92 ± 0.70 versus 3.40 ± 0.41, P = 0.0047; right kidney (RK): 7.08 ± 0.49 versus 3.95 ± 0.41, P = 0.0012). However, there were no significant differences in interstitial PI, although mean values were ∼40% higher in heterozygous mice compared with wild-type (LK: 3.69 ± 0.60 versus 2.61 ± 0.32; RK: 3.88 ± 0.51 versus 2.89 ± 0.23).
Cell Proliferation Following IRI in Pkd2 Heterozygous and Wild-Type Kidneys
Following IRI, Pkd2+/− kidneys demonstrated on average a 1.5- to 2-fold greater increase in proliferating cells (Ki-67 positive) compared with wild-type kidneys at 2 days postinjury (Figure 1; Supplemental Table 1, see http://ajp.amjpathol.org). This increase was observed in both tubular and interstitial compartments. In addition, the magnitude and duration of the tubular and interstitial proliferative response of the injured LK and uninjured RK was clearly different between wild-type and heterozygous animals (Figure 1). In wild-type mice, tubular PI peaked at 2 days in the injured kidney but was not significantly elevated at 7 days post reperfusion compared with the uninjured or control kidneys (Figure 1, A and C). In contrast, tubular PI in the injured heterozygous kidneys showed an exaggerated peak in tubular PI at 2 days. In addition, tubular PI between injured and uninjured or control kidneys was significantly different up to 28 days postinjury (Figure 1, A, C, E). Similar differences were observed for interstitial proliferation in both wild-type and heterozygous kidneys (Figure 1, B, D, F). Of interest, the tubular and interstitial PI of wild-type and heterozygous injured kidneys remained significantly elevated compared with control kidneys at 28 days but were significantly higher in heterozygous kidneys (Figure 1, E and F; Supplemental Table 1, see http://ajp.amjpathol.org). PCNA expression by immunoblotting was approximately threefold higher in heterozygous than wild-type injured kidneys at 2 days, consistent with the Ki-67 expression data (Figure 1G). Representative images of Ki-67-positive cells postischemia for both genotypes at each time point are shown in Supplemental Fig S2 (see http://ajp.amjpathol.org).
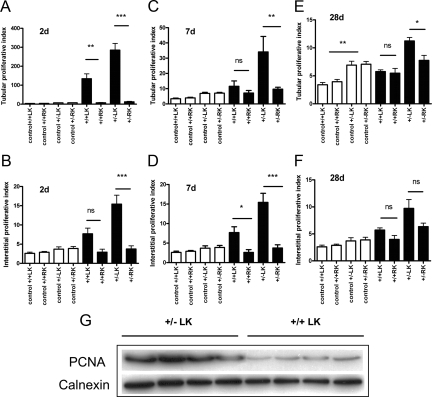
Figure 1.
Proliferative responses of tubular and interstitial compartments in Pkd2 heterozygous and wild-type mice following IRI. The PI (Ki-67 positive cells per mm2) for tubular and interstitial compartments at different postreperfusion periods is shown: 2 days (A and B); 7 days (C and D); and 28 days (E and F). The contralateral RK served as a nonischemic control for the procedure. Values from control age and sex matched kidneys are shown as open bars: baseline tubular PI was twofold higher in heterozygous kidneys compared with wild type (significance only shown at 28 days for clarity). Tubular and interstitial PI were significantly higher at all time points in the injured LKs of heterozygous (+/−) and wild type (+/+) compared with control values (data not shown). In addition, values for the heterozygous LK were significantly greater than those for wild-type LK at all time points (except for interstitial PI at 7 days, P = 0.06). Data shown are means ± SEM of cell counts per mm2 per kidney (n = 5–7). ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. G: Mean PCNA (∼36 kDa) expression at 2 days was threefold higher in +/−LK compared with +/+LK. Calnexin (∼90 kDa) served as a control for loading and protein integrity.
The response of the uninjured wild-type and heterozygous kidneys were similar following IRI. We observed a small but significant rise in tubular PI at 2 days only for both genotypes compared with controls (Figure 1; Supplemental Table 1, see http://ajp.amjpathol.org). No significant changes were detected for interstitial PI in the uninjured kidneys for both genotypes compared with controls.
Leukocyte Accumulation and Macrophage Infiltration Following IRI
Following IRI, we also noted a marked increase in leukocyte (CD45) and macrophage (F4/80) accumulation in the renal interstitium at all time periods examined (Figure 2, A–C). Once again, quantitative differences of two- to threefold were noted between both genotypes with a peak in the number of CD45 and F4/80 cells at 7 days (Table 1). The number of F4/80 cells in injured heterozygous kidneys was significantly greater than that seen in wild-type kidneys at 2 days (Figure 2, A–C). At 7 days, the proportion of proliferating macrophages (F4/80 cells that were also PCNA-positive) was significantly greater in injured heterozygous kidneys (Figure 2, D–F). Highly significant correlations were found especially between the F4/80 (r = 0.87) or CD45 (r = 0.83) counts and the fibrosis score at 7 days (Table 2). There were no significant differences in the number of F4/80 cells (2 days), SMA-positive cells (28 days) and fibrosis (28 days) between the uninjured kidneys of both genotypes (Supplemental Fig S3, see http://ajp.amjpathol.org).
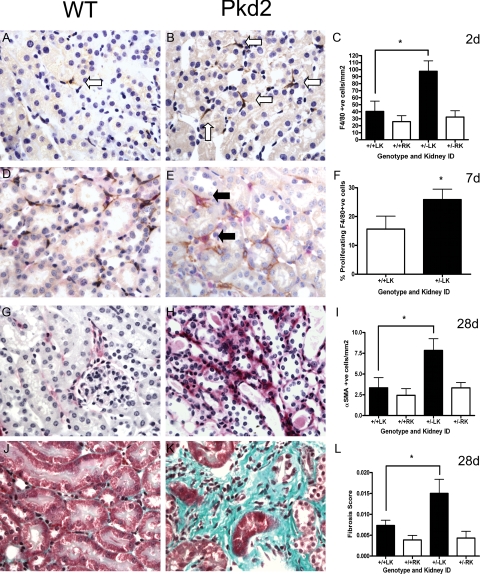
Figure 2.
Changes in macrophage infiltration, activated fibroblasts and interstitial fibrosis in Pkd2 heterozygotes and wild-type mice following IRI. Wild-type (A, D, G, and J) and heterozygous (B, E, H, and K) kidneys were examined for F4/80 macrophages (A and B), the percentage of PCNA-positive F4/80 macrophages (D and E), α-SMA-positive fibroblasts (G and H), and collagen deposition (J and K). In heterozygous kidneys, the number of F4/80 macrophages was significantly increased at 2 days (C), and the number of doubly positive PCNA (red) - F4/80 (brown) cells at 7 days (F), respectively. At 28 days, both α-SMA-positive fibroblasts (I) and the overall fibrosis score (L) were significantly greater in heterozygous compared with wild-type LKs. The white arrows indicate typical F4/80-positive cells and the black arrows double PCNA and F4/80-positive cells. Magnification, ×400. Data shown are means ± SEM (n = 5–7). ∗P < 0.05.
Table 1.
Effect of IRI on Apoptosis (TUNEL), Macrophage Infiltration (F4/80), and Leukocyte Accumulation (CD45)
| Parameter | LK+/− | RK+/− | LK+/+ | RK+/+ | Mann-Whitney P value |
|---|---|---|---|---|---|
| 2 days | |||||
| TUNEL (tubular cells/mm2) | 1.9 ± 0.6 | 0.15 ± 0.04 | 0.86 ± 0.27 | 0.18 ± 0.06 | 0.2949 |
| F4/80 (cells/mm2) | 97.7 ± 14.8 | 32.4 ± 9.0 | 40.4 ± 14.6 | 25.8 ± 8.6 | 0.0350 |
| CD45 (cells/mm2) | 315.8 ± 93.4 | 146.8 ± 34.04 | 175.6 ± 35.9 | 182.1 ± 75.8 | 0.366 |
| 7 days | |||||
| TUNEL (tubular cells/mm2) | 3.3 ± 1.9 | 0.03 ± 0.03 | 1.5 ± 1.2 | 0.05 ± 0.03 | 0.5884 |
| TUNEL (interstitial cells/mm2) | 1.17 ± 0.4 | 0.1 ± 0.1 | 1.0 ± 0.7 | 0.0 ± 0.0 | 0.4848 |
| F4/80 (cells/mm2) | 400.9 ± 163.6 | 99.1 ± 44.5 | 236.0 ± 113.1 | 72.4 ± 30.5 | 0.4848 |
| CD45 (cells/mm2) | 816.9 ± 304.8 | 184.9 ± 79.7 | 446.6 ± 170.4 | 213.0 ± 80.7 | 0.4848 |
| 28 days | |||||
| TUNEL (tubular cells/mm2) | 0.4 ± 0.1 | 0.05 ± 0.03 | 0.1 ± 0.04 | 0.02 ± 0.02 | 0.0727 |
| TUNEL (interstitial cells/mm2) | 0.3 ± 0.01 | 0.02 ± 0.02 | 0.04 ± 0.02 | 0.02 ± 0.02 | 0.2303 |
| F4/80 (cells/mm2) | 330.7 ± 79.5 | 179.7 ± 34.4 | 142.0 ± 29.7 | 137.6 ± 32.5 | 0.1636 |
| CD45 (cells/mm2) | 387.3 ± 95.3 | 193.3 ± 33.1 | 168.2 ± 36.7 | 150.7 ± 49.7 | 0.1091 |
LK+/− versus LK+/+.
Table 2.
Correlations between Fibrosis Score and Other Cellular Responses at 7 and 28 days Postreperfusion
| Parameter | Fibrosis score
|
|
|---|---|---|
| P | r | |
| 7 days | ||
| α-SMA (cells/mm2) | 0.0207* | 0.4693 |
| F4/80 (cells/mm2) | <0.0001*** | 0.8653 |
| CD45 (cells/mm2) | <0.0001*** | 0.8287 |
| Tubular PI (cells/mm2) | 0.001** | 0.6284 |
| Interstitial PI (cells/mm2) | 0.0278* | 0.4489 |
| 28 days | ||
| α-SMA (cells/mm2) | 0.0078** | 0.5516 |
| F4/80 (cells/mm2) | 0.0185* | 0.4975 |
| CD45 (cells/mm2) | 0.0035** | 0.5942 |
| Tubular PI (cells/mm2) | 0.0008*** | 0.6623 |
| Interstitial PI (cells/mm2) | 0.0059** | 0.5675 |
∗P < 0.05, number of ∗ indicates level of significance.
Fibroblast Activation and Interstitial Fibrosis Following IRI
α-SMA expression has been found to correlate with a more activated fibroblastic phenotype and may precede the onset of interstitial fibrosis.23 Occasional α-SMA-positive cells were detected at 2 days postreperfusion in either interstitium or glomeruli. There were more α-SMA-positive cells by 7 days in heterozygous kidneys following injury and this was significantly different at 28 days (Figure 2, G–I). At this time point, we also detected a significant difference in the fibrosis score between heterozygous and wild-type kidneys (Figure 2, J–L) and a highly significant correlation between α-SMA expression and the fibrosis score (Table 2). An increase in macrophage and proliferating macrophage infiltration as well as myofibroblast (SMA) accumulation has been previously correlated with chronic kidney injury in the rat remnant kidney model.23
Polycystin-1and Polycystin-2 Expression Following IRI
In a previous study using a rat model of unilateral IRI, we reported that renal Pc2 expression was increased following ischemia.22 In that study, we were unable to assess Pc1 expression because our PC1 antibody did not cross-react with rat Pc1. In the present study, we found a differential rise in Pc1 expression of between 1.4- and 1.7-fold in the LK compared with RK at 2 days—the magnitude of this increase was not different between the genotypes (Figure 3, A and B). Although baseline Pc2 expression was lower in the heterozygotes, a similar level of increase in Pc2 expression (1.7- to 1.9-fold) was observed in LK of both genotypes compared with the nonischemic RK at 2 days.
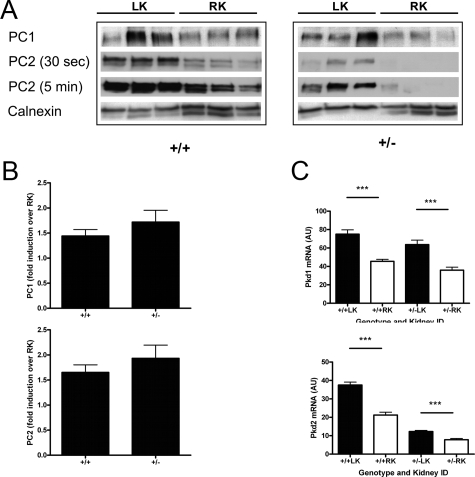
Figure 3.
Expression of PC1 and PC2 in Pkd2 heterozygotes and wild-type mice following IRI. A: Immunoblotting for PC1 and PC2 in membrane fractions prepared from both kidneys at 2 days postinjury (n = 3). Highly specific antibodies to PC1 (7e12) and PC2 (p30) were used. Representative blots for PC2 for short (30 seconds) and long (5 minutes) exposures are shown to illustrate the differences in PC2 protein levels between both genotypes most apparent at shorter exposures. Calnexin (∼90 kDa) was used to demonstrate equivalent loading between lanes. B: PC1 expression (∼500 kDa) was increased by up to twofold in the postischemic LK compared with the nonischemic RK in both genotypes (+/+1.44 ± 0.13; +/−1.72 ± 0.58). Although baseline PC2 (∼110 kDa) was lower in Pkd2 heterozygous kidneys, a similar fold increase in PC2 expression was observed in both genotypes following IRI (+/+1.65 ± 0.46; +/−1.93 ± 0.79). Bars represent mean ± SEM values from three separate experiments. C: Renal Pkd1 mRNA and Pkd2 mRNA levels were significantly increased at 2 days reperfusion in the ischemic LK relative to the nonischemic RK. However, as predicted, mean levels of Pkd2 were at least 50% lower in both heterozygous kidneys compared with wild-type equivalents. AU, arbitrary units. Values are means ± SEM (n = 9). ∗∗∗P < 0.001.
By quantitative PCR, pkd2 mRNA levels at 2 days reperfusion were significantly lower in heterozygous nonischemic RK compared with wild-type RK reflecting the difference in gene dosage (Figure 3C). Pkd1 mRNA levels were the same in both genotypes. Both Pkd1 and Pkd2 mRNA levels were significantly increased in both wild-type and heterozygous LK. Relative to the RK, the increases were between 1.6 and 1.8 times, values similar to that found by immunoblotting (Figure 3B). This indicates that the increase in Pc1 and Pc2 expression following IRI is likely due to increased gene transcription and/or mRNA stability. Nevertheless, Pkd2 mRNA levels in the heterozygous LK were still ∼33% that of the wild-type LK at 2 days postischemia.
p21 Expression Following IRI
p21 expression was examined by immunohistochemistry in ischemic and control kidneys from both genotypes. There was a significant difference in the pattern and magnitude of tubular p21 expression between wild-type and heterozygous ischemic kidneys (Figure 4, A and B; Supplemental Table 1, see http://ajp.amjpathol.org). Wild-type mice showed a significantly greater increase in p21 expression in the injured kidney at 2 days, and this rise was maintained at 7 and 28 days postinjury. In contrast, p21 expression in heterozygous mice showed a smaller increase reaching significance only at 7 days (Figure 4B). At each time point, the increase in p21 expression postinjury was lower in heterozygous kidneys (Supplemental Table 1, see http://ajp.amjpathol.org). We examined age-matched control sections (n = 7) from both genotypes and found significantly fewer p21 positive cells in both kidneys of heterozygous (∼50%) compared with wild-type mice (Figure 4B; Supplemental Table 1, see http://ajp.amjpathol.org). These results are the exact reverse of our findings with Ki-67-positive cells in control mice.
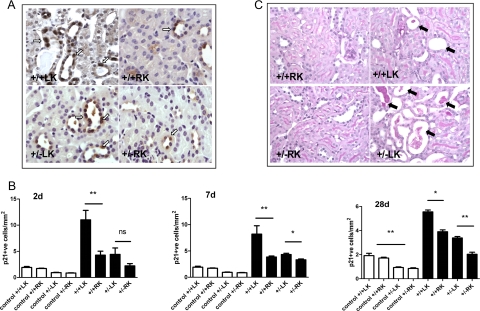
Figure 4.
The severity of tubular injury is increased in Pkd2 heterozygous mice compared with wild-type following IRI. A: Representative panels showing p21-positive tubular cells for both genotypes at 2 days post-IRI. Some background apical and cytoplasmic staining was seen with this antibody, but only nuclear positive cells (arrows) were counted. Magnification, ×400. B: The number of p21 nuclear positive tubular cells was quantified in age-matched control kidneys of both genotypes (white bars) and at 2, 7, and 28 days following IRI (black bars). There were significantly fewer p21-positive cells in heterozygous control kidneys compared with wild-type (significance shown on 28 days only for clarity). At all time points following reperfusion, tubular p21 expression was significantly lower in the heterozygous LK compared with wild-type LK. ∗P < 0.05, ∗∗P < 0.01. C: The panels show representative periodic acid-Schiff-stained kidney sections from the most severely affected animals of both genotypes (n = 6–7) with arrows indicating dilated tubules, some with cell debris and proteinaceous casts. Magnification, ×400. The mean percentage of necrotic tubules per section at 2 days post-IRI was greater in heterozygous mice but did not reach statistical significance.
Tubular Injury Following IRI
The severity of tubular injury at 2 days post-IRI was assessed by the number of necrotic tubules per section expressed as a percentage of the total number of tubules counted. Examples of the most severely affected animals from both genotypes are shown in Figure 4C. The mean necrosis scores were 1.7 times higher in the ischemic heterozygotes compared with wild-type kidneys (+/−21.4 ± 6.1%, +/+12.9 ± 3.4%; P > 0.05), although a wide range of severity was detected in both groups.
Cytokine Release Following IRI
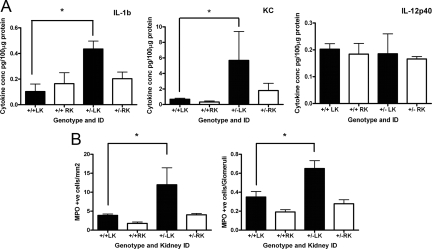
Because of the prominent inflammatory changes and resultant increase in interstitial fibrosis, we assayed for a variety of cytokines in kidney tissue at 2 days reperfusion using a commercial multiplex bead assay. Of 23 cytokines measured, 7 (granulocyte-CSF, keratinocyte-derived chemokine, regulated on activation normal T cell expressed and secreted, IL-1β, IL-2, IL-9, IL-12p40) were within the detectable range. Of these, IL-1β and KC (or CXCL1) were both significantly elevated in injured heterozygous kidneys compared with wild-type (Figure 5A). The levels in nonoperated age-matched wild-type and heterozygous control kidney lysates (n = 3–4) were largely below the limit of detection (data not shown).
Figure 5.
Cytokine expression and neutrophil infiltration is increased in Pkd2 heterozygous mice compared with wild-type following IRI. A: Significantly greater increases in IL-1β and KC were detectable in the postischemic LK of heterozygous mice compared with their wild-type littermates at 2 days. Levels of other detectable cytokines, including IL-12p40, were not different between genotypes and unchanged by ischemia. B: There were a greater number of neutrophils (myeloperoxidase-positive cells) at 2 days in both interstitium and glomeruli of postischemic LK of heterozygous mice compared with their wild-type littermates. ∗P < 0.05 (n = 5–7).
Neutrophil Infiltration Following IRI
In view of the increase in IL-1β and KC observed, we also evaluated the degree of interstitial and glomerular neutrophil infiltration detectable. As shown in Figure 5B, significantly more neutrophils were detected in the glomeruli and interstitium of heterozygous compared with wild-type LK at 2 days postinjury.
Discussion
Recent studies have highlighted subtle phenotypic changes in Pkd1 and Pkd2 heterozygous mice that appear to correlate to a reduction in gene dosage without the requirement for somatic mutation. For instance, there is an increase in tubular PI in Pkd1 and Pkd2 kidneys that precedes cyst formation.15,17,18 For Pkd2, a highly significant correlation was observed between the tubular PI and the presence of interstitial fibrosis in the kidneys of heterozygous mice.18 A syndrome of inappropriate antidiuresis has been reported in Pkd1 heterozygous mice.24 Similarly, Pkd2 heterozygous mice are more prone to developing intracranial vascular abnormalities when rendered hypertensive by salt-loading; freshly dissociated vascular smooth muscle cells isolated from these mice have altered Ca2+ regulation.25 Finally, pkd2 heterozygous mice have been reported to have a shortened life span compared with their wild-type littermates although the cause of their reduced longevity is unexplained.26
Our present study adds further evidence that the haploinsufficient state in PKD2 is not benign but may render the kidney more susceptible to tubular injury and an exaggerated repair response (Figure 6). The repair response in terms of proliferation, apoptosis, and inflammation was increased both in magnitude and duration following a single-shot ischemic stimulus. This in turn led to permanent structural (fibrosis) and presumably functional changes in the Pkd2+/− kidney. A likely interpretation of these results is that normal levels of PC2 are required to regulate tissue repair following injury.22 Although both Pkd1 and Pkd2 gene expression were similarly increased by up to twofold in both genotypes at 2 days following IRI, levels of Pkd2 mRNA remained significantly lower in both heterozygous kidneys (Figure 3C). We hypothesize that both the baseline level of Pc2 in the kidney at the time of the injury (see later) as well as an appropriate magnitude of increase in response to injury is critical to the regulation of the subsequent repair response. In this study, we observed a twofold difference in baseline tubular PI between wild-type and heterozygous kidneys, a lower difference than what we previously reported (5- to 10-fold) in the same model.18 However, the latter study was performed on older mice (9 to 12 months of age) of both genders, factors that could explain the differences observed. Of interest, two studies in Pkd1 heterozygous mice have reported increases in baseline tubular PI of two- to fourfold in animals of up to 5 months of age, results similar to ours.15,17 These results and ours support the idea that dosage of Pkd1 or Pkd2 controls the level of basal cell turnover in the kidney.
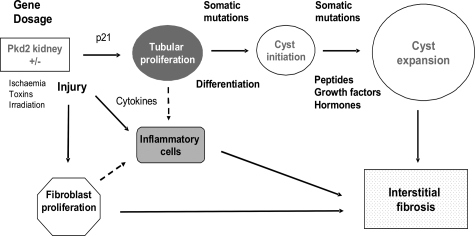
Figure 6.
A multistep model of disease pathogenesis in ADPKD. Hypothetical scheme of the interaction between gene dosage (Pkd2 haploinsufficiency) and environmental factors (injury) in the development of cysts and interstitial fibrosis. The dotted lines represent likely recruitment of inflammatory cells resulting from cytokines release by injured and/or proliferating tubular, fibroblast, and endothelial cells. Not shown are potential pathogenic links between proliferating tubular cells and fibroblast proliferation (paracrine growth factors) or interstitial fibrosis (interstitial collagen secretion and degradation). Baseline tubular proliferation was increased by twofold in Pkd2 heterozygous kidneys correlating to a proportional reduction in p21 expression. Increased cell cycling could predispose cells to acquiring somatic mutations and susceptibility to additional stochastic events necessary to trigger cyst initiation. However, overall susceptibility is likely to depend on the state of cellular differentiation and/or proliferative potential. Cyst expansion may depend on the acquisition of somatic mutations in other nonallelic genes and the influence of other soluble factors.
In this study, we chose to compare the cellular responses of injured to the uninjured kidney between two genotypes by using a model of unilateral renal ischemia. Our results demonstrate that differences in magnitude and duration for the tubular and interstitial proliferative response following injury can be related to gene dosage. A potential disadvantage of our approach is that the uninjured kidneys could respond to injury of the contralateral kidney in a genotype-dependent manner. Indeed, although there were still differences in tubular PI between the uninjured kidneys from both wild-type and heterozygous mice, they were smaller than that observed in control mice, suggesting that this could be the case. Nonetheless, none of these differences was statistically significant (Supplemental Table 1 and Supplemental Figure 3, see http://ajp.amjpathol.org). An alternative approach would have been to use age- and sex-matched sham-operated mice for both wild-type and heterozygous mice without renal injury at each time point.
One possible link between PC2 and tissue repair is through the cyclin-dependent kinase inhibitor, p21, which has multiple effects on proliferation and apoptosis in different cells. p21 has been reported to regulate cell cycle progression from G1 to S, to inhibit DNA polymerase activity by direct binding to PCNA, and to inhibit apoptosis.27 An important link to PKD pathogenesis was the finding that p21 is transcriptionally regulated by PC1 (and dependent on PC2) via the Janus kinase-signal transducers and activators of transcription 1 pathway. At E15.5, p21 levels in Pkd1 null embryos were greatly reduced compared with wild-type controls, whereas basal p21 levels in Pkd1 heterozygotes were unchanged.28 p21 has been reported to be induced by IRI (bilateral renal clamping) in C57BL/6 mice primarily in tubular cells.29 The induction of p21 following IRI may be protective because kidney function and mortality were worse in p21-knockout mice despite an increase in PCNA-positive cells.27 In this study, we found that p21 expression was 50% lower in control kidneys from Pkd2 heterozygotes compared with wild-type mice (Figure 4B). In addition, tubular p21 expression remained significantly lower at all times postreperfusion in Pkd2 heterozygotes at a time when the tubular PI was two to three times higher. Suboptimal increases in p21 following injury (such as in Pkd2 mice) could also lead to inappropriate entry of damaged cells into the cell cycle rather than cell cycle arrest with consequent cell loss by apoptosis and necrosis. To our knowledge, this is the first study to report p21 expression in Pkd2 kidneys and that basal p21 expression is influenced by Pkd2 dosage.
The increase in kidney fibrosis at 28 days following IRI in Pkd2 heterozygote kidneys was an unexpected finding. Fibrosis was preceded by an early and persistent increase in interstitial infiltrates (neutrophils, macrophages, and leukocytes), which are likely to have been of pathogenic relevance. There was a significant increase in neutrophils and F4/80 macrophages by 2 days postinjury, indicating that elevated cytokine release by resident or infiltrating cells could be important (Figures 2 and 5). Using cytokine assays, we found significant differences between heterozygous and wild-type kidneys at 2 days postinjury for IL-1β and KC (Figure 5). Both cytokines are induced early following IRI and known to be critical for neutrophil recruitment in the postischemic kidney.30,31 These findings suggest that the transcriptional regulation of key cytokine genes such as IL-1β and KC could be altered in Pkd2-deficient animals and be unmasked by IRI. Future studies will seek to clarify the time course of these events and also the underlying mechanism. Our results are consistent with previous reports of detectable cytokines in human PKD cyst fluid, including IL-1β and KC/Gro-α.32,33
Three recent articles have highlighted a clear difference in phenotypic severity depending on the timing of tissue-specific Pkd1 inactivation using Cre-Lox technology.15,16,17 If Pkd1 is inactivated during development, cyst development is severe. Conversely, a mild phenotype is observed if Pkd1 is deleted in the mature organ. These studies differ in their evaluation of the critical window of susceptibility. Nonetheless, they demonstrate that the absence of Pc1 is insufficient by itself to trigger a cystic phenotype. An additional stimulus such as injury seems to be required to trigger cystic transformation in cells predisposed by their particular differentiation status or proliferative potential.16,34,35 On the basis of our results and that of others, we propose a multistep model for cystogenesis as summarized in Figure 6. In this model, there is increased cell cycling as a result of heterozygosity of Pkd2 or Pkd1 (first hit) in part related to p21 deficiency. This could lead to an increased frequency of somatic mutation (second hit) and/or susceptibility to stochastic events during the cell cycle. Environmental triggers such as ischemia, irradiation, or toxic injury could exaggerate this process by further stimulating the cycle of proliferation and repair. Moreover, the exaggerated release of cytokines could lead independently to inflammation and fibrosis, prominent features of other rodent cystic models.36,37 Finally, we propose that somatic mutations in other nonallelic genes (third hit) could be important modifiers of cyst expansion.38 Their interactions with stochastic events and other stimuli (eg, growth factors, peptides, hormones) could further influence the rate of cyst expansion.20,39 We did not observe an increase in cyst formation in Pkd2 heterozygous kidneys up to 28 days following injury, although in some mice, marked tubular dilatation was seen (Figure 4C). In this model, it seems likely that cyst formation occurs exclusively on a cellular recessive background as previously shown.18,19 It would be interesting to examine if adult Pkd2 conditional null kidneys develop more cysts in response to tubular injury. During the submission and review of this article, two groups have reported that tubular injury (IRI or toxic) does exacerbate cystic disease in adult Pkd1 conditional knockout mice.40,41
Recent studies in PKD1 and PKD2 pedigrees have highlighted the relatively minor role of specific alleles but emphasized the major roles of modifying genes, gender, environmental factors, and their interactions in determining individual phenotypic severity.5,6,42 Our data highlight for the first time the critical role that a single environmental factor can play in determining disease severity on a predisposing genetic background in a syntenic ADPKD model.
Supplementary Material
Acknowledgments
We thank Linghong Huang for technical assistance, Stefan Somlo for the gift of Pkd2 mutant mice, John Shortland for access to archival nephrectomy tissue and Meguid El Nahas for helpful comments on the manuscript.
Footnotes
Address reprint requests to Dr. Albert Chee Meng Ong, D.M. F.R.C.P., Kidney Genetics Group, Academic Nephrology Unit, The Henry Wellcome Laboratories for Medical Research, University of Sheffield Medical School, Beech Hill Road, Sheffield S10 2RX, UK. E-mail: a.ong@sheffield.ac.uk.
Supported by Kidney Research UK (RP16-1-07) and the Sheffield Kidney Research Foundation. S.P. was supported by a University of Sheffield Ph.D. scholarship. A.C.M.O. is a Wellcome Trust Research Leave Senior Fellow.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005;67:1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- Rossetti S, Burton S, Strmecki L, Pond GR, San Millan JL, Zerres K, Barratt TM, Ozen S, Torres VE, Bergstralh EJ, Winearls CG, Harris PC. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol. 2002;13:1230–1237. doi: 10.1097/01.asn.0000013300.11876.37. [DOI] [PubMed] [Google Scholar]
- Paterson AD, Magistroni R, He N, Wang K, Johnson A, Fain PR, Dicks E, Parfrey P, St George-Hyslop P, Pei Y. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:755–762. doi: 10.1681/ASN.2004090758. [DOI] [PubMed] [Google Scholar]
- Fain PR, McFann KK, Taylor MR, Tison M, Johnson AM, Reed B, Schrier RW. Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int. 2005;67:1256–1267. doi: 10.1111/j.1523-1755.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Ong AC, Harris PC. Molecular basis of renal cyst formation—one hit or two? Lancet. 1997;349:1039–1040. doi: 10.1016/S0140-6736(05)62286-6. [DOI] [PubMed] [Google Scholar]
- Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, Strmecki L, Walker D, Ward CJ, Alpers CE, Zhou J, Wood WG, Harris PC. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet. 2000;9:2617–2627. doi: 10.1093/hmg/9.18.2617. [DOI] [PubMed] [Google Scholar]
- Thivierge C, Kurbegovic A, Couillard M, Guillaume R, Cote O, Trudel M. Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol. 2006;26:1538–1548. doi: 10.1128/MCB.26.4.1538-1548.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- Jiang ST, Chiou YY, Wang E, Lin HK, Lin YT, Chi YC, Wang CK, Tang MJ, Li H. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol. 2006;168:205–220. doi: 10.2353/ajpath.2006.050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JR, Maheshwar MM, Aspinwall R, Thompson P, Cheadle JP, Ravine D, Roy S, Haan E, Bernstein J, Harris PC. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am J Hum Genet. 1997;61:843–851. doi: 10.1086/514888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AC, Harris PC, Davies DR, Pritchard L, Rossetti S, Biddolph S, Vaux DJ, Migone N, Ward CJ. Polycystin-1 expression in PKD1, early-onset PKD1, and TSC2/PKD1 cystic tissue. Kidney Int. 1999;56:1324–1333. doi: 10.1046/j.1523-1755.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007;16:3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura A, Contrino L, Beck AW, Zhou J. Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol. 2008;19:2351–2363. doi: 10.1681/ASN.2007101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MY, Parker E, Ibrahim S, Shortland JR, Nahas ME, Haylor JL, Ong AC. Haploinsufficiency of Pkd2 is associated with increased tubular cell proliferation and interstitial fibrosis in two murine Pkd2 models. Nephrol Dial Transplant. 2006;21:2078–2084. doi: 10.1093/ndt/gfl150. [DOI] [PubMed] [Google Scholar]
- Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- Chang MY, Parker E, El Nahas M, Haylor JL, Ong AC. Endothelin B receptor blockade accelerates disease progression in a murine model of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:560–569. doi: 10.1681/ASN.2006090994. [DOI] [PubMed] [Google Scholar]
- Ong AC, Ward CJ, Butler RJ, Biddolph S, Bowker C, Torra R, Pei Y, Harris PC. Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1, in normal and cystic tissue. Am J Pathol. 1999;154:1721–1729. doi: 10.1016/S0002-9440(10)65428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Haylor JL, Ong AC. Polycystin-2 expression is increased following experimental ischaemic renal injury. Nephrol Dial Transplant. 2002;17:2138–2144. doi: 10.1093/ndt/17.12.2138. [DOI] [PubMed] [Google Scholar]
- Yang N, Wu LL, Nikolic-Paterson DJ, Ng YY, Yang WC, Mu W, Gilbert RE, Cooper ME, Atkins RC, Lan HY. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dial Transplant. 1998;13:1967–1974. doi: 10.1093/ndt/13.8.1967. [DOI] [PubMed] [Google Scholar]
- Ahrabi AK, Terryn S, Valenti G, Caron N, Serradeil-Le Gal C, Raufaste D, Nielsen S, Horie S, Verbavatz JM, Devuyst O. PKD1 haploinsufficiency causes a syndrome of inappropriate antidiuresis in mice. J Am Soc Nephrol. 2007;18:1740–1753. doi: 10.1681/ASN.2006010052. [DOI] [PubMed] [Google Scholar]
- Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet. 2003;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24:75–78. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 2001;60:2164–2172. doi: 10.1046/j.1523-1755.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 Induces p21waf1 and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Hochegger K, Koppelstaetter C, Tagwerker A, Huber JM, Heininger D, Mayer G, Rosenkranz AR. p21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2007;292:F762–F768. doi: 10.1152/ajprenal.00084.2006. [DOI] [PubMed] [Google Scholar]
- Molls RR, Savransky V, Liu M, Bevans S, Mehta T, Tuder RM, King LS, Rabb H. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2006;290:F1187–F1193. doi: 10.1152/ajprenal.00342.2005. [DOI] [PubMed] [Google Scholar]
- Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol. 1998;9:614–619. doi: 10.1681/ASN.V94614. [DOI] [PubMed] [Google Scholar]
- Gardner KD, Jr, Burnside JS, Elzinga LW, Locksley RM. Cytokines in fluids from polycystic kidneys. Kidney Int. 1991;39:718–724. doi: 10.1038/ki.1991.87. [DOI] [PubMed] [Google Scholar]
- Amura CR, Brodsky KS, Gitomer B, McFann K, Lazennec G, Nichols MT, Jani A, Schrier RW, Doctor RB. CXCR2 agonists in ADPKD liver cyst fluids promote cell proliferation. Am J Physiol Cell Physiol. 2008;294:C786–C796. doi: 10.1152/ajpcell.00457.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet JP. Injury and development in polycystic kidney disease. Curr Opin Nephrol Hypertens. 1994;3:340–348. doi: 10.1097/00041552-199405000-00017. [DOI] [PubMed] [Google Scholar]
- Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrug M, Zhou J, Woo Y, Cui X, Szalai AJ, Novak J, Churchill GA, Guay-Woodford LM. Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int. 2008;73:63–76. doi: 10.1038/sj.ki.5002627. [DOI] [PubMed] [Google Scholar]
- Riera M, Burtey S, Fontes M. Transcriptome analysis of a rat PKD model: importance of genes involved in extracellular matrix metabolism. Kidney Int. 2006;69:1558–1563. doi: 10.1038/sj.ki.5000309. [DOI] [PubMed] [Google Scholar]
- Gogusev J, Murakami I, Doussau M, Telvi L, Stojkoski A, Lesavre P, Droz D. Molecular cytogenetic aberrations in autosomal dominant polycystic kidney disease tissue. J Am Soc Nephrol. 2003;14:359–366. doi: 10.1097/01.asn.0000046963.60910.63. [DOI] [PubMed] [Google Scholar]
- Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O'Hare MJ, Ong AC. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 2007;72:157–165. doi: 10.1038/sj.ki.5002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet. 2009;18:2523–2531. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet. 2009;18:2532–2542. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- Magistroni R, He N, Wang K, Andrew R, Johnson A, Gabow P, Dicks E, Parfrey P, Torra R, San-Millan JL, Coto E, Van Dijk M, Breuning M, Peters D, Bogdanova N, Ligabue G, Albertazzi A, Hateboer N, Demetriou K, Pierides A, Deltas C, St George-Hyslop P, Ravine D, Pei Y. Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:1164–1174. doi: 10.1097/01.asn.0000061774.90975.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.