Abstract
Reduced immune function is frequently a consequence of serious injury such as trauma-hemorrhage (T-H). Injury may lead to reduced T-cell activation, resulting in decreased engagement of costimulatory molecules after antigen recognition and in subsequent immunological compromise and anergy. We hypothesized that inhibition of CD28 expression is one possible mechanism by which immune functions are suppressed after T-H. Male C3H/HeN mice (with or without ovalbumin immunization) were subjected to sham operation or T-H and sacrificed after 24 hours. Splenic T cells were then stimulated with concanavalin A or ovalbumin in vivo or in vitro, and CD28, cytotoxic T-lymphocyte antigen 4 (CTLA-4), CD69, and phospho-Akt expression was determined. T-cell proliferation/cytokine production was measured in vitro. Stimulation-induced CD69, CD28, and phospho-Akt up-regulation were significantly impaired after T-H compared with sham-operated animals; however, CTLA-4 expression was significantly higher in the T-H group. Over a 3-day span, stimulated T cells from sham-operated animals showed significantly higher proliferation compared with the T-H group. IL-2 and IFN-γ were elevated in sham-operated animals, whereas IL-4 and IL-5 rose in the T-H group, revealing a shift from TH1 to TH2 type cytokine production after T-H. Dysregulation of the T-cell costimulatory pathway is therefore likely to be a significant contributor to post-traumatic immune suppression.
It is now generally accepted that serious injury in humans or experimental animal models may be followed by systemic inflammatory response syndrome and may frequently lead to subsequent multiple organ dysfunction syndrome, which remains a major cause of death despite numerous advances in trauma research.1,2,3,4,5 Perturbations of both the innate and adaptive immune systems after trauma-hemorrhage such as hyperactivity of macrophages and Kupffer cells, depressed dendritic cell functions, and impaired T-cell activities have been widely recognized as the cause of multiple organ dysfunction syndrome.6,7,8,9 However, interactions between innate and adaptive immune systems and their relative contribution to multiple organ dysfunction syndrome after trauma-hemorrhage are not completely understood.
The immune system has the remarkable ability to defend against various microbial pathogens and yet not to respond to self. This is due to the tightly regulated activation of T cells. T-cell activation requires two signals that are delivered by antigen-presenting cells (APCs).10 The first signal is the recognition by T-cell receptors of antigen presentation in the context of major histocompatability complex. The subsequent secondary or costimulatory signal is provided by molecules on APCs that engage cognate receptors on T cells. It is known that T cells will fail to recognize an antigen or enter a state of anergy in the absence of costimulation. The best-characterized T-cell costimulatory pathway involves the CD28 receptor, which binds to CD80 and CD86.11 The interaction of CD80 and CD86 with CD28 promotes the expansion of activated T cells and up-regulates cell survival protein expression.
Our previous studies revealed that the antigen-presenting capacity of dendritic cells is suppressed after trauma-hemorrhage because of the down-regulation of major histocompatability complex II expression and the decreased ability to stimulate T-cell proliferation.8 However, in that study, we also found that the expression of CD80 and CD86 on dendritic cells was not influenced by trauma-hemorrhage. However, in keeping with an overall pattern of immune unresponsiveness, we showed that T-cell activities were suppressed after trauma-hemorrhage.9,12 Hence, it is not clear whether posttraumatic T-cell suppression is secondary to suppression of APC functions or alternatively whether costimulatory molecules on T cells also play an important role in producing T-cell dysfunction. We hypothesized that the costimulatory factor CD28 is down-regulated after trauma-hemorrhage even though the expression of CD80 and CD86 on APCs is not affected under those conditions, and the decreased expression of CD28 on T-cell surface is another key mechanism that explains the impaired T-cell function after trauma-hemorrhage.
Materials and Methods
Animals, Experimental Groups, and Study Design
Male C3H/HeN mice 8 weeks old and weighing 22 to 25 g (Charles River Laboratories, Wilmington, MA) were fasted overnight before the experiment but were allowed water ad libitum. All experiments were performed in adherence with National Institutes of Health (Bethesda, MD) Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (Birmingham, AL). Animals were subjected to either the trauma-hemorrhage procedure or a sham operation. To study the impact of trauma-hemorrhage on T-cell activation and CD28 expression, T cells were activated either in vivo or in vitro and were activated either by concanavalin A (ConA) or by ovalbumin (OVA) immunization followed by OVA challenge.
Trauma-Hemorrhagic Shock Model
Animals were anesthetized with isoflurane (Minrad, Bethlehem, PA) and restrained in a supine position.13 A midline laparotomy (2 cm) was performed, which was then closed in two layers with sutures (Ethilon 6/0, Ethicon, Somerville, NJ). Both femoral arteries and a femoral vein were cannulated with polyethylene-10 tubing (Becton Dickinson, Sparks, MD). Blood pressure was measured via one of the arterial catheters using a blood pressure analyzer (Micro-Med, Louisville, KY). The animals were then restrained in a supine position in a small plastic box covered with a dark cloth to prevent the mice from seeing the surrounding environment and thus keeping them calm. On awakening, the mice were bled rapidly through the other arterial catheter to a mean arterial blood pressure of 35 ± 5 mmHg within 10 minutes. The rapid bleeding puts the animals in a state of depressed sensibility. Blood was further withdrawn slowly to reach a blood loss of 60% of the total circulating blood volume while still maintaining mean arterial pressure at 35 ± 5 mmHg. The entire hemorrhagic shock time lasted for 90 minutes. At the end of that interval, the animals were resuscitated with four times the shed blood volume with Ringer’s lactate over 30 minutes via the venous line. After resuscitation, the restraint was removed, and the animals were lightly anesthetized with isoflurane. After the blood vessels were ligated, catheters were removed, and the incision sites were bathed with lidocaine and closed with sutures. The animals were then allowed to awaken and were returned to their cages. Sham-operated animals underwent the same surgical procedures but were neither subjected to hemorrhage nor resuscitated. The animals were sacrificed at 24 hours after resuscitation or sham operation.
In Vivo Stimulation of Lymphocytes
For in vivo ConA stimulation, a nonhepatotoxic dosage of Con A (3 mg/kg b.wt.14) dissolved in 100 μl of Ringer’s lactate was injected via the venous line at the end of resuscitation. Sham-operated animals were also injected at the same time point. For OVA immunization, each mouse was injected s.c. with 0.1 ml of complete Freund’s adjuvant mixed with 0.1 mg of OVA 14 days before trauma-hemorrhage. After trauma-hemorrhage, mice were challenged with 0.1 mg of OVA again at the end of resuscitation or at the respective time point for sham-operated animals. ConA, OVA, and complete Freund’s adjuvant were all purchased from Sigma-Aldrich (St. Louis, MO).
Harvesting of Spleen and Isolation of Splenocytes
The animals were anesthetized with isoflurane 24 hours after resuscitation or sham operation. Spleens were removed aseptically and were isolated as described previously.15 In brief, spleens were gently ground to produce a single cell suspension, which was centrifuged at 400 × g for 10 minutes at 4°C. The erythrocytes were lysed with lysis buffer (BD Biosciences, San Jose, CA), and the remaining cells were washed with PBS by centrifugation (400 × g, 10 minutes, 4°C). After centrifugation, cells were resuspended in PBS to get a final concentration of 1 × 107 cells/ml. Splenocyte suspensions were divided into two parts for either flow cytometric analysis or further purification of CD4+ T cells and macrophages by magnetic separation.
Magnetic Separation of CD4+ T Cells and CD11b+ Macrophages
Isolated splenocytes were resuspended in MACS buffer (Miltenyi Biotec, Auburn, CA) and incubated with CD4 or CD11b microbeads (Miltenyi Biotec) at 4°C for 15 minutes according to the manufacturer’s instructions. After washing steps, CD4+ T cells and CD11b+ macrophages were positively selected by applying splenocytes onto MS columns that were placed on a MACS separator (all from Miltenyi Biotec). The magnetically labeled CD4+ T cells and CD11b+ macrophages were eluted and collected. The CD11b+ macrophages were used as antigen-presenting cells for experiments involving in vitro stimulation of T cells (see below). Only macrophages of sham animals were isolated.
Isolation of CD4+ T Cells and Regulatory T Cells
A regulatory T-cell staining kit was used to stain CD4+ T cells and regulatory T cells (Tregs) (eBioscience, San Diego, CA). The fraction of Tregs in CD4+ T cells was analyzed for splenocytes in the sham and trauma-hemorrhage groups. Furthermore, Tregs and CD4+ T cells were purified from splenocytes by using a CD4+CD25+ regulatory T-cell isolation kit and anti-CD4 microbeads according to the manufacturer’s instruction (all from Miltenyi Biotec, Auburn, CA).
Cell Surface Marker Staining and Flow Cytometric Analysis
Three anti-mouse antibodies against surface markers were used in this analysis: APC anti-CD4 antibody (Ab), phycoerythrin (PE) anti-CD28 Ab, and PE Cy7-anti-CD69 Ab (all from eBioscience). Splenocytes were washed and resuspended in staining buffer (PBS containing 0.5% bovine serum albumin and 0.09% sodium azide, Sigma-Aldrich) at a concentration of 1 × 107 cells/ml and were stained as described previously.8 In brief, after blocking with anti-CD16/32 Ab (eBioscience) for 15 minutes on ice, cells were stained with the above indicated antibodies for 45 minutes at 4°C in the dark. Cells were then washed, resuspended in staining buffer, and analyzed using a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA). A total of 50,000 events were collected for analysis.
Staining of Cytotoxic T-Lymphocyte Antigen-4 and Phospho-Akt for Flow Cytometric Analysis
A PE-conjugated American hamster anti-mouse cytotoxic T-lymphocyte antigen-4 (CTLA-4) monoclonal antibody (eBioscience) and an Alexa Fluor 488-conjugated anti-phospho-Akt (p-Akt) rabbit monoclonal antibody (Cell Signaling Technology, Danvers, MA) were used in this study. Cells were surface-stained with APC anti-CD4 Ab as described in the previous section and were then fixed in 2% formaldehyde for 20 minutes at room temperature. After washing, cells were resuspended in permeabilization buffer (eBioscience) and incubated with either anti-p-Akt Ab or anti-CTLA4 Ab for 30 minutes. After 2 additional washes, cells were resuspended in staining buffer and analyzed on a flow cytometer. A total of 50,000 events were collected for analysis.
In Vitro Stimulation and Determination of CD4+ T-Cell Proliferation
To determine any changes in proliferative capacity of T cells after trauma-hemorrhage or sham operation, T cells were cultured and challenged with either ConA or OVA. For ConA stimulation, purified CD4+ T cells from sham or trauma-hemorrhage animals were resuspended in complete RPMI 1640 medium (10% fetal bovine serum, 2 mmol/L glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 20 μg/ml gentamicin, all from Invitrogen, Carlsbad, CA) with or without 5 μg/ml of ConA and plated in a 96-well plate at a cell density of 1 × 105 cells/well. For OVA challenge, isolated splenic macrophages from a sham mouse were first incubated for 30 minutes (37°C, 5% CO2, in the dark) with 50 μg/ml mitomycin C (Sigma-Aldrich) to serve as APCs. After washing, these APCs were cultured with T cells (1 × 105 cells/well at a 1:1 ratio) purified from OVA-immunized sham or trauma-hemorrhage animals in culture medium with or without 0.1 mg/ml of OVA. The proliferation capacities of T cells were measured after 24, 48, and 72 hours of culture, respectively. Cell proliferation was determined using a Cell Proliferation Biotrak ELISA System (Amersham Biosciences Inc., Piscataway, NJ) as described previously.8 In brief, 5-bromo-2′-deoxyuridine was added to the cell suspension, which was then given 3-hour exposure to the substrate. The cells were fixed, and their DNA was denatured and then incubated with peroxidase-labeled anti-5-bromo-2′-deoxyuridine Ab according to the manufacturer’s instructions. The optical density of each well was determined by a spectrophotometer at 450 nm after the peroxidase substrate 3,3′,5,5′-tetramethylbenzide was added to the cells.
Determination of CD28 Expression and Akt Activation on CD4+ T Cells after in Vitro Stimulation
CD28 and Akt activation were measured in cultured T cells by flow cytometry. Cells were cultured in the same way as those for measuring cellular proliferation capacity. After 3 days of culture, cells were harvested, stained for CD4, CD28, and p-Akt, and analyzed using flow cytometry as described in the previous section. The mean fluorescence intensity (MFI) of CD28 and p-Akt of those events gated as CD4+ were measured.
Flow Cytometric Analysis of Cytokine Concentration
The cytokine concentrations in culture supernatant were determined with a cytometric bead array using flow cytometry according to the manufacturer’s instructions (BD Pharmingen, San Diego, CA) as described previously.16 In brief, 50 μl of mixed capture beads were incubated with 50 μl of sample for 1 hour at 25°C, and then 50 μl of mixed PE-conjugated detection antibody was added. After incubation for 1 hour at 25°C in the dark, the complexes were washed twice and analyzed using the LSRII flow cytometer. Data analysis was performed using the FACSDiva and FCAP array software (BD Biosciences).
Statistics
Statistical analysis was performed using Sigma-Stat computer software (SPSS, Chicago, IL). Statistical significance was assumed when probability values of less than 0.05 were obtained. Comparisons between groups were performed using one-way analysis of variance followed by Tukey’s test. Results are expressed as mean ± SE.
Results
Cellular Activation and Expression of Costimulatory Molecules on in Vivo-Stimulated T Cells after Trauma-Hemorrhage
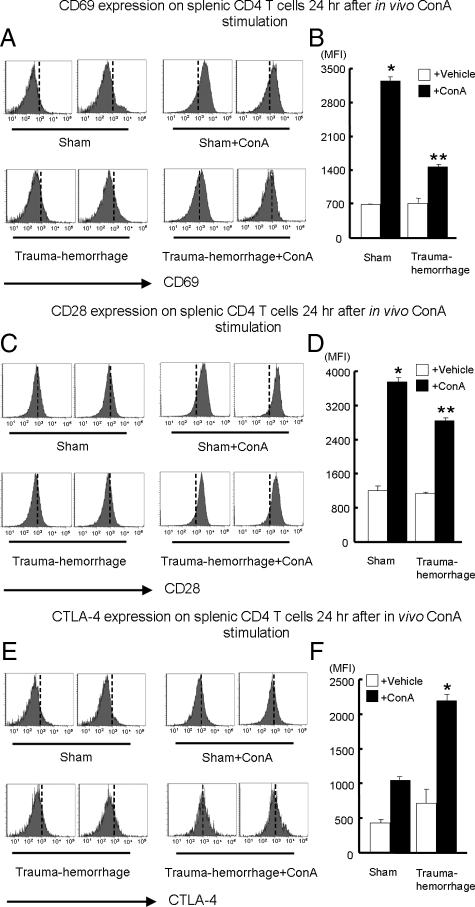
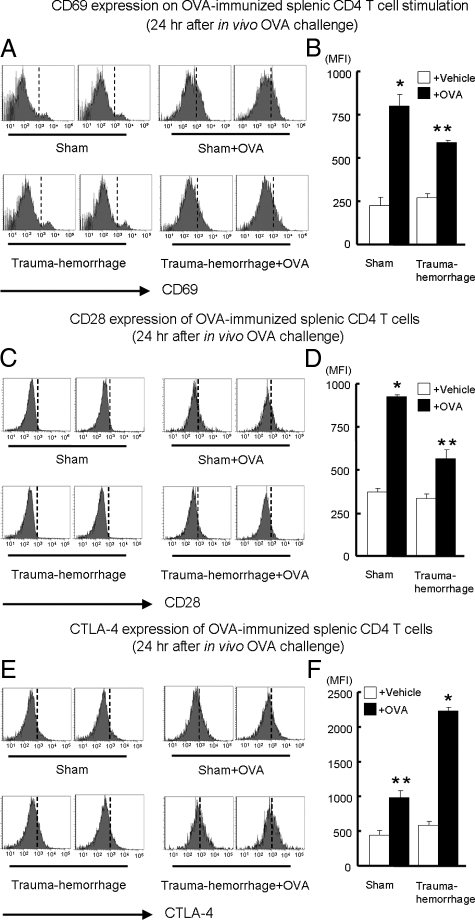
The expression of CD69, CD28, and CTLA-4 on splenic CD4+ T cells was analyzed 24 hours after in vivo stimulation. As shown in Figure 1, A and B, CD69 expression was significantly increased after ConA stimulation compared with that for matched unstimulated controls. In addition, when one compares both stimulated groups, CD69 expression by sham-treated animals was significantly higher than that in the trauma-hemorrhage group. There were no differences between unstimulated sham and trauma-hemorrhage groups. The expression of CD28 with or without ConA stimulation showed a similar pattern as observed for CD69. As shown in Figure 1, C and D, although ConA stimulation induced a significant increase in CD28 expression, such an increase was suppressed by the trauma-hemorrhage insult. No differences in CD28 expression were noted between the unstimulated groups. In contrast, as shown in Figure 1, E and F, the expression of CTLA-4 was significantly higher in the ConA-stimulated trauma-hemorrhage group than in all other groups. The OVA challenge induced increased CD69 and CD28 expression on T cells similar to those stimulated by ConA; however, such increases were suppressed by the trauma-hemorrhage insult (Figure 2, A–D). On the other hand, although CTLA-4 expression was elevated after OVA challenge in sham and trauma-hemorrhage groups, trauma-hemorrhage was found to induce a further increase in CTLA-4 compared with that in the sham group (Figure 2, E and F).
Figure 1.
Expression of CD69, CD28, and CTLA-4 on splenic CD4+ T cells after in vivo ConA stimulation. For in vivo stimulation, 3 mg/kg of ConA were injected i.v. at the end of resuscitation in trauma-hemorrhage groups or at corresponding time points in sham groups. Twenty-four hours after the end of resuscitation, splenocytes with or without stimulation were isolated and stained with APC anti-CD4 Ab plus either PE Cy7-anti-CD69 Ab, PE anti-CD28 Ab, or PE anti-CTLA-4 Ab as described in Materials and Methods. Representative histograms were gated on CD4+ T cells and showed MFIs of CD69 (A), CD28 (C), and CTLA-4 (E). MFI data for CD69 (B), CD28 (D), and CTLA-4 (F) are shown as mean ± SEM. *P < 0.05 versus all of the other groups. **P < 0.05 versus vehicle groups. n = 5/group. The vertical dotted line in each histogram is intended for better recognition of the shift of MFI among different treatment groups.
Figure 2.
Expression of CD69, CD28, and CTLA-4 on splenic CD4+ T cells after OVA immunization and in vivo OVA challenge. In this experiment, all of the animals were immunized with OVA 14 days before trauma-hemorrhage by injection of 0.1 ml of complete Freund’s adjuvant mixed with 0.1 mg of OVA. Another 0.1 mg of OVA or vehicle (PBS) was injected at the end of resuscitation in trauma-hemorrhage groups or at corresponding time points in sham groups. Twenty-four hours after the end of resuscitation, splenocytes with or without OVA challenge were isolated and stained with APC anti-CD4 Ab plus either PE Cy7-anti-CD69 Ab, PE anti-CD28 Ab, or PE anti-CTLA-4 Ab as described in Materials and Methods. Representative histograms were gated on CD4+ T cells and showed MFI of CD69 (A), CD28 (C), and CTLA-4 (E). MFI data for CD69 (B), CD28 (D), and CTLA-4 (F) are shown as mean ± SEM. *P < 0.05 versus all of the other groups. **P < 0.05 versus vehicle groups. n = 5/group. The vertical dotted line in each histogram is intended for better recognition of the shift of MFI among different treatment groups.
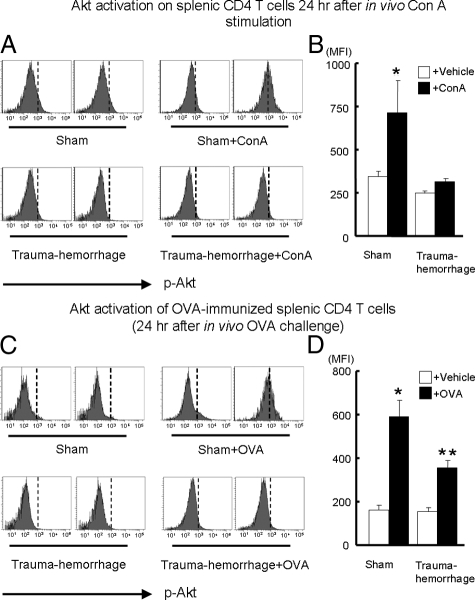
Activation of Akt after in Vivo Stimulation
As shown in Figure 3, A and B, the expression of p-Akt was significantly increased in ConA-stimulated sham animals compared with that in other groups. However, in the ConA-stimulated trauma-hemorrhage animals, it was significantly lower than in sham-operated animals and was similar to that in those without stimulation. In the experiments in which animals were challenged with OVA in vivo, the p-Akt levels increased significantly after OVA challenge compared with those without OVA challenge. Moreover, sham animals challenged with OVA had significantly higher p-Akt levels compared with trauma-hemorrhage animals challenged with OVA (Figure 3, C and D).
Figure 3.
Expression of phospho-Akt on splenic CD4+ T cells after stimulation with ConA or OVA in vivo. Splenic T cells were activated either by in vivo ConA stimulation or by OVA challenge after prior OVA immunization as described in Materials and Methods. Twenty-four hours after the end of resuscitation, splenocytes with or without stimulation were isolated and surface stained with APC anti-CD4 Ab. Cells were than fixed, permeabilized and stained with Alexa Fluor 488-conjugated anti-p-Akt Ab as described in Materials and Methods. Representative histograms were gated on CD4+ T cells and showed MFI of p-Akt after ConA stimulation (A) or OVA challenge (C). MFI data for p-Akt after ConA stimulation (B) or OVA challenge (D) are shown as mean ± SEM. *P < 0.05 versus. all of the other groups. **P < 0.05 versus vehicle groups. n = 5/group. The vertical dotted line in each histogram is intended for better recognition of the shift of MFI among different treatment groups.
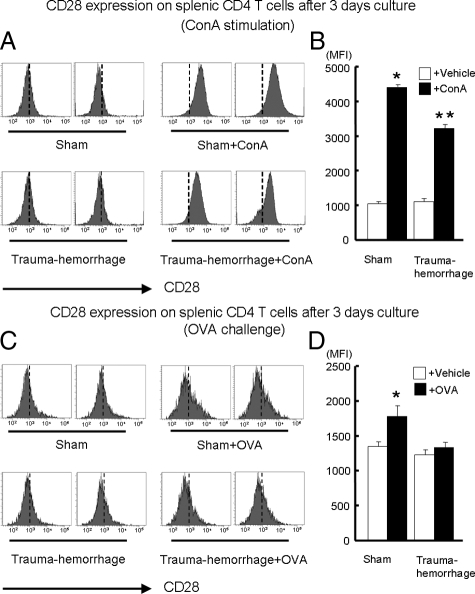
Expression of CD28 on in Vitro Stimulated T Cells after Trauma-Hemorrhage
Although we demonstrated that trauma-hemorrhage suppressed the responses of T cells to in vivo activation, we further examined whether this suppression also occurred in vitro. As shown in Figure 4, A and B, ConA significantly induced an increase in CD28 expression in both sham and trauma-hemorrhage groups compared with unstimulated groups. We also found that cells of the ConA-stimulated sham group had higher CD28 expression compared with that of the ConA-stimulated trauma-hemorrhage group. Moreover, OVA stimulation induced a marked increase in CD28 expression in cells of the sham group, an effect not seen in the OVA-stimulated trauma-hemorrhage group (Figure 4, C and D).
Figure 4.
Expression of CD28 on cultured splenic CD4+ T cells after in vitro ConA or OVA stimulation. For ConA stimulation, purified CD4+ T cells from sham or trauma-hemorrhage animals were resuspended in complete RPMI 1640 medium with or without 5 μg/ml of ConA and plated in a 96-well plate at a cell density of 1 × 105 cells/well. For OVA stimulation, isolated splenic macrophages from a sham mouse were first incubated for 30 minutes with 50 μg/ml mitomycin C to serve as APCs. After washing, these APCs were cultured with CD4+ T cells (1 × 105 cells/well at a 1:1 ratio) purified from OVA-immunized sham or trauma-hemorrhage animals in RPMI 1640 medium with or without 0.1 mg/ml of OVA. After 3 days of culture, cells were harvested and stained with APC anti-CD4 Ab and PE anti-CD28 Ab as described in Materials and Methods. Representative histograms were gated on CD4+ T cells and showed MFI of CD28 after ConA stimulation (A) or OVA challenge (C). MFI data for CD28 after ConA stimulation (B) or OVA challenge (D) are shown as mean ± SEM. *P < 0.05 versus all other groups. **P < 0.05 versus vehicle groups. n = 5/group. The vertical dotted line in each histogram is intended for better recognition of the shift of MFI among different treatment groups.
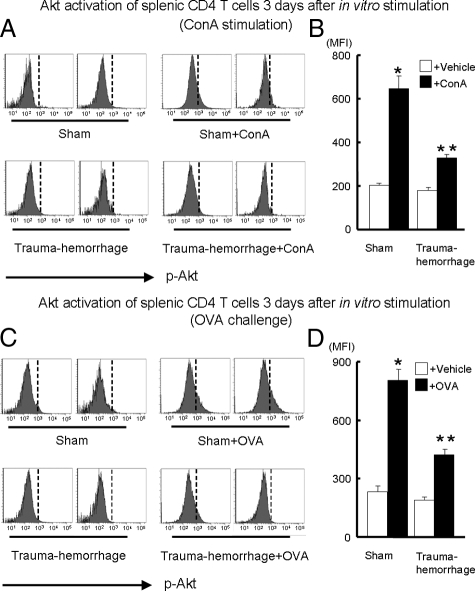
Activation of Akt after in Vitro Activation
As shown in Figure 5, A and B, comparison between the stimulated and unstimulated subsets, ConA treatment induced a marked increase in p-Akt in both sham and trauma-hemorrhage groups. Moreover, the increase was more pronounced in the sham group compared with that in the trauma-hemorrhage group. There was no difference in p-Akt expression in unstimulated groups. In the experiments in which the cells were stimulated by OVA, a result similar to that in the ConA-stimulated group was observed. Whereas p-Akt levels of T cells were increased after OVA stimulation, they were significantly elevated in the sham versus the trauma-hemorrhage group (Figure 5, C and D).
Figure 5.
Expression of p-Akt on cultured splenic CD4+ T cells after in vitro ConA or OVA stimulation. For ConA stimulation, purified CD4+ T cells from sham or trauma-hemorrhage animals were resuspended in complete RPMI 1640 medium with or without 5 μg/ml of ConA and plated in a 96-well plate at a cell density of 1 × 105 cells/well. For OVA stimulation, isolated splenic macrophages from a sham mouse were first incubated for 30 minutes with 50 μg/ml mitomycin C to serve as APCs. After washing, APCs were cultured with CD4+ T cells (1 × 105 cells/well at a 1:1 ratio) purified from OVA-immunized sham or trauma-hemorrhage animals in RPMI 1640 medium with or without 0.1 mg/ml of OVA. After 3 days of culture, cells were harvested and stained with APC anti-CD4 Ab. Cells were then fixed, permeabilized, and stained with Alexa Fluor 488-conjugated anti-p-Akt Ab as described in Materials and Methods. Representative histograms were gated on CD4+ T cells and showed MFI of p-Akt after ConA stimulation (A) or OVA challenge (C). MFI data for p-Akt after ConA stimulation (B) or OVA challenge (D) are shown as mean ± SEM. *P < 0.05 versus all of the other groups. **P < 0.05 versus vehicle groups. n = 5/group. The vertical dotted line in each histogram is intended for better recognition of the shift of MFI among different treatment groups.
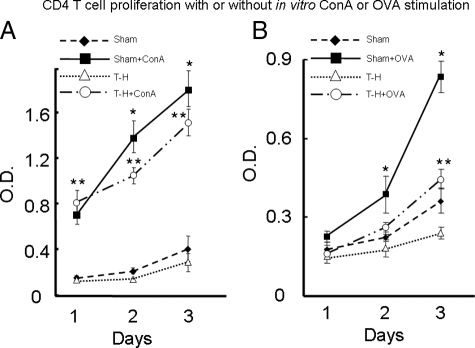
T-Cell Proliferation Capacities With or Without Stimulation
As shown in Figure 6A, T cells proliferated minimally throughout 3 days of culture in nonstimulated groups. Beginning 24 hours after culture, the proliferation of ConA-stimulated T cells was markedly increased compared with that in those without stimulation. Moreover, although the extent of T-cell proliferation in the stimulated sham and trauma-hemorrhage groups was similar after 24 hours of culture, the ConA-stimulated sham T cells showed increased proliferative capacity after 48 and 72 hours of culture compared with ConA-stimulated trauma-hemorrhage T cells. For those T cells stimulated by APC-processed OVA (Figure 6B) after 48 hours in culture, T cells of the OVA-stimulated sham group proliferated more compared with all of the other groups. However, by 72 hours after culture the proliferation of T cells in OVA-stimulated trauma-hemorrhage groups was significantly increased compared with that in the nonstimulated groups.
Figure 6.
Proliferation of splenic CD4+ T cells after in vitro ConA or OVA stimulation. The isolation, stimulation, and culture of T cells were the same as in Figures 4 and 5 and are described in Materials and Methods. The proliferation capacities of T cells were measured after 24, 48, and 72 hours of culture, respectively, and were determined using a 5-bromo-2′-deoxyuridine-based, commercially available ELISA kit as described in Materials and Methods. A: T-cell proliferation with or without ConA stimulation. B: T cell proliferation with or without OVA stimulation. Data are shown as mean ± SEM. *P < 0.05 compared with all of the other groups on the same day. **P < 0.05 compared with nonstimulated groups on the same day. n = 5/group.
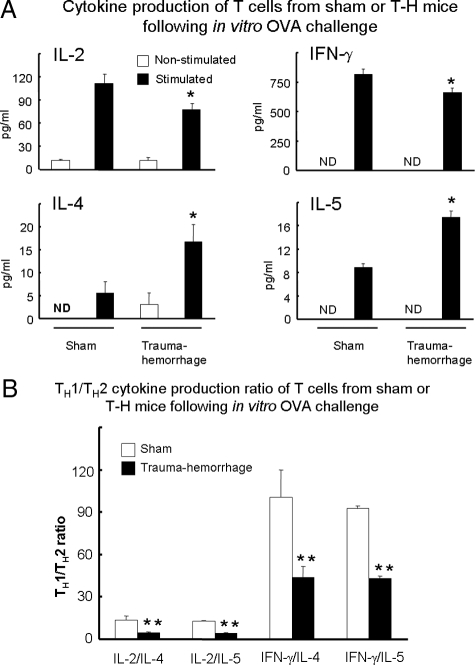
Cytokine Production Capacities of T Cells With or Without Ova Stimulation
As shown in Figure 7A, unstimulated T cells produced minimal amounts of interleukin (IL)-2, interferon (IFN)-γ, IL-4, and IL-5. The cytokine response of T cells to OVA stimulation was different between sham and trauma-hemorrhage groups. T cells in the sham group produced significantly more IL-2 compared with those in the trauma-hemorrhage group. In contrast, cells in the trauma-hemorrhage group produced more IL-4 and IL-5 compared with those in the sham group. Furthermore, TH1 (IL-2 and IFN-γ)/TH2 (IL-4 and IL-5) cytokine production ratios in the trauma-hemorrhage group were significantly lower than those in the sham group, indicating that T-cell cytokine production shifted from TH1 type to TH2 type after trauma-hemorrhage (Figure 7B). In addition, to exclude the possibility that the increased IL-2 and IFN-γ production in the sham group was due to increased numbers of T cells being generated during culture, intracellular cytokine staining was performed to determine cytokine production on a per cell basis. These measurements revealed that intracellular cytokine production of IL-2 and IFN-γ was significantly suppressed after trauma-hemorrhage compared with those of the sham group (supplementary Figure 1, A and B, see http://ajp.amjpathol.org).
Figure 7.
Cytokine production capacities of splenic CD4+ T cells with or without OVA stimulation. Purified OVA-immunized splenic CD4+ T cells were cultured with mitomycin-treated macrophages with or without OVA challenge as described in Materials and Methods. After 3 days of culture, cytokine concentrations of culture supernatant were determined with cytometric bead arrays. A: Concentrations of IL-2, IFN-γ, IL-4, and IL-5. B: ratios of IL-2 or IFN-γ to IL-4 or IL-5. Data are shown as mean ± SEM. *P < 0.05 compared with other groups in A. **P < 0.05 compared with sham animal of the same group in B. n = 5/group; ND, nondetectable.
Discussion
The immune system is well designed to defend against diverse pathogens, while avoiding a response to self. In their role as the key mediators of the immune system, T-cell activation is tightly regulated to maintain immune homeostasis.17 As an example of a fail-safe mechanism, T-cell activation requires two signals that are delivered by APCs. Whereas the first signal mediates the recognition of a specific antigen by T-cell receptors, the second signal (costimulatory signal) stimulates the expansion and differentiation of T cells.17 CD28 interaction with either of its two ligands (CD80 and CD86) borne by APCs is widely recognized as the major T-cell costimulatory pathway and thus is important for initiating T-cell responses, such as enhancing the production of IL-2 as well as T-cell proliferation.11 Therefore, there is great interest in manipulating these costimulatory signals to either enhance antimicrobial immunity or treat autoimmune diseases.18,19,20,21
In contrast, trauma-hemorrhagic shock induces serious and widespread dysregulation of immune functions,22 including suppression of T-cell activation in response to a variety of stimuli.9 In this study, the up-regulation of CD69 expression (a marker of early-stage activation) in stimulated T cells was suppressed after trauma-hemorrhage. This observation indicates that trauma-hemorrhage inhibited T-cell activation shortly after the insult at an upstream level of signal transduction. Whereas CD28 costimulatory factor plays an important role in enhancing the efficiency of T-cell activation, our results suggest that one of the mechanisms by which trauma-hemorrhage suppresses T-cell activation is by altering the balance between costimulatory and regulatory signals. It is well known that to maintain immune homeostasis and prevent harmful inflammatory responses, the expression of CD28 also leads to the up-regulation and expression of CTLA-4,10 a competitive negative T-cell regulator. CTLA-4 antagonizes the major events mediated by CD28 signal cascades, including T-cell proliferation and IL-2 production, therefore preventing or dampening uncontrolled T-cell activities.10 In keeping with this role, when we compared the stimulated T cells of the sham group with those from the trauma-hemorrhage group, we observed a decreased level of CD28 expression and a corresponding increased level of CTLA-4 expression. This finding suggests that the balance of CD28/CTLA-4 signaling had shifted in favor of inducing T-cell anergy after trauma-hemorrhage.
It should be reiterated that, in contrast to the surface-stained CD28, the level of CTLA-4 expression was determined by intracellular staining. CTLA-4 is primarily an intracellular antigen whose surface expression is tightly regulated by restricted trafficking to the cell surface and rapid internalization.23 As minor changes in surface expression levels may have major effects on the outcome of T-cell activation, CTLA-4 levels are known to rapidly decrease in the absence of new protein translation, and the molecule is quickly degraded on internalization.24,25 Although our results revealed increased intracellular CTLA-4 expression after trauma-hemorrhage, we were not able to demonstrate increased surface expression of CTLA-4 under those conditions (supplemental Figure 2, see http://ajp.amjpathol.org). One possible explanation for this observation is that CTLA-4 was up-regulated and expressed on the T-cell surface, accounting for T-cell suppression soon after trauma-hemorrhage but was subsequently rapidly internalized within 24 hours after the insult.
Our results were consistent with the works of Laudanski et al,26 who conducted a genome-wide expression analysis to reveal the alteration of T-cell signaling pathways of human subjects after severe blunt trauma. In that study, decreased CD28 gene expression of T cells was noted in trauma patients compared with those from healthy individuals.26 Moreover, our previous studies showed that the expression of the CD28 ligands CD80 and CD86 on dendritic cells was not decreased after trauma-hemorrhage.8 Taken together, these results further emphasize the significance of CD28 down-regulation as the likely mechanism that, through negatively affecting T-cell activity (rather than APCs), accounts at least in part for the reduced immunological responsiveness after trauma-hemorrhage. In addition, other than CD28 and CTLA-4, numerous other pathways and molecules have been proposed to be involved in the suppression of T-cell responsiveness after trauma-hemorrhage.27 To further clarify how these pathways interact with each other, an extension of the current study may be to determine the relative contribution of each molecule important to T-cell suppression. More specifically, it will be illuminating to study the effects of hypoxia per se on T cells in vitro with the specific objective of observing the effects of hypoxia/hypoxia-inducible factor-1α on CD28 expression.
It could be argued that because the animals were sacrificed 24 hours after trauma-hemorrhage, it was unclear whether this posttraumatic period had a different impact on cellular responses compared with those immediately after trauma-hemorrhage. Because trauma-hemorrhage induces physiological derangements such as systemic inflammatory response syndrome and multiple organ dysfunction syndrome within hours and days after the insult, our current results suggest that the dysregulation of CD28 expression and associated altered T-cell activities were also part of such immune derangements and were a direct result of the trauma-hemorrhage insult. Furthermore, our previous findings demonstrated a continuity of alteration in cellular responses, ie, if a depression or enhancement of cellular function was evident at 2 hours, it was also evident at 24 hours after injury.12,13 Based on these observations, although we only investigated one time point (24 hours after injury) in the current study, it would be reasonable to assume that the differences in T-cell activation and regulation after sham and trauma-hemorrhage would be sustained between 2 and 24 hours.
Recently, the phosphatidylinositol 3-kinase/Akt pathway has been recognized as an important regulatory mechanism involving cell survival, activation, and glucose metabolism.28,29,30,31,32 Previous studies from our laboratory have shown that Akt activation in various tissues and cell types such as intestine, cardiomyocytes, hepatocytes, and Kupffer cells was decreased after trauma-hemorrhage.33,34,35,36 This investigation showed consistently that the ConA- or OVA-stimulated T cells expressed less p-Akt after trauma-hemorrhage than with sham animals. Although studies have shown that Akt is one of the key molecules that mediate CD28 costimulatory signaling,37,38 several regulatory mechanisms may account for the down-regulation of p-Akt after trauma-hemorrhage. First, phosphatidylinositol 3-kinase is known to be the upstream molecule that regulates Akt activation. Our previous publications revealed that inhibition of phosphatidylinositol 3-kinase activity could suppress the activation of Akt.34,35 Furthermore, Parry et al39 demonstrated that the Akt-mediated CD28 signaling was phosphatidylinositol 3-kinase-dependent. In addition, up-regulated CTLA-4 is able to block Akt activity by inhibiting Akt phosphorylation,40,41,42 which could be mediated by engaging inhibitory signal transduction machinery such as protein phosphatase type 2A and SHP-2.43,44,45 Our data therefore suggest that the suppressed T-cell activities after trauma-hemorrhage could be accounted for by a shift from a pattern dominated by CD28 expression into one favoring CTLA-4, which subsequently down-regulates Akt activity.
Helper T cells (TH) are divided into two opposing phenotypes: TH1 cells are characterized by the production of IL-2 and IFN-γ, and, conversely, TH2 cells produce IL-4 and IL-5. Our results revealed that the cytokine production profiles were different between stimulated T cells in the sham and trauma-hemorrhage groups. Those in the sham group produced significantly higher amounts of TH1-type cytokines (IL-2 and INF-γ), whereas those in the trauma-hemorrhage group produced significantly higher amounts of TH2-type cytokines (IL-4 and IL-5). The observed dysregulation of TH1/TH2 phenotype and cytokine production balance after trauma-hemorrhage has also been seen in human/animal subjects after major injuries or surgery.46,47 Studies of Kane et al41 showed that Akt mediated the CD28 costimulatory signal for up-regulation of IL-2 and IFN-γ but not TH2 cytokines,41 indicating that the suppressed p-Akt expression after trauma-hemorrhage might be a contributor to the decreased TH1 cytokine production of T cells under those conditions. Furthermore, because IL-2 is a well recognized promoter of T-cell activation and proliferation,48,49 it was not surprising that the increased concentration of IL-2 resulted in the progressive increase in proliferation capacities of sham group T cells over a 3-day span compared with those of the trauma-hemorrhage group.
Although splenic CD4+ T cells exhibited a diminished proliferative capacity and altered cytokine production profile after trauma-hemorrhage, one significant question is whether there were changes in splenic CD4+ T-cell numbers under those conditions. Although splenic CD4+ T-cell numbers could be affected by different events such as acute, severe blood loss with attendant lymphopoietic compensation, as well as inflammatory responses after trauma-hemorrhage, we noticed that there were no significant differences in the number of total splenocytes and T cells from each spleen harvested, either from sham or trauma-hemorrhage animals (data not shown). Consistent with our observation, Abraham and Freitas50 reported that there were no changes in the total number of splenocytes or of splenic B cells after hemorrhage; however, the number of B cells secreting immunoglobulins was significantly decreased during the period from 2 to 96 hours after hemorrhage. Taken together, these results indicate that the most plausible explanation for the response patterns of splenic CD4+ T cells to trauma-hemorrhage is adversely altered proliferation competence combined with reduced cytokine production capacity, whereas splenic CD4+ T-cell numbers would remain unchanged within the 24 hour interval after trauma-hemorrhage.
One may also argue as to whether there are differential responses to trauma-hemorrhage among each subset of CD4+ T cells, such as the differences between Tregs and T helper cells, as well as differences among naive, memory, and effector T cells. Enhanced Treg activity has been reported to be an element of immune suppression after injury.51,52 Our ongoing study of splenic Tregs has revealed that the activities of the Tregs were enhanced after trauma-hemorrhage. We found that although the IL-2 and IFN-γ production capacities of T-helper cells alone were decreased after trauma-hemorrhage, they were further suppressed by approximately 50% if T-helper cells were co-cultured with Tregs (supplemental Figure 3, A and B, see http://ajp.amjpathol.org). Furthermore, because it is known that naive CD4+ T cells are less sensitive to antigenic stimulation than memory T cells and that the cytokine production capacities are different among naive, central memory T cells and effector memory T cells,53,54 it is possible that suppression of effector T-cell function(s) correlates with the decreased cytokine production after trauma-hemorrhage. Thus, it would be of great interest to determine the differential effects of trauma-hemorrhage on the various CD4+ T-cell subsets to gain insights into their roles in immune suppression after injury.
ConA is a polyvalent tetrameric lectin that binds to oligosaccharides on the cell surface and triggers a direct but nonspecific activation of T cells.55 In contrast, T cells in the animals immunized with OVA would be activated via a classic antigen-specific pathway, involving antigen presentation by APC and T-cell receptor recognition, followed by costimulatory factor engagement and signal transduction. Our results showed that regardless of whether T cells were specifically or nonspecifically activated, the expression of CD28 on T cells after trauma-hemorrhage was suppressed compared with that of sham T cells. Thus, in a hierarchical sense, these findings suggest that the insult of trauma-hemorrhage could suppress T-cell activities globally and lead to widespread immunological dysfunction. Furthermore, the relative difference in T-cell proliferative capacity between OVA-stimulated sham and trauma-hemorrhage groups was greater than that seen in the respective ConA-stimulated groups, even though the magnitude of response was greater with ConA. This result probably occurs because ConA is regarded as a strong mitogen56 and thus operates outside of the normal physiology of T-cell activation in adaptive immunity (ie, antigen presentation via the T-cell receptor and second signals). Thus, we speculate that mitogen stimulation may circumvent some of the vulnerability to trauma-hemorrhage compared with that of the sham group.
Our results demonstrate that the suppression of T-cell activation after trauma-hemorrhage was apparent in cells immediately after harvesting or 3 days after culture. The inhibition of CD28 expression and Akt activation followed a similar temporal pattern. It is thus interesting to note that, although the trauma-hemorrhage insult occurred only in vivo, we showed that its effects on the explanted cells persisted for at least 3 days after culture. This matches the clinical experience in that patients are at high risk of immune suppression and subsequent infection even though their major injury has been successfully treated.57 In addition to the well known fact that post-traumatic suppression of adaptive immunity is due to reduced major histocompatability complex II expression and impaired antigen presentation,8,58,59 our current study indicated that dysregulation of T-cell costimulatory signaling may also play a crucial role in the induction of T-cell anergy after trauma-hemorrhage.
Supplementary Material
Acknowledgments
We thank Bobbi Smith for her editorial skill and assistance.
Footnotes
Address reprint requests to Dr. Irshad H. Chaudry, Ph.D. Center for Surgical Research, University of Alabama at Birmingham, G094 Volker Hall, 1670 University Blvd., Birmingham, AL 35294-0019. E-mail: irshad.chaudry@ccc.uab.edu.
Supported by National Institutes of Health (grant R01 GM37127).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of C.-H.H.: Department of Trauma and Emergency Surgery and Department of Surgery, China Medical University Hospital, China Medical University, Taichung, Taiwan; current address of J.T.-H.: Department of Surgery, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan.
References
- Noel G, Guo X, Wang Q, Schwemberger S, Byrum D, Ogle C. Postburn monocytes are the major producers of TNF-α in the heterogeneous splenic macrophage population. Shock. 2007;27:312–319. doi: 10.1097/01.shk.0000239753.75088.5e. [DOI] [PubMed] [Google Scholar]
- Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 1996;87:4261–4275. [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand F, Hubbard WJ, Choudhry MA, Frink M, Pape HC, Kunkel SL, Chaudry IH. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am J Pathol. 2006;169:784–794. doi: 10.2353/ajpath.2006.060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–4520. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- Schneider CP, Schwacha MG, Chaudry IH. Influence of gender and age on T-cell responses in a murine model of trauma-hemorrhage: differences between circulating and tissue-fixed cells. J Appl Physiol. 2006;100:826–833. doi: 10.1152/japplphysiol.00898.2005. [DOI] [PubMed] [Google Scholar]
- Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7. doi: 10.1023/a:1014256417651. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Chaudry IH. Salutary effects of 17β-estradiol on T-cell signaling and cytokine production after trauma-hemorrhage are mediated primarily via estrogen receptor-α. Am J Physiol Cell Physiol. 2007;292:C2103–C2111. doi: 10.1152/ajpcell.00488.2006. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Chaudry IH. The role of MIP-1α in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–2812. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun R, Wei H, Dong Z, Tian Z. Pre-activation of T lymphocytes by low dose of concanavalin A aggravates toll-like receptor-3 ligand-induced NK cell-mediated liver injury. Int Immunopharmacol. 2006;6:800–807. doi: 10.1016/j.intimp.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, Chaudry IH. Are the protective effects of 17β-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-α or ER-β? J Leukoc Biol. 2006;79:1173–1180. doi: 10.1189/jlb.0106029. [DOI] [PubMed] [Google Scholar]
- Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol. 2007;44:2625–2630. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Martins GA, Campanelli AP, Silva RB, Tadokoro CE, Russo M, Cunha FQ, Rizzo LV, Silva JS. CD28 is required for T cell activation and IFN-γ production by CD4+ and CD8+ T cells in response to Trypanosoma cruzi infection. Microbes Infect. 2004;6:1133–1144. doi: 10.1016/j.micinf.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Newell KA, He G, Guo Z, Kim O, Szot GL, Rulifson I, Zhou P, Hart J, Thistlethwaite JR, Bluestone JA. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163:2358–2362. [PubMed] [Google Scholar]
- Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Beyersdorf N, Ding X, Blank G, Dennehy KM, Kerkau T, Hunig T. Protection from graft versus host disease with a novel B7 binding site-specific mouse anti-mouse CD28 mAb. Blood. 2008;112:4328–4336. doi: 10.1182/blood-2008-03-146662. [DOI] [PubMed] [Google Scholar]
- Coimbra R, Melbostad H, Loomis W, Porcides RD, Wolf P, Tobar M, Hoyt DB. LPS-induced acute lung injury is attenuated by phosphodiesterase inhibition: effects on proinflammatory mediators, metalloproteinases. NF-κB, and ICAM-1 expression. J Trauma. 2006;60:115–125. doi: 10.1097/01.ta.0000200075.12489.74. [DOI] [PubMed] [Google Scholar]
- Valk E, Rudd CE, Schneider H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008;29:272–279. doi: 10.1016/j.it.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- Maszyna F, Hoff H, Kunkel D, Radbruch A, Brunner-Weinzierl MC. Diversity of clonal T cell proliferation is mediated by differential expression of CD152 (CTLA-4) on the cell surface of activated individual T lymphocytes. J Immunol. 2003;171:3459–3466. doi: 10.4049/jimmunol.171.7.3459. [DOI] [PubMed] [Google Scholar]
- Laudanski K, Miller-Graziano C, Xiao W, Mindrinos MN, Richards DR, De A, Moldawer LL, Maier RV, Bankey P, Baker HV, Brownstein BH, Cobb JP, Calvano SE, Davis RW, Tompkins RG. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci USA. 2006;103:15564–15569. doi: 10.1073/pnas.0607028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, Bankey P, Miller-Graziano C. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35:794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- Summers SA, Yin VP, Whiteman EL, Garza LA, Cho H, Tuttle RL, Birnbaum MJ. Signaling pathways mediating insulin-stimulated glucose transport. Ann NY Acad Sci. 1999;892:169–186. doi: 10.1111/j.1749-6632.1999.tb07795.x. [DOI] [PubMed] [Google Scholar]
- Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor κB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of estrogen-mediated attenuation of hepatic injury following trauma-hemorrhage: Akt-dependent HO-1 upregulation. J Leukoc Biol. 2007;82:1019–1026. doi: 10.1189/jlb.0607355. [DOI] [PubMed] [Google Scholar]
- Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann Surg. 2007;245:971–977. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: upregulation of the PI-3K/Akt pathway. J Leukoc Biol. 2007;82:774–780. doi: 10.1189/jlb.0307182. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Nickel EA, Chen J, Schwacha MG, Choudhry MA, Bland KI, Chaudry IH. Mechanism of the salutary effects of estrogen on Kupffer cell phagocytic capacity following trauma-hemorrhage: pivotal role of Akt activation. J Immunol. 2009;182:4406–4414. doi: 10.4049/jimmunol.0803423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garçon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109:295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Reif K, Smith G, Sansom DM, Hemmings BA, Ward SG. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol. 1997;27:2495–2501. doi: 10.1002/eji.1830271006. [DOI] [PubMed] [Google Scholar]
- Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for upregulation of IL-2 and IFN-γ but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengère LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr JM, Vander Heiden MG, Gardner JP, Hambor JE, Neveu MJ, Thompson CB. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg. 1999;177:55–60. doi: 10.1016/s0002-9610(98)00299-2. [DOI] [PubMed] [Google Scholar]
- Hope JC, Campbell F, Hopkins SJ. Deficiency of IL-2 or IL-6 reduces lymphocyte proliferation, but only IL-6 deficiency decreases the contact hypersensitivity response. Eur J Immunol. 2000;30:197–203. doi: 10.1002/1521-4141(200001)30:1<197::AID-IMMU197>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lord JD, McIntosh BC, Greenberg PD, Nelson BH. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- Abraham E, Freitas AA. Hemorrhage in mice induces alterations in immunoglobulin-secreting B cells. Crit Care Med. 1989;17:1015–1019. doi: 10.1097/00003246-198910000-00010. [DOI] [PubMed] [Google Scholar]
- MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Okada R, Kondo T, Matsuki F, Takata H, Takiguchi M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int Immunol. 2008;20:1189–1199. doi: 10.1093/intimm/dxn075. [DOI] [PubMed] [Google Scholar]
- Berger SL. Lymphocytes as resting cells. Methods Enzymol. 1979;58:486–494. doi: 10.1016/s0076-6879(79)58163-4. [DOI] [PubMed] [Google Scholar]
- Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275:251–255. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Nathens AB, Frankel HL, Vanek SL, Moore EE, Maier RV, Minei JP. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008;36:1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- Mayr S, Walz CR, Angele P, Hernandez-Richter T, Chaudry IH, Loehe F, Jauch KW, Angele MK. Castration prevents suppression of MHC class II (Ia) expression on macrophages after trauma-hemorrhage. J Appl Physiol. 2006;101:448–453. doi: 10.1152/japplphysiol.00166.2006. [DOI] [PubMed] [Google Scholar]
- Aguilar MM, Battistella FD, Owings JT, Olson SA, MacColl K. Posttraumatic lymphocyte response: a comparison between peripheral blood T cells and tissue T cells. J Trauma. 1998;45:14–18. doi: 10.1097/00005373-199807000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.