Abstract
Hepatic steatosis and insulin resistance are factors that aggravate the progression of liver disease caused by hepatitis C virus (HCV) infection. In the pathogenesis of liver disease and metabolic disorders in HCV infection, oxidative stress due to mitochondrial respiratory chain dysfunction plays a pivotal role. Tacrolimus (FK506) is supposed to protect mitochondrial respiratory function. We studied whether tacrolimus affects the development of HCV-associated liver disease using HCV core gene transgenic mice, which develop hepatic steatosis, insulin resistance, and hepatocellular carcinoma. Administration of tacrolimus to HCV core gene transgenic mice three times per week for 3 months led to a significant reduction in the amounts of lipid in the liver as well as in serum insulin. Tacrolimus treatment also ameliorated oxidative stress and DNA damage in the liver of the core gene transgenic mice. Tacrolimus administration reproduced these effects in a dose-dependent manner in HepG2 cells expressing the core protein. The intrahepatic level of tumor necrosis factor-α, which may be a key molecule for the pathogenesis in HCV infection, was significantly decreased in tacrolimus-treated core gene transgenic mice. Tacrolimus thus reversed the effect of the core protein in the pathogenesis of HCV-associated liver disease. These results may provide new therapeutic tools for chronic hepatitis C, in which oxidative stress and abnormalities in lipid and glucose metabolism contribute to liver pathogenesis.
Hepatitis C virus (HCV) is a major cause of liver disease; approximately 170 million people are chronically infected worldwide. Persistent HCV infection leads to the development of chronic hepatitis, cirrhosis, and, eventually, hepatocellular carcinoma (HCC), thereby being a serious problem from both medical and socioeconomic viewpoints.1,2 Recently, a growing amount of evidence showing that HCV infection induces alteration in lipid3,4,5,6,7 and glucose metabolism has accumulated.8,9 Augmentation of oxidative stress is also substantiated in HCV infection by a number of clinical and basic studies.10,11,12,13
We demonstrated previously that the core protein of HCV induces HCC in transgenic mice that have marked hepatic steatosis in the absence of inflammation.14 In this animal model for HCV-associated HCC, there is augmentation of oxidative stress in the liver during the incubation period.10 Also noted is an accumulation of lipid droplets that are rich with carbon 18 monounsaturated fatty acids such as oleic and vaccenic acids, which is also observed in liver tissues of patients with chronic hepatitis C compared with those in patients with fatty liver due to simple obesity.15 Recently, we have also shown, using the HCV transgenic mouse model, that the ability of insulin to lower plasma glucose levels is impaired in association with HCV infection,16 which would be the basis for the frequent development of type 2 diabetes in patients with chronic hepatitis C.8,9
Disturbances in lipid and glucose metabolism are notable features of HCV infection and may be profoundly involved in the pathogenesis of liver diseases. Although the mechanism underlying these phenomena is not yet well understood, the development of clues to correct these metabolic disturbances occurring in HCV infection, which have been recently connected to the poor prognosis of patients with chronic hepatitis C, is awaited. Moreover, a key role for oxidative stress in the pathogenesis of hepatitis C,11,12 which may be closely associated with the aforementioned metabolic disorders, has been identified. The association of oxidative stress augmentation in HCV infection with mitochondrial respiratory dysfunction10,13,17 suggests that one possibility to ameliorate such a condition is the use of agents that can protect the mitochondrial respiratory function.
We have conducted information retrieval and screening for agents that can protect the mitochondrial respiratory function. Tacrolimus (FK506), which is widely used in organ transplantation, is one such agent with evidence showing protection of the mitochondrial respiratory function,18,19,20,21 although it shows no antiviral effect. We explored, using transgenic mouse and cultured cell models that express the HCV core protein, whether tacrolimus improves metabolic disturbances including lipid and glucose homeostases as well as oxidative stress augmentation through a possible involvement of mitochondrial function.
Materials and Methods
Transgenic Mouse and Cultured Cells
The production of HCV core gene transgenic mice has been described previously.6 Mice were cared for according to institutional guidelines with the approval by the institutional review board of the animal care committee, fed an ordinary chow diet (Oriental Yeast Co., Ltd., Tokyo, Japan), and maintained in a specific pathogen-free state. Because there is a sex preference in the development of liver lesions in the transgenic mice, we used only male mice. At least five mice were used in each experiment, and the data were subjected to statistical analysis. HepG2 cell lines expressing the HCV core protein under the control of the CAG promoter (Hep39J, Hep396, and Hep397) or a control HepG2 line (Hepswx) carrying the empty vector were described previously.22,23 Bulk HepG2 cells were also used as a control.
Reagents
Cholesterol esters and lipid standards were purchased from Sigma-Aldrich (St. Louis, MO), and glycogen and amyloglucosidase were obtained from Seikagaku Kogyo (Tokyo, Japan). Other chemicals were of analytical grade and were purchased from Wako Chemicals (Tokyo, Japan). Tacrolimus (FK506) was kindly provided by Astellas Pharma Inc. (Tokyo, Japan). Cyclosporine A (CyA) was purchased from Sigma-Aldrich.
Administration of Tacrolimus and Cyclosporine A
Tacrolimus (0.1 mg/kg b.wt., suspended in mannitol and hydroxychlorinated caster oil [HCO-60]), or vehicle only was administered to the core gene transgenic or control mice i.p., three times per week for 3 months beginning at 3 months of age. For in vitro experiments, tacrolimus was added to the culture medium at the final concentration of 0 nmol/L, 10 nmol/L, 100 nmol/L, or 1 μmol/L. CyA was also added to the culture medium at the same concentrations.
Assessment of Glucose Homeostasis
Blood was drawn at different time points from the tail vein, and plasma glucose concentrations were measured using an automatic biochemical analyzer (DRI-CHEM 3000V, Fuji Film, Tokyo, Japan). The levels of serum insulin were determined by radioimmunoassay (Biotrak, Amersham Pharmacia Biotech, Piscataway, NJ) using rat insulin as a standard. For the determination of the fasting plasma glucose level, the mice were fasted for >16 hours before the study. An insulin tolerance test was performed as described previously.16
Lipid Extraction, Measurement of Triglyceride Content, and Analysis of Fatty Acid Compositions
Lipid extraction from the mouse liver tissues or cultured cells was performed as described previously.15,24 For the analysis of fatty acid compositions, the residue was methanolysed by the modified Morrison and Smith method with boron trifluoride as a catalyst.25 Fatty acid methyl esters were analyzed using a Shimadzu GC-7A gas chromatograph (Shimadzu Corp., Kyoto, Japan) equipped with a 30-m-long × 0.3-mm diameter support coated with ethylene glycol succinate.24
Evaluation of Oxidative and Antioxidative System
Lipid peroxidation was estimated spectrophotometrically using thiobarbituric acid-reactive substances and is expressed in terms of malondialdehyde formed per milligram protein. Reduced glutathione and oxidized glutathione levels were measured as described previously.10 The total amount of glutathione was calculated by adding the amounts obtained for glutathione and oxidized glutathione. For the evaluation of DNA damage in cells, apurinic/apyrimidinic sites were determined using a DNA Damage Quantification Kit (Dojindo Molecular Technologies, Inc., Tokyo, Japan) following the manufacturer’s protocol.
Determination of Reactive Oxygen Species
Cells were plated onto glass coverslips and examined for reactive oxygen species (ROS) production as a marker for oxidative stress. They were loaded for 2 hours with chloromethyl 2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes Inc., Eugene, OR) at a final concentration of 10 μmol/L.26 Results were expressed as relative fluorescence intensity and normalized to the control cells. In some experiments, ROS was measured after the incubation with tacrolimus or CyA.
Measurement of Ketone Body Ratio
For the determination of ketone body ratio (KBR), cells were cultured to confluence on a 3.5-cm dish, and the medium was replaced with 700 μl of fresh medium. For arterial KBR, the mice were fasted for >16 hours, followed by the drawing of arterial blood. After a 24-hour incubation, acetoacetate and β-hydroxybutyrate in the medium were measured by monitoring the production or consumption of NADH with a Ketorex kit (Sanwa Chemical, Nagoya, Japan).27 The KBR was calculated as the acetoacetate/β-hydroxybutyrate ratio.
Microarray Analysis
An Affymetrix GeneChip analysis cDNA array system (Mouse Genome 430A 2.0, Kurabo, Osaka, Japan) was used for the analysis. Two thousand species of mouse DNA fragments were spotted on the filter. Genes that were 1.5-fold increased or decreased in both of the two tacrolimus-treated mice compared with mice treated with vehicle were defined as up-regulated or down-regulated, respectively.
Real-Time PCR and Western Blotting
RNA was prepared from mouse liver tissues using TRIzol LS (Invitrogen, Carlsbad, CA). The first-strand cDNAs were synthesized with a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech, Franklin Lakes, NJ). The fluorescent signal was measured with an ABI Prism 7000 system (Applied Biosystems, Tokyo, Japan).
The genes encoding mouse tumor necrosis factor (TNF)-α, sterol regulatory element binding protein (SREBP)-1c, resistin, stearoyl-CoA desaturase (SCD)-1, and hypoxanthine phosphoribosyltransferase were amplified with the primer pairs 5′-GACAAGGTGGGCTACGGGCTTG-3′ and 5′-TCCCAAATGGGCTCCCTCT-3′, 5′-ACGGAGCCATGGATTGCACATTTG-3′ and 5′-TACATCTTTAAAGCAGCGGGTGCCGATGGT-3′, 5′-GAAGGCACAGCAGTCTTGA-3′ and 5′-GCGACCTGCAGCTTACAG-3′, 5′-TTCCCTCCTGCAAGCTCTAC-3′ and 5′-CGCAAGAAGGTGCTAACGAAC-3′, and 5′-CCAGCAAGCTTGCAACCTTAACCA-3′ and 5′-GTAATGATCAGTCAACGGGGGAC-3′, respectively. The sense and antisense primers were located in different exons to avoid false-positive amplification from contaminated genomic DNA. Each PCR product was confirmed as a single band of the correct size by agarose gel electrophoresis (data not shown).
Reporter Assay for SREBP-1c Promoter Activity
A plasmid encoding firefly luciferase under the control of the SREBP-1c promoter (pGL3-srebp-1cPro) and a control plasmid encoding Renilla luciferase (Promega, Madison, WI) were transfected into 293T cells. Tacrolimus was added at a final concentration of 100 nmol/L to the culture medium of 293T cells transfected with pGL3-srebp-1cPro with or without an expression plasmid of HCV core protein at 24 hours after transfection. Cells were harvested 24 hours after treatment. Luciferase activity was measured by using the dual-luciferase reporter assay system (Promega). Firefly luciferase activity was standardized with that of Renilla luciferase, and the results are expressed as the fold-increase in relative luciferase units.
Statistical Analysis
Data are presented as the mean ± SE. The significance of the difference in means was determined by a Mann-Whitney U test wherever appropriate. P < 0.05 was considered significant.
Results
Effect of Tacrolimus on Insulin Resistance Induced by HCV
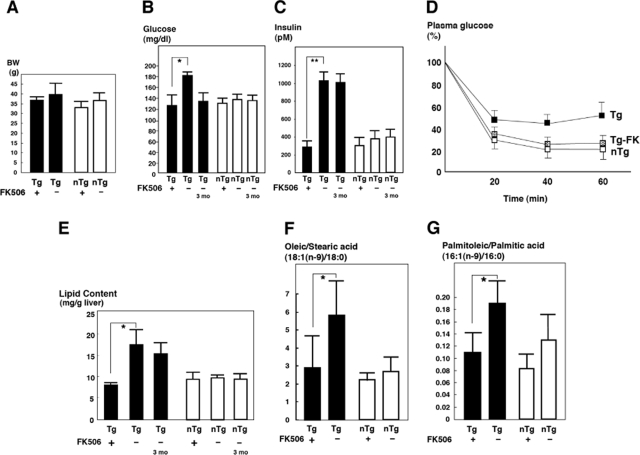
The core gene transgenic mice exhibit insulin resistance in the absence of obesity from the age of 2 months.16 In tacrolimus-treated mice, there was a slight, but not significant, reduction in body weight compared with control mice at the end of tacrolimus administration at 6 months of age (Figure 1A). Tacrolimus administration to the core gene transgenic mice restored the plasma glucose levels to within normal limit (Figure 1B) (P < 0.05), whereas it caused no significant reduction in the control mice. The plasma glucose levels in the vehicle-treated core gene transgenic mice were higher than those in the core gene transgenic mice reported previously,16 probably owing to the older age of mice in the current study than in the previous one. The levels of serum insulin were also significantly reduced by the treatment with tacrolimus for 3 months in the core gene transgenic mice, whereas there was no significant change in the control mice (Figure 1C). The reduction in both plasma glucose and serum insulin levels indicates that the administration of tacrolimus restored the resistance to insulin action, which is attributed to the suppression of insulin action in the liver by the core protein.16 Actually, an insulin tolerance test (1 U/kg b.wt.) demonstrated the improvement of insulin action in the tacrolimus-treated core gene transgenic mice (Figure 1D).
Figure 1.
Effect of tacrolimus (FK506) on glucose and lipid metabolism in the core gene transgenic mice. Tacrolimus (0.1 mg/kg b.wt.) or vehicle was administered to core gene transgenic or control mice i.p., three times weekly for 3 months beginning at 3 months of age. A: Body weight at the baseline and end of treatment. B: Plasma glucose level. C: Serum insulin level. D: Insulin tolerance test. Black boxes represent core gene transgenic mice; white boxes represent control mice; gray boxes represent core gene transgenic mice treated with tacrolimus (Tg-FK). E: Total lipid content in the liver. F: Ratio of oleic/stearic acid [18:1(n-9)/18:0]. G: Ratio of palmitoleic/palmitic acid [16:1(n-9)/16:0]. black bars represent transgenic mice; white bars represent control mice. Tg 3 mo indicates 3-month-old transgenic mice showing the baseline state just before FK treatment, and Tg indicates 6-month-old transgenic mice, either with or without tacrolimus treatment for 3 months. Values represent the mean ± SE, n = 5 in each group. *P < 0.05. Tg, transgenic mice; nTg, nontransgenic control mice. **P < 0.01.
Tacrolimus Improves Lipid Metabolism Disorders in Mice
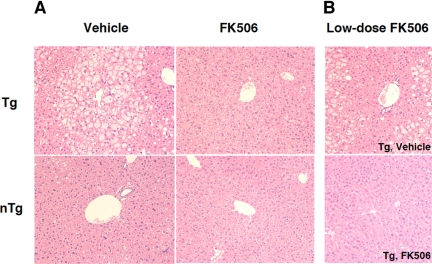
We then studied whether tacrolimus administration affects lipid metabolism in the mice. The core gene transgenic mice developed a marked hepatic steatosis.6,14 In addition, the composition of accumulated lipid was different from that in the fatty liver as a result of simple overnutrition: carbon 18 or 16 monounsaturated fatty acid levels were significantly increased.15 As shown in Figure 1E, the tacrolimus treatment significantly reduced the lipid content in liver tissues compared with the vehicle treatment of the core gene transgenic mice (P < 0.05, n = 5 each), whereas there was no change in the control mice. The increased ratios of oleic to stearic acid [18:1 (n-9)/18:0] and palmitoleic to palmitic acid [16:1(n-9)/16:0] in the core gene transgenic mice returned to levels similar to those in control mice (Figure 1, F and G) (P < 0.05). Thus, the administration of tacrolimus for 3 months restored the abnormalities in lipid metabolism that were induced by the core protein of HCV. Histologically, tacrolimus significantly improved steatosis in the liver of core gene transgenic mice, in which micro- and macrovesicular lipid droplets were accumulated in hepatocytes, chiefly around the central veins of the liver (Figure 2A). There was no sign of inflammation in the liver with or without the tacrolimus treatment.
Figure 2.
Morphological analysis of the liver of the core gene transgenic mice. Representative cases are shown either treated with tacrolimus (FK506) or vehicle (H&E staining). A: There is a prominent improvement of steatosis in the 3-month tacrolimus-treated core gene transgenic mice compared with the vehicle-treated mice. B: A prominent improvement in steatosis was also obtained by the administration of one-fifth dose of tacrolimus for 1 month beginning at 3 months of age. For histological analysis, two independent researchers evaluated 40 microscopic fields each, and a representative picture is shown for each category. Original magnification, ×125. Tg, transgenic mice; nTg, nontransgenic control mice.
Effect of Tacrolimus on Lipid Metabolism in HepG2 Cells Expressing HCV Core Protein
To further prove the ameliorating effect of tacrolimus on lipid metabolism, we then performed experiments using HepG2 cells that express the core protein.22,23 HepG2 cells, the lipid metabolism of which is somewhat different from that in normal hepatocytes,28 show a significant increase in the level of 5,8,11-eicosatrienoic acid [20:3 (n-9)], as a result of activations of the fatty acid enzymes, Δ9-, Δ6-, and Δ5-desaturases, by the core protein (H. Miyoshi and K. Koike, unpublished data). Incubation of the core-expressing HepG2 cells with tacrolimus at 100 nmol/L and 1 μmol/L for 48 hours significantly reduced the accumulation of 20:3(n-9), whereas CyA treatment increased the level of 20:3(n-9) in a dose-dependent manner in the core-expressing HepG2 cells (Figure 3, A and B). Neither tacrolimus nor CyA changed the 20:3(n-9) content in HepG2 cells that do not express the core protein.
Figure 3.
Effect of tacrolimus (FK506) or CyA on fatty acid compositions in HepG2 cells expressing the core protein. The fatty acid compositions of the total cell lipids were analyzed, and the percentage of 5,8,11-eicosatrienoic acid [20:3(n-9)] in the core-expressing and control HepG2 cells was calculated. A: Treatment with tacrolimus at 0 nmol/L, 100 nmol/L, or 1 μmol/L. B: Treatment with CyA at 0 nmol/L, 100 nmol/L, or 1 μmol/L. Black bars represent core-expressing cells; white bars represent control cells. Because similar results were obtained by using Hep39J, Hep396, and Hep397 cell lines, representative results using the Hep39J cell line are shown. Values represent the mean ± SE; n = 5 in each group. *P < 0.05 and **P < 0.01.
Low Dose of Tacrolimus Also Ameliorates Steatosis and Insulin Resistance
Because the usual dose of tacrolimus for liver transplantation naturally induces an immunosuppressed state in patients, we conducted a mouse study with a tacrolimus dose lower than that in the aforementioned study. In this low-dose experiment, tacrolimus at 0.02 mg/kg b.wt. (one-fifth of the previous one) was administered to mice for 1 month from the age of 3 months. Similar to the results with the dose of 0.1 mg/kg b.wt., there were significant decreases in the lipid content in the liver (9.5 ± 0.8 [0.02 mg/kg b.wt. tacrolimus] versus 18.7 ± 4.4 [vehicle only] mg/g liver; P < 0.05) and serum insulin concentration (96.6 ± 16.9 [0.02 mg/kg b.wt. tacrolimus] versus 1137.1 ± 88.0 [vehicle only] pmol/L; P < 0.05) in the core gene transgenic mice treated with tacrolimus. Histological changes are shown in Figure 2B.
Effect of Tacrolimus on Oxidative Stress and Antioxidative System in Mice
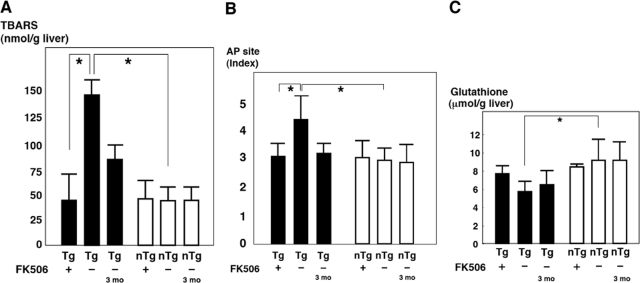
We next examined whether the 3-month administration of tacrolimus affects the redox state in the core gene transgenic mice. In the liver of the core gene transgenic mice, the ROS level was higher than that in the liver of control mice as determined by lipid peroxidation.10 Treatment with tacrolimus significantly reduced the level of thiobarbituric acid-reactive substances in the liver of the core gene transgenic mice (Figure 4A) (P < 0.05). As a result of oxidative stress overproduction, there was damage in the DNA of hepatocytes of the core gene transgenic mice from a young age.10 To evaluate the effect of tacrolimus on the nuclear DNA damage, the apurinic/apyrimidinic site index was determined in liver tissues from the core gene transgenic mice. As shown in Figure 4B, the apurinic/apyrimidinic site index in the liver of the core gene transgenic mice, which was significantly higher than that in the control mice, was significantly decreased by the tacrolimus treatment to a level similar to that in the control mice (P < 0.05).
Figure 4.
Effect of tacrolimus (FK506) on oxidative stress in the core gene transgenic mice. Tacrolimus (0.1 mg/kg b.wt.) or vehicle only was administered to the core gene transgenic or control mice for 3 months. A: Lipid peroxidation in the liver. B: apurinic/apyrimidinic (AP) site in the liver as a marker of nuclear DNA damage; C: Total glutathione level in the liver. Black bars represent transgenic mice; white bars represent control mice. Tg 3 mo indicates 3-month-old transgenic mice, showing the baseline state just before tacrolimus treatment, and Tg indicates 6-month-old transgenic mice, either with or without 3 months of tacrolimus treatment. Values represent the mean ± SE; n = 5 in each group. *P < 0.05. Tg, transgenic mice; nTg, nontransgenic control mice. TBARS, thiobarbituric acid-reactive substances.
The level of glutathione, one of the antioxidant systems, was significantly decreased in the liver of the core gene transgenic mice presumably as a result of oxidative stress overproduction but returned to a level similar to that in the control mice after the 3-month administration of tacrolimus, although the difference was not statistically significant (P = 0.063) (Figure 4C). Thus, the oxidative stress augmentation induced by the core protein of HCV was reduced by tacrolimus.
Effect of Tacrolimus on Oxidative Stress in Core-Expressing HepG2 Cells
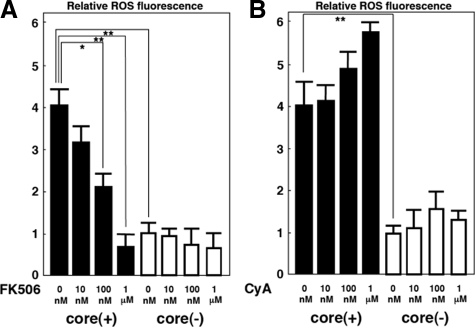
Evidence for scavenging ROS by the administration of tacrolimus to the mice prompted us to validate this finding using cultured cells. For this purpose, tacrolimus or CyA was added to the culture medium of HepG2 cells that express or do not express the core protein. After 24 hours of incubation, tacrolimus decreased the ROS production level in the core-expressing HepG2 cells in a dose-dependent manner (Figure 5A). In contrast, no decrease but rather an augmentation of ROS production was observed by the treatment with CyA at various concentrations (Figure 5B).
Figure 5.
Effect of tacrolimus (FK506) or CyA on ROS production in HepG2 cells expressing the core protein. Results are expressed as relative brightness and normalized to control cells. A: Treatment with tacrolimus at 0 nmol/L, 10 nmol/L, 100 nmol/L, or 1 μmol/L. B: Treatment with CyA at 0 nmol/L, 10 nmol/L, 100 nmol/L, or 1 μmol/L. Black bars represent transgenic mice; white bars represent control cells. Because similar results were obtained by using Hep39J, Hep396, and Hep397 cell lines, representative results using the Hep39J cell line are shown. Values represent the mean ± SE; n = 5 in each group. *P < 0.05; **P < 0.01.
Because dysfunction of the mitochondrial respiratory chain complex 1 is suspected to be the reason for the ROS production associated with HCV infection (H. Miyoshi and K. Koike, unpublished data),12,13,17 an increase in the NADH/NAD+ ratio, which is caused by the repression of the complex 1 NADH dehydrogenase activity, would be a good marker for the mitochondrial complex 1 dysfunction. Therefore, we evaluated the effect of tacrolimus on the accumulation of NADH in the core-expressing HepG2 cells. The NADH/NAD+ ratio, which is strictly estimated from a reciprocal of KBR,26,29 was significantly higher in the core gene transgenic mice than in control mice (1/atrial KBR) and in HepG2 cells expressing the core protein than in control cells (1/KBR) (Figure 6A). By the treatment with 1 μmol/L tacrolimus, the ratio significantly decreased compared with the baseline (Figure 6B), whereas CyA treatment caused no effect in the core-expressing HepG2 cells (Figure 6C), as was the case in the determination of ROS by chloromethyl 2′,7′-dichlorodihydrofluorescein diacetate.
Figure 6.

Effect of tacrolimus (FK506) or CyA on NADH accumulation in HepG2 cells expressing the core protein. A: NADH/NAD+ was determined in mice (left) or HepG2 cells (right) with or without the core protein. B: The ketone body ratio was determined in HepG2 cells with or without the core protein after incubation with tacrolimus for 24 hours at 0 nmol/L, 10 nmol/L, 100 nmol/L, or 1 μmol/L. C: The ketone body ratio was determined in HepG2 cells with or without the core protein after incubation with CyA for 24 hours at 0 nmol/L, 10 nmol/L, 100 nmol/L, or 1 μmol/L. Black bars represent transgenic mice; white bars represent control cells. Because similar results were obtained by using Hep39J, Hep396 and Hep397 cell lines, representative results using the Hep39J cell line are shown. Values represent the mean ± SE; n = 5 in each group. *P < 0.05. AKBR, arterial KBR; Tg, transgenic mice; nTg, nontransgenic mice.
Changes in Gene Expression by Tacrolimus Treatment of Mice
We then performed a comprehensive microarray analysis of gene expression in the liver, which was up- or down-regulated by tacrolimus. For this analysis, the tacrolimus-treated mice were compared with the vehicle-treated mice, in two pairs of the core gene transgenic and control mice, respectively. Genes that were 1.5-fold increased or decreased in both of the two tacrolimus-treated mice compared with those treated with vehicle were defined as up-regulated or down-regulated, respectively. As shown in Table 1, several genes were found to be up-regulated or down-regulated in both the core gene transgenic and control mice after the treatment with tacrolimus for 3 months. A number of genes including that for TNF-α were up- or down-regulated both in the core gene transgenic and control mice. In contrast, the expressions of some genes including that for resistin were differentially regulated between the core gene transgenic and control mice. The expressions of these genes were confirmed by real-time PCR analysis.
Table 1.
Genes Whose Expression Levels in the Mouse Liver Were Altered by the Treatment with FK506
| Up-regulated in Tg | Down-regulated in Tg | |
|---|---|---|
| Up-regulated in nTg | Nuclear factor, erythroid derived 2 | Resistin |
| DNA segment, human D6S2654E | Resistin like alpha | |
| Fatty acid binding protein 5 epidermal squalene epoxidase | Nuclear receptor subfamily 4, group A, member insulin-like growth factor binding protein 1 calcium and integrin binding family member 3 | |
| Zinc finger protein 69 | ||
| Down-regulated in nTg | X-linked lymphocyte-regulated 4 | Tumor necrosis factor alpha |
| Cytochrome P450, family 2, subfamily b, polypeptide 9 | Cytochrome P450, family 17, subfamily a, polypeptide 1 | |
| X-linked lymphocyte-regulated 3a | B-cell leukemia/lymphoma 6 | |
| Signal sequence receptor, delta |
Genes with altered expression in Tg (columns) or in nTg (rows) are described in a 4 × 4 table. Genes that were 1.5-fold increased or decreased in both of the two FK506-treated mice compared with those treated with placebo were defined as up-regulated or down-regulated, respectively. Tg, core gene transgenic mouse; nTg, nontransgenic control mouse.
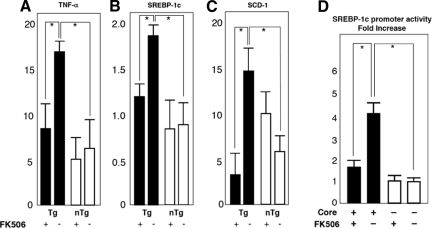
Then, to explore the mechanism by which tacrolimus reverses the pathological effect of the core protein in the liver, we examined, by real-time PCR analysis, the expression of some cellular genes including TNF-α, SREBP-1c, SCD-1, and proteasome activator 28-γ. These genes or gene products have been suggested to play a pivotal role in the pathogenesis of HCV-associated liver disease.30,31 TNF-α and SREBP-1c genes have been shown to be up-regulated in the liver of the core gene transgenic mice and considered to play a role in the development of insulin resistance and steatosis.30,31 By the treatment of the core gene transgenic mice with tacrolimus for 3 months, there was a significant decrease in the mRNA level of both TNF-α and SREBP-1c (Figure 7, A–C) (P < 0.05). The SCD-1 mRNA level was also reduced in the tacrolimus-treated core gene transgenic mice. Because down-regulation of SREBP-1c expression by tacrolimus was observed only in the core gene transgenic mice but not in control mice, it is estimated that tacrolimus antagonizes the action of core protein in its transactivating function of the SREBP-1c promoter. The down-regulation of SREBP-1c, then, would lead to the suppression of SCD-1 expression and amelioration of steatosis. We confirmed this by conducting luciferase assays using cultured cells. As shown in Figure 7D, tacrolimus cancelled the effect of the core protein on the activation of SREBP-1c gene promoter. The level of the proteasome activator 28-γ protein, which is indispensable for the action of the core protein in the pathogenesis of HCV-associated liver lesion,31 was determined by Western blotting, but there was no change caused by the tacrolimus treatment (data not shown).
Figure 7.
A–C: Effect of tacrolimus (FK506) on mRNA levels of cellular genes. The mRNA levels of TNF-α (A), SREBP-1c (B), and SCD-1 (C) genes were determined by real-time PCR analysis in the tacrolimus- or vehicle-treated mouse livers. The transcriptions of the genes were normalized with that of hypoxanthine phosphoribosyltransferase, and the values are expressed as relative activities. D: Effect of tacrolimus on the transactivating function of the core protein on the SREBP-1c promoter. A luciferase assay was performed using a plasmid encoding firefly luciferase under the control of the SREBP-1c promoter with or without the expression of HCV core protein. Tacrolimus was added at a final concentration of 100 nmol/L to the culture medium. Black bars represent transgenic mice; white bars represent control cells. Values represent the mean ± SE; n = 5 in each group. *P < 0.05. Tg, transgenic mice; nTg, nontransgenic mice.
Discussion
Antiviral treatment for chronic hepatitis C has advanced markedly. Nearly 50% of patients with chronic hepatitis C with HCV genotype 1 and high viral loads achieve a sustained virological response as a result of ribavirin/peginterferon combination therapy.32,33 However, the remaining patients who could not achieve sustained virological response continue to experience progression of chronic hepatitis and have a high probability for development of HCC. Although therapies with new agents such as viral protease or RNA polymerase inhibitors are being developed, there is hope for development of the means to retard the progression of chronic hepatitis.
Recently, evidence showing that hepatic steatosis and insulin resistance are crucial determinants of the progression of liver fibrosis has accumulated.34,35,36,37 Moreover, the importance of oxidative stress, which is closely associated with metabolic disorders such as insulin resistance and steatosis, is implicated in the pathogenesis of HCV-associated liver disease. Given the suggested association of oxidative stress augmentation with the dysfunction of mitochondrial respiration in HCV infection,12,13,17 one possibility to ameliorate such a condition is the use of agents that can protect the mitochondrial respiratory function. Tacrolimus is one such agent with evidence of providing protection of the mitochondrial respiratory function,18,19,20,21 although it does not show an antiviral effect.
In the current study, the administration of tacrolimus significantly improved the disturbances in lipid and glucose metabolism both in vivo and in vitro. As disorders of lipid metabolism associated with HCV infection, hepatic steatosis and increases in monounsaturated fatty acid levels have been demonstrated.3,4,6,7,15 The latter is caused by the activation of fatty acid enzymes such as Δ9- or Δ6-desaturase, resulting in increases in 18:1(n-9)/18:0 and 16:1(n-9)/16:0 ratios (H. Miyoshi and K. Koike, unpublished data).15 Tacrolimus ameliorated these lipid alterations associated with HCV infection with no impact on mouse body weight. Tacrolimus also improved the insulin resistance in the HCV mouse model, in which tyrosine phosphorylation of insulin receptor substrate-1 is impaired by the HCV core protein.16
Moreover, tacrolimus treatment ameliorated oxidative stress augmentation, which is considered to play a pivotal role in the progression of liver disease or the development of HCC in HCV infection.10,11,12,13 In mice transgenic for the HCV core gene, in which DNA damage develops because of oxidative stress augmentation,13 tacrolimus decreased the levels of peroxylipid and DNA damage formations. Dysfunction of the mitochondrial respiratory chain complex 1 is suspected to be a source of ROS overproduction in HCV infection.12,13,17 To assess changes in mitochondrial complex 1 function caused by tacrolimus, the NADH/NAD+ ratio, which reflects the complex 1 NADH dehydrogenase activity, was determined in HepG2 cells expressing the core protein. The NADH/NAD+ ratio, which is strictly estimated from a reciprocal of KBR (1/atrial KBR),26,29 was significantly reduced by the addition of tacrolimus but not CyA. Thus, tacrolimus protected the mitochondrial respiratory chain complex 1 function from the impact of the core protein, decreased oxidative stress, and improved steatosis and insulin resistance.
Some of features induced by the core protein including steatosis, insulin, and DNA damage were already present in the core gene transgenic mice at 3 months of age as the baseline, and those were improved by tacrolimus treatment. This fact indicates that tacrolimus is not only preventing the development of core-induced features but also reversing such changes in the mouse liver.
The tacrolimus dose used in the current study was 0.1 mg/kg b.wt. This is the same dose as that used in recipients of liver or kidney transplantation. The result of a subexperiment with a lower tacrolimus dose of 0.02 mg/kg b.wt. was similar to that with the dose of 0.1 mg/kg b.wt. This finding is promising because it indicates that the “anti-core protein effect” may be achievable at such a low dose of tacrolimus without provoking strong immunosuppression. The tacrolimus concentration (100 nmol/L) that caused the anti-core protein effect in the cultured cell study is similar to that in the blood of recipients of liver transplantation and much lower than those used in previous studies.19,38 In the current study, tacrolimus was administered only i.p., although it tacrolimus is administered i.v. or p.o. in humans. Therefore, a concern may arise regarding the administration route. Because the bioavailability of tacrolimus is approximately 25% (range from 5 to 93%) in human patients,39 a difference in the concentrations of tacrolimus may be possible between i.p. and p.o. administration. However, in human patients, target levels of tacrolimus concentration are generally achieved by p.o. administration as the maintenance therapy. Therefore, the target concentration would be achieved in mouse models by p.o. administration for 3 months as it is in human patients. Our current results strongly support the notion that tacrolimus can protect the mitochondrial respiratory function, resulting in a reduction of ROS production.
There is also a controversy concerning the effect of tacrolimus on glucose homeostasis. Post-transplantation diabetes is a complication in kidney or liver transplantation.40,41 In vivo and in vitro studies have shown that tacrolimus may inhibit insulin secretion from the pancreatic β-cells.40 Thus, tacrolimus may have a potential to induce diabetes. However, there have been no well designed studies on this specific point: in one study, corticosteroid withdrawal from tacrolimus-based immunosuppression reduced insulin resistance without changing insulin secretion.41 In our study using the HCV mouse model, tacrolimus administration at the dose similar to those in organ transplant recipients decreased serum insulin levels without increasing plasma glucose levels. These results point toward the future use of tacrolimus in vivo for the amendment of metabolic abnormalities, such as steatosis and insulin resistance, associated with HCV infection. However, it should be noted that there is a difference between our mouse model and human patients. Organ transplant recipients generally have injury to other bodily organs after a prolonged course of illness, whereas the mouse model we have exploited does not. In addition, our mouse model originally has insulin resistance with the presence of hyperplasia of Langerhans islands.16 Therefore, the effect of tacrolimus on glucose homeostasis in the current mouse study may not be exactly applicable to human patients.
The results of the gene expression analysis by microarray and subsequent real-time PCR were of considerable interest. Tacrolimus reduced the mRNA levels of TNF-α, SCD-1, and SREBP-1c genes, which are elevated in both patients with chronic hepatitis C and HCV core gene transgenic mice.30,31 The elevation in the TNF-α level causes insulin resistance in vivo, which is also observed in HCV core gene transgenic mice.16 The elevations in SREBP-1c and SCD-1 gene mRNA levels cause the overproduction of triglycerides, leading to the development of steatosis. The reductions in the expression levels of these genes may explain the effect of tacrolimus on the improvement of steatosis, insulin resistance, and oxidative stress in these HCV models. Although recent investigations have shown that the immunosuppressive drugs tacrolimus and rapamycin inhibit the expression of different inflammatory mediators,42,43 the anti-inflammatory functions of these drugs are not well established. Our in vitro and in vivo experiments confirmed that tacrolimus inhibited the induction of ROS generation, which is mediated by the core protein. Our data indicate that the inhibition of ROS formation may explain part of the favorable effect of immunosuppressive agents on inflammatory conditions.
In conclusion, our results demonstrate that tacrolimus has protective potential against damage caused by the HCV core protein including the induction of steatosis, insulin resistance, and oxidative stress, both in mice and cultured cells. Although more studies are required to elucidate the precise mechanism underlying the potential of tacrolimus in reversing the pathogenesis in HCV infection, these results may provide new therapeutic tools for chronic hepatitis C, in which oxidative stress and abnormalities in lipid and glucose metabolism contribute to liver pathogenesis.
Footnotes
Address reprint requests to Kazuhiko Koike, M.D., Ph.D., Department of Gastroenterology, Internal Medicine, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan. E-mail: kkoike-tky@umin.ac.jp.
Supported in part by a Grant-in-Aid for Scientific Research on Priority Area from the Ministry of Education, Science, Sports and Culture of Japan, by Health Sciences research grants from the Ministry of Health, Labour and Welfare (Research on Hepatitis), and by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan.
References
- Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, Choo Q, Houghton M, Kuo G. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti RG, Camma C, Fiorello F, Cottone M, Rapicetta M, Marino L, Fiorentino G, Craxì A, Ciccaglione A, Giuseppetti R, Stroffolini T, Pagliaro L. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis. Ann Intern Med. 1992;116:97–102. doi: 10.7326/0003-4819-116-2-97. [DOI] [PubMed] [Google Scholar]
- Scheuer PJ, Ashrafzadeh P, Sherlock S, Brown D, Dusheiko GM. The pathology of chronic hepatitis C. Hepatology. 1992;15:567–571. doi: 10.1002/hep.1840150402. [DOI] [PubMed] [Google Scholar]
- Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572–577. doi: 10.1002/hep.1840150403. [DOI] [PubMed] [Google Scholar]
- Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, Bréchot C. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527–1531. doi: 10.1099/0022-1317-78-7-1527. [DOI] [PubMed] [Google Scholar]
- Lerat H, Honda M, Beard MR, Loesch K, Sun J, Yang Y, Okuda M, Gosert R, Xiao SY, Weinman SA, Lemon SM. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–365. doi: 10.1053/gast.2002.31001. [DOI] [PubMed] [Google Scholar]
- Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O'Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. 2006;290:G847–G851. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- Koike K, Miyoshi H. Oxidative stress and hepatitis C viral infection. Hepatol Res. 2006;34:65–76. doi: 10.1016/j.hepres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T, Imai K, Miyamura T, Kimura S, Koike K. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365–4370. [PubMed] [Google Scholar]
- Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- Moriya K, Todoroki T, Tsutsumi T, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Takayama T, Makuuchi M, Watanabe K, Miyamura T, Kimura S, Koike K. Increase in the concentration of carbon 18 monounsaturated fatty acids in the liver with hepatitis C: analysis in transgenic mice and humans. Biophys Biochem Res Commun. 2001;281:1207–1212. doi: 10.1006/bbrc.2001.4523. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Kimura S, Moriya K, Koike K. Hepatitis C virus and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Piccoli C, Scrima R, Quarato G, D'Aprile A, Ripoli M, Lecce L, Boffoli D, Moradpour D, Capitanio N. Hepatitis C virus protein expression causes calcium-mediated mitochondrial bioenergetic dysfunction and nitro-oxidative stress. Hepatology. 2007;46:58–65. doi: 10.1002/hep.21679. [DOI] [PubMed] [Google Scholar]
- Cetinkale O, Konukoğlu D, Senel O, Kemerli GD, Yazar S. Modulating the functions of neutrophils and lipid peroxidation by FK506 in a rat model of thermal injury. Burns. 1999;25:105–112. doi: 10.1016/s0305-4179(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Kaibori M, Inoue T, Tu W, Oda M, Kwon AH, Kamiyama Y, Okumura T. FK506, but not cyclosporin A, prevents mitochondrial dysfunction during hypoxia in rat hepatocytes. Life Sci. 2001;69:17–26. doi: 10.1016/s0024-3205(01)01098-0. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Chander B, Hasan C, Griffin JW, McArthur JC, Hoke A. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann Neurol. 2003;53:57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- Kaymaz M, Emmez H, Bukan N, Dursun A, Kurt G, Paçsaoğlu H, Paçsaoğlu A. Effectiveness of FK506 on lipid peroxidation in the spinal cord following experimental traumatic injury. Spinal Cord. 2005;43:22–26. doi: 10.1038/sj.sc.3101621. [DOI] [PubMed] [Google Scholar]
- Ruggieri A, Murdolo M, Harada T, Miyamura T, Rapicetta M. Cell cycle perturbation in a human hepatoblastoma cell line constitutively expressing hepatitis C virus core protein. Arch Virol. 2004;149:61–74. doi: 10.1007/s00705-003-0202-x. [DOI] [PubMed] [Google Scholar]
- Aizaki H, Harada T, Otsuka M, Seki N, Matsuda M, Li YW, Kawakami H, Matsuura Y, Miyamura T, Suzuki T. Expression profiling of liver cell lines expressing entire or parts of hepatitis C virus open reading frame. Hepatology. 2002;36:1431–1438. doi: 10.1053/jhep.2002.36937. [DOI] [PubMed] [Google Scholar]
- Todoroki T, Imai K, Matsumoto K, Kano S. Initial deactivation of Florisil adsorbent for column chromatographic separation of lipids. Analyst. 1983;108:1267–1269. [Google Scholar]
- Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- Gelasco AK, Raymond JR. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1551–F1558. doi: 10.1152/ajprenal.00281.2004. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Mellanby J, Krebs HA. Enzymic determination of d(−)-β-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Park Y, Pariza MW, Ntambi JM. Regulation of stearoyl-CoA desaturase activity by the trans-10,cis-12 isomer of conjugated linoleic acid in HepG2 cells. Biochem Biophys Res Commun. 2001;284:689–693. doi: 10.1006/bbrc.2001.5036. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi T, Suzuki T, Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Matsuura Y, Kimura S, Koike K, Miyamura T. Intrahepatic cytokine expression and AP-1 activation in mice transgenic for hepatitis C virus core protein. Virology. 2002;304:415–424. doi: 10.1006/viro.2002.1702. [DOI] [PubMed] [Google Scholar]
- Moriishi K, Mochizuki R, Moriya K, Miyamoto H, Mori Y, Abe T, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Critical role of PA28γ in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc Natl Acad Sci USA. 2007;104:1661–1666. doi: 10.1073/pnas.0607312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- Koike K. Antiviral treatment of hepatitis C: present status and future prospects. J Infect Chemother. 2006;12:227–232. doi: 10.1007/s10156-006-0460-0. [DOI] [PubMed] [Google Scholar]
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- Patton HM, Patel K, Behling C, Tripodi MF, Utili R, Ruggiero G. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. J Hepatol. 2003;39:1042–1048. doi: 10.1016/s0168-8278(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Han SY, Chang EJ, Choi HJ, Kwak CS, Suh SI, Bae JH, Park SB, Kim HC, Mun KC. Effect of tacrolimus on the production of oxygen free radicals in hepatic mitochondria. Transplant Proc. 2006;38:2242–2243. doi: 10.1016/j.transproceed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- Penfornis A, Kury-Paulin S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006;32:539–546. doi: 10.1016/s1262-3636(06)72809-9. [DOI] [PubMed] [Google Scholar]
- van Hooff JP, Christiaans MH, van Duijnhoven EM. Evaluating mechanisms of post-transplant diabetes mellitus: Nephrol Dial Transplant. 2004;19(Suppl 6):vi8–vi12. doi: 10.1093/ndt/gfh1063. [DOI] [PubMed] [Google Scholar]
- Vigil SV, de Liz R, Medeiros YS, Fröde TS. Efficacy of tacrolimus in inhibiting inflammation caused by carrageenan in a murine model of air pouch. Transpl Immunol. 2008;19:25–29. doi: 10.1016/j.trim.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Pereira R, Medeiros YS, Fröde TS. Antiinflammatory effects of tacrolimus in a mouse model of pleurisy. Transpl Immunol. 2006;16:105–111. doi: 10.1016/j.trim.2006.04.001. [DOI] [PubMed] [Google Scholar]