Abstract
Muscle-specific tyrosine kinase (MuSK) is essential for clustering of acetylcholine receptors (AChRs) at embryogenesis and likely also important for maintaining synaptic structure in adult muscle. In 5 to 7% of myasthenia gravis (MG) cases, the patients’ blood contains antibodies to MuSK. To investigate the effect of MuSK-MG antibody on synapse regeneration, notexin was used to induce damage to the flexor digitorum brevis muscle. We administered aliquots of MuSK-MG patients’ plasma to the flexor digitorum brevis twice daily for a period up to 21 days, and muscles were investigated ex vivo in contraction experiments. AChR levels were measured with 125I-α-bungarotoxin, and endplates were studied with quantitative immunohistochemistry. In normal muscles and in 14-day regenerated muscles, MuSK plasma caused impairment of nerve stimulus-induced contraction in the presence of 0.35 and 0.5 mmol/L Ca2+ with or without 100 to 400 nmol/L tubocurarine. Endplate size was decreased in regenerated muscles relative to controls; however, we did not observe such differences in muscle not treated with notexin. MuSK plasma had no effect on the amount and turnover rate of AChRs. Our results suggest that anti-MuSK antibodies influence the activity of MuSK molecules without reducing their number, thereby diminishing the size of the endplate and affecting the functioning of AChRs.
Weakness of skeletal muscle in myasthenia gravis (MG) is generally caused by loss of acetylcholine receptors (AChRs) due to circulating autoantibodies against the α-subunit of the AChRs at the motor endplate (AChR-MG).1,2,3 These antibodies act mainly through a combination of increased turnover of AChRs, following divalent antibody binding to adjacent AChRs, and complement-mediated attack on the postsynaptic membrane leading to further loss of AChRs and probably other important components of the neuromuscular junction.3,4,5 In approximately 15% of the cases with symptoms of MG, autoantibodies to AChRs are absent (“seronegative” MG), although some patients may not be truly seronegative because their sera contain antibodies that bind to concentrated, rapsyn-clustered AChRs expressed in human embryonic kidney cells.6 Passive immunization of mice with seronegative MG serum causes a reduction of the miniature endplate potentials (MEPP) amplitude, although the number of AChRs is usually not reduced.7,8,9
In many seronegative MG patients with generalized MG, there are antibodies against extracellular regions of the muscle-specific receptor tyrosine kinase, MuSK10; in central Europe, the proportion is approximately 40%, but in some countries, this fraction may be lower, e.g., 22% in The Netherlands.11 Most MuSK-MG patients have predominantly bulbar symptoms, often associated with muscle atrophy.12,13,14 MuSK antibodies belong predominantly to the IgG4 class,15,16 which does not activate the classical pathway of the complement system. Furthermore, it is functionally monovalent because IgG4 exchanges Fab arms in vivo with nonpathogenic IgG4.17 Consequently, anti-MuSK IgG4 should not be able to cause a complement-mediated attack on the postsynaptic membrane and may not be able to reduce the number of MuSK molecules by a cross-linking/endocytosis mechanism, because for this functionally, divalent antibodies would be required. Thus, it seems likely, assuming the MuSK antibody is the pathogenic agent responsible for the disease, that antibodies must interfere directly with the physiological function(s) of MuSK.
Most of what we know about the function of MuSK comes from in vitro and developmental studies. Studies on cell cultures have revealed that agrin activates the receptor lrp4, which then triggers MuSK to induce the clustering of AChRs during synapse formation through interaction with rapsyn.18,19,20,21 Gene disruptions of agrin, lrp4, MuSK, or rapsyn are lethal because AChRs do not get clustered, and synapses fail to be formed.18,20,22 It has been suggested that MuSK is essential for maintenance of ultrastructure and anchored AChRs in the adult endplate, because injection and electroporation of dsRNA targeting MuSK causes a reduction of the level of MuSK and disintegration of mouse endplates within 6 weeks.23 MuSK appears also to have a stabilizing effect on the turnover of AChRs in adult muscle, because exposure of denervated muscles to agrin increases the half-life time of AChRs from 1 to 10 days.24 Therefore, we hypothesized that the MuSK antibodies that arise in patients after endplates have formed act by interference with the lrp4-agrin-MuSK-rapsyn-AChR pathway, resulting in degeneration of neuromuscular junctions and disturbance of AChR turnover. We explored this idea by testing the effect of MuSK-MG plasma on regenerating instead of normal endplates to see whether under this condition the antibody has an especially pronounced effect. To this end, we first developed a mouse model for regenerating endplates in the flexor digitorum brevis (FDB) muscle based on the reversible myotoxic action of notexin, which is similar to a model earlier described for the soleus muscle of the rat.25 We found among other things that endplates in regenerated muscles from mice treated with MuSK-MG plasma were smaller than those from mice treated with control plasma and that neuromuscular transmission in these muscles was more sensitive to low Ca2+ concentration and tubocurarine.
Materials and Methods
Patients and Blood Plasma
Plasma samples from five MuSK-MG patients were used, four obtained through plasmapheresis. Titers of anti-MuSK antibody, assayed as described earlier,27 were in the range of 4.6 to 50.4 nmol/L (Table 1). The collected blood plasma was divided into 50-ml aliquots and kept at −20°C until further use. For controls, plasma was used from patients without a neuromuscular disorder, also after plasmapheresis.
Table 1.
Anti-MuSK-Positive Patients
| Patients | Sex | Age at onset (years) | Severity of disease* | Plasma anti-MuSK+ titre (nM) |
|---|---|---|---|---|
| 1 | F | 33 | IIIb | 50.4 |
| 2 | M | 29 | IVb | 46.2 |
| 3 | F | 29 | IIb | 12.3 |
| 4 | F | 3 | IIIb | 4.6 |
| 5 | F | 28 | IIb | 47.9 |
Quantitative MG score.26
All patients were treated with prednisone; patient 2 received azathioprine as well.
Five-milliliter aliquots of plasma were dialyzed overnight at 4°C against modified Ringer’s solution (M-Ringer), composition: 136 mmol/L NaCl, 4.6 mmol/L KCl, 1 mmol/L MgSO4, 2 mmol/L CaCl2, 1 mmol/L NaH2PO4, 2.5 mmol/L NaHCO3, and 11 mmol/L glucose.28 The dialysis procedure served to standardize the ionic content of the plasma and to wash away the drugs in the plasma, mostly steroids (Table 1). Any traces of drugs remaining would be greatly diluted from the region of injection (see below) by systemic washout (ratio injection volume/body weight was approximately 10−3).
Animals and Muscles
Female Swiss mice (8-week-old) were injected subcutaneously into the foot sole with 0.125 μg notexin (Sigma-Aldrich) dissolved in 25 μl M-Ringer. Mice received similar injections of 40-μl of dialyzed plasma from MuSK-MG or control patients, twice daily for a period of up to 21 days (not during weekends), starting on the day after the notexin injection. For experiments with normal muscle, the mice received plasma up to 14 days. The experimental procedures had been approved by the Animal Ethics Committee at the Leiden University Medical Centre (approval no. 01094), as required by the Dutch Law on Animal Experiments.
Mice were sacrificed by cervical dislocation and the FDB muscles were dissected, together with their nerve branch leading to the sciatic when appropriate.29 For some experiments, hemidiaphragms were dissected with the phrenic nerve attached.
For contraction experiments, muscles were mounted, bathed at 24 to 28°C in Ringer’s medium (116 mmol/L NaCl, 4.5 mmol/L KCl, 1 mmol/L MgSO4, 2 mmol/L CaCl2, 1 mmol/L NaH2PO4, 23 mmol/L NaHCO3, and 11 mmol/L glucose, pH 7.4, bubbled with carbogene), and stimulated at the nerve or directly at the muscle as explained elsewhere.29 Muscles were exposed to tubocurarine and decreased concentrations of calcium.29
The AChR binding sites for α-bungarotoxin (α-BuTX) were measured by incubation of muscles for 2 hours in the presence of 1 μg/ml 125I-α-BuTX in M-Ringer.28 Excess label was washed away overnight in M-Ringer at 4°C, and subsequently, the muscles were carefully cleaned from all adherent tissue and placed in a gamma scintillation counter. Correction for nonspecific binding of α-BuTX by measuring binding of radioactivity to endplate-free parts of the FDB muscle was not possible,28 the FDB fibers being only 1–2 mm long, which precluded the division into endplate-containing and endplate-free parts of muscle. However, because the fibers of the FDB are so short, the extrajunctional nonspecific 125I-α-BuTX binding is relatively little compared with, for instance, the diaphragm muscle with a very much larger extrajunctional area.28 On the basis of our earlier work on the diaphragm, we estimate the nonspecific binding in the FDB to be approximately 10% of the total amount of 125I-α-BuTX bound.
For turnover studies of AChRs, 125I-α-BuTX was injected into the foot sole (40 μl with a total of 5 μg/ml α-BuTX). In each experiment, a correction was made for the radioactive decay of 125I.
Histology and Histochemistry
Cryostat sections (hematoxylin and eosin) of normal and notexin-treated muscles were prepared. For histochemistry, the FDB muscles were quickly frozen in melting isopentane, precooled in liquid nitrogen. Cryosections of 10 μm were cut and stained for AChR (Alexa 594-conjugated α-BuTX) and the vesicular acetylcholine transporter (rabbit anti-VAChT) and analyzed quantitatively as described previously.30,31 The murine IgGs were blocked with a mouse on mouse (M.O.M.) immunodetection kit (Vector Laboratories, Burlingame, CA) following manufacturer’s recommendations. Additionally, mouse anti-rapsyn was used as described,30,31 with M.O.M.-biotinylated anti-mouse streptavidin Alexa-350 1/500 (Invitrogen). The fluorescence intensities of rapsyn labeling correlate well with histochemical measurements of the AChR in the same endplates (data not shown). Pictures were analyzed using the ImageJ software (version 1.37v; National Institutes of Health, Bethesda, MD). The fluorescence intensities of α-BuTX labeling correlate well with biochemical measurements of the AChR, with the advantage that the histochemical analysis can be confined to the area of the neuromuscular junction without measuring extrajunctional AChR.30 Endplate areas were identified as regions of vesicular acetylcholine transporter (VAChT) staining and the mean intensity of VAChT and AChR staining was measured in the corresponding area. The threshold intensity of VAChT staining for defining the margin of junctional areas was automatically calculated for each picture. The ratios of AChR:VAChT were calculated as a relative measure for the postsynaptic AChR concentration for 25 to 200 endplates per muscle. All sections were stained and processed in parallel to avoid interassay variations.
Statistics
The data are presented as the mean ± SEM. Possible statistical differences were analyzed with a paired or unpaired Student’s t-test and two-way analysis of variance wherever appropriate.
Results
A Model for Regenerating Endplates
The supplemental data explain the notexin model that has been used for most experiments in this study. Subcutaneous injection of notexin into the foot sole led to complete destruction of FDB muscle fibers within 2 days, leaving debris and connective tissue (Supplemental Figure S1, see http://ajp.amjpathol.org). However, by 15 days after the notexin injection, the muscle fibers had regenerated and the FDB nuclei, which are normally situated peripherally (Supplemental Figure S1A, see http://ajp.amjpathol.org), were found in the center of the fiber, characteristic of muscle regeneration (Supplemental Figure S1C, see http://ajp.amjpathol.org). This present pattern of notexin-induced degeneration/regeneration of the FDB muscle is similar to that reported earlier for the soleus muscle of the rat after injection of notexin25 or the myotoxic drug bupivacaine.32
By 3 days after notexin treatment and labeling of AChRs by injection of radioactive α-BuTX, most radioactivity was lost from the muscle (Supplemental Figure S1D, see http://ajp.amjpathol.org). When notexin-treated muscles were exposed in vitro to 125I-α-BuTX, an extra amount of radioactivity was bound starting at day 4 (Supplemental Figure S1D, see http://ajp.amjpathol.org). Nerve stimuli-induced contraction of the FDB was first restored also at day 4.
To examine the half-life of the new AChRs, we injected radioactive α-BuTX in vivo at various times after the notexin treatment. The half-life of 125I-α-BuTX-labelled AChR in notexin-treated muscle was only approximately 2 days when the toxin was injected just after the new AChRs had formed in the regenerated muscle (Supplemental Figure S1E, see http://ajp.amjpathol.org). When the label was injected at 14 days, the half-life time had increased to 5 days. On the other hand, the half-life time was 10 days when the label was given to normal muscle not treated with notexin.
The diaphragm is known to have a great safety factor of neuromuscular transmission, see for instance Ref. 33. The normal FDB was almost as resistant to paralysis by tubocurarine as the hemidiaphragm (Supplemental Figure S1F, see http://ajp.amjpathol.org). Supplemental data S1G (see http://ajp.amjpathol.org) show that newly formed fibers in the FDB after notexin treatment were paralyzed at 400 nmol/L tubocurarine to about the same degree as normal muscle.
The Effect of MuSK-MG Plasma
We treated mice with notexin and subsequently injected plasma from MuSK-MG patients into the foot sole for 2 weeks. None of the plasma samples of the patients caused impairment of contraction in the presence of normal physiological solution (with 2 mmol/L Ca2+) on delivery of single pulses to the nerve. It was thought possible that MuSK-MG plasma caused a relatively small decrease in the efficiency of neuromuscular transmission, eg, due to a decrease in the number of AChRs or the amount of ACh released by the nerve impulse. If such a defect would still allow endplate potentials that are still larger than the firing threshold, muscle contraction would still not be affected. Such “subclinical” defects can be made visible by decreasing ACh release by lowering the concentration of calcium in the medium or otherwise by decreasing the number of available AChRs by adding tubocurarine to the bath.
Indeed, when the concentration of Ca2+ was decreased from 2 to 0.5 mmol/L or 0.35 mmol/L, plasma samples from five patients caused an approximately twofold, statistically significant, decrease in nerve stimulus-induced muscle contraction relative to muscles derived from mice treated with control plasma (Table 2, P < 0.05, n = 5 patients). In patients 1 and 2, plasma under the condition of stimulation in 0.35 or 0.5 mmol/L Ca2+ caused repeatedly a highly significant decrease of the contraction relative to controls (Table 2, P < 0.005, n = 15–18 mice).
Table 2.
Effect of MuSK-MG Plasma on Muscle Contraction in Regenerating FDB Muscles
| Medium | Single pulses | 40 Hz pulses
|
||
|---|---|---|---|---|
| 2 mmol/L Ca2+ | 0.35 or 0.5 mmol/L Ca2+ | 2 mmol/L Ca2+ 0 nmol/L tubocurarine | 2 mmol/L Ca2+ 400 nmol/L tubocurarine | |
| MuSK-MG patients, regenerating muscles | ||||
| 1 | 118 ± 15 (8) | 40 ± 10 (10)* | 117 ± 9 (7) | 99 ± 8 (8) |
| 2 | 102 ± 12 (7) | 59 ± 13 (8)* | 110 ± 13 (7) | 97 ± 13 (7) |
| 3 | 137 (1) | 69 (1) | 185 (1) | 203 (1) |
| 4 | 111 ± 2 (2) | 49 (1) | 110 ± 1 (2) | 110 ± 20 (2) |
| 5 | 86 ± 24 (2) | 73 ± 8 (2) | 81 (1) | 123 ± 43 (2) |
| Mean ± SEM | 111 ± 8 | 58 ± 17** | 121 ± 17 | 126 ± 20 |
Contractions induced by nerve stimulation are presented as a percentage of matching contractions of muscles from human control plasma-injected mice. The experiments were performed 14 to 16 days after notexin injections with MuSK-MG and control plasma injections starting at day 2. Means ± SEM with the number of tested mice between parentheses.
P < 0.005, compared with controls; statistically significant difference compared with values in 2 mmol/L Ca2+,
P < 0.05, n = 5 patients, Student’s paired t-tests.
When the nerve was stimulated at 40 Hz instead of single pulses, muscles from mice treated with patients’ plasma contracted as well as those from control plasma-treated mice in the presence of 2 mmol/L Ca2+. The addition of 400 nmol/L tubocurarine to the medium caused in the muscles from control plasma-treated mice an approximately 20% decrease in nerve stimulus-induced contraction (data not shown). In the presence of 400 nmol/L tubocurarine, MuSK muscles did not show a further reduction in contraction force, relative to controls (Table 2).
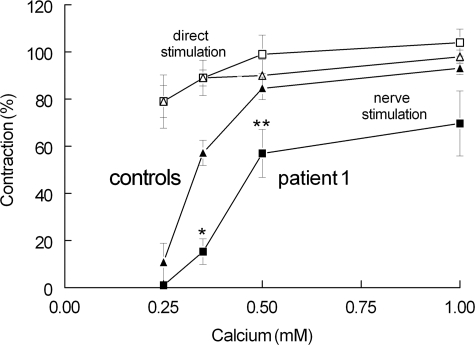
For subsequent experiments, we focused on patient 1 plasma because this had the highest anti-MuSK antibody titer and caused the strongest effect on contraction. Figure 1 shows that the nerve stimulus-induced contractions of muscles from MuSK-MG plasma-treated mice as a function of Ca2+ concentration, at 14 days after notexin injection, were more affected than the contractions of control muscles. This effect of MuSK plasma must have been due to an effect on neuromuscular transmission per se because the contraction as a result of direct stimulation of the muscle fibers was hardly affected by Ca2+ and was not different between MuSK and controls. In other experiments (data not shown), the effect of MuSK plasma was tested already after 7–8 days after notexin injections. The contraction was weak in these muscles, but this was due to the immature condition of the (2- to 3-day-old) fibers rather than to the efficiency of neuromuscular transmission, which appeared already rather good when the nerve stimulus-induced contractions were compared with those resulting from direct muscle stimulation. Because of the weakness of the contractions, it was difficult to ascertain the effect of the MuSK plasma treatment, although the impression was that there was, relative to controls, not a marked effect of MuSK plasma on the contractions in 0.35 mmol/L Ca2+.
Figure 1.
Effect of calcium and MuSK-MG plasma treatment (patient 1) on the contraction of regenerating FDB muscle (14 to 16 days after notexin). Plasma injections were started 1 day after notexin. Nerve stimulation with single pulses in muscles treated with patient’s plasma (squares) and control plasma (triangles). Direct stimulation of the same muscles with single pulses (open squares and triangles for patient and control, respectively). Means ± SEM of values from six to eight muscles. *P < 0.005; **P < 0.05 (Student’s t-test).
Unexpectedly, the aforementioned effect of MuSK plasma on muscle contraction appeared not to be directly related to an effect on AChRs and/or ACh release as shown in two reference models of defective neuromuscular transmission where the level of AChRs or the level of ACh release was moderately affected, namely in the α-BuTX-induced MG model with a decreased number of AChRs28 and the Rn/Rn mutation with a decreased number of active presynaptic calcium channels.34 In these models, both low calcium and curare caused a very marked reduction in contraction relative to controls (P. C. Molenaar, unpublished observations).
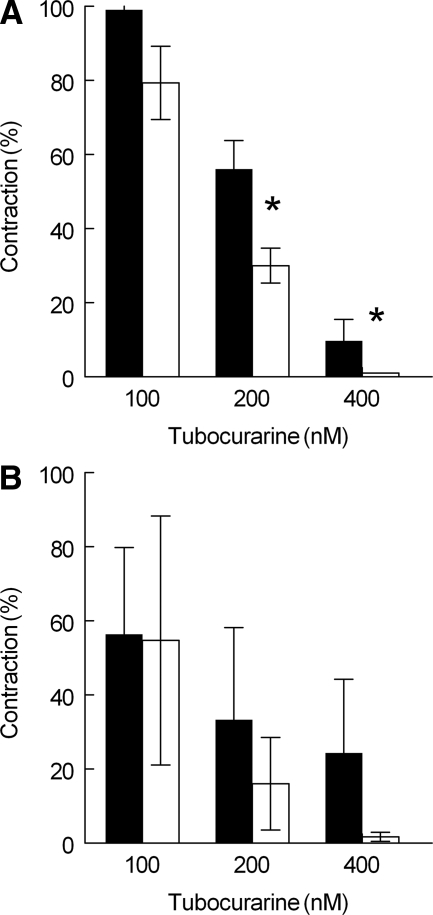
Because MuSK plasma had an effect on nerve stimulus-induced muscle contraction under the condition of low [Ca2+] in the medium and not in the presence of 2 mmol/L Ca2+ in combination with 400 nmol/L tubocurarine, the effect of tubocurarine was reinvestigated but now in medium with low [Ca2+]. For this purpose, a concentration of 0.5 mmol/L Ca2+ was chosen because in the presence of 0.25 or 0.35 mmol/L Ca2+ the contractions had become too small to evaluate quantitatively any additional effect of tubocurarine. Figure 2, upper panel, shows that in normal FDB muscles, the effect of tubocurarine was, relative to controls, increased in muscles derived from mice treated with MuSK plasma (P < 0.05). In notexin-treated muscles (Figure 2, lower panel), MuSK plasma showed a similar trend, but this was not statistically significant (P = 0.09).
Figure 2.
Effect of anti-MuSK+ plasma (patient 1) and tubocurarine on the contraction of normal (upper panel) and notexin-treated (14 days, lower panel) FDB muscles in the presence of 0.5 mmol/L (upper panel) or 0.35 mmol/L Ca2+ (lower panel). Nerve stimulation at 40 Hz. Ordinate, the contractions of muscles are expressed as a fraction of the contractions caused by direct stimulation of the muscle. Data derived from mice treated with control plasma (black bars) and patient 1 plasma (white bars). Means ± SEM of three muscles. *P < 0.05 (ANOVA two-way test).
The number of AChRs was measured by in vitro binding of 125I-α-BuTX (Table 3). It can be seen that 14 days after notexin treatment the FDB muscle had bound the same amount of radiolabel as control muscles. On the other hand, at 8 days there was approximately 30% more binding in the regenerated muscle, which may be due to the fact that the endplates were larger at this time of regeneration (see below), in contrast to the situation at 14 days after notexin when this effect had subsided. Injection of MuSK plasma for 10 days in normal muscle had no effect on 125I-α-BuTX binding. Similarly after notexin, the treatment with MuSK plasma for 1 to 14 days and 1 to 21 days did not cause a change in the binding of 125I-α-BuTX.
Table 3.
Measurement of AChRs in Regenerating and Normal FDB Muscles
| Time after toxin or PBS injection | Binding of 125I- α-BuTX (cpm)
|
||
|---|---|---|---|
| Notexin | Control | Notexin/control | |
| 8 days | 8900 ± 440 (4) | 7100 ± 350 (4) | 1.30 ± 0.08* |
| 14 days | 7700 ± 570 (4) | 7400 ± 510 (4) | 1.00 ± 0.02 |
| MuSK patient 1 plasma, day 1–10 | Control patient plasma, day 1–10 | MuSK/Control | |
| No notexin | 6100 ± 240 (4) | 5300 ± 250 (4) | 1.10 ± 0.07 (4) |
| MuSK patient 1 plasma, day 1–14 | Control patient plasma, day 1–14 | MuSK/Control | |
| 14 days after notexin | 1500 ± 90 (10) | 1500 ± 50 (10) | 1.00 ± 0.07 (10) |
| MuSK patient 1 plasma, day 1–21 | Control patient plasma, day 1–21 | MuSK/Control | |
| 21 days after notexin | 6300 ± 180 (4) | 6300 ± 260 (4) | 1.00 ± 0.04 (4) |
Muscles were incubated for 2 hours in the presence of 1 μg/ml 125I- α-BuTX. Values of radioactivity have not been corrected for the specific radioactivity of the radiolabel used, so the values of the binding between different experiments cannot be compared.
Means ± SEM with number of muscles between parentheses.
P < 0.05, Student’s t-test.
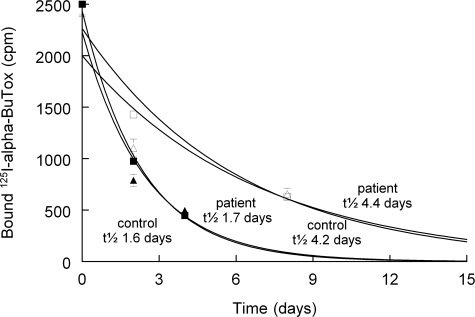
Figure 3 shows that MuSK plasma had no effect on the fast turnover of newly formed AChRs after notexin injection; the half-life was 1.7 and 1.6 days, for patient and control plasma, respectively. When 125I-α-BuTX was injected at 14 days, corresponding to the condition of Figure 1 and Table 2, these values had increased to 4.4 and 4.2 days for MuSK and control plasma, respectively.
Figure 3.
Loss of bound 125I-α-BuTX from notexin-treated FDB muscle treated with plasma from patient 1 (triangles) and control plasma (squares). Injections of 125I-α-BuTX were given (at t = 0 in the figure) either at day 7 or at day 13 (open symbols) after notexin-treatment. The continuous lines were exponentially fitted to the data points, with the values of half-life time as indicated in the figure. Values at t = 0 were normalized to 2500 cpm. Means ± SEM of four to eight muscles.
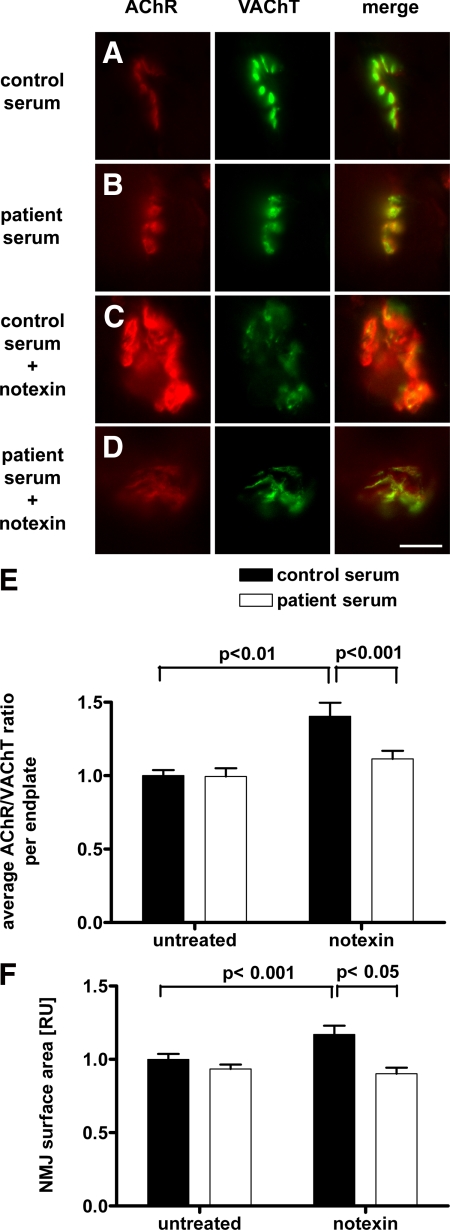
Quantitative histochemistry showed that endplates were enlarged after notexin treatment compared with normal endplates (Figure 4, A–D). Moreover, in notexin-treated muscles the levels of AChRs were increased relative to presynaptic VAChT levels (Figure 4E). Figure 4D also shows that this increased ratio of the AChR versus VAChT staining by notexin was prevented by injection of MuSK plasma, thereby reducing the AChR/VAChT ratio to the value observed in normal FDB muscles. Similarly, the notexin-induced increase of synapse area was prevented by MuSK plasma (Figure 4F). No AChR clusters were observed outside the areas that had stained for VAChT. Similar results were obtained when the endplates were stained for rapsyn; the rapsyn/VACHT ratio was reduced by MuSK plasma in notexin-treated muscles (Supplemental Figure S2, see http://ajp.amjpathol.org).
Figure 4.
Immunohistochemical analysis of the NMJ using fluorescent microscopy. Muscles were analyzed after 7 days’ treatment with patient’s or control plasma. AChR (postsynaptic membrane) is stained in red, VAChT (nerve bouton) is stained in green; merge on the right. Scale bar is 10 μm. A: Normal endplate in a muscle treated with control serum. B: Endplate in a muscle treated with patient 1 serum appears normal. C: In a muscle treated with control serum, new endplates are formed 7 days after notexin treatment. These endplates are enlarged compared with normal adult endplates. D: Endplate in a muscle treated with patient 1 serum 7 days after notexin treatment. The enlargement of newly formed endplates is not present in this condition. E: Measurement of fluorescence intensities of AChR relative to VAChT staining in an average of 105 endplates per muscle. The average AChR/VAChT staining was significantly decreased at endplates of patient serum treated animals compared with control serum treated muscles in the group of 7 days after notexin. F: Surface area of photographed endplates of identically treated muscles. The average surface area was significantly decreased at endplates of patient serum treated animals compared with control serum treated muscles in 7 days after notexin treatment. Means ± SEM of four to six muscles. The level of statistical significance (unpaired t-test) is as indicated in the figure.
Discussion
The main outcome of the present investigation using the notexin model of regenerating synapses is that MuSK-MG patient’s plasma causes a reduction in endplate size, whereas minimal effect was found in normal adult muscle where no such endplate regeneration takes place. Second, MuSK plasma reduces the efficacy of neuromuscular transmission under conditions of low external calcium concentration, but this effect is no greater than the effect of the MuSK-plasma in normal muscle. It will be discussed below whether or not these two effects of MuSK-MG plasma are two sides of the same coin or really different effects. Third, the magnitude of the effects of MuSK antibody on regenerating synapses proves to be rather moderate, in contrast to our prediction that the results would be relatively pronounced during the formation of synaptic contacts. Two possible explanations are that the bound antibody leaves the MuSK to exert still some of its trophic influence on synapse formation or that not enough molecules of MuSK have antibody bound to block endplate formation. It has been reported that endocytosis of MuSK is required for its physiological function,35 and it is quite possible that binding of MuSK antibody blocks this process.
Notexin Model of Regenerating Synapses
The time course of degeneration and regeneration in the FDB muscle is similar to results obtained earlier by others in the soleus muscle of the rat by application of the myotoxic agents bupivacaine and notexin.25,32 After 7 to 8 days, the regenerated muscle contracted, unfortunately, still too weakly for reliable recordings to be made so that this early time point of regeneration could not be compared with the quantitative histochemical data of Figure 4 that were obtained on day 7.
In the present experiments, the amount of 125I-α-BuTX binding at 8 days after notexin injection was approximately 1.3 times higher than in controls, whereas at 7 days after notexin the histochemical staining of AChRs and rapsyn, relative to controls, was 1.5 times higher. On the other hand, at 14 days after notexin the 125I-α-BuTX binding was the same as in controls not treated with notexin. This suggests that the size of the endplates had, at 7 days after notexin, transiently increased during regeneration of the fibers. This is compatible with the results by Grubb and Harris25 who found that after an initial increase at 7 days the amplitude of MEPPs and the output of ACh had become normal again at 14 days; although, the effect on the amplitude of the MEPPs could be largely attributed to changes in the impendance of the membrane.25
The finding that the sensitivity to tubocurarine in notexin-treated, 14-days-regenerated FDB muscles was comparable with that of normal FDB muscle indicates that the neuromuscular transmission was as efficient as in control muscles. However, not all was “normal” in regenerated FDB muscle. First, the turnover of AChRs at 6 days was remarkably fast (t1/2 = 2 days), and at 14 days, the turnover, although slowed down, was still two times faster than in normal muscles (t1/2 = 5 to 6 days). Second, the regenerated fibers were rather twitchy and sometimes relaxed more slowly than controls after a stimulus. Although this was not investigated further, the picture that emerged is that regenerated muscle contained embryonic-like AChRs36 and embryonic rSkM2 sodium channels instead of the normal sodium channels being responsible for the muscle action potential.37 Shyng and Salpeter38,39 have demonstrated that in denervated muscle the AChRs have a very fast turnover (approximately 1 day) when they are recently inserted at the endplate, whereas on reinnervation, a stabilization of the AChRs takes place to a half-life time of approximately 14 days. Although denervated muscle contains extrajunctional AChRs with a rapid turnover and with the gamma instead of the epsilon subunit, it is uncertain as to whether the endplate AChRs with a rapid turnover in the present regeneration experiments also had receptors with the gamma subunit and whether this was replaced gradually by epsilon subunit containing AChRs when the muscle regenerated further in 14 to 20 days.
It should be borne in mind that in regenerated FDB muscle there is not really a de novo formation of synapses like in embryonic muscle because the basal lamina probably largely survives the notexin treatment, so that regeneration of junctions is much faster than would be possible in the absence of extracellular matrix specialization at the site of the old endplate.
Effect of MuSK-MG Plasma on Endplates
Two main findings of this study were the effects of MuSK-MG plasma on the size of endplates and on the muscle contraction at low [Ca2+]. The effect on endplate size was found only in regenerating muscle, whereas the effect on contraction was found in regenerating as well as in normal muscles. It is likely that the effect on endplate size is because of a direct, negative effect of antibody on the functioning of MuSK protein, such as the interaction between lrp4 and MuSK.21 Whether this effect is of the same type as the fragmentation of endplates caused by the human MuSK mutation studied by Chevessier et al40 remains to be seen; at any rate, it is a much more modest effect. These effects on morphology and contraction may be due to different mechanisms that are as yet unclear. Although the number and density of AChRs at the endplate was not influenced by the MuSK-MG plasma injections at 14 days, it is possible that the function of one of the channel properties of the AChRs was diminished by the combination of lowering [Ca2+] and the MuSK-antibody interaction. An alternative possibility is that there was a presynaptic effect of MuSK antibody, eg, on terminal size. However, it is difficult to see how this can be reconciled with the results of the experiments with tubocurarine in the presence of 2 mmol/L Ca2+; because under this condition, it would be expected that there is less ACh release from nerve terminals because of their subnormal size, and thus, the contraction of the muscle would have been more sensitive to tubocurarine than controls. In fact this was not the case. It is unlikely, therefore, that the increased sensitivity in low [Ca2+] is simply due to a morphological effect of MuSK antibody treatment, ie, a decreased size of the synapse. In other words, because of the lack of the effect of tubocurarine at 2 mmol/L Ca2+, it is likely that in the MuSK-plasma-treated muscle the safety factor of neuromuscular transmission was not affected at all at least at 14 days’ regeneration of the muscle, in contrast to the situation at 7 days’ regeneration when the endplace size seemed to be reduced as shown in the experiments with the histochemical staining of the AChR. However, in low [Ca2+] the safety factor of neuromuscular transmission was affected more than in control muscles, possibly for reasons as outlined above, and tubocurarine compromised the safety factor then even further.
The turnover of AChRs was increased during regeneration after notexin but otherwise not influenced as a result of MuSK-MG plasma injections. It has been reported that stimulation of the MuSK-rapsyn pathway by agrin stabilizes AChRs in the endplate of denervated muscle even when the muscle is not reinnervated.24 Therefore, it was expected that MuSK-MG plasma would exercise a negative effect on the recovery of the half-life time of the AChRs, but this turned out not to be the case.
Sera from patients with seronegative MG (including in retrospect some MuSK-MG patients) have some effect on AChRs in tissue culture or whole muscle, eg, Refs.9 and.41 Exposure of TE671 cells to MuSK-MG sera reduced AChR expression by approximately 20% but had no effect on AChR subunit or MuSK mRNA expression.42 The present results with passive immunization using MuSK-MG plasma are different from those obtained by Jha et al,43 who used the approach of active immunization with recombinant MuSK in mice, and by Cole et al,44 who administered i.p. injections of IgG to mice in relatively large amounts (compared with total mouse IgGs). Jha and collegues43 found weight loss, decrease of compound muscle action potentials, and a reduction of MEPP amplitudes in isolated muscles. It is possible that the differences with the present results have been due to the production of anti-MuSK immunoglobulin IgG2 in the mice with resulting complement attack to the endplate membrane near the sites where AChRs are inserted. Cole and collegues44 found decrement in the EMG on repetitive nerve stimulation and reduced staining of AChRs in diaphragm muscle. The reason of the discrepancy between these and our results remains to be established. Cole and collegues44 reported that C57BL/6J mice were more susceptible than FVB/NJ mice to MuSK-MG antibody, but in our experiments, C57BL/6J mice are not particularly susceptible in contraction experiments to MuSK-MG plasma (unpublished observation).
Implications for the Pathophysiology of MuSK-MG
An interesting case of a severe congenital myasthenic syndrome has been presented in which the cause was demonstrated to be a V790M mutation of the gene coding for MuSK resulting in clusters of very small endplates; further that this abnormality could be transferred within weeks to mice after transfection of the corresponding human cDNA mutant by electroporation into the tibialis anterior muscle.40 This unequivocally shows that malfunctioning MuSK can have rapid and severe consequences in adult muscle, even though the structure of the neuromuscular junction appears to be fairly stable during life.45,46 Silencing of MuSK in animal studies by injection and electroporation of small interfering RNAs against MuSK-mRNA causes deterioration of the structure of endplates within 6 weeks.23 The question arises as to whether anti-MuSK antibodies from patients can affect the function of MuSK as severely as in the abovementioned mutation, even when the amount of MuSK remains constant. The question is apparently not an easy one to answer. There also seems to be a paradox in the pathophysiology; on the one hand, symptoms in MuSK-MG can be very severe while on the other hand the laboratory findings are ambiguous, with abnormalities that seem relatively innocuous, such as slightly subnormal MEPP amplitudes with normal AChR numbers.47,48,49
In summary, the present results in mice suggest the possibility that MuSK MG autoantibodies indeed influence the molecular function of MuSK, possibly by hampering the interaction between lrp4 and MuSK,21 with the capability thereby to diminish the size of endplates and the functioning of the AChRs without reducing their number. Obviously, such effects might be much larger in patients than in the present experimental mice because antibodies would circulate at a higher concentration and for a very much longer period than could be tested in our mice.
Supplementary Material
Acknowledgments
We thank Mr. Henk Veldman and Prof. John H.J. Wokke (University Medical Center Utrecht, Utrecht, The Netherlands) for helpful discussions.
Footnotes
Address reprint requests to Dr. Peter C. Molenaar, Department of Neuroscience, School of Mental Health and Neuroscience, University of Maastricht, Universiteitssingel 50, PO Box 616, 6200 MD Maastricht, The Netherlands. E-mail: p.molenaar@maastrichtuniversity.nl.
Support of W.P.t.B. by the Prinses Beatrix Fonds is gratefully acknowledged (project MAR 02-0204 to P.C.M.). P.M.-M. and M.L. were supported by grants from the Prinses Beatrix Fonds, L’Association Française contre les Myopathies, the European Union Sixth Framework Program (FP6) MYASTAID LSHM-CT-2006-037833, and Genmab.
W.P.t.B. and P.M.-M. contributed equally to the work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180:871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Drachman DB. Myasthenia gravis: immunobiology of a receptor disorder. Trends Neurosci. 1983;6:446–451. [Google Scholar]
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- Losen M, Martinez-Martinez P, Phernambucq M, Schuurman J, Parren PW, De Baets MH. Treatment of myasthenia gravis by preventing acetylcholine receptor modulation. Ann NY Acad Sci. 2008;1132:174–179. doi: 10.1196/annals.1405.034. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Merlie J, Lindstrom J. Modulation of acetylcholine receptor in rat diaphragm by anti-receptor sera. Nature. 1978;274:65–68. doi: 10.1038/274065a0. [DOI] [PubMed] [Google Scholar]
- Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, Beeson D, Willcox N, Vincent A. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain. 2008;131(Pt 7):1940–1952. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DB, de Silva S, Ramsay D, Pestronk A. Humoral pathogenesis of myasthenia gravis. Ann NY Acad Sci. 1987;505:90–105. doi: 10.1111/j.1749-6632.1987.tb51285.x. [DOI] [PubMed] [Google Scholar]
- Mossman S, Vincent A, Newsom-Davis J. Myasthenia gravis without acetylcholine-receptor antibody: a distinct disease entity. Lancet. 1986;1:116–119. doi: 10.1016/s0140-6736(86)92259-2. [DOI] [PubMed] [Google Scholar]
- Burges J, Vincent A, Molenaar PC, Newsom-Davis J, Peers C, Wray D. Passive transfer of seronegative myasthenia gravis to mice. Muscle Nerve. 1994;17:1393–1400. doi: 10.1002/mus.880171208. [DOI] [PubMed] [Google Scholar]
- Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- Niks EH, Kuks JB, Verschuuren JJ. Epidemiology of myasthenia gravis with anti-muscle specific kinase antibodies in The Netherlands. J Neurol Neurosurg Psychiatry. 2007;78:417–418. doi: 10.1136/jnnp.2006.102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, Marino M, Bartoccioni E. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–2311. doi: 10.1093/brain/awg223. [DOI] [PubMed] [Google Scholar]
- Farrugia ME, Robson MD, Clover L, Anslow P, Newsom-Davis J, Kennett R, Hilton-Jones D, Matthews PM, Vincent A. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain. 2006;129:1481–1492. doi: 10.1093/brain/awl095. [DOI] [PubMed] [Google Scholar]
- Lavrnic D, Losen M, Vujic A, De Baets M, Hajdukovic LJ, Stojanovic V, Trikic R, Djukic P, Apostolski S. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. 2005;76:1099–1102. doi: 10.1136/jnnp.2004.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, McConville J, Farrugia ME, Bowen J, Plested P, Tang T, Evoli A, Matthews I, Sims G, Dalton P, Jacobson L, Polizzi A, Blaes F, Lang B, Beeson D, Willcox N, Newsom-Davis J, Hoch W. Antibodies in myasthenia gravis and related disorders. Ann NY Acad Sci. 2003;998:324–335. doi: 10.1196/annals.1254.036. [DOI] [PubMed] [Google Scholar]
- Niks EH, van Leeuwen Y, Leite MI, Dekker FW, Wintzen AR, Wirtz PW, Vincent A, van Tol MJ, Jol-van der Zijde CM, Verschuuren JJ. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195:151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JG, Aalberse RC, Parren PW. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Kong XC, Barzaghi P, Ruegg MA. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep. 2004;5:183–188. doi: 10.1038/sj.embor.7400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezakova G, Rabben I, Sefland I, Fumagalli G, Lomo T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc Natl Acad Sci USA. 2001;98:9924–9929. doi: 10.1073/pnas.171539698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BD, Harris JB, Schofield IS. Neuromuscular transmission at newly formed neuromuscular junctions in the regenerating soleus muscle of the rat. J Physiol. 1991;441:405–421. doi: 10.1113/jphysiol.1991.sp018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, Vincent A. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- Molenaar PC, Oen BS, Plomp JJ, Van Kempen GT, Jennekens FG, Hesselmans LF. A nonimmunogenic myasthenia gravis model and its application in a study of transsynaptic regulation at the neuromuscular junction. Eur J Pharmacol. 1991;196:93–101. doi: 10.1016/0014-2999(91)90413-k. [DOI] [PubMed] [Google Scholar]
- Molenaar PC. A relative weak leg muscle in the rolling Nagoya mouse as a model for Lambert-Eaton myasthenic syndrome. J Neuroimmunol. 2008;201–202:166–171. doi: 10.1016/j.jneuroim.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Losen M, Stassen MH, Martinez-Martinez P, Machiels BM, Duimel H, Frederik P, Veldman H, Wokke JH, Spaans F, Vincent A, De Baets MH. Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis. Brain. 2005;128:2327–2337. doi: 10.1093/brain/awh612. [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez P, Losen M, Duimel H, Frederik P, Spaans F, Molenaar P, Vincent A, De Baets MH. Overexpression of rapsyn in rat muscle increases acetylcholine receptor levels in chronic experimental autoimmune myasthenia gravis. Am J Pathol. 2007;170:644–657. doi: 10.2353/ajpath.2007.060676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirmanova I, Thesleff S. Ultrastructural study of experimental muscle degeneration and regeneration in the adult rat. Z Zellforsch Mikrosk Anat. 1972;131:77–97. doi: 10.1007/BF00307202. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, van den Maagdenberg AM, Ferrari MD, Frants RR, Molenaar PC. Transmitter release deficits at the neuromuscular synapse of mice with mutations in the Cav2.1 (α1A) subunit of the P/Q-type Ca2+ channel. Ann NY Acad Sci. 2003;998:29–32. doi: 10.1196/annals.1254.005. [DOI] [PubMed] [Google Scholar]
- Zhu D, Yang Z, Luo Z, Luo S, Xiong WC, Mei L. Muscle-specific receptor tyrosine kinase endocytosis in acetylcholine receptor clustering in response to agrin. J Neurosci. 2008;28:1688–1696. doi: 10.1523/JNEUROSCI.4130-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter MM, Loring RH. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog Neurobiol. 1985;25:297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Zhang H, Barchi RL, Kallen RG. Molecular cloning and functional analysis of the promoter of rat skeletal muscle voltage-sensitive sodium channel subtype 2 (rSkM2): evidence for muscle-specific nuclear protein binding to the core promoter. DNA Cell Biol. 1994;13:9–23. doi: 10.1089/dna.1994.13.9. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Salpeter MM. Degradation rate of acetylcholine receptors inserted into denervated vertebrate neuromuscular junctions. J Cell Biol. 1989;108:647–651. doi: 10.1083/jcb.108.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Salpeter MM. Effect of reinnervation on the degradation rate of junctional acetylcholine receptors synthesized in denervated skeletal muscles. J Neurosci. 1990;10:3905–3915. doi: 10.1523/JNEUROSCI.10-12-03905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevessier F, Faraut B, Ravel-Chapuis A, Richard P, Gaudon K, Bauche S, Prioleau C, Herbst R, Goillot E, Ioos C, Azulay JP, Attarian S, Leroy JP, Fournier E, Legay C, Schaeffer L, Koenig J, Fardeau M, Eymard B, Pouget J, Hantai D. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum Mol Genet. 2004;13:3229–3240. doi: 10.1093/hmg/ddh333. [DOI] [PubMed] [Google Scholar]
- Barrett-Jolley R, Byrne N, Vincent A, Newsom-Davis J. Plasma from patients with seronegative myasthenia gravis inhibit nAChR responses in the TE671/RD cell line. Pflugers Arch. 1994;428:492–498. doi: 10.1007/BF00374570. [DOI] [PubMed] [Google Scholar]
- Farrugia ME, Bonifati DM, Clover L, Cossins J, Beeson D, Vincent A. Effect of sera from AChR-antibody negative myasthenia gravis patients on AChR and MuSK in cell cultures. J Neuroimmunol. 2007;185:136–144. doi: 10.1016/j.jneuroim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jha S, Xu K, Maruta T, Oshima M, Mosier DR, Atassi MZ, Hoch W. Myasthenia gravis induced in mice by immunization with the recombinant extracellular domain of rat muscle-specific kinase (MuSK). J Neuroimmunol. 2006;175:107–117. doi: 10.1016/j.jneuroim.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Ann Neurol. 2008;63:782–789. doi: 10.1002/ana.21371. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Breedlove SM, Bernstein S, Lichtman JW. Neuromuscular junctions shrink and expand as muscle fiber size is manipulated: in vivo observations in the androgen-sensitive bulbocavernosus muscle of mice. J Neurosci. 1990;10:2660–2671. doi: 10.1523/JNEUROSCI.10-08-02660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Magrassi L, Purves D. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci. 1987;7:1215–1222. doi: 10.1523/JNEUROSCI.07-04-01215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcen D, Fukuda T, Shen XM, Engel AG. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology. 2004;62:1945–1950. doi: 10.1212/01.wnl.0000128048.23930.1d. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Motomura M, Yoshimura T, Fukudome T, Fukuda T, Nakao Y, Tsujihata M, Vincent A, Eguchi K. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol. 2005;57:289–293. doi: 10.1002/ana.20341. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Is “seronegative” MG explained by autoantibodies to MuSK? Neurology. 2004;62:1920–1921. doi: 10.1212/01.wnl.0000129702.41868.a5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.