Abstract
The α7β1 integrin, dystrophin, and utrophin glycoprotein complexes are the major laminin receptors in skeletal muscle. Loss of dystrophin causes Duchenne muscular dystrophy, a lethal muscle wasting disease. Duchenne muscular dystrophy-affected muscle exhibits increased expression of α7β1 integrin and utrophin, which suggests that these laminin binding complexes may act as surrogates in the absence of dystrophin. Indeed, mice that lack dystrophin and α7 integrin (mdx/α7−/−), or dystrophin and utrophin (mdx/utr−/−), exhibit severe muscle pathology and die prematurely. To explore the contribution of the α7β1 integrin and utrophin to muscle integrity and function, we generated mice lacking both α7 integrin and utrophin. Surprisingly, mice that lack both α7 integrin and utrophin (α7/utr−/−) were viable and fertile. However, these mice had partial embryonic lethality and mild muscle pathology, similar to α7 integrin-deficient mice. Dystrophin levels were increased 1.4-fold in α7/utr−/− skeletal muscle and were enriched at neuromuscular junctions. Ultrastructural analysis revealed abnormal myotendinous junctions, and functional tests showed a ninefold reduction in endurance and 1.6-fold decrease in muscle strength in these mice. The α7/utr−/− mouse, therefore, demonstrates the critical roles of α7 integrin and utrophin in maintaining myotendinous junction structure and enabling force transmission during muscle contraction. Together, these results indicate that the α7β1 integrin, dystrophin, and utrophin complexes act in a concerted manner to maintain the structural and functional integrity of skeletal muscle.
Duchenne muscular dystrophy (DMD) is a lethal neuromuscular disease that affects 1 in every 3500 live male births. Patients with DMD have impaired mobility, are restricted to a wheelchair by their teens, and die from cardiopulmonary failure in their early twenties.1,2 Currently, there is no cure or effective treatment for this devastating disease. Mutations in the dystrophin gene resulting in loss of the dystrophin protein are the cause of disease in DMD patients and the mdx mouse model.3,4,5,6,7
The dystrophin glycoprotein complex links laminin in the extracellular matrix to the actin cytoskeleton. The N-terminal region of dystrophin interacts with cytoskeletal F-actin8 and the C-terminal region associates with the dystrophin-associated protein complex, which include α- and β-dystroglycan, α- and β-syntrophin, the sarcoglycans, and sarcospan.9 In DMD, the absence of dystrophin leads to disruption of the dystrophin glycoprotein complex, resulting in increased muscle fragility and altered cell signaling.9 Loss of this critical transmembrane linkage complex in DMD patients and mdx mice results in progressive muscle damage and weakness, inflammation, necrosis, and fibrosis. Lack of dystrophin also leads to abnormalities at myotendinous and neuromuscular junctions (MTJ and NMJ), which further contribute to skeletal muscle damage.10,11,12,13,14,15,16,17 In addition, defective muscle repair in DMD patients eventually results in muscle degeneration exceeding the rate of regeneration.18 Overall, dystrophin is critical for muscle function, structure, and stability, and its absence results in progressive muscle wasting and severe muscular dystrophy. In the absence of dystrophin two additional laminin-binding receptors, the α7β1 integrin and utrophin, are up-regulated in the skeletal muscle of DMD patients and mdx mice, which may compensate for the loss of the dystrophin glycoprotein complex.19,20,21
The α7β1 integrin is a heterodimeric laminin receptor involved in bidirectional cell signaling and is localized at junctional and extrajunctional sites in skeletal muscle.22,23 At least six α7 integrin isoforms produced by developmentally regulated RNA splicing are expressed in skeletal muscle.24 Mutations in the α7 integrin gene (ITGA7) cause myopathy in humans.25 Mice lacking the α7 integrin develop myopathy, exhibit vascular smooth muscle defects and have altered extracellular matrix deposition.26,27,28,29,30 The observation that the α7β1 integrin is elevated in the muscle of DMD patients and mdx mice led to the hypothesis that the α7β1 integrin may compensate for the loss of dystrophin.19 Enhanced expression of the α7 integrin in the skeletal muscle of severely dystrophic mice reduced muscle pathology and increased lifespan by threefold.10,11 In contrast, loss of both dystrophin and α7 integrin in mice results in severe muscular dystrophy and premature death by 4 weeks of age.28,31 The α7β1 integrin is therefore a major modifier of disease progression in DMD.
The utrophin glycoprotein complex is a third major laminin receptor in skeletal muscle. Utrophin has significant sequence homology to dystrophin.32,33 In normal adult muscle utrophin is restricted to neuromuscular and myotendinous junctions.34 During development or in damaged or diseased muscle, utrophin expression is increased and becomes localized at extrajunctional sites.35,36 Utrophin interacts with the same proteins as dystrophin, but binds to actin filaments at different sites.37 In mice, loss of utrophin results in a mild form of myasthenia with reduced sarcolemmal folding at the postsynaptic membrane of the neuromuscular junction.12,15 Transgenic overexpression of utrophin has been shown to rescue mdx mice.38 Mice that lack both dystrophin and utrophin exhibit severe muscular dystrophy and die by 14 weeks of age.13,14 Thus, utrophin is also a major laminin receptor that modifies disease progression in DMD.
To understand the functional overlap between the α7β1 integrin and utrophin in skeletal muscle, we produced mice that lack both α7 integrin and utrophin (α7/utr−/−). Since both complexes are highly enriched at the MTJ and NMJ, we hypothesized that α7/utr−/− mice may have severe abnormalities at these critical junctional sites. Our study demonstrates α7/utr−/− mice exhibit partial embryonic lethality comparable with that observed in α7−/− mice. Dystrophin is increased in these animals and enriched at the NMJ but not the MTJ. α7/utr−/− mice display ultrastructural defects in their MTJ and compromised force transmission. Together, these results indicate that the α7β1 integrin, dystrophin and utrophin laminin binding complexes provide continuity between laminin in the extracellular matrix and the cell cytoskeleton, which are necessary for the normal structural and functional properties of skeletal muscle.
Materials and Methods
Generation of α7/utr−/− Mice
Male α7−/− mice (C57BL6 × 129 strain) generated in the Nevada Transgenic Center26 were mated to female utr−/− mice (C57BL6 × 129 strain and a kind gift from Dr. Joshua Sanes, Harvard University, MA). The resulting male offspring were backcrossed to utr−/− females. To generate α7/utr−/− mice, F2 generation mice heterozygous at the α7 integrin locus were mated. Wild-type mice (C57BL6 × 129) were used as controls for this study. Age-matched male mice were used for all experiments.
Mice were genotyped using genomic DNA isolated from tail clips. Tail clips were incubated in TNES buffer (200 mmol/L TrisHCl pH 8.0, 5 mmol/L EDTA pH8.0, 400 mmol/L NaCl, and 1% SDS) and 100 μg proteinase K overnight at 55°C. 5 M NaCl was added to the samples and DNA precipitated using ice-cold 100% ethanol. The DNA pellet was washed with 70% ethanol and resuspended in TE buffer (10 mM Tris-HC1, 1 mM EDTA pH 8.0). The wild-type and mutant α7 integrin alleles were detected using a multiplex PCR as previously described.28 A multiplex PCR was done for utrophin using the following primers: 553 (5′-TTGCAGTGTCTCCCAATAAGG TATGAAC-3′), 554 (5′-CTGAGTCAAACAGCTTGGAAGCCTCC-3′), 22803 (5′-TGCCAAGTTCTAATTCCA TCAGAAGCTG-3′). The PCR conditions were: 95°C for 2 minutes, 40 cycles of 95°C for 1 minute, 62°C for 1 minute, and 72°C for 1 minute. The utrophin wild-type band produced a 640 bp and the utrophin targeted allele produced a 450 bp band.
Tissue Isolation
Five week-old wild-type, α7−/−, utr−/−, and α7/utr−/− mice were euthanized according to a protocol approved by the University of Nevada, Reno, Animal Care and Use Committee. Gastrocnemius, triceps, and tibialis anterior muscles were dissected from mice and flash frozen in liquid nitrogen cooled isopentane.
Immunoblotting
To detect α7 integrin, protein was extracted from gastrocnemius muscle as previously reported.28 The anti-α7A integrin (A2 345) and anti-α7B integrin (B2 347) rabbit polyclonal antibodies (a gift from Dr. Stephen Kaufman, University of Illinois) were used to detect the α7A and α7B integrin isoforms at a dilution of 1:500 and 1:2000 respectively. Utrophin was detected using the MANCHO3 8A4 anti-utrophin mouse monoclonal antibody (a kind gift of Glenn Morris, Center for Inherited Neuromuscular Disease, Shropshire, UK) as previously described.28 For dystrophin immunoblots, protein was extracted from gastrocnemius using a 10% SDS extraction buffer (100 mmol/L Tris-HCl pH 8.0, 10% SDS, 10 mmol/L EDTA, 10% glycerol). Samples were boiled for 10 minutes and centrifuged for 20 minutes. 10 μg of protein was loaded on 5% polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. The mouse monoclonal antibody Dys-2 (Novocastra Laboratories Ltd, Newcastle Upon Tyne, UK) was used at a dilution of 1:350 to detect dystrophin. Blots were incubated with Alexa Fluor 680-coupled goat anti-rabbit IgG (Molecular Probes, Eugene, OR) to detect the primary antibody. The Odyssey Imaging System (LiCor Biosciences, Lincoln, NE) was used to quantify band intensities. Myosin heavy chain was used as the loading control and analysis performed in the linear range for the detection of these antibodies.
For acetylcholine receptor (AChR)-β subunit immunoblots, protein was extracted from gastrocnemius using RIPA buffer (50 mmol/L Hepes pH 7.4, 150 mmol/L NaCl, 1 mmol/L Na3VO4, 10 mmol/L NaF, 0.5% Triton X-100, 0.5% NP40, 10% glycerol, 2 mmol/L phenylmethylsulfonyl fluoride, and 1:200 Protease Inhibitor Cocktail Set III [Calbiochem, EMD Biosciences, San Diego, CA]). 50 μg of protein was loaded on 7.5% Tris-HCL gels (Bio-Rad, Hercules, CA). To detect the AChR-β, the rabbit polyclonal antibody AChR-β (Epitomics, Burlingame, CA) was used at a dilution of 1:2000. Primary antibodies were detected, and blots were normalized for protein loading, as previously published.28
Immunofluorescence
Ten-micron-thick cryosections from triceps or tibialis anterior muscles were placed on Surgipath slides (Surgipath Medical Supplies, Richmond, IL). Sections were fixed with methanol, acetone, or 4% paraformaldehyde for 2 minutes. α7 integrin and utrophin were detected as reported28 using the rat monoclonal antibody CA5.5 (Sierra Biosource, Morgan Hill, CA) and the mouse monoclonal antibody MANCHO7 7F3. Dystrophin was detected using the mouse monoclonal antibody MANDRA1 (Sigma Aldrich, St. Louis, MO) (1:100) after using the M.O.M. kit (Vector Laboratories, Burlingame, CA) to block endogenous mouse immunoglobulin. Tenascin C was detected using a rat monoclonal anti-tenascin C antibody (Sigma Aldrich, St. Louis, MO) at 1:200 on unfixed TA muscle. Fluorescein isothiocyanate-conjugated secondary antibodies were used to detect primary antibodies at a dilution of 1:500. Rhodamine-labeled α-bungarotoxin (Molecular Probes, Eugene, OR) was used at a 1:1000 dilution to identify AChRs at the NMJ. Images were captured with Zeiss Axioskop 2 Plus fluorescent microscope, AxioCam HRc digital camera and Axiovision 5.0 software.
Histology
Ten-micron-thick cryosections from triceps muscle were placed on glass slides and fixed in ice-cold 95% ethanol for 2 minutes, placed in 70% ethanol for 2 minutes, and rinsed in running water for 5 minutes. Slides were immersed in Gill’s hematoxylin (Fisher Scientific, Fair Lawn, NJ) for 3 minutes and rinsed in running water for 5 minutes. Slides were then incubated in Scott’s solution for 3 minutes and rinsed in running water for 5 minutes. Slides were immersed in 1× eosin for 2 minutes and progressively dehydrated in ice cold 70% ethanol for 30 seconds, 95% ethanol for 30 seconds, and 100% ethanol for 2 minutes. Slides were placed in xylene for 5 minutes and mounted in DePeX mounting medium (Electron Microscopy Sciences, Washington, PA). Using H&E-stained sections from the triceps muscle, the number of centrally located nuclei in 1000 fibers was counted per mouse (N = 6). The results were expressed as the percentage of fibers containing centrally located nuclei.
Evan’s Blue Dye Uptake
Wild-type, α7−/−, utr−/−, and α7/utr−/− mice at 5 weeks of age were injected intraperitoneally with 50 μl sterile Evan’s blue dye (500 μg/50 μl PBS) per 10g of body weight. After 3 hours, tissues were frozen in liquid nitrogen cooled isopentane, cryosectioned (10 μm), and placed on slides. Tissue sections were fixed in 4% paraformaldehyde. To define muscle fibers, wheat-germ agglutinin was used at a concentration of 1 μg/ml. A Zeiss Axioscope II fluorescence microscope was used to visualize sections. Fibers positive for Evan’s blue dye in 1000 random fibers per mouse were counted at a magnification of ×630 (N = 5).
Muscle Endurance Test
Five-week-old wild-type, α7−/−, utr−/−, and α7/utr−/− mice were allowed to grasp wire cages and remain suspended. The recorded time was terminated when the mice released their grip. Every mouse was subjected to two tests and results from both tests were averaged. Muscle endurance was determined using the ratio of time suspended over mouse weight.
Grip Strength Test
The forelimb grip strength was measured using a SDI Grip Strength System and a Chatillon DFE Digital Force Gauge (San Diego Instruments, Inc., San Diego, CA). Five-week-old wild-type, α7−/−, utr−/−, and α7/utr−/− mice were allowed to grasp a horizontal platform with their forelimbs and then pulled backwards. The peak tension (grams of force) was recorded just before the mice released their grip. Five consecutive tests were performed for each mouse and the data were averaged. At least nine mice per group were analyzed.
Electron Microscopy
Tibialis anterior muscles from 5-week-old wild-type and α7/utr−/− mice were fixed in 2% gluteraldehyde and 2.5% paraformaldehyde in a 0.2 M/L phosphate buffer overnight. Tissues were rinsed with PBS and postfixed with 1% osmium tetroxide in phosphate buffer for one hour. Samples were successively dehydrated in 70%, 95%, and 100% ethanol, incubated in propylene oxide for 30 minutes, in 1:1 propylene oxide and LX-112 epoxy embedding resin for 12 hours. 0.1 μm sections were cut on a Reichert Ultracut E Ultramicrotome. Sections were stained with uranyl acetate and lead citrate. The MTJ were viewed on a Hitachi H-600 transmission electron microscope at ×20,000 magnification.
Statistical Analysis
All averaged data are reported as the mean ± SD unless otherwise stated. To compare multiple groups, one-way and two-way analysis of variance was performed using SigmaStat 1.0 software (Jandel Corporation, San Rafael, CA). Chi-squared analysis was used to determine statistical significance for the Mendelian inheritance pattern. P < 0.05 was considered statistically significant unless otherwise stated.
Results
Production of α7 Integrin and Utrophin Double Knockout Mice
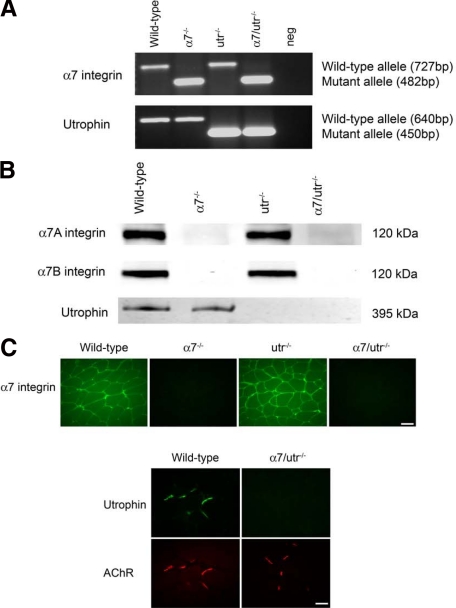
To determine the contributions of the α7 integrin and utrophin as modifiers of DMD disease progression, α7 integrin null mice were bred with utrophin-deficient animals to produce mice that lack both the α7 integrin and utrophin (α7/utr−/−). Genotyping was used to confirm mutant alleles for α7 integrin and utrophin (Figure 1A). PCR was used to identify the mutant α7 integrin allele at 482 bp while the wild-type allele was detected at 727 bp (Figure 1A). PCR identified the targeted utrophin allele at 450 bp, whereas the wild-type allele produced a 640 bp product (Figure 1A). Loss of both α7 integrin and utrophin proteins was confirmed by immunoblotting and immunofluorescence (Figure 1, B and C).
Figure 1.
Generating α7 integrin and utrophin (α7/utr−/−) double knockout mice. A: α7 integrin null mice were mated with utrophin deficient mice and the resultant heterozygous animals bred to generate α7/utr−/− double knockout animals. Multiplex PCR was performed to detect the wild-type and mutant α7 integrin and utrophin alleles. As expected, a 727-bp wild-type α7 integrin allele was detected in wild-type and utr−/− mice. A 482-bp mutant α7 integrin allele was detected in α7−/− and α7/utr−/− mice. A 640-bp wild-type utrophin allele was detected in wild-type and α7−/− mice, while a 450-bp mutant utrophin allele was identified in utr−/− and α7/utr−/− mice. Neg = no template DNA control. B: Immunoblotting was used to confirm loss of α7A and α7B integrin isoforms and utrophin in the skeletal muscle of 5-week-old α7−/− and α7/utr−/− mice. C: Absence of the α7 integrin in α7−/− and α7/utr−/− mice and loss of utrophin in α7/utr−/− mice was confirmed by immunofluorescence. Scale bar = 50 μm.
α7/utr−/− Mice Exhibit Partial Embryonic Lethality Similar to α7 Integrin Null Mice
To determine whether loss of both α7 integrin and utrophin results in partial embryonic lethality, the Mendelian inheritance patterns of wild-type, α7−/−, utr−/−, and α7/utr−/− mice were analyzed. Matings of α7+/−/utr+/− mice to α7−/−/utr+/− animals showed that 7.6% of α7−/−/utr−/− mice were born instead of the expected 12.5% (Table 1), indicating that 40% of the α7/utr−/− animals died in utero. This embryonic lethality is similar to that observed in α7 integrin null mice in which about 50% of the mice die before birth (Table 1).26,29 These results indicate α7/utr−/− mice exhibit partial embryonic lethality as a result of loss of the α7 integrin alone and loss of utrophin did not further reduce the viability.
Table 1.
Mendelian Inheritance Pattern from Breeding α7+/−/utr+/− × α7−/−/utr+/− mice*
| Genotype | Expected | Observed |
|---|---|---|
| Wild-type | 37.5% | (50/92) 54.3% |
| α7−/− | 37.5% | (17/92) 18.5%** |
| utr−/− | 12.5% | (18/92) 19.6% |
| α7/utr−/− | 12.5% | (7/92) 7.6%* |
Chi-squared analysis.
P < 0.0001.
α7/utr−/− Mice Are Viable and Fertile
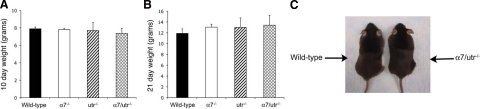
The absence of α7 integrin or utrophin in mdx mice results in reduced viability, joint contractures, kyphosis, and impaired mobility.13,14,28,31 To examine if loss of both utrophin and α7 integrin affected the ability of animals to thrive, male mice of various genotypes at 10 and 21 days were weighed. The average weights of 10-day-old wild-type, α7−/−, utr−/−, and α7/utr−/− mice were 7.9 ± 0.20 g, 7.8 ± 0.17 g, 7.7 ± 0.90 g, and 7.4 ± 0.58 g, respectively (Figure 2A). At 21 days, the mice averaged 11.89 ± 0.83 g, 13.04 ± 0.54 g, 13.0 ± 1.76 g, and 13.4 ± 1.8 g, respectively (Figure 2B). At 5-weeks of age, no differences in size or weight were observed in α7/utr−/− compared with wild-type mice (Figure 2C). In addition, α7/utr−/− mice were fertile, did not exhibit joint contractures, kyphosis, or reduced mobility. Thus, absence of utrophin in α7 integrin null mice did not have any effect on viability, fertility, or weight gain.
Figure 2.
α7/utr−/− mice exhibit normal weight gain and viability. A: The average weights of 10-day-old male wild-type, α7−/−, utr−/−, and α7/utr−/− mice were not significantly different. B: The average weights of 21-day-old male wild-type, α7−/−, utr−/−, and α7/utr−/− mice were not statistically different. C: Five-week-old α7/utr−/− mice were phenotypically indistinguishable from wild-type animals.
Mild Muscle Pathology in Mice that Lack α7 Integrin and Utrophin
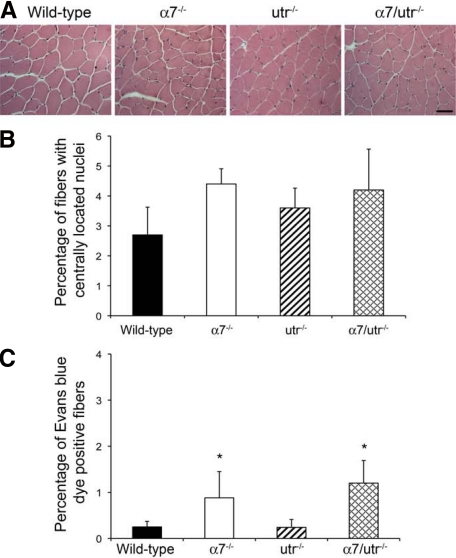
Loss of α7 integrin or utrophin in mdx mice results in severe muscle pathology.13,14,28,31 To determine whether loss of both α7 integrin and utrophin results in more severe skeletal muscle disease, H&E-stained sections of triceps muscles were examined for centrally located nuclei and changes in myofiber diameter. Skeletal muscle from 5-week-old wild-type and utr−/− mice contained few centrally located nuclei, while α7−/− and α7/utr−/− mice had myofibers with centrally located nuclei (Figure 3A). To investigate if the observed changes were significant, the percentage of myofibers in the triceps muscle containing centrally located nuclei were quantified. As expected, 5-week-old wild-type and utr−/− mice contained few myofibers with centrally located nuclei (Figure 3B). α7−/− mice showed 4.4% of fibers with centrally located nuclei and α7/utr−/− mice had 4.2% centrally located nuclei, however, these values were not statistically different from the wild-type controls (Figure 3B). Analysis of myofiber cross-sectional area in the triceps muscle revealed no significant difference between all four strains of mice (data not shown). These results indicate that at 5 weeks of age, loss of α7 integrin and utrophin results in mild muscle pathology.
Figure 3.
α7/utr−/− mice exhibit a subtle muscle phenotype. A: H&E staining of triceps muscle from 5-week-old mice was used to examine muscle pathology. Wild-type, α7−/−, utr−/−, and α7/utr−/− contained few muscle fibers with centrally located nuclei. Scale bar = 50 μm. B: Quantitation of centrally located nuclei in triceps muscle of wild-type, α7−/−, utr−/−, and α7/utr−/− mice. Wild-type and utrophin null mice showed few centrally located nuclei. α7−/− and α7/utr−/− mice showed a slight increase in the percentage of myofibers with centrally located nuclei but these were not statistically different from wild-type controls (N = 6 mice/genotype). C: Evan’s blue dye uptake confirmed that sarcolemmal integrity was not further compromised in α7/utr−/− mice. Wild-type, α7−/−, and utr−/−, α7/utr−/− skeletal muscle showed fewer than 2% of muscle fibers that were Evan’s blue dye positive. (*P < 0.05, N = 5 mice/genotype).
Sarcolemmal Integrity Is Not Compromised in Mice that Lack α7 Integrin and Utrophin
To determine whether loss of α7 integrin and utrophin results in reduced sarcolemmal integrity, 5-week-old wild-type, α7−/−, utr−/−, and α7/utr−/− were injected with Evan’s blue dye. The triceps muscles from wild-type and utr−/− mice were negative for Evan’s blue dye uptake (Figure 3C). A small increase in myofibers containing Evan’s blue dye uptake in the Triceps muscles of α7−/− (0.87%) and α7/utr−/− (1.24%) mice was observed (Figure 3C). These results indicate that loss of utrophin and α7 integrin did not result in a further reduction in sarcolemmal integrity.
Altered Expression and Localization of Dystrophin to the NMJ of α7 Integrin Null Mice
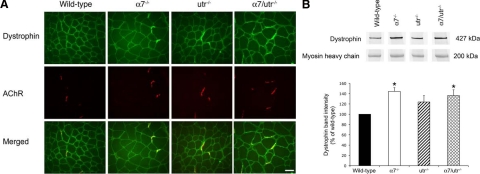
To investigate if the mild muscle phenotype observed in α7/utr−/− mice was due to compensation by dystrophin, immunofluorescence was performed using the TA muscle to examine dystrophin localization. As expected, dystrophin was localized to the sarcolemma in 5-week-old wild-type mice. In contrast, dystrophin was enriched at the NMJ in α7−/−, utr−/−, and α7/utr−/− mice (Figure 4A). These results indicate that loss of α7 integrin and/or utrophin leads to a compensatory relocalization and enrichment of dystrophin at the NMJ.
Figure 4.
Increased dystrophin at the NMJ of α7/utr−/− mice. A: Immunofluorescence was used to detect dystrophin (green) and acetylcholine receptors (red) at the neuromuscular junction of 5-week-old triceps muscle. Dystrophin was detected throughout the sarcolemma in wild-type muscle, but dystrophin localization was enhanced at the neuromuscular junction of α7−/−, utr−/−, and α7/utr−/− mice. Scale bar = 50 μm. B: Dystrophin was quantified by immunoblotting using protein extracted from the gastrocnemius muscle of wild-type, α7−/−, utr−/−, and α7/utr−/− muscle. Mice that lacked the α7 integrin (α7−/− and α7/utr−/− animals) showed a 1.4-fold increase in dystrophin protein compared with wild-type. (*P < 0.05, N = 4 mice/genotype).
Immunoblotting was performed to confirm and quantify changes in dystrophin expression. No significant increase in dystrophin was observed in utr−/− mice compared with wild-type controls (Figure 4B). In contrast, a statistically significant 1.4-fold increase in dystrophin protein was observed in α7−/− and α7/utr−/− skeletal muscle (Figure 4B). The above results demonstrate that loss of α7 integrin results in increased dystrophin expression in skeletal muscle.
To determine whether α7/utr−/− mice have defects at the NMJ, immunofluorescence using rhodamine-labeled bungarotoxin was performed to detect AChR. No changes in the fluorescence intensity or organization of AChR at the NMJ of α7/utr−/− mice were observed compared with control animals (data not shown). To investigate if loss of both the α7 integrin and utrophin resulted in fewer NMJ, immunoblotting was performed using an AChRβ antibody. Immunoblotting analysis revealed no significant difference in the expression of AChRβ subunit in α7/utr−/− compared with wild-type controls (data not shown). These results indicate loss of utrophin and α7 integrin does not lead to significant neuromuscular junction defects.
Severe MTJ Defects in α7 Integrin and Utrophin-Deficient Mice
Since the α7β1 integrin and utrophin are localized at the MTJ, we examined if loss of both protein complexes resulted in the enrichment of dystrophin at this critical junctional site.
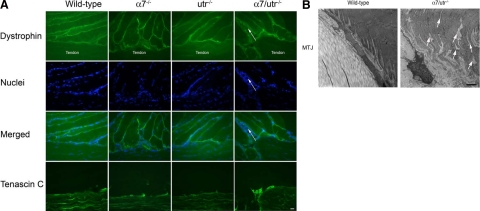
Immunofluorescence using antibodies against dystrophin and tenascin C (a myotendinous junction marker) revealed no change in the localization of these proteins to the MTJ of α7−/−, utr−/−, and α7/utr−/− mice compared with wild-type (Figure 5A). Images revealed separation of the myofibers at the myotendinous junction and inflammatory cell infiltrate (Figure 5A arrows). These data indicate that enrichment and possible compensation by dystrophin at the NMJ did not take place at the MTJ in the absence of utrophin and/or α7β1 integrin.
Figure 5.
MTJ defects in α7/utr−/− mice. A: Dystrophin was detected at the MTJ of wild-type, α7−/−, utr−/−, and α7/utr−/− mice by immunofluorescence. Tenascin C was used to identify the MTJ in muscle sections. α7/utr−/− mice exhibited inflammatory cell infiltrate at the MTJ (arrows). Scale bar = 20 μm. B: Transmission electron microscopy was used to examine the ultrastructure of the MTJ. As expected, the MTJs of wild-type mice were highly folded and were maintained as cohesive myofibers at the MTJ. In contrast, α7/utr−/− mice exhibited reduced myofiber cohesion and frayed myofibers at the MTJ that extended several microns into the muscle (arrows). Scale bar = 1 μm.
To investigate if loss of both α7 integrin and utrophin result in ultrastructural defects at the MTJ, transmission electron microscopy was performed. As expected wild-type mice showed extensive sarcolemmal folding at the MTJ (Figure 5B). In contrast 5-week-old α7/utr−/− mice exhibited abnormal myotendinous junctions (Figure 5B). Previous studies have demonstrated reduced folding at the MTJ of α7 integrin null mice,28 but loss of both utrophin and α7 integrin resulted in more severe MTJ defects. Myofibers of α7/utr−/− mice exhibited a frayed appearance with loss of adhesive contact between myofiber bundles extending several microns into the muscle fiber (Figure 5B). Together these results indicate that loss of both α7 integrin and utrophin results in more severe ultrastructural defects at the MTJ compared with mice lacking either α7 integrin or utrophin.
α7/utr−/− Mice Exhibit Reduced Muscle Endurance and Strength
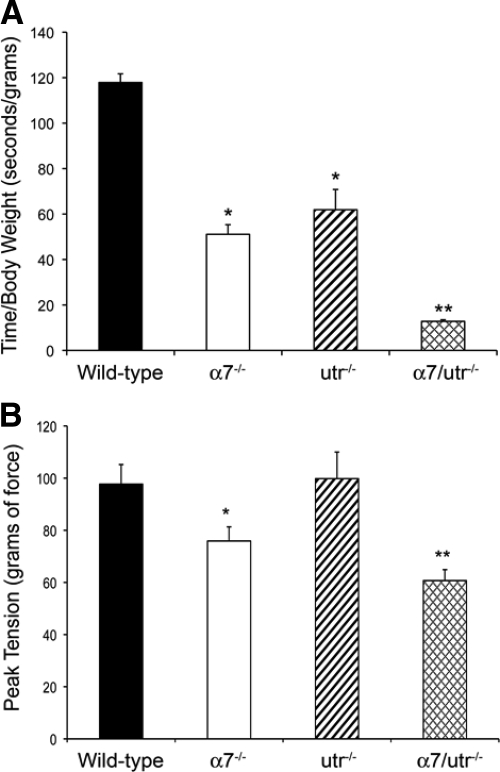
To determine whether loss of the α7 integrin and utrophin leads to decreased muscle function, endurance time and peak muscle tension tests were performed on 5-week-old wild-type, α7−/−, utr−/−, and α7/utr−/− mice. Wild-type mice had an average endurance time of at least 45 minutes (Figure 6A). A 2.3- and 1.9-fold reduction in endurance time was observed in α7−/− and utr−/− mice respectively compared with the wild-type controls (Figure 6A). α7/utr−/− mice showed a ninefold reduction in endurance time compared with wild-type (Figure 6A).
Figure 6.
α7/utr−/− mice exhibit reduced endurance and force transmission. A: Endurance tests were performed using 5-week-old male wild-type, α7−/−, utr−/−, and α7/utr−/− mice. To determine endurance, the ratio of the grip time over the body weight was measured. A 2.3- and 1.9-fold decrease in endurance was observed in α7−/− and utr−/− mice compared with the wild-type controls. The endurance was further reduced in α7/utr−/− compared with the other animal groups (*P < 0.05, **P < 0.001, N = 5 mice/genotype). B: A grip strength meter was used to record the muscle strength of 5-week-old mice. Wild-type and utr−/− mice showed grip strength of 97.7 and 89.6 gf, respectively and were not statistically different from each other. Loss of the α7 integrin resulted in a reduced grip strength to 74.0 gf, which was statistically significant from wild-type. A further reduction in grip strength was observed in α7/utr−/− mice at 59.8 gf and was significantly different from all animal groups (*P < 0.001, **P < 0.0001, N = 9 mice/genotype).
Forelimb muscle strength was assessed in 5-week-old wild-type, α7−/−, utr−/−, and α7/utr−/− mice using a grip strength meter. A 1.3- and 1.6-fold decrease in muscle strength was observed in α7−/− and α7/utr−/− mice, respectively (Figure 6B). Data from α7/utr−/− mice indicated that their muscle strength was significantly lower compared with the other groups (Figure 6B). These results suggest that loss of α7 integrin results in reduced force transmission that is further compromised by the absence of utrophin.
Discussion
The α7β1 integrin, dystrophin, and utrophin glycoprotein complexes are the major laminin receptors providing structural and functional integrity to skeletal muscle. Studies have suggested that these three complexes may have overlapping functional roles in DMD.10,11,19 All of the different combinations of dystrophin, utrophin, and α7 integrin knockout mice have been developed with exception of the α7/utr−/− mice. To complete the investigation of these laminin receptors and to examine the functional overlap between α7 integrin and utrophin, we generated mice that lacked both proteins. Due to the localization of the α7β1 integrin and utrophin to critical junctional sites in skeletal muscle,23,39,40 we hypothesized that α7/utr−/− mice might exhibit severe muscle disease. Surprisingly, α7/utr−/− mice were viable and fertile although they did have embryonic lethality. The embryonic lethality in these mice was most likely due to vascular defects identified previously in α7 integrin null mice that cause embryonic vascular hemorrhage.26,27,42 Conversely, mdx/utr−/− and mdx/α7−/− mice develop severe muscular dystrophy and die by 20 weeks and 4 weeks of age, respectively.13,14,28 In addition, mdx/utr−/− and mdx/α7−/− mice exhibit reduced weights at 10 and 21 days, severe skeletal muscle pathology, kyphosis and contractures.13,14,28 Table 2 summarizes the phenotypic data from various mouse models of muscular dystrophy.
Table 2.
Overview of the Phenotypes Exhibited in Single and Double Knockout Mice for Major Skeletal Muscle Laminin Receptors
| mdx | α7−/− | utr−/− | mdx/utr−/− | mdx/α7−/− | α7/utr−/− | |
|---|---|---|---|---|---|---|
| Lifespan | Almost normal | >1 year | Normal | 20 weeks | 4 weeks | >1 year |
| Embryonic lethality | None | Partial | None | None | Partial | Partial |
| Weight at 21 days | Normal | Normal | Normal | Reduced | Reduced | Normal |
| Skeletal muscle pathology | Moderate | Minor | None | Severe | Severe | Minor |
| Joint contractures | None | None | None | Severe | Severe | None |
| Kyphosis | None | None | None | Severe | Severe | None |
| MTJ | Reduced sarcolemmal folds | Reduced sarcolemmal folds | No | Reduced sarcolemmal folds | Reduced sarcolemmal folds | Reduced myofiber adhesion |
| NMJ defects | None | None | Mild | Severe | Not reported | None |
| References | 4, 11, 43, 44 | 26, 29 | 12, 15 | 11, 13, 14 | 28, 31 | This paper |
Studies show mice that lack both dystrophin and utrophin have increased expression of α7 integrin, while mice deficient in both dystrophin and α7 integrin do not show altered expression of utrophin but relocalization to extrajunctional sites.13,14,28 To determine whether the mild muscle phenotype observed in α7/utr−/− mice might be attributed to compensation by dystrophin, we investigated its expression and localization in skeletal muscle. Enrichment of dystrophin at the NMJ was observed in α7−/−, utr−/−, and α7/utr−/− mice and overall dystrophin levels in skeletal muscle were increased significantly in α7−/− and α7/utr−/− mice. These results suggest that the enhanced expression of dystrophin compensates for the lack of α7 integrin and/or utrophin, resulting in the mild muscle pathology observed in both α7−/− and α7/utr−/− mice.
Given that α7 integrin and utrophin are located at the NMJ and MTJ, we hypothesized that loss of both α7 integrin and utrophin would result in defects at these critical junctional sites. Surprisingly, no major defects were observed at the NMJ of α7/utr−/− mice, which may be related to the increase of dystrophin localization at this critical junctional site. In contrast, the MTJ of α7/utr−/− mice showed significant ultrastructural defects that included frayed muscle ends and absence of a compensatory change in dystrophin localization. These results indicate that although dystrophin compensates for the loss of α7β1 integrin and/or utrophin at the NMJ, it is unable to act as a surrogate for the deficiency at the MTJ. Similarly α7−/−, mdx, mdx/utr−/−, and mdx/α7−/− mice also exhibit structural defects at the MTJ. While the mdx and mdx/utr−/− mice exhibit fewer folds at the MTJ, α7−/− mice show almost no sarcolemmal folding at the MTJ.13,28,30,42,43 In contrast, overexpression of α7 integrin in skeletal muscle of mdx/utr−/− mice can restore sarcolemmal folding at the MTJ indicating a critical role for the α7 integrin in maintaining the normal architecture of the MTJ.11 MTJ ultrastructural defects have also been identified in mice lacking extracellular matrix proteins or members of the dystrophin glycoprotein complex. For example, mice lacking laminin β2 showed structural and functional disruptions at the MTJ, while α-dystrobrevin knockout mice have shorter folds at the MTJ.44,45
Although the pathology observed in α7/utr−/− mice is similar to α7−/− mice, there is a distinct difference at the MTJ, with the former having more severe structural defects. These observations suggest that utrophin compensates for loss of α7 integrin in the α7−/− mice resulting in milder MTJ abnormalities and that the additional loss of utrophin results in severe MTJ structural defects. Interestingly, muscles with MTJ injury have elevated levels of both utrophin and α7 integrin suggesting both proteins are critical in modulating force stresses at this junctional site during injury.46 Together these data implicate important roles for the α7β1 integrin and utrophin in maintaining the structural and functional integrity of the MTJ.
Since the MTJ is a critical site for force transmission between muscle and tendon, we hypothesized that defects in MTJ structure would manifest as reduced muscle function. Our data confirmed that α7 integrin null mice exhibit reduced forelimb grip strength, which was further decreased in α7 integrin null mice that lack utrophin. These data indicate that both the α7 integrin and utrophin are important for normal force transmission between the muscle and tendon at the MTJ. Mice lacking utrophin exhibited reduced endurance, but normal forelimb muscle strength, indicating that the α7β1 integrin may play a more critical role in force transmission at the MTJ.
Together, our results show that α7 integrin and utrophin are required for normal ultrastructure and force transmission at the MTJ. Each laminin receptor knockout mouse exhibits a distinct phenotype and severity of muscle pathology demonstrating the complexity of the functional overlap between α7 integrin, utrophin, and dystrophin glycoprotein complexes in skeletal muscle.
Acknowledgments
We thank Dr. Joshua Sanes (Harvard University, Boston, MA) for the utrophin null mice, Dr. Stephen Kaufman (University of Illinois, Urbana-Champaign, IL) for the anti-α7 integrin antibodies, and Dr. Glenn Morris (Center for Inherited Neuromuscular Disease, Shropshire, UK) for the MANCHO7 anti-utrophin antibody. We thank Ms. Naomi Lange for technical assistance and Dr. Heather Burkin for critically reading the manuscript.
Footnotes
Address reprint requests to Dean J. Burkin, Ph.D., Department of Pharmacology, University of Nevada School of Medicine, Reno, NV 89557. E-mail: dburkin@medicine.nevada.edu.
Supported by NIH/NIAMS R01AR053697-01 to D.J.B.
References
- Emery AEH. Duchenne muscular dystrophy–Meryon’s disease. Neuromuscular Disorders. 1993;3:263–266. doi: 10.1016/0960-8966(93)90018-f. [DOI] [PubMed] [Google Scholar]
- Moser H. Duchenne muscular dystrophy—pathogenetic aspects and genetic prevention. Human Genetics. 1984;66:17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton- extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome—linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin—the protein product of the Duchenne Muscular Dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tome FMS, Collin H, Leturcq F, Jeanpierre M, Kaplan JC, Fardeau M, Campbell KP. Expression of dystrophin-associated proteins in dystrophin-positive muscle-fibers (revertants) in Duchenne muscular dystrophy. Neuromusc Dis. 1994;4:115–120. doi: 10.1016/0960-8966(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Monaco AP, Neve RL, Collettifeener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Senter L, Luise M, Presotto C, Betto R, Teresi A, Ceoldo S, Salviati G. Interaction of dystrophin with cytoskeletal proteins—binding to talin and actin. Biochem Biophys Res Comm. 1993;192:899–904. doi: 10.1006/bbrc.1993.1500. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152:1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of alpha7beta1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol. 2005;166:253–263. doi: 10.1016/s0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deconinck AE, Potter AC, Tinsley JM, Wood SJ, Vater R, Young C, Metzinger L, Vincent A, Slater CR, Davies KE. Postsynaptic abnormalities at the neuromuscular junctions of utrophin-deficient mice. J Cell Biol. 1997;136:883–894. doi: 10.1083/jcb.136.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- Grady RM, Merlie JP, Sanes JR. Subtle neuromuscular defects in utrophin-deficient mice. J Cell Biol. 1997;136:871–882. doi: 10.1083/jcb.136.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- Rafael JA, Townsend ER, Squire SE, Potter AC, Chamberlain JS, Davies KE. Dystrophin and utrophin influence fiber type composition and post-synaptic membrane structure. Hum Mol Genet. 2000;9:1357–1367. doi: 10.1093/hmg/9.9.1357. [DOI] [PubMed] [Google Scholar]
- Heydemann A, McNally EM. Regenerating more than muscle in muscular dystrophy. Circulation. 2004;110:3290–3292. doi: 10.1161/01.CIR.0000149847.84152.0B. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110 (Pt 22):2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Weir AP, Burton EA, Harrod G, Davies KE. A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J Biol Chem. 2002;277:45285–45290. doi: 10.1074/jbc.M205177200. [DOI] [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Morris GE, Davies KE, Guerin C, Holland P. Localization and quantitation of the chromosome 6-encoded dystrophin-related protein in normal and pathological human muscle. J Neuropathol Exp Neurol. 1993;52:119–128. doi: 10.1097/00005072-199303000-00004. [DOI] [PubMed] [Google Scholar]
- Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7A, and alpha7B integrins with the neuromuscular junction. Dev Biol. 1996;174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of alpha 7 integrin cytoplasmic domains during skeletal muscle development: alternate forms, conformational change, and homologies with serine/threonine kinases and tyrosine phosphatases. J Cell Sci. 1993;106 (Pt 4):1139–1152. doi: 10.1242/jcs.106.4.1139. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- Welser JV, Lange ND, Flintoff-Dye N, Burkin HR, Burkin DJ. Placental defects in alpha7 integrin null mice. Placenta. 2007;28:1219–1228. doi: 10.1016/j.placenta.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der MH, Miosge N, Poschl E, von der MK. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- Nawrotzki R, Willem M, Miosge N, Brinkmeier H, Mayer U. Defective integrin switch and matrix composition at alpha 7-deficient myotendinous junctions precede the onset of muscular dystrophy in mice. Hum Mol Genet. 2003;12:483–495. doi: 10.1093/hmg/ddg047. [DOI] [PubMed] [Google Scholar]
- Guo C, Willem M, Werner A, Raivich G, Emerson M, Neyses L, Mayer U. Absence of alpha 7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:989–998. doi: 10.1093/hmg/ddl018. [DOI] [PubMed] [Google Scholar]
- Pearce M, Blake DJ, Tinsley JM, Byth BC, Campbell L, Monaco AP, Davies KE. The utrophin and dystrophin genes share similarities in genomic structure. Hum Mol Genet. 1993;2:1765–1772. doi: 10.1093/hmg/2.11.1765. [DOI] [PubMed] [Google Scholar]
- Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, Knight AE, Kendrick-Jones J, Suthers GK, Love DR. Primary structure of dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K, Ervasti JM, Matsumura K, Kahl SD, Leveille CJ, Campbell KP. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991;7:499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Watkins SC, Chafey P, Chelly J, Tome FM, Fardeau M, Kaplan JC, Kunkel LM. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord. 1991;1:185–194. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Pons F, Nicholson LV, Robert A, Voit T, Leger JJ. Dystrophin, and dystrophin-related protein (utrophin) distribution in normal and dystrophin-deficient skeletal muscles. Neuromuscul Disord. 1993;3:507–514. doi: 10.1016/0960-8966(93)90106-t. [DOI] [PubMed] [Google Scholar]
- Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2007;1772:108–117. doi: 10.1016/j.bbadis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons F, Robert A, Fabbrizio E, Hugon G, Califano JC, Fehrentz JA, Martinez J, Mornet D. Utrophin localization in normal and dystrophin-deficient heart. Circulation. 1994;90:369–374. doi: 10.1161/01.cir.90.1.369. [DOI] [PubMed] [Google Scholar]
- Welser JV, Lange N, Singer CA, Elorza M, Scowen P, Keef KD, Gerthoffer WT, Burkin DJ. Loss of the alpha7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res. 2007;101:672–681. doi: 10.1161/CIRCRESAHA.107.151415. [DOI] [PubMed] [Google Scholar]
- Law DJ, Tidball JG. Dystrophin deficiency is associated with myotendinous junction defects in prenecrotic and fully regenerated skeletal muscle. Am J Pathol. 1993;142:1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Ridge JC, Tidball JG, Ahl K, Law DJ, Rickoll WL. Modifications in myotendinous junction surface morphology in dystrophin-deficient mouse muscle. Exp Mol Pathol. 1994;61:58–68. doi: 10.1006/exmp.1994.1025. [DOI] [PubMed] [Google Scholar]
- Grady RM, Akaaboune M, Cohen AL, Maimone MM, Lichtman JW, Sanes JR. Tyrosine-phosphorylated and nonphosphorylated isoforms of alpha-dystrobrevin: roles in skeletal muscle and its neuromuscular and myotendinous junctions. J Cell Biol. 2003;160:741–752. doi: 10.1083/jcb.200209045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Go G, Cunningham J, Patton BL, Jarad G. Transgenic isolation of skeletal muscle and kidney defects in laminin beta 2 mutant mice: implications for Pierson syndrome. Development. 2006;133:967–975. doi: 10.1242/dev.02270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks GB, Combs AC, Chamberlain JR, Chamberlain JS. Molecular and cellular adaptations to chronic myotendinous strain injury in mdx mice expressing a truncated dystrophin. Hum Mol Genet. 2008;17:3975–3986. doi: 10.1093/hmg/ddn301. [DOI] [PMC free article] [PubMed] [Google Scholar]