Abstract
Cartilage oligomeric matrix protein (COMP) is a pentameric extracellular protein expressed in cartilage and other musculoskeletal tissues. Mutations in the COMP gene cause pseudoachondroplasia (PSACH), a severe dwarfing condition that has a growth plate chondrocyte pathology. PSACH is characterized by intracellular retention of COMP and other extracellular matrix (ECM) proteins, which form an ordered matrix within large rough endoplasmic reticulum cisternae. This accumulation is cytotoxic and causes premature chondrocyte cell death, thereby depleting chondrocytes needed for normal long bone growth. Research to define the underlying molecular mechanisms of PSACH has been hampered by the lack of a suitable model system. In this study, we achieved robust expression of human mutant (MT) or wild-type (WT) COMP in mice by using a tetracycline-inducible promoter. Normal growth plate distribution of ECM proteins was observed in 1-month-old WT-COMP and C57BL\6 control mice. In contrast, the structure of the MT-COMP growth plate recapitulated the findings of human PSACH growth plate morphology, including (1) retention of ECM proteins, (2) intracellular matrix formation in the rER cisternae, and (3) increased chondrocyte apoptosis. Therefore, we have generated the first mouse model to show extensive intracellular retention of ECM proteins recapitulating the human PSACH disease process at the cellular level.
Cartilage oligomeric matrix protein (COMP) is an extracellular matrix (ECM) protein expressed primarily in cartilage, tendon, and ligament.1,2,3,4,5,6,7,8,9,10 COMP is the fifth member of the thrombospondin gene family (TSP-5) belonging to the TSP pentameric subgroup B, which consists of adhesive glycoproteins that are incorporated into the extracellular matrix of a variety of cells.2,11,12,13 Experimental evidence suggests that COMP has a variety of functions including interacting with other ECM molecules in the matrix, increasing cellular attachment, and regulating collagen fibril assembly and proliferation of chondrocytes.14,15,16,17,18,19,20
Mutations in COMP cause two skeletal dysplasias pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED/EDM1), both of which are associated with a distinctive chondrocyte cellular pathology.2,16,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 This pathology was recognized long before mutations in COMP were identified and consists of giant rER cisternae that occupy the entire chondrocyte and contains lamellar appearing material.26 COMP, type IX collagen and matrilin 3 (MATN3) are retained in the rER cisternae and form an ordered intracellular matrix.35,36,40,43 The presence of an insoluble intracellular matrix may explain why this material remains in the growth plate even after chondrocyte death.43 Interestingly, mutations in MATN3 and type IX collagen, which are co-retained with mutant COMP (MT-COMP), also cause MED suggesting a common cellular pathology.21,44,45,46,47
Information obtained from studies of the PSACH growth plate has been used to develop a pathophysiologic model of PSACH.48 In PSACH, 3% or less of the COMP pentamers are postulated to contain all wild-type COMP (WT-COMP) subunits, so that most of the pentamers contain one or more mutant subunits.41 These mutant subunits are unable to fold properly and exert a dominant negative effect.41,48 Secondarily, the mutant COMP cannot be processed through the rER to the Golgi and becomes trapped along with other ECM proteins, initiating premature formation of matrix.16,24,29,32,33,39,40,43,48 The rER enlarges in response to the expanding retained matrix, eventually encompassing most of the cell volume and causing premature chondrocyte death.
Other PSACH growth plate findings include a large resting zone with fewer chondrocytes in the proliferative and hypertrophic zones.43 The growth plate is divided into the following three zones: resting; proliferative; and hypertrophic. The chondrocytes in the resting zone are relatively inactive and supply the proliferative zone with chondrocytes as the cells in the proliferative zone divide and mature. In the hypertrophic zone chondrocytes enlarge and produce calcified cartilage that will be later converted into bone. The PSACH growth plate has few chondrocytes in the proliferative and hypertrophic zones that produce the cartilage anlagen necessary for linear bone growth. Chondrocytes that are normally found in ordered columns appear in clusters in the PSACH growth plate with many acellular gaps between clusters.43 Many dead chondrocytes and cell remnants are present throughout the growth plate suggesting that the acellular gaps result from the loss of chondrocytes.43 Depletion of chondrocytes from the growth plate results in decreased linear growth and growth retardation that manifests as dwarfism.37,42,49
Although the outcome of COMP intracellular accumulation is well described, very little is known about the in vivo progression of the disease pathology. Most of the information about the disease pathology is from cross-sectional growth plate biopsy studies that provide information at only one time point, thus demonstrating the need for a physiological PSACH mouse model.49 While several in vivo PSACH mouse models have been reported, none adequately mimics the characteristic PSACH chondrocyte pathology.49,50,51 An in vivo model that recapitulates the disease pathology is important to define the establishment and progression of the chondrocyte pathology, including information about the pre- and postnatal timing of protein accumulation and its effect on joint formation. This information cannot be obtained from human PSACH patients or from the in vitro chondrocyte cartilage models that do not emulate the growth plate. Here, we describe a transgenic inducible mutant COMP mouse model that recapitulates the cellular hallmark of PSACH pathology.
Materials and Methods
Generation of Constructs
Two plasmids, pTRE-COMP and pTET-On-ColII, were generated and used to produce bigenic WT-COMP and MT-COMP mice. The pTRE-COMP construct contains the coding sequence of human COMP gene driven by the tetracycline responsive element (TRE) promoter. The WT and MT del469D constructs were generated by removing the COMP coding sequence with the FLAG tag (DYKDDDDK) from COMP-pcDNA 3.1 and placing it into the complementary NheI and EcoRV sites of pTRE-Tight vector (Clontech Inc., Mountain View, CA).25 The orientation of the construct was confirmed by PCR and final constructs were verified by sequencing. The pTET-On-ColII construct contains the tetracycline transactivator (rtTA) coding sequence driven by a type II collagen promoter.52 A fragment from pTET-On (rtTA) was amplified and NotI and SpeI restriction endonuclease sites were inserted by using the following primers: pTET-On- NotI 3′-CCGCGGCCGCGGATTCATATGTCTAGATTAGATAAAAGTAAAGTG-5′ and pTET-On-SpeI 3′-CTCCATACTAGTTTCCTTAGCTCCTGAAAATCTCGCC-5′. The rtTA-SV40 polyA fragment was inserted into the NotI and XbaI restriction endonuclease sites of pNK185 vector replacing the β-galactosidase gene and verified by sequencing.52 Immunoblot analysis using antibodies directed against COMP or FLAG showed that the apparent molecular mass of WT- and MT-COMP-FLAG expressed from these plasmids was identical to the molecular mass of human COMP (data not shown). Vector sequences were removed from both constructs before injection into C57BL\6 mouse blastocysts.
Generation of Bigenic Mice
Founder mice were screened for the transgenes by using tail biopsy DNA and PCR primers specific for each transgene. COMP primers, 5′-GGAAGGGAGATCGTGCAGAC-3′ and 5′-CTCGGCCACAGCACGGAAGG-3′, were used to genotype the TRE-COMP transgenic mice. Custom commercial assay was used to determine whether animals were homozygous or heterozygous (Embark Scientific, Austin, TX). rtTA primers, 5′-ATGCCCTTGGAATTGACGAGTACGG-3′ and 5′-CGAGGCTTGCAGGATCATAATCAG-3′, were used to genotype the Col II-rtTA transgenic animals and commercial assay was used to determine whether animals were homozygous or heterozygous (Charles Rivers, Wilmington, MA). Standard breeding was used to generate bigenic animals. Mice were administered 100 or 500 ng/ml doxycycline (DOX) (via drinking water + 5% sucrose) pre- and postnatally. Various organs (heart, lung, brain, and liver) from progeny of three positive lines were tested for transgenic human COMP expression by using real-time quantitative RT-PCR (qRT-PCR) as a means to verify tissue specificity of expression. No detectable expression was found in any of these tissues (data not shown). qRT-PCR of embryonic day 15 mouse embryos showed steady-state levels of human WT- and MT-COMP were approximately four to six-fold higher than steady-state levels of mouse COMP mRNA (data not shown).
Histology and Immunohistochemistry
Hind limbs from 1-month-old MT-COMP, WT-COMP, and C57BL\6 control mice were collected and tibial growth plates were analyzed. The limbs were fixed in 95% ethanol for COMP, calreticulin (CRT), FLAG, MATN3, types II and IX collagens immunostaining, or in 10% formalin for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining. CRT is a resident rER protein and was used here to indicate whether protein accumulation was occurring in the subcellular rER compartment. The COMP polyclonal antibody cross-reacts with both endogenous mouse and transgenic human COMP (Table 1). FLAG specifies the recombinant human WT- or MT-tagged COMP. All samples were decalcified for 2 days in immunocal formic acid decalcifier solution (American Master Tech Scientific, Inc., Lodi, CA) and embedded in paraffin.
Table 1.
Reagents Used in Growth Plate Immunostaining
| Co-staining Parameters | Primary | Secondary | Tertiary |
|---|---|---|---|
| COMP and CRT co-staining | |||
| COMP (human and mouse) | Rabbit polyclonal 1:300 (Kamiya) | Anti-rabbit Alexa fluor 488 1:400 (Molecular Probes) | — |
| CRT (ER) | Goat polyclonal calregulin (C-17) (Santa Cruz) | Anti-goat Alexa fluor 594 1:400 (Molecular Probes) | — |
| COMP and FLAG co-staining | |||
| COMP (human and mouse) | Rabbit polyclonal 1:300 (Kamiya) | Anti-rabbit Alexa fluor 488 1:400 (Molecular Probes) | — |
| FLAG (Tg. human COMP) | Goat polyclonal FLAG 1:200 (Abcam) | Anti-goat Alexa fluor 594 1:400 (Molecular Probes) | — |
| COMP, Collagen Type IX, MATN3, and Collagen Type II co-staining | |||
| Step I | |||
| COMP (human and mouse) | Rabbit polyclonal 1:300 (Kamiya) | Anti-rabbit Alexa fluor 488 1:500 (Molecular Probes) | — |
| Collagen Type IX | Mouse monoclonal 1:300 (Iowa Hybridoma Bank) | Anti-mouse Alexa fluor 594 1:500 (Molecular Probes) | — |
| MATN3 | Goat polyclonal 1:400 (R&D Systems) | Biotinylated anti-goat 1:600 (Chemicon) | Streptavidin Alexa fluor 405 (Molecular Probes) |
| Step II | |||
| Collagen Type II | Goat polyclonal 1:200 (Santa Cruz) | Zenon Alexa fluor 647 goat IgG labeling kit (Molecular Probes) | — |
Suppliers: Kamiya, Thousand Oaks, CA, Santa Cruz, Santa Cruz, CA, Abcam, Cambridge, MA, Iowa Hybridoma Bank, Iowa City, IA, and R&D Systems, Minneapolis, MN.
For immunostaining, 5 μm sections were digested with pepsin A (Sigma-Aldrich, St. Louis, MO) at 1 mg/ml in 0.1 N HCl for 30 minutes at room temperature. The sections were rinsed in distilled H2O and then washed in PBS containing 0.05% Tween-20 (PBS-T) for 5 minutes, followed by 20 minutes of blocking incubation with 10% donkey serum in PBS-T. All sections were then incubated overnight at 4°C with the primary antibodies listed in Table 1. Primary antibodies were detected by using fluorochrome conjugated secondary (and tertiary) antibodies listed in Table 1. Co-localization immunostaining was performed as previously described.35,36 Coverslips were mounted by using ProLong Gold anti-fade reagent (Molecular Probes, Carlsbad, CA). TUNEL staining was performed following the manufacturer’s protocol (Promega, Madison, WI). DAPI (4′-6-Diamidino-2-phenylindole) prolong gold anti-fade reagent staining and mounting solution was used to detect nuclei (Molecular Probes). All sections were examined and images were captured by using an Olympus (Melville, NY) BX51 inverted microscope attached to a Spot RT camera version 4.6 and images were merged by using Adobe Photoshop. To ensure consistency and so that samples could be compared, immunostaining of all samples was performed simultaneously and identical exposure times were used.
Human Chondrocyte Nodule Preparation
Primary human costochondral chondrocytes were collected from patients undergoing pectus excavatum surgery, isolated and cultured in a 3-D environment as previously described.2,36
Protein Modeling of Subcelluar Structure
Deconvolution microscopy was preformed as previously described.36
Results
Growth Plate Analysis of MT-COMP Mice
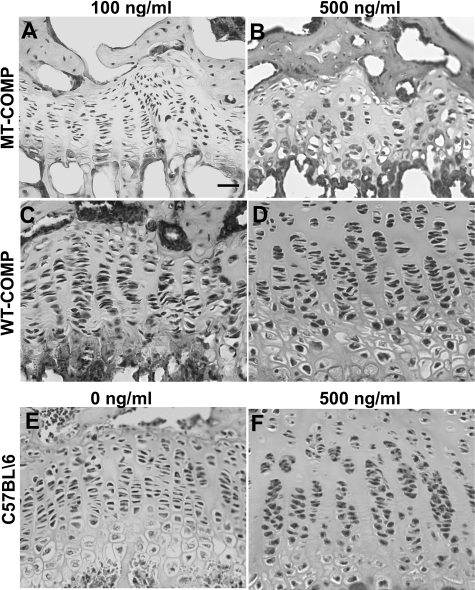
The bigenic WT- or MT-COMP lines were evaluated at 1 month of age, which corresponds to human adolescence and an age when the PSACH chondrocyte pathology is present.26,37,43,53 All analyses were performed on mice that were heterozygous for either the bigenic WT- or MT-COMP transgenes because the human PSACH disease is caused by a dominant COMP mutation.22,41 We first determined whether there were any growth plate abnormalities due to expression of the transgenes. Upper tibial growth plate sections were stained with H&E and were compared with the background C57BL\6 control strain (Figure 1, E and F). Growth plate organization was disrupted in mice expressing MT-COMP with the disorganization increasing as doxycycline (DOX) concentration increased from 100 to 500 ng/ml (Figure 1, A and B). Few hypertrophic chondrocytes were observed in the MT-COMP growth plates (Figure 1, A and B). In contrast, mice expressing WT-COMP (Figure 1, C and D) showed more chondrocytes, which were better organized into chondrocyte columns and a wider growth plate (Figure 1). In addition, RT-PCR using human COMP-specific primers verified expression of the transgenic human MT- and WT-COMP in mice at embryonic day 15 (data not shown). Taken together, these observations suggest that MT-COMP expression is associated with fewer growth plate chondrocytes, disruption of chondrocyte columns, thinner growth plate, fewer hypertrophic chondrocytes and this cellular phenotype is modulated by DOX dosage.
Figure 1.
Growth plate abnormalities are associated with overexpression of MT-COMP. H&E staining of growth plates from MT-COMP (A, B) and WT-COMP (C, D) mice at 1 month that were fed 100 or 500 ng/ml of DOX, respectively. C57BL\6 age-appropriate control growth plates exposed to 0 and 500 ng/ml DOX are shown in E and F. Increasing concentration of DOX is associated with more chondrocyte disorganization and acellular areas in MT-COMP growth plate. Scale bar = 25 μmol/L.
MT-COMP Expression Is Associated with Increased Apoptosis
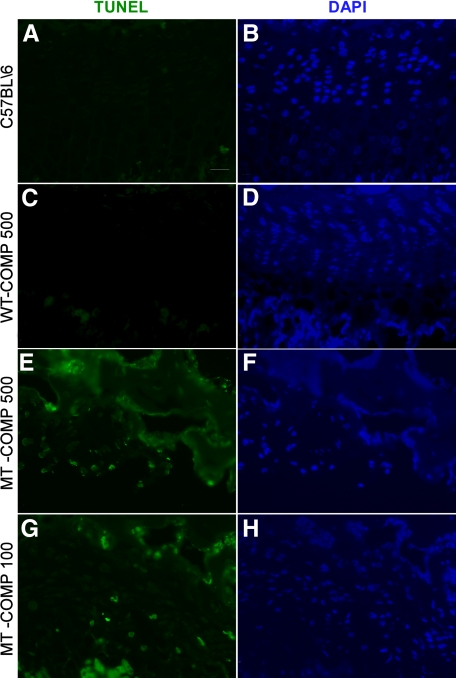
Several studies of MT-COMP suggest that apoptosis is increased in PSACH chondrocytes because intracellular retention negatively impacts chondrocyte function.30,43,54 To determine whether expression of MT-COMP stimulates a higher level of apoptosis in the growth plate, TUNEL staining was preformed. The cells in each field were localized by using DAPI staining (Figure 2, B, D, F, and H). As shown in Figure 2, E and G, similar positive TUNEL staining in the MT-COMP growth plates exposed to 100 and 500 ng/ml of DOX is observed, whereas the WT-COMP and C57BL\6 control growth plates show negligible staining (Figure 2, A and C). Similar levels of apoptosis were observed in three different MT-COMP mouse lines. These results suggest that intracellular retention of MT-COMP induces increased apoptosis and this association is similar to that seen in human PSACH chondrocytes. Since exposure to 100 and 500 ng/ml of DOX elicits similar levels of apoptosis in the growth plates, the lower level of DOX was used in following studies to minimize any possible adverse physiological effects of high levels of COMP.
Figure 2.
Increased apoptosis in growth plate chondrocytes is associated with mutant COMP expression. TUNEL staining was performed on growth plates from 1-month-old mice fed 100 or 500 ng/ml of DOX. DAPI staining is shown to indicate all of the chondrocytes in the growth plate section (B, D, F and H). MT-COMP expression is associated with an increase in the number of TUNEL positive cells (E and G) in the growth plate compared with relatively few TUNEL positive cells in the WT-COMP (C) and the C57BL\6control (A) mice. Scale bar = 25 μmol/L.
Human MT-COMP Expression Results in COMP Retention in Growth Plate Chondrocytes
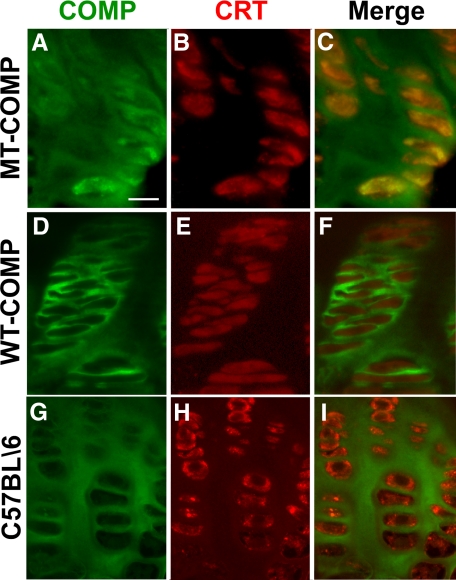
We next determined if MT-COMP is retained in the rER of growth plate chondrocytes of the mice as observed in human PSACH.2 In these studies, the COMP antibody recognizes both the endogenous murine and transgenic human COMP-FLAG protein, whereas the FLAG antibody binds only to the transgenic WT- or MT-COMP-FLAG protein. To delineate the rER, CRT, the resident chaperone protein of the rER, was used as a marker.55 As shown in Figure 3A, while some COMP is found in the ECM, strong intracellular localization is observed in the MT-COMP growth plate. When the COMP and CRT images were merged, co-localization was observed indicating that COMP is being retained in the rER (Figure 3, A–C). In contrast, neither human WT-COMP (Figure 3, D–F) nor endogenous mouse COMP (Figure 3, G–I) is retained in the chondrocyte, and both show similar pericellular localization.
Figure 3.
Mutant human COMP accumulates in the rER of mouse chondrocytes. Growth plates from MT-COMP (A–C), WT-COMP (D–F) and C57BL/6 (G–I) mice at 1 month were fed 100 ng/ml of DOX and were immunostained for COMP (green) and CRT (red) as described in the methods and the images were merged. CRT is a resident ER chaperone protein and the COMP antibody recognizes both transgenic human COMP and endogenous mouse COMP. Mice expressing MT-COMP show intracellular retention of COMP co-localizing with CRT indicating that they are in the rER of the growth plate chondrocytes (C). No co-localization is observed with WT-COMP and CRT (F). Scale bar = 20 μmol/L.
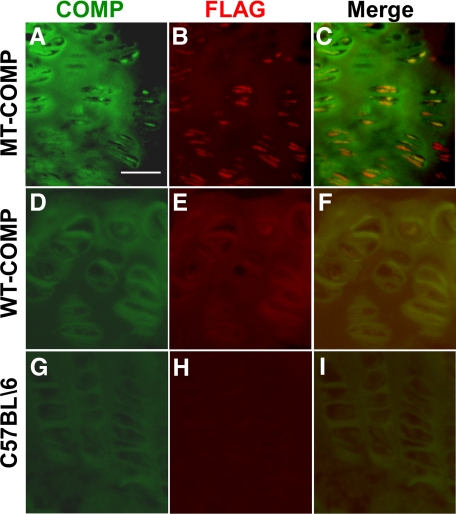
Since human WT- and MT-COMP expressed in these animals has a FLAG moiety, FLAG immunostaining was used to confirm that MT-COMP was being retained and WT-COMP was being secreted. As shown in Figure 4, A–C, co-localization of COMP and FLAG was observed indicating that MT-COMP is being retained. Extracellular COMP was observed and did not co-localize with FLAG showing that endogenous mouse COMP was exported to the ECM (Figure 4C). In contrast, WT-COMP co-localized with FLAG in the ECM (Figure 4, D–F) indicating secretion to the ECM. This latter pattern is similar to that observed in the control C57BL\6 mice (Figure 4, G–I).
Figure 4.
COMP and FLAG co-localize in the transgenic mouse growth plate. Growth plates from MT-COMP (A–C), WT-COMP (D–F), and control C57BL\6 (G–I) mice at 1 month were fed 100 ng/ml of DOX and were immunostained for COMP (green) and FLAG (red) as described in the methods. COMP antibody binds to both endogenous mouse and recombinant human COMP, whereas the antibody to FLAG is specific for the recombinant human COMP-FLAG protein. Endogenous and recombinant COMP expression is extracellular in the WT-COMP (D–F) and control mice (G–I). Both endogenous and recombinant COMP expression is observed extracellular and intracellular compartments of the MT-COMP mouse growth plate (A–C). Intracellular COMP is recognized by the FLAG antibody indicating that the retained material is recombinant MT-COMP (B and C). There appears to be less MT-COMP in the matrix as compared with the WT-COMP (B and E). Scale bar = 50 μmol/L.
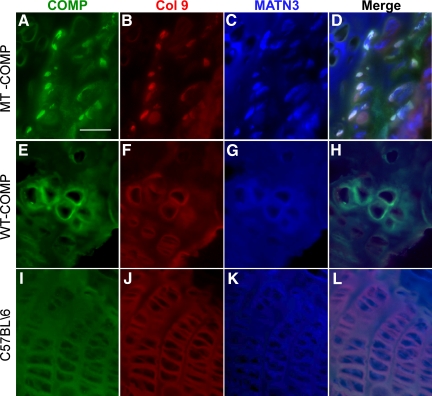
Type IX Collagen and MATN3 Are Retained with MT-COMP in rER of Growth Plate Chondrocytes
Both type IX collagen and MATN3 are retained in the rER of human PSACH chondrocytes.35,36,38,43 To assess whether MT-COMP affects the secretion of type IX collagen and MATN3, these proteins were immunolocalized in the mouse growth plates. As shown in Figure 5, A–D, MT-COMP immunolocalized with type IX collagen and MATN3 (white fluorescence foci in merge D) indicating that all three proteins are being retained in the rER. Less type IX collagen and MATN3 were found in the MT-COMP ECM (Figure 5, B and C). In comparison, human WT- (Figure 5, E–H) and endogenous mouse COMP (Figure 5, I–L) co-localized in the ECM with type IX collagen and MATN3.
Figure 5.
MT-COMP co-localizes with type IX collagen and MATN3. Growth plates from 1-month WT-COMP, MT-COMP, and control mice were sectioned and stained for COMP (green), type IX collagen (red), and MATN3 (blue) as described in the methods. All mice were fed 100 ng/ml of DOX. In the MT-COMP growth plate, MATN3 and types II and IX collagen are observed intracellularly (A–D), whereas these proteins are observed in the ECM of the WT-COMP and control growth plates, respectively (E–H and I–L). Both WT-COMP and MT-COMP growth plates are disorganized compared with the C57BL\6 control (A–L). Scale bar = 50 μmol/L.
Intracellular Matrix Is Formed in the rER of MT-COMP Mouse Growth Plate Chondrocytes
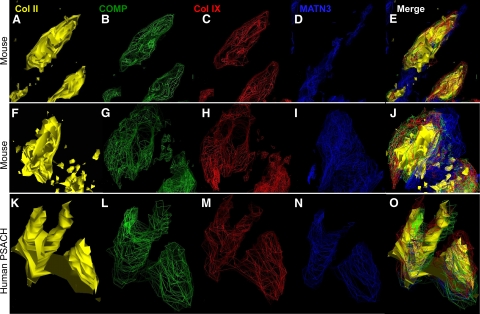
We have recently shown that mutant COMP retained in the rER of human chondrocytes supports the formation of intracellular matrix.36 This intracellular matrix network, comprised of mutant COMP, type IX collagen and MATN3, was organized around a type II pro-collagen core. This novel finding was previously shown in human PSACH chondrocytes with different COMP mutations.36 To determine whether a similar mechanism occurs in these mice, we used the same fluorescent deconvolution microscopy technique to model the architecture of the intracellular COMP, types II and IX collagen, and MATN3 proteins.36 The images shown in Figure 6 are from three different rER chondrocyte cisternae, two from MT-COMP mice (Figure 6, A–J) and one from a human PSACH patient (Figure 6, K–O). The results of the modeling in all three cisternae show that COMP, type IX collagen, and MATN3 co-localize and form a similar intracellular matrix surrounding a type II pro-collagen core (Figure 6). This result demonstrates that expression of MT-COMP supports intracellular matrix formation in the mouse growth plate chondrocytes and mimics the human PSACH chondrocyte disease pathology that we have previously described.36
Figure 6.
Intracellular matrix is present in MT-COMP growth plate chondrocytes. Images of rER were captured by using fluorescence deconvolution microscopy and modeled as described in methods. Images are shown in solid or wire-frame renditions with type II collagen (yellow), type IX collagen (red), COMP (green), and MATN3 (blue). The retained proteins in the rER have a similar intracellular matrix structure (E, J, and O) composed of a central core of type II procollagen (A and F) that is surrounded by COMP (B and G), type IX collagen (C and H), and MATN3 (D and I). This intracellular matrix pattern is identical to that which we previously described36 and show again in human PSACH chondrocytes with the D469del mutation (K–O).
Discussion
In this study, we show that the characteristic human PSACH chondrocyte pathology of rER intracellular retention of COMP, types II and IX collagens and MATN3, premature intracellular ECM assembly and growth plate abnormalities are recapitulated in growth plate chondrocytes of transgenic mice expressing MT-COMP. Cartilage-specific expression of MT–COMP with the D469del mutation was accomplished by using heterozygous bigenic TET-inducible transgenes. Unlike other reported mouse PSACH models, this one manifests the dominant negative effect of COMP mutations and has many of the cellular hallmarks of human PSACH chondrocytes.2,26,36,38,39,43
The MT-COMP chondrocyte phenotype resulting from intracellular retention of MT-COMP and other ECM proteins is present throughout the growth plate by 4 weeks of age when the mice are fed DOX pre- and postnatally. In contrast, WT-COMP transgenic mice treated similarly do not demonstrate this chondrocyte phenotype because the endogenous mouse and transgenic human COMP is exported to the ECM. Three mouse models expressing mutant COMP have been reported; however, none have shown massive intracellular retention of MT-COMP in growth plate chondrocytes or any significant growth plate pathology.49,50,51 One model showed only minimal intracellular retention in the wild-type background expressing endogenous COMP but intracellular retention increased when mutant COMP was expressed in the COMP null background.51 No intracellular MT-COMP was found in two other models.49,50 Finally, a very mild MED phenotype was reported in a mouse expressing T585M mutant COMP from homozygous knock-in COMP mutation.50 Based on these mouse models, we reasoned that expression levels of MT-COMP need to be very high to achieve the PSACH chondrocyte pathology in the context of murine physiology. Using an inducible system to facilitate MT-COMP expression, we observed significant intracellular retention of MT-COMP in the presence of endogenous wild-type mouse COMP, a phenotypic result that mimics the heterozygous human PSACH condition. In comparison, we show that induced human WT-COMP expression has an extracellular matrix distribution and no intracellular retention of MATN3 and type IX collagen. Finally, we demonstrate that human MT-COMP induces apoptosis in the mouse growth plate.
Previous in vitro human chondrocyte studies have shown that types II and IX collagen and MATN3 are co-retained with mutant COMP, and interact prematurely thereby initiating matrix assembly in the rER.35,36 This novel observation was found in PSACH chondrocytes having different COMP mutations. We now demonstrate that human MT-COMP in the mouse background interacts with endogenous mouse collagens and MATN3. This premature interaction results in the same intracellular chondrocyte matrix architecture in the rER cisternae as that found in human PSACH chondrocytes (Figures 5 and 6). These results confirm our previous observation that MT-COMP appears to catalyze intracellular ECM matrix assembly, suggesting that this is an important and significant role of COMP in the matrix.36,43
We had previously suggested that linear growth in PSACH was significantly impacted by the loss of chondrocytes in the growth plate.49,56 This was based on the in vitro observation that apopotosis was increased in patient chondrocytes expressing different COMP mutations.29,30,54,57,58 We have now shown that apoptosis is significantly increased in growth plate chondrocytes expressing MT-COMP (Figure 2) and that there are acellular areas in these growth plates (Figure 1).
In other studies, we have shown that complete loss of COMP has minimal effect on the growth plate while loss of type IX collagen results in widened growth plates particularly at 1 month of age.59 Compound loss of these ECM proteins results in limbs that are 11% shorter than the wild-type control mice. In the MT-COMP mouse, we find that there is diminished type IX collagen, and MATN3 in the ECM, which is due to intracellular retention and matrix assembly of these proteins, leading to their relative deficiency in the ECM. Based on these and previous results, the loss of chondrocytes, as well as the relative deficiency of these proteins in the matrix, may synergistically contribute to the profound short stature seen in PSACH. We can now assess the roles of COMP, type IX collagen, and MATN3 by crossing our MT-COMP mouse into these null mouse lines and evaluate the effect of MT-COMP in these single and compound knock-out mice. This will allow determination of the role of these proteins in matrix assembly.
The clinical phenotype of PSACH and MED can be quite variable. With our novel PSACH MT-COMP mouse model we can modulate the PSACH cellular phenotype on a homogenous genetic background, allowing us to determine whether clinical phenotypic variability is due to different modifier genes or the amount of MT-COMP trapped in the rER and/or apoptosis in the growth plate. Additionally, the ability to regulate MT-COMP expression temporally by the addition or withdrawal of DOX at different stages of development will offer insight into the appropriate timing of delivery of future therapies.
Global expression of MT-COMP may have consequences beyond the PSACH phenotype and restricting expression to cartilage circumvents the potential side effects of inappropriate MT-COMP expression. Using this tissue-specific model, the PSACH phenotype can be examined pre- and postnatally to assess changes throughout development and at various ages. Furthermore, the effect of exercise on joints expressing MT-COMP can be studied in detail as the skeletal elements mature since joint disease/erosion is a serious and painful long-term problem in human PSACH. In summary, this PSACH mouse model displays the human PSACH phenotype in vivo and will allow experiments to be preformed that are not possible with in vitro systems.
Acknowledgments
We thank Trung Le, Sujatha Kakuru, Shawn Tang, and Fego Galvan for their technical assistance.
Footnotes
Address reprint requests to Jacqueline T. Hecht, Ph.D., Department of Pediatrics, University of Texas Medical School, 6431 Fannin, Houston, TX 77030. E-mail: Jacqueline.T.Hecht@uth.tmc.edu.
Supported by NIH grant (R21 #1R01AR053364-02) to J.T.H. and J.L.A., the Shriners Hospital for Children grant to J.T.H., and the Leah Lewis Foundation.
References
- DiCesare PE, Morgelin M, Mann K, Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur J Biochem. 1994;223:927–937. doi: 10.1111/j.1432-1033.1994.tb19070.x. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17:269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heinegard D. Cartilage matrix proteins: an acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6136. [PubMed] [Google Scholar]
- Urban JP, Maroudas A, Bayliss MT, Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16:447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- Kempson GE, Freeman MA, Swanson SA. Tensile properties of articular cartilage. Nature. 1968;220:1127–1128. doi: 10.1038/2201127b0. [DOI] [PubMed] [Google Scholar]
- Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- Adams JC, Tucker RP, Lawler J. The thrombospondin gene family. Adams JC, editor. Austin, TX: R.G. Landes Co.; 1995:191 p. [Google Scholar]
- Fang C, Carlson CS, Leslie MP, Tulli H, Stolerman E, Perris R, Ni L, Di Cesare PE. Molecular cloning, sequencing, and tissue and developmental expression of mouse cartilage oligomeric matrix protein (COMP). J Orthop Res. 2000;18:593–603. doi: 10.1002/jor.1100180412. [DOI] [PubMed] [Google Scholar]
- Fang C, Johnson D, Leslie MP, Carlson CS, Robbins M, Di Cesare PE. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res. 2001;19:634–641. doi: 10.1016/S0736-0266(00)00039-5. [DOI] [PubMed] [Google Scholar]
- Posey KL, Davies S, Bales ES, Haynes R, Sandell LJ, Hecht JT. In vivo human Cartilage oligomeric matrix protein (COMP) promoter activity. Matrix Biol. 2005;24:539–549. doi: 10.1016/j.matbio.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Smith RK, Zunino L, Webbon PM, Heinegard D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997;16:255–271. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- Fife RS, Brandt KD. Identification of a high-molecular-weight (greater than 400 000) protein in hyaline cartilage. Biochim Biophys Acta. 1984;802:506–514. doi: 10.1016/0304-4165(84)90370-2. [DOI] [PubMed] [Google Scholar]
- DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354:237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- Holden P, Meadows RS, Chapman KL, Grant ME, Kadler KE, Briggs MD. Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J Biol Chem. 2001;276:6046–6055. doi: 10.1074/jbc.M009507200. [DOI] [PubMed] [Google Scholar]
- Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins: implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279:25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- Thur J, Rosenberg K, Nitsche DP, Pihlajamaa T, Ala-Kokko L, Heinegard D, Paulsson M, Maurer P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- Kipnes J, Carlberg AL, Loredo GA, Lawler J, Tuan RS, Hall DJ. Effect of cartilage oligomeric matrix protein on mesenchymal chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:442–454. doi: 10.1016/s1063-4584(03)00055-4. [DOI] [PubMed] [Google Scholar]
- Xu K, Zhang Y, Ilalov K, Carlson CS, Feng JQ, Di Cesare PE, Liu CJ. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282:11347–11355. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J Biol Chem. 2005;280:32655–32661. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MD, Chapman KL. Pseudoachondroplasia and multiple epiphyseal dysplasia: mutation review, molecular interactions, and genotype to phenotype correlations. Hum Mutat. 2002;19:465–478. doi: 10.1002/humu.10066. [DOI] [PubMed] [Google Scholar]
- Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- Briggs MD, Mortier GR, Cole WG, King LM, Golik SS, Bonaventure J, Nuytinck L, De Paepe A, Leroy JG, Biesecker L, Lipson M, Wilcox WR, Lachman RS, Rimoin DL, Knowlton RG, Cohn DH. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am J Hum Genet. 1998;62:311–319. doi: 10.1086/301713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Deere M, Hecht JT, Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein, and a mutation in its type 3 repeats causes conformational changes. J Biol Chem. 2000;275:26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- Chen TL, Stevens JW, Cole WG, Hecht JT, Vertel BM. Cell-type specific trafficking of expressed mutant COMP in a cell culture model for PSACH. Matrix Biol. 2004;23:433–444. doi: 10.1016/j.matbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Cooper RR, Ponseti IV, Maynard JA. Pseudoachondroplastic dwarfism: a rough-surfaced endoplasmic reticulum storage disorder. J Bone Joint Surg Am. 1973;55:475–484. [PubMed] [Google Scholar]
- Delot E, Brodie SG, King LM, Wilcox WR, Cohn DH. Physiological and pathological secretion of cartilage oligomeric matrix protein by cells in culture. J Biol Chem. 1998;273:26692–26697. doi: 10.1074/jbc.273.41.26692. [DOI] [PubMed] [Google Scholar]
- DiCesare PE, Morgelin M, Carlson CS, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein: isolation and characterization from human articular cartilage. J Orthop Res. 1995;13:422–428. doi: 10.1002/jor.1100130316. [DOI] [PubMed] [Google Scholar]
- Dinser R, Zaucke F, Kreppel F, Hultenby K, Kochanek S, Paulsson M, Maurer P. Pseudoachondroplasia is caused through both intra- and extracellular pathogenic pathways. J Clin Invest. 2002;110:505–513. doi: 10.1172/JCI14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke J, Montufar-Solis D, Underwood S, Lalani Z, Hecht JT. Apoptosis staining in cultured pseudoachondroplasia chondrocytes. Apoptosis. 2003;8:191–197. doi: 10.1023/a:1022926811397. [DOI] [PubMed] [Google Scholar]
- Ikegawa S, Ohashi H, Nishimura G, Kim KC, Sannohe A, Kimizuka M, Fukushima Y, Nagai T, Nakamura Y. Novel and recurrent COMP (cartilage oligomeric matrix protein) mutations in pseudoachondroplasia and multiple epiphyseal dysplasia. Hum Genet. 1998;103:633–638. doi: 10.1007/s004390050883. [DOI] [PubMed] [Google Scholar]
- Kleerekoper Q, Hecht JT, Putkey JA. Disease-causing mutations in cartilage oligomeric matrix protein cause an unstructured Ca2+ binding domain. J Biol Chem. 2002;277:10581–10589. doi: 10.1074/jbc.M109944200. [DOI] [PubMed] [Google Scholar]
- Maddox BK, Mokashi A, Keene DR, Bachinger HP. A cartilage oligomeric matrix protein mutation associated with pseudoachondroplasia changes the structural and functional properties of the type 3 domain. J Biol Chem. 2000;275:11412–11417. doi: 10.1074/jbc.275.15.11412. [DOI] [PubMed] [Google Scholar]
- McKeand J, Rotta J, Hecht JT. Natural history study of pseudoachondroplasia. Am J Med Genet. 1996;63:406–410. doi: 10.1002/(SICI)1096-8628(19960517)63:2<406::AID-AJMG16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Merritt TM, Alcorn JL, Haynes R, Hecht JT. Expression of mutant cartilage oligomeric matrix protein in human chondrocytes induces the pseudoachondroplasia phenotype. J Orthop Res. 2006;24:700–707. doi: 10.1002/jor.20100. [DOI] [PubMed] [Google Scholar]
- Merritt TM, Bick R, Poindexter BJ, Alcorn JL, Hecht JT. Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am J Pathol. 2007;170:293–300. doi: 10.2353/ajpath.2007.060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger S, Hecht JT. Pseudoachondroplasia and multiple epiphyseal dysplasia: New etiologic developments. Am J Med Genet. 2001;106:244–250. [PubMed] [Google Scholar]
- Hecht JT, Hayes E, Haynes R, Cole WG. COMP mutations, chondrocyte function and cartilage matrix. Matrix Biol. 2005;23:525–533. doi: 10.1016/j.matbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Hayes E, Snuggs M, Decker G, Montufar-Solis D, Doege K, Mwalle F, Poole R, Stevens J, Duke PJ. Calreticulin. PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachondroplasia chondrocytes. Matrix Biol. 2001;20:251–262. doi: 10.1016/s0945-053x(01)00136-6. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Montufar-Solis D, Decker G, Lawler J, Daniels K, Duke PJ. Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol. 1998;17:625–633. doi: 10.1016/s0945-053x(98)90113-5. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M, Lawler J. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- Posey KL, Hayes E, Haynes R, Hecht JT. Role of TSP-5/COMP in pseudoachondroplasia. Int J Biochem Cell Biol. 2004;36:1005–1012. doi: 10.1016/j.biocel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Makitie O, Hayes E, Haynes R, Susic M, Montufar-Solis D, Duke PJ, Cole WG. Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate. J Orthop Res. 2004;22:759–767. doi: 10.1016/j.orthres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Stove J, Heber F, Brenner RE. Clinical phenotype and molecular diagnosis of multiple epiphyseal dysplasia with relative hip sparing during childhood (EDM2). Am J Med Genet. 2002;112:144–153. doi: 10.1002/ajmg.10554. [DOI] [PubMed] [Google Scholar]
- Chapman KL, Mortier GR, Chapman K, Loughlin J, Grant ME, Briggs MD. Mutations in the region encoding the von Willebrand factor A domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat Genet. 2001;28:393–396. doi: 10.1038/ng573. [DOI] [PubMed] [Google Scholar]
- Jackson GC, Barker FS, Jakkula E, Czarny-Ratajczak M, Makitie O, Cole WG, Wright MJ, Smithson SF, Suri M, Rogala P, Mortier GR, Baldock C, Wallace A, Elles R, Ala-Kokko L, Briggs MD. Missense mutations in the beta strands of the single A-domain of matrilin-3 result in multiple epiphyseal dysplasia. J Med Genet. 2004;41:52–59. doi: 10.1136/jmg.2003.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert AK, Dijkstra PF, Jansen BR, van Horn JR, de Graaf B, Heutink P, Lindhout D. Familial multiple epiphyseal dysplasia due to a matrilin-3 mutation: further delineation of the phenotype including 40 years follow-up. Am J Med Genet A. 2003;120:490–497. doi: 10.1002/ajmg.a.20034. [DOI] [PubMed] [Google Scholar]
- Hou J, Putkey JA, Hecht JT. Delta 469 mutation in the type 3 repeat calcium binding domain of cartilage oligomeric matrix protein (COMP) disrupts calcium binding. Cell Calcium. 2000;27:309–314. doi: 10.1054/ceca.2000.0125. [DOI] [PubMed] [Google Scholar]
- Posey KL, Yang Y, Veerisetty AC, Sharan SK, Hecht JT. Model systems for studying skeletal dysplasias caused by TSP-5/COMP mutations. Cell Mol Life Sci. 2008;65:687–699. doi: 10.1007/s00018-007-7485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirog-Garcia KA, Meadows RS, Knowles L, Heinegard D, Thornton DJ, Kadler KE, Boot-Handford RP, Briggs MD. Reduced cell proliferation and increased apoptosis are significant pathological mechanisms in a murine model of mild pseudoachondroplasia resulting from a mutation in the C-terminal domain of COMP. Hum Mol Genet. 2007;16:2072–2088. doi: 10.1093/hmg/ddm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Niehoff A, Miosge N, Smyth N, Paulsson M, Zaucke F. Transgenic mice expressing D469Delta mutated cartilage oligomeric matrix protein (COMP) show growth plate abnormalities and sternal malformations. Matrix Biol. 2007;27:67–85. doi: 10.1016/j.matbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux P, Lamy M. Pseudo-achondroplastic forms of spondylo-epiphyseal dysplasias. Presse Med. 1959;67:383–386. [PubMed] [Google Scholar]
- Hashimoto Y, Tomiyama T, Yamano Y, Mori H. Mutation (D472Y) in the type 3 repeat domain of cartilage oligomeric matrix protein affects its early vesicle trafficking in endoplasmic reticulum and induces apoptosis. Am J Pathol. 2003;163:101–110. doi: 10.1016/S0002-9440(10)63634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Chen TL, Posey KL, Hecht JT, Vertel BM. COMP mutations: domain-dependent relationship between abnormal chondrocyte trafficking and clinical PSACH and MED phenotypes. J Cell Biochem. 2008;103:778–787. doi: 10.1002/jcb.21445. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Becker A, Schmitz A, Weirich C, Paulsson M, Zaucke F, Dinser R. Disruption of extracellular matrix structure may cause pseudoachondroplasia phenotypes in the absence of impaired cartilage oligomeric matrix protein secretion. J Biol Chem. 2006;281:32587–32595. doi: 10.1074/jbc.M601976200. [DOI] [PubMed] [Google Scholar]
- Weirich C, Keene DR, Kirsch K, Heil M, Neumann E, Dinser R. Expression of PSACH-associated mutant COMP in tendon fibroblasts leads to increased apoptotic cell death irrespective of the secretory characteristics of mutant COMP. Matrix Biol. 2007;26:314–323. doi: 10.1016/j.matbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT. Skeletal abnormalities in mice lacking extracellular matrix proteins: thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol. 2008;172:1664–1674. doi: 10.2353/ajpath.2008.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]