Abstract
Neuroinflammation is a hallmark of several neurodegenerative diseases, including Alzheimer’s disease (AD). Strong epidemiological and experimental evidence supports the use of nonsteroidal anti-inflammatory drugs to reduce AD risk. However, poor outcome in clinical trials and toxicity in a prevention trial have shifted focus away from these cyclooxygenase (COX) inhibitors to seek additional therapeutic targets in the prostaglandin pathway. Previously, the prostaglandin E2 receptor, EP2, was shown to regulate neuroinflammation and reduce Aβ plaque burden in transgenic mice. Unfortunately, widespread EP2 distribution and a direct effect on COX2 induction make EP2 a less desirable target. In this study, we link dedicator of cytokinesis 2 (DOCK2) to the prostaglandin pathway in the brain. Additionally, we show that DOCK2 regulates microglial innate immunity independent of COX2 induction and that DOCK2+ microglia are associated with human AD pathology. Together, these results suggest DOCK2 as a COX2 expression-independent therapeutic target for neurodegenerative diseases such as AD.
Innate immune activation of the central nervous system is associated with several neurodegenerative diseases including Alzheimer’s disease (AD).1,2,3,4 The major cellular component of this response, activated microglia, demonstrates both beneficial and deleterious effects on surrounding neurons.4,5,6,7,8,9 The deleterious effects include microglial secretion of a variety of molecules including prostaglandins (PGs) that can mediate paracrine neurotoxicity. Indeed, activation of the PG pathway has been linked with neurotoxicity in a number of cell culture and in vivo models.2,6,10,11,12,13 This is especially compelling because there are existing drugs that target the PG pathway, such as the cyclooxygenase (COX) inhibitors that inhibit PG production.
Strong epidemiological evidence supports the efficacy of COX isozyme suppression in PG signaling by nonsteroidal anti-inflammatory drugs (NSAIDs) for AD therapy (reviewed in14). Recently, hard-gained knowledge about COX2 toxicity associated with NSAIDs has led academic and industry laboratories to pursue more specific targets.15,16,17 Through a series of studies we and others have demonstrated the pro-inflammatory, pro-oxidative, and pro-amyloidogenic nature of the prostaglandin E2 receptor (EP2) in mouse brain or primary cultures from mouse brain, suggesting it as a potentially beneficial therapeutic target for AD.4,9,18,19,20 While work to date with EP2 highlights a promising approach to PG-related therapeutics in neurodegenerative diseases, widespread organ and cellular distribution of EP make it a nonspecific therapeutic target. Moreover, EP2 activation regulates COX2 expression, at least in microglia, and so EP2 targeting may lead to a similar toxicity profile as relatively COX2-selective NSAIDs.20,21,22,23
The aims of this study were to discover and evaluate EP2-dependent regulators of microglial innate immune response that did not regulate COX2 expression. Indeed, we identified dedicator of cytokinesis 2 (DOCK2) expression as being nearly completely dependent on EP2 expression in microglia. DOCK2 was identified in 1999 as a member of the CDM family of proteins, which includes Caenorhabditis Elegans CED-5, human DOCK180, and Drosophila Melanogaster Myoblast City.24 To date, the majority of studies concerning DOCK2 have shown it to act as a guanyl-nucleotide exchange factor (GEF), which positively regulates Rac- (a Rho family small GTPase) mediated cellular processes such as lymphocyte migration.24,25,26,27,28,29,30,31 The Rho family of small GTPases, including Rac, is known to be intimately associated with actin cytoskeleton processes as well as oxidative processes in phagocytic cells. In primary and immortalized microglial cell cultures, Rac1 activation has been shown to promote phagocytosis, including Aβ1-42 clearance.32,33 With the exception of DOCK2’s role in neutrophil chemotaxis, there has been no literature describing its function in phagocytic cells even though it has been implicated in macrophage phagocytosis and NADPH oxidation.24,34,35
Following our discovery, we further investigated the role of DOCK2 in microglial function relating to phagocytosis and neurotoxicity as well as regulation of COX2 expression. We also sought to establish relevance of DOCK2 expression to AD pathogenesis by evaluating its expression pattern in human brain. To date, there has been no literature providing evidence for DOCK2 expression or function in the brain. Our identification and subsequent characterization of DOCK2 in brain may highlight its potential as a microglial-specific, COX2-expression independent therapeutic target for neurodegenerative diseases, such as AD.
Materials and Methods
Animal and Human Use
Wild-type C57Bl/6 mice (Jackson, Bar Harbor, Maine), Dock2−/− mice (Dr. Yoshinori Fukui, Kyushu University, Japan), and EP2−/− mice (Dr. Richard Breyer, Vanderbilt University) were used with approval by the University of Washington Institutional Animal Care and Use Committee. Well-characterized human tissue was obtained from the University of Washington’s Alzheimer’s Disease Research Center in complete compliance with Institutional Review Board-approved protocols (Postmortem intervals <8 hours).
Primary Cell Culture
Primary mouse microglia and neurons were cultured as previously described.6,20 Microglia at DIV 7–15 and neurons at DIV 8 were used for experiments.
Pharmacological Cell Treatment
Soluble Aβ1-42 (Bachem, Torrance, CA) was freshly prepared as previously described.6,20 Primary microglia in 6-well plates (1 well = 1 × 106 cells) were treated with Dulbecco’s modified Eagle’s medium (DMEM) containing Aβ1-42 (10 μmol/L)/interferon-γ (10 pg/ml) for 6 hours. DMEM containing 10% fetal bovine serum and 100 ng/ml of lipopolysaccharide (EMD Chemicals, Gibbstown, NJ) was added to microglia in 96-well plates (1 well = 5 × 104 cells) for 6- or 24-hour treatment.
Gene Expression
Total Microglial RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) and purified using an RNeasy Mini column (Qiagen, Valencia, CA). RNA quality was verified using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). Total RNA was labeled and hybridized to Mouse Genome 430 2.0 Arrays (Affymetrix, Santa Clara, CA). Four independent gene chips were hybridized. The intensity value for each probe was calculated using GeneChip operating software (GCOS) version 1.4 (Affymetrix, Santa Clara, CA), which was followed by data quality validation. Raw microarray data were processed and analyzed using GeneTraffic (Iobion Informatics, La Jolla, CA) along with Statistical Analysis of Microarray methodology to produce pair comparison lists containing Affymetrix gene probe identifications, their relative fold-change, and the statistical indicator False Determining Rate. From these data, the False Determining Rate of 5% was used as the cutoff for gene probe significance. Validation by quantitative real-time PCR was done using validated TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) and analyzed through relative quantitation in an ABI 7500 Real-Time PCR instrument (SDS v1.3.1). The comparative threshold cycle method (ΔΔCT), with glyceraldehyde-3-phosphate dehydrogenase as the endogenous reference, was used to determine relative message abundance.
Western Blotting
Standardized protein concentrations from cellular lysate were determined by BCA protein assay (Pierce, Rockford, IL) and subjected to SDS-polyacrylamide gel electrophoresis. Anti-DOCK2 (Millipore, Temecula, CA) and anti-COX2 (Calbiochem, Gibbstown, NJ) antibodies were used at 1:1000. Detection was done using HRP-based enhanced chemiluminescence.
Immunohistochemistry
Brains were dissected out from PBS-perfused adult mice frozen in cryopreservative. Postmortem human brain tissue was placed directly into cryopreservative and stored at −80°C. For all tissue, 10 μm sections were cut using a cryostat. Sections were placed in cold acetone for 1 to 2 minutes followed by a 1-hour room temperature block. Primary antibodies (anti-DOCK2 [Millipore, Temecula, CA], anti-NeuN [Millipore, Temecula, CA], anti-CD68 [Serotec, Raleigh, NC], anti-glial fibrillary acidic protein [Dako, Capinteria, CA], anti-paired helical filament-Tau AT100 [Pierce, Rockford, IL], anti-Aβ 6E10 [Covance, Berkeley, CA]) were diluted 1:100 and incubated for 1 hour at room temperature. Sections were rinsed with Tris-saline. Secondary antibodies (Invitrogen, Carlsbad, CA) (1:200), tomato lectin (1:100), or Ricinus communis agglutinin 1(RCA1) lectin (Vector Laboratories, Burlingame, CA) (1:100) were added for 1 hour at room temperature. Sections were rinsed followed by 1 to 2 minutes in 70% ethanol. Saturated sudan black was added for 3 minutes. Sections were then rinsed with 70% ethanol and ddH2O. Tissue was mounted in ProLong Gold Antifade with 4,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA). Imaging was done using an Olympus FV-1000 confocal microscope.
Cytokine Induction
Multiplex analysis of mouse cytokines (tumor necrosis factor [TNF]-α, monocyte chemoattractant protein [MCP-1]) from culture medium was done using immunobead-based multiplex assays (Millipore, Billerica, MA) according to manufacturer instructions and data were collected using the LiquiChip Workstation from Qiagen. Standard curves were constructed from authentic kit standards. Data are represented as a percent induction after standardizing the amount of cytokine containing media by dividing this by the total amount of protein from its respective cellular lysate.
Phagocytosis
Microglia were plated overnight in 96-well plates (3 × 104 cells/well). Medium was replaced with DMEM containing 2 μm fluorescent microspheres (Invitrogen, Carlsbad, CA) (1:1700). Following a 6-hour incubation, cells were rinsed with cold PBS and collected after brief trypsinization. Cells were re-suspended in 4% paraformaldehyde-PBS and fixed for 1 hour at 4°C. Cells were subjected to flow cytometry (FACScan, BD Biosciences, San Jose, CA) with threshold values set at FSC 50, SSC 52, and FL1–3 52. Data were collected as the mean fluorescence intensity for each measure.
Co-Culture Neurotoxicity
Primary microglia were seeded in 24-well collagen-treated transwell inserts (Fisher Scientific, Hanover Park, IL) at 1 × 105 cells/well in DMEM. From primary neurons, all but 500 μl conditioned media was removed and replaced with fresh serum-free Neurobasal Medium (0.5 mmol/L glutamine, 1× B27) with or without 1 μg/ml LPS. Butaprost or vehicle was added at 10 μmol/L. Co-cultures were incubated for 24 hours. Medium was collected and neurotoxicity was assessed using a lactate dehydrogenase (LDH) cytotoxicity assay (Sigma, St. Louis, MO) according to manufacturer’s protocol.
Statistics
Statistical analyses were performed as described in each results section using GraphPad Prism software (GraphPad Software Inc., San Diego CA). All experiments were performed with at least n = 4 unless otherwise specified.
Results
DOCK2 Expression Is EP2-Dependent
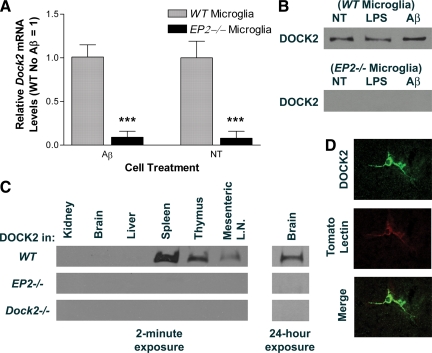
First, we sought observable associated transcriptional changes in activated EP2−/− mouse primary microglia. Using cDNA microarray analysis followed by qRT-PCR validation, we screened for transcriptional events related to the EP2 pathway that may lead to a candidate gene to study further. From this analysis, a total of 17 genes were identified as significantly differentially expressed between EP2−/− and wild-type microglia after exposure to soluble Aβ1-42 (Table 1). One of these genes, DOCK2, was selected for further study because existing literature suggest that its expression might be microglia specific. Indeed, DOCK2 was validated as being significantly down-regulated by tenfold in EP2−/− microglia (Figure 1A). Next, we determined if the observed difference in Dock2 mRNA was due to Aβ1-42 exposure or a property of the EP2−/− genotype itself. We performed quantitative real-time PCR for wild-type and EP2−/− primary microglia either not treated (NT) or exposed to Aβ1-42 (Figure 1A). Dock2 gene expression level from NT wild-type cells were set to a relative value of 1 for normalization. Dock2 transcriptional levels were about tenfold lower in EP2−/− microglia compared with wild-type, regardless of treatment conditions (***P < 0.001 determined by Two-way analysis of variance with Bonferroni posttest correction, n = 4). DOCK2 protein expression was evaluated by Western blot analysis to see if protein levels correlate with that of the transcript. Cells challenged by LPS were also observed for extended testing of possible treatment effects. Corresponding with transcript, DOCK2 protein was only observed in wild-type microglia, again regardless of treatment type (Figure 1B). These findings indicate that regulation of DOCK2 transcription and protein expression is a consequence of genetic ablation of EP2, and not a result of microglial activation.
Table 1.
Discovery of EP2-Dependent Dock2 mRNA Expression in Microglia
| Gene symbol | Gene title | GenBank | Transcript ratio (WT:EP2−/−) |
|---|---|---|---|
| BC020077 | cDNA sequence BC020077 | NM_145549 | 2.11 |
| Nnt | Nicotinamide nucleotide transhydrogenase | NM_008710 | 2.05 |
| Rnf13 | Ring finger protein 13 | NM_011883 | 0.77 |
| Serinc1 | Serine incorporator 1 | NM_019760 | 0.76 |
| Cyp51 | Cytochrome P450, family 51 | NM_020010 | 0.74 |
| Idi1 | Isopentenyl-diphosphate delta isomerase | NM_145360 | 0.71 |
| Phf20l1 | PHD finger protein 20-like 1 | XM_484476 | 0.69 |
| Hnrpab | Heterogeneous nuclear ribonucleoprotein A/B | NM_010448 | 0.65 |
| Scoc | Short coiled-coil protein | NM_001039137 | 0.64 |
| 9330182L06Rik | RIKEN cDNA 9330182L06 gene | NM_172706 | 0.63 |
| A830039H10Rik | RIKEN cDNA A830039H10 gene | NM_172153 | 0.53 |
| Peli2 | Pellino 2 | NM_033602 | 0.42 |
| Zfp236 | Zinc finger protein 236 | XM_484752 | 0.24 |
| Gmfb | Glia maturation factor, beta | NM_022023 | 0.19 |
| 5031439G07Rik | RIKEN cDNA 5031439G07 gene | NM_001033273 | 0.16 |
| Ang1 | Angiogenin, ribonuclease A family, member 1 | NM_007447 | 0.11 |
| Dock2 | Dedicator of cytokinesis 2 | NM_033374 | 0.11 |
Microarray gene expression data shows that Dock2 mRNA is decreased by approximately 10-fold in EP2−/− primary microglia stimulated by Aβ in vitro when compared with wild-type (WT).
Figure 1.
Microglial DOCK2 expression is EP2-dependent. A: Quantitative real-time PCR analysis of Dock2 mRNA in EP2−/− primary mouse microglia shows significantly lower levels than those in wild-type (WT), either with or without Aβ treatment (***P < 0.001). B: Western blot images for DOCK2 show a complete absence of DOCK2 protein in EP2−/− primary microglia with LPS, Aβ, or No Treatment (NT). C: Wb images for DOCK2 in several organs show expression in wild-type mice primary lymphoid tissue (2-minute film exposure), but not in EP2−/− or Dock2−/− mice. There is a low level of DOCK2 expression in wild-type brain (24-hour film exposure). (LN = Lymph Node). D: Confocal images showing microglial staining for both DOCK2 and tomato lectin (magnification, ×600).
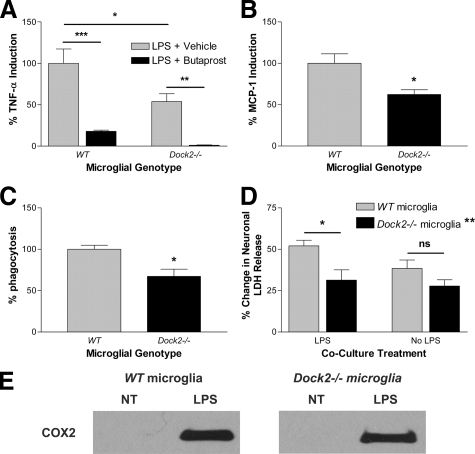
One nonphysiological interpretation of the in vitro findings is that DOCK2 expression in wild-type microglia changes as a result of cell culture technique, possibly due to loss of inhibition by interacting neurons or astrocytes in the brain. To test if this difference in DOCK2 expression also exists in vivo, we harvested several organs from adult mice and determined DOCK2 expression (Figure 1C). Dock2−/− mice were used as a negative control for antibody specificity. Confirming the results of others, DOCK2 was observed in the primary lymphoid tissue of wild-type mice24; however, no DOCK2 was detected in lymphoid tissue harvested from EP2−/− mice. Much longer Western blot film exposure time demonstrated for the first time that DOCK2 is indeed present at low levels in wild-type brain (Figure 1C). DOCK2 was not detected in EP2−/− or Dock2−/− brain. To test if the loss of Dock2 leads to loss of EP2, we did a functional assay for the presence of EP2 in Dock2−/− microglia using the selective EP2 agonist butaprost. For Dock2−/− microglia, butaprost effectively decreases LPS-stimulated TNF-α induction, providing evidence that EP2 activity is not DOCK2-dependent (Figure 2A) (**P < 0.01 determined by one-way analysis of variance with Bonferroni corrected comparisons, n = 4). Together, these experiments confirm that DOCK2 is transcriptionally down-regulated in the absence of EP2.
Figure 2.
DOCK2 is a COX2-independent regulator of microglial innate immune responses. A: The EP2 agonist butaprost shows that Dock2−/− microglia have EP2 functionally present (**P < 0.01 and ***P < 0.001). A–D: Quantification of in vitro primary mouse microglial response to LPS stimulation shows Dock2−/− microglia to have decreased TNF-α cytokine induction (A) (*P < 0.05), decreased MCP-1 chemokine induction (B) (*P < 0.05), decreased phagocytosis (C) (*P < 0.05), and decreased paracrine neurotoxicity (D) (*P < 0.05) when compared with wild-type (WT) (LDH=lactate dehydrogenase). (No significance = n.s.). Total significant difference between wild-type and Dock 2−/− micrological genotype (**P < 0.01, n = 4). E: Western blot images show COX2 induction following LPS stimulation of both wild-type and Dock2−/− primary microglia.
DOCK2 Localizes to Microglia in Mouse Brain
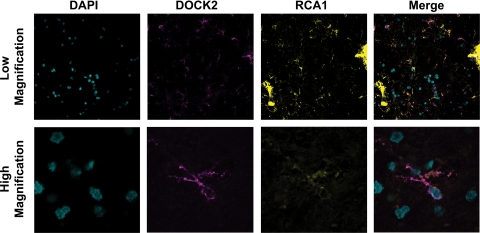
Having discovered that DOCK2 is expressed in wild-type microglia in vitro and weakly in wild-type brain in vivo, we used immunohistochemistry to determine whether DOCK2 is expressed by microglia in vivo. Double-labeling immunohistochemistry using cryopreserved wild-type mouse hippocampus revealed that DOCK2 cellular staining is present and that it co-localized exclusively with established microglial markers tomato lectin (Figure 1D) and CD68. DOCK2 did not co-localize with the neuronal marker NeuN or the astrocytic marker glial fibrillary acidic protein. Immunohistochemistry did not reveal cellular staining for DOCK2 in EP2−/− or Dock2−/− hippocampus. Therefore, DOCK2 expression in mouse brain is restricted to wild-type microglia.
DOCK2 Regulates Microglial Response
We tested if DOCK2 down-regulation had physiological relevance to activated microglial innate immune response, namely cytokine induction, phagocytosis, paracrine neurotoxicity, and COX2 induction. Primary microglia derived from wild-type and Dock2−/− mice underwent 24-hour LPS exposure and cell culture media was collected for quantification of pro-inflammatory cytokines. TNF-α and MCP-1 were of special interest because of their important roles in neurotoxicity and microglial chemotaxis, respectively.8,36,37,38,39,40,41,42,43 Dock2−/− microglia demonstrated a concomitant significant reduction in the induction of TNF-α and MCP-1 (Figure 2, A and B) by approximately 40% (*P < 0.05 for TNF-α, *P < 0.05 for MCP-1, determined by two-tailed t-test analysis, n = 5). As with cytokine induction, Dock2−/− microglia demonstrated a significant approximately 30% decrease in phagocytosis of 2 μm fluorescent spheres (Figure 2C) (*P < 0.05 determined by two-tailed t-test analysis, n = 5). For a direct measure of microglial DOCK2 effect on paracrine neurotoxicity, we co-cultured wild-type primary neurons with either wild-type or Dock2−/− primary microglia, with or without LPS. Lactate dehydrogenase release by neurons was used as a surrogate marker of total neuronal cell death. LPS exposure to neurons alone did not induce lactate dehydrogenase release. Similarly, wild-type and Dock2−/− microglia alone without neurons did not induce lactate dehydrogenase release, with or without LPS. LPS-stimulated Dock2−/− microglia show a significant reduction in bystander neuronal damage when compared with wild-type (Figure 2D) (*P < 0.05 determined by two-way analysis of variance with Bonferroni corrected comparisons, n = 4). There was no significant difference between wild-type and Dock2−/− cells without LPS (P > 0.19, n = 4). Furthermore, two-way analysis of variance analysis for microglial genotype demonstrated a total significant difference between wild-type and Dock2−/− microglial genotype (**P < 0.01, n = 4). Others have presented evidence that activated microglia-mediate neuron damage is COX-dependent.44,45,46 In addition, given the importance of NSAID toxicity in prevention trials, we explored whether or not COX2 induction depends on microglial DOCK2. Indeed, Dock2−/− cells stimulated with LPS do demonstrate COX2 induction like that of wild-type (Figure 2E), indicating a COX2 expression-independent role for DOCK2 in innate immune regulation and paracrine neuron damage.
DOCK2 in Normal and AD Human Brain
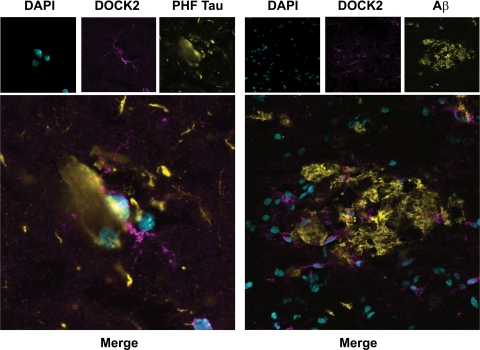
Following the promising studies highlighting the benefits of targeting DOCK2, we set to establish the relevance of DOCK2 as a therapeutic target in humans by immunohistochemistry survey of normal and AD brain. Six AD patients and six age-matched controls were used for analysis. To see if DOCK2 expression was restricted to microglia in human brain, tissue was probed for DOCK2 along with the lectin RCA1 as an established microglial marker.47,48 For each case and brain region, cells from three microscopic fields were counted and averaged together to establish a group total (Table 2). These data led to three separate findings. First, the average number of DOCK2+ cells divided by the average number of DOCK2+/RCA1+ double-labeled cells reveals that DOCK2 was expressed nearly exclusively in microglia (>95%) in both human frontal cortex and hippocampus. (In addition, DOCK2+ cells demonstrate an established ramified profile characteristic for microglia; Figure 3). Second, the number of DOCK2+ cells was significantly increased in AD brain compared with normal controls (two-way analysis of variance for average number of DOCK2+ cells shows a significant difference for normal versus AD brain (**P < 0.01) but not for hippocampus versus frontal cortex; P > 0.30). Third, the total number of microglia determined by DOCK2+/RCA1+ double-labeling was increased in AD compared with normal controls (two-way analysis of variance for average number of DOCK2+/RCA1+ cells shows a significant difference for normal versus AD brain [**P < 0.01] but not for hippocampus versus frontal cortex [P > 0.26]). This last point confirms previous reports about the increase of microglia in tissue density associated with AD.49,50,51,52 This increase in DOCK2+ microglia, likely reflective of the well-characterized microgliosis that accompanies AD, and is important because it shows that activated microglia in AD retain DOCK2 expression.53 Finally, we wanted to see if DOCK2+ microglia were associated with characteristic AD pathological changes. Indeed, DOCK2+ microglia were associated with paired helical filament-Tau+ neurofibrillary tangles and Aβ+ plaques (Figure 4). Together these data suggest that DOCK2 may be targeted as a way to reduce AD associated neuroinflammation, including bystander neuronal toxicity, without altering COX2 expression.
Table 2.
Quantification of DOCK2 Expression in Normal and Alzheimer’s Disease-Affected (AD) Human Brain
| Age (Sex) | Diagnosis | Brain region | Avg # of DOCK2+ cells per field (±SD) | Avg # of DOCK2+/RCA1+ cells per field (±SD) | % of DOCK2+ cells that are double labeled |
|---|---|---|---|---|---|
| 70 (F), 73 (M), 77 (F), 78 (M), 80 (F), 85 (F) | Normal | Hippocampus | 33.0 ± 3.7 | 32.0 ± 3.7 | 97.0 |
| Frontal cortex | 27.9 ± 9.8 | 26.7 ± 9.7 | 95.4 | ||
| 68 (M), 70 (F), 75 (F), 75 (M), 80 (F), 88 (F) | AD | Hippocampus | 41.9 ± 11.0* | 41.2 ± 10.7* | 98.4 |
| Frontal cortex | 39.8 ± 7.3* | 38.6 ± 7.6* | 96.9 |
DOCK2 is expressed almost exclusively in microglia (>95%) and DOCK2+ microglia are found at a higher tissue density in AD compared with normal brain (*P < 0.01 for DOCK2+ and DOCK2+/RCA1+).
Figure 3.
DOCK2 expression localizes to microglia in human brain. Confocal images showing 4,6-diamidino-2-phenylindole (nucleus), DOCK2, and the lectin microglial marker Ricinus Communis Agglutinin 1 (RCA1) in normal human hippocampus (magnification, top ×200, bottom ×600).
Figure 4.
Reactive human microglia express DOCK2 in Alzheimer’s disease. Confocal images show DOCK2+ microglia associate with paired helical filament-Tau+ neurofibrillary tangles as well Aβ(6E10)+ senile plaques in Alzheimer’s disease human hippocampus (magnification, left ×600, right ×200).
Discussion
The major aim of this study was to identify and characterize an EP2-dependent effector of microglial activation that may play a role in neurodegeneration. Indeed, we have shown that genetically ablating the prostaglandin receptor EP2 in mice leads to markedly reduced DOCK2 expression. For the first time, we show that DOCK2 is expressed in mouse and human brain. DOCK2 expression is localized to cerebral microglia, consistent with its proposed restricted expression to hematopoeitic lineage.25 Furthermore, the well-characterized microgliosis of AD includes DOCK2+ microglia associated with AD pathological structures in brain. This increase in DOCK2+ microglia is likely reflective of the role microglia contribute to AD pathogenesis. In the AD brain extracellular Aβ damages neurons and forms amyloid plaques, which leads to recruitment of microglia for Aβ clearance. These microglia release cytotoxic molecules to damage nearby neurons and release pro-inflammatory molecules, both of which recruit more microglia and contribute to the cycle glial–neuronal interaction believed to contribute to the pathogenesis of AD (recently reviewed in53). Finally, we have functionally characterized DOCK2 as playing a microglial COX2 expression-independent regulatory role in the neuroinflammatory response.
Our work establishes a link between PGE2 signaling and DOCK2 expression. Indeed, our data clearly demonstrated that DOCK2 expression is critically dependent on EP2 expression, presumably acting via its well-characterized role as a Rac-specific unconventional GEF, however the signaling mechanism remains to be worked out.24,25,26,27,28,29,30,31,34 More broadly, this is the first report of a role for DOCK2 in innate immunity outside of neutrophil and dendritic cell migration.34,54,55 Our findings demonstrate that DOCK2 regulates microglia function consistent with what is known about Rac signaling and activity of its conventional GEF, Vav1.27,32,33,56,57,58,59,60,61,62 Rac1 signaling in microglia also has been shown to promote NADPH oxidase formation of reactive oxygen species, as well as inducible nitric-oxide synthase and COX2 expression.60,61,62 DOCK2 binds Rac1, along with its conventional GEF, Vav, to form a part of a signaling complex.27 Interestingly, in microglia and monocytes, Vav is necessary for induction of respiratory burst and phagocytosis of Aβ.62 However, important detailed roles for differential regulation by DOCK2 and Vav1 of Rac-mediated signaling key to microglial processes remain to be clarified. Our finding that lack of DOCK2 expression led to decreased bead phagocytosis is in contrast to our previous finding that lack of EP2 results increased amyloid phagocytosis.6 We attribute the EP2 observation to suppression of phagocytosis by as yet undetermined mechanism that is abolished in the absence of EP2 expression. Our results here strongly suggest DOCK2 is not the mediator of phagocytic suppression.
Our results show that lack of DOCK2 leads to significant alterations in cultured microglia function with respect to several processes proposed to be important to neurodegeneration in AD: cytokine secretion, phagocytosis, paracrine neurotoxicity, and COX2 induction. The net effect of these DOCK2-dependent changes in microglia activity in vivo will be examined in future work. There may be concern over total suppression of DOCK2 related to the immunological phenotype of Dock2−/− mice, which includes defects in T and B lymphocyte migration, T lymphocytopenia, and architectural defects in the spleen, lymph nodes, and Peyer’s patches.25 However, it is difficult to predict the effects of partial pharmacological suppression of DOCK2 based on a developmental phenotype from genetic ablation of Dock2. While it is not yet known whether targeting DOCK2 would generally suppress inflammation or produce side effects, our data suggest that DOCK2 would lead to general inflammation suppression independent of COX2 suppression. In addition to a possible decrease in immunocompetence, there may be concern over a target such as DOCK2, which when suppressed may lead to a decrease in microglial phagocytosis of Aβ peptides There are reasons to propose that the benefits of suppressing microglial inflammation supercede those of suppressing phagocytosis. For example, strong epidemiological evidence supports the use of NSAIDs to suppress immune-mediated damage in brain, however NSAIDs are themselves known to decrease microglial phagocytosis.14,63 Moreover, microglia that accumulate later in AD pathogenesis, likely when therapy would be initiated, show decreased phagocytosis of Aβ.53
Our findings highlight both the limitations and advantages of pursuing candidate genes from microarray analysis. While we established that DOCK2 is down-regulated in EP2−/− cells, we do not as yet know why this occurs or what the mechanism of this down-regulation might be. There are also unanswered questions about the association of either EP2 or DOCK2 with AD. Resolving these issues by further studies using additional methods such as knockdown and reintroduction will move this area forward and provide mechanistic insight into our initial findings. That said, there are also advantages to identifying novel immune effectors in the brain through an unbiased approach and discovering new players in neuroinflammation that might not otherwise be considered. Using this approach requires caution and restraint to not over-interpret, but may also yield connections that might not otherwise have been uncovered.
In summary, this study identified DOCK2 as a novel COX2 expression-independent, microglial-specific regulator of innate immunity in the brain. This point is important given the important concerns regarding NSAID toxicity in clinical trials with AD patients.15,16,17 The expression and functional findings presented in this study are consistent and complementary to those of DOCK2 in other model systems. This study provides relevance to AD pathogenesis by examination of DOCK2 expression human brain. Taken together, these results suggest that DOCK2 as a potential therapeutic target for neurodegenerative disease, such as AD, that warrants further investigation.
Acknowledgments
We thank the antibody development scientists at Millipore for collaborating with us during the generation of Anti-DOCK2, Cat Number 09-454.
Footnotes
Address reprint requests to Patrick Cimino, Division of Neuropathology, University of Washington, Box 359645, Harborview Medical Center, Seattle, WA 98104. E-mail: pjjc@u.washington.edu.
Supported by National Institutes of Health grants AG24011, AG05136, GM007266, and AG13280.
References
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino PJ, Keene CD, Breyer RM, Montine KS, Montine TJ. Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr Med Chem. 2008;15:1863–1869. doi: 10.2174/092986708785132915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Shie FS, Breyer RM, Montine TJ. Microglia lacking E prostanoid receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005;166:1163–1172. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad AS, Yun YT, Ahmad M, Maruyama T, Dore S. Selective blockade of PGE2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation. Neurotox Res. 2008;14:343–351. doi: 10.1007/BF03033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Shie FS, Liu J, Wang Y, Davis J, Schantz AM, Montine KS, Montine TJ, Zhang J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55:668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- Wu L, Wang Q, Liang X, Andreasson K. Divergent effects of prostaglandin receptor signaling on neuronal survival. Neurosci Lett. 2007;421:253–258. doi: 10.1016/j.neulet.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Group AR, Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, Piantadosi S, Sabbagh M. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Lehmann DF. The cardiovascular toxicity of selective and nonselective cyclooxygenase inhibitors: comparisons, contrasts, and aspirin confounding. J Clin Pharmacol. 2005;45:742–750. doi: 10.1177/0091270005278202. [DOI] [PubMed] [Google Scholar]
- Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Clerman A, Dore S. Stimulation of PGE receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following beta-amyloid exposure. Eur J Neurosci. 2005;22:2199–2206. doi: 10.1111/j.1460-9568.2005.04427.x. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002;83:463–470. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- Yu Y, Fan J, Chen XS, Wang D, Klein-Szanto AJ, Campbell RL, FitzGerald GA, Funk CD. Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat Med. 2006;12:699–704. doi: 10.1038/nm1412. [DOI] [PubMed] [Google Scholar]
- Yu Y, Fan J, Hui Y, Rouzer CA, Marnett LJ, Klein-Szanto AJ, FitzGerald GA, Funk CD. Targeted cyclooxygenase gene (ptgs) exchange reveals discriminant isoform functionality. J Biol Chem. 2007;282:1498–1506. doi: 10.1074/jbc.M609930200. [DOI] [PubMed] [Google Scholar]
- Nishihara H, Kobayashi S, Hashimoto Y, Ohba F, Mochizuki N, Kurata T, Nagashima K, Matsuda M. Non-adherent cell-specific expression of DOCK2, a member of the human CDM-family proteins. Biochim Biophys Acta. 1999;1452:179–187. doi: 10.1016/s0167-4889(99)00133-0. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2004;2:E6. doi: 10.1371/journal.pbio.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H, Maeda M, Oda A, Tsuda M, Sawa H, Nagashima K, Tanaka S. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100:3968–3974. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- Nishihara H, Maeda M, Tsuda M, Makino Y, Sawa H, Nagashima K, Tanaka S. DOCK2 mediates T cell receptor-induced activation of Rac2 and IL-2 transcription. Biochem Biophys Res Commun. 2002;296:716–720. doi: 10.1016/s0006-291x(02)00931-2. [DOI] [PubMed] [Google Scholar]
- Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, Fukui Y. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119–129. doi: 10.1016/s1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- Sanui T, Inayoshi A, Noda M, Iwata E, Stein JV, Sasazuki T, Fukui Y. DOCK2 regulates Rac activation and cytoskeletal reorganization through interaction with ELMO1. Blood. 2003;102:2948–2950. doi: 10.1182/blood-2003-01-0173. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Pasvolsky R, Woolf E, Grabovsky V, Feigelson SW, Erez N, Fukui Y, Alon R. DOCK2 regulates chemokine-triggered lateral lymphocyte motility but not transendothelial migration. Blood. 2006;108:2150–2158. doi: 10.1182/blood-2006-04-017608. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shibagaki K, Takata K, Tsuchiya D, Taniguchi T, Gebicke-Haerter PJ, Miki H, Takenawa T, Shimohama S. Involvement of Wiskott-Aldrich syndrome protein family verprolin-homologous protein (WAVE) and Rac1 in the phagocytosis of amyloid-beta(1-42) in rat microglia. J Pharmacol Sci. 2003;92:115–123. doi: 10.1254/jphs.92.115. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113 (Pt 17):3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Tanaka Y, Sanui T, Inayoshi A, Noda M, Nakayama T, Harada M, Taniguchi M, Sasazuki T, Fukui Y. DOCK2 is required in T cell precursors for development of Valpha14 NK T cells. J Immunol. 2006;176:4640–4645. doi: 10.4049/jimmunol.176.8.4640. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Mir M, Tolosa L, Asensio VJ, Llado J, Olmos G. Complementary roles of tumor necrosis factor alpha and interferon gamma in inducible microglial nitric oxide generation. J Neuroimmunol. 2008;204:101–109. doi: 10.1016/j.jneuroim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Zhou C, Gravanis I, Rogove AD, Wu YP, Bogenhagen DF, Tsirka SE. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J Neurosci. 2007;27:1738–1745. doi: 10.1523/JNEUROSCI.4987-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Thompson WL, Karpus WJ, Van Eldik LJ. MCP-1-deficient mice show reduced neuroinflammatory responses and increased peripheral inflammatory responses to peripheral endotoxin insult. J Neuroinflammation. 2008;5:35. doi: 10.1186/1742-2094-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-beta peptide degradation by human mononuclear phagocytes. J Immunol. 2008;181:3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging. 2002;23:787–794. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Levi G, Minghetti L, Aloisi F. Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie. 1998;80:899–904. doi: 10.1016/s0300-9084(00)88886-0. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Mannoji H, Yeger H, Becker LE. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71:341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Hao C, Munoz DG. Role of microglia in senile plaque formation. Neurobiol Aging. 1995;16:797–804. doi: 10.1016/0197-4580(95)00092-s. [DOI] [PubMed] [Google Scholar]
- Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24:321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Haroutunian V, Ho L, Purohit D, Pasinetti GM. Microglia activation in the brain as inflammatory biomarker of Alzheimer’s disease neuropathology and clinical dementia. Dis Markers. 2006;22:95–102. doi: 10.1155/2006/276239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharmacol Sci. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Gotoh K, Tanaka Y, Nishikimi A, Inayoshi A, Enjoji M, Takayanagi R, Sasazuki T, Fukui Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, Tanaka Y, Shibasaki M, Kanaho Y, Sasaki T, Frohman MA, Fukui Y. Sequential Regulation of DOCK2 Dynamics by Two Phospholipids during Neutrophil Chemotaxis. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff K, Rasmussen I, Sawada M, Cudre-Maroux C, Salmon P, Bokoch G, van Deurs B, Vilhardt F. Stimulus-dependent regulation of the phagocyte NADPH oxidase by a VAV1. Rac1, and PAK1 signaling axis. J Biol Chem. 2008;283:7983–7993. doi: 10.1074/jbc.M708281200. [DOI] [PubMed] [Google Scholar]
- Shah VB, Ozment-Skelton TR, Williams DL, Keshvara L. Vav1 and PI3K are required for phagocytosis of beta-glucan and subsequent superoxide generation by microglia. Mol Immunol. 2009;46:1845–1853. doi: 10.1016/j.molimm.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Vilhardt F, Plastre O, Sawada M, Suzuki K, Wiznerowicz M, Kiyokawa E, Trono D, Krause KH. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J Biol Chem. 2002;277:42136–42143. doi: 10.1074/jbc.M200862200. [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Ye SK, Cho IH, Jung JE, Kim DH, Choi S, Kim YS, Park CG, Kim TY, Lee JW, Chung MH. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic Biol Med. 2006;41:1392–1403. doi: 10.1016/j.freeradbiomed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud-Sawin DA, Banach L, Harry GJ. Raft aggregation with specific receptor recruitment is required for microglial phagocytosis of Abeta42. Glia. 2009;57:320–335. doi: 10.1002/glia.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]