Abstract
Kidneys are the second most frequent site for chemically induced cancers in rats. However, there is still limited information on direct effects of carcinogens on pathways involved in the development of kidney tumors. Since transformed tumor cells have different characteristics than their cell of origin, it was hypothesized that healthy tissue and progressing stages of preneoplastic lesions are differentially influenced by chemical carcinogens. To elucidate this question, TSC2−/− Eker rats were gavaged with genotoxic aristolochic acid or nongenotoxic ochratoxin A for 3 and 6 months, respectively. Histopathology and cell proliferation analysis demonstrated a compound- and sex-specific onset of preneoplastic lesions. In contrast, comparable gene expression profiles of laser-microdissected preneoplastic lesions from carcinogen-treated and control rats, including reduced expression of genes involved in carcinogen uptake and metabolism, point to a compound-independent lesion progression. Gene expression profiles and additional immunostaining suggested that clonal expansion of renal lesions appears primarily driven by disturbed mammalian target of rapamycin complex 1 and mammalian target of rapamycin complex 2 pathway regulation. Finally, prolonged carcinogen exposure resulted in only marginal gene expression changes in tubules with normal morphology, indicating that some tubules may have adapted to the treatment. Taken together, these findings indicate that the final outcome of in vivo carcinogenicity studies is primarily determined by time-restricted initial events, while lesion progression may be a compound-independent process, involving deregulated mTOR signaling in the Eker rat model.
Renal tumors experimentally induced in rodent bioassays following exposure to chemicals, hormones, viruses, and radiation1,2,3 are phenotypically comparable with tumor types observed in humans.4 Thus, it may be assumed that the deregulation of some cellular pathways, eg, the AKT-tuberous sclerosis complex 2-mammalian target of rapamycin (AKT-TSC2-mTOR) pathway, appears critical for renal tumor development in both humans and rats.5,6,7 Indeed, inactivating mutations of the TSC2 tumor suppressor gene were shown in both rodent and human renal tumors8,9 and were accompanied by activation of the raptor containing mTOR complex 1 (TORC1) and its downstream effectors involved in the control of the translational machinery.5,7 Proteins activated by TORC1 target several processes involved in cancer such as cell growth and proliferation, angiogenesis, and energy metabolism.10,11 Recent findings suggest that mTOR can also interact with rictor and SIN1 instead of raptor to form a second complex (TORC2).12 This complex was previously shown to function as an important regulator of the cytoskeleton,13,14 and to activate the proto-oncogene AKT by phosphorylating AKT at Ser473.15 However, the role of TSC2 in TORC2-dependent signaling remains elusive.
Despite the gain of knowledge on pathways involved in kidney cancer, their distinct participation in the onset and/or progression of tumors is not well understood. Furthermore, the interaction with pathways involved in the development of renal tumors by genotoxic and nongenotoxic carcinogens remains elusive. Novel and sensitive tools like gene expression profiling have been used to study renal carcinogenesis and a number of recent publications have focused on the molecular classification of the different subtypes of kidney cancers in humans.16,17,18 However, none of these studies have analyzed gene expression profiles of early preneoplastic lesions and of pathways involved in preneoplastic to neoplastic progression. In addition, chronic effects of genotoxic and nongenotoxic carcinogens in morphologically unaffected tissue or tumor tissue have not been distinguished.
Using a novel protocol allowing microarray analyses of microdissected renal preneoplastic lesions from carcinogen-treated rats,19 it was hypothesized that unaffected tissue as well as different stages of preneoplastic lesions can be distinguished by their gene expression profiles, therefore allowing to study pathways involved in the onset and progression of preneoplastic lesions. In addition, it was hypothesized that renal carcinogens, based on their compound class-specific mode of action (genotoxic versus nongenotoxic), differentially affect pathways in preneoplastic lesions (eg, the mTOR pathway).
To elucidate the above hypotheses, Eker rats, carrying a heterozygous mutation in the TSC2 tumor suppressor gene and thus predisposed for the early development of renal lesions,20,21 appeared an ideal model. Their hereditary basophilic tumors are morphologically comparable with chemically induced tumors in other rat strains as well as to human basophilic epithelial adenomas and carcinomas.22 Moreover, Eker rat renal tumors have a hyperactive TORC1 pathway,7 similar to the situation assumed to predominate in human renal tumors.23,24 More importantly, Eker rats are highly susceptible toward genotoxic and nongenotoxic renal carcinogens.25,26,27 This animal model thus should allow the evaluation of the influence of genotoxic and nongenotoxic carcinogens in the development and progression of preneoplastic and neoplastic renal lesions. Furthermore, the Eker rat model helps to delineate the involvement and importance of the TSC2-mTor pathway in different stages of preneoplastic renal lesions.
Accordingly, male and female Eker rats were treated for 3 and 6 months with relatively low yet carcinogenic doses of the genotoxic plant toxin aristolochic acid (AA), and the nongenotoxic mycotoxin, ochratoxin A (OTA). Both compounds are known potent renal carcinogens in rats.28,29,30 Furthermore, they are assumed to be involved in the etiology of Balkan endemic nephropathy, associated with renal fibrosis and urothelial tumors in humans.31,32,33
Compound-induced nonneoplastic and neoplastic renal pathology, site-specific renal cell proliferation, incidence, phenotype, and progression stage of preneoplastic and neoplastic lesions were determined at the 3- and 6-months time point in both sexes. Finally, microdissected preneoplastic lesions and healthy tissue of AA- and OTA-treated as well as control male Eker rats were analyzed using microarrays. Thus, pathways specific for various progression stages of preneoplastic lesion and gene expression changes specific for AA and OTA exposure could be evaluated. Activation of TORC1 and TORC2 pathways in carcinogen-treated and control rats were visualized via immunohistochemical detection of respective phosphorylated downstream targets.
Materials and Methods
Compounds
AA sodium salt mixture (AA I: 41% and AA II: 56%) was purchased from Sigma-Aldrich Germany. OTA (≥98% purity) was provided by M. E. Stack (U.S. Food and Drug Administration, Washington DC).
Animals
Eker rats were purchased at 6–8 weeks of age from the University of Texas MD Anderson Cancer Center. Groups of females and males were randomly assigned to dose groups (10 rats per compound (or vehicle) and time point) and acclimatized for 4 weeks.
Groups of 10 Eker rats per sex were gavaged with OTA (210 μg/kg body weight) or AA (1 mg/kg body weight) at 5 days a week. Similar dose regimens have previously been used to induce renal tumors in other non-Eker rat strains (F344; Wistar).28,30 Time-matched controls were gavaged with the vehicle 0.1 M NaHCO3. Five days before sacrifice 5 of 10 rats per dose group and sex were s.c. implanted with osmotic ALZET pumps (model 2ML1; Charles River Laboratories, Germany) containing 5-bromo-2-deoxyuridine (BrdU; 20 mg/ml sterile saline; Sigma-Aldrich, Germany) to allow postmortem immunohistochemical analysis of cell proliferation. After 3 and 6 months of treatment, anesthetized rats were sacrificed by retrograde PBS perfusion-induced exsanguination. Kidneys were collected following retrograde perfusion. An overview of the overall experimental design and sample collection is given in supplemental Figure S1 on http://ajp.amjpathol.org.
Sample Collection
A freshly isolated kidney was cut in half. One half was cross-sectioned to 5-mm slices, which were snap frozen in liquid nitrogen and stored at −80°C for subsequent cryosectioning. The other, half of the same kidney was fixed in PBS-buffered fixative (2% paraformaldehyde and 1% glutaraldehyde) for subsequent paraffin embedding and sectioning.
Histopathology
H&E-stained paraffin sections from 10 rats per group were randomized for histopathological evaluation. Nonneoplastic pathology was classified as none (0), very mild (1), mild (2), moderate (3), pronounced (4), and severe (5), whereas preneoplastic and neoplastic lesions were classified according to the lesion type3 and quantified (total number of each lesion type per section).
Immunohistochemistry
Cell proliferation was evaluated via BrdU immunohistochemistry. Immunostaining was performed as previously described,34 yet using a monoclonal mouse anti-BrdU primary antibody (MU247-UC; Biogenex) diluted 1:100 in Power Block (BioGenex) in an overnight application at 4°C. Cell proliferation was quantified on randomized sections. Twenty microscopic fields (×10 ocular, ×40 objective) were randomly chosen within the area of the renal outer cortex and inner cortex/outer medulla. Proximal and distal tubules and collecting ducts were counted separately per field, distinguishing between negative and positive BrdU-stained nuclei. Nuclear labeling indices for BrdU (LI%) (BrdU-positive nuclei/total number of nuclei counted) were determined. For LI determination, at least 1000 nuclei were counted in proximal tubules of the outer part and 1000 nuclei in the inner part of the corticomedullary section. In addition, a total number of 500 nuclei in distal tubules and 500 nuclei in collecting ducts were counted. This approach of counting could also include areas with regenerating tubules and earliest preneoplastic lesions, however avoiding overtly morphologically changed areas (tumors or chronic progressive nephropathy) for counting. In an additional approach at least 500 BrdU-positive and -negative nuclei were counted specifically only in morphologically unaffected proximal tubules, in the outer as well as the inner cortex separately.
Immunohistochemistry of phospho-S6 ribosomal protein (pS6RP), phospho-Akt (pAKT), and FOXO1 in paraffin sections was performed according to the manufacturer’s instructions (Cell Signaling Technology). Briefly, primary rabbit anti-pS6RP (Ser235/236) (catalog no. 2211) was diluted 1:100, whereas rabbit anti-p-AKT (Ser473) (catalog no. 3787) was diluted 1:10. Rabbit anti-FOXO1 (FOXO1A, FKHR, Abcam ab39656) was diluted 1:200 in 5% normal goat serum. Antigen–antibody complexes were visualized using the Super Sensitive (BioGenex) alkaline phosphatase-labeled, biotin–streptavidin amplified detection system with Fast Red as chromogen.
Laser Microdissection and RNA Isolation
For microdissection of preneoplastic lesions from H&E-stained renal cryosections, a laser microdissection and pressure catapulting system (P.A.L.M. Microlaser Technologies, Germany) was used. Basophilic atypical tubules (bAT), basophilic atypical hyperplasia (bAH), or tubules with normal morphology (NT) were micodissected, according to a previously established protocol.19 For this approach, preneoplastic lesions and NT were isolated from male 6 months treated and control rats (N = 3). From each replicate rat per dose group bATs, bAHs, and NT were separately pooled from the corticomedullary section until an overall area of 2 mm2 was reached. RNA isolation from pooled samples and subsequent Affymetrix Rat Genome RAE_230A_2.0 chip hybridization was performed as described previously.19
Microarray Data Processing and Analysis
Microarray quality control was performed as previously described,35 and gene expression data were submitted to the National Center for Biotechnology Information- Gene Expression Omnibus (GEO) repository (GSE 10608). For statistical analysis, Expressionist Analyst software (Genedata AG, Switzerland) was used. Genes with significantly increased or decreased expression values per compound and lesion type when compared with the expression levels of NTs of control rats (NT(C)) were selected by Student’s t-test with a P value cutoff of 0.005, combined with a 2.0-fold deregulation threshold. Identical cut-offs were applied for comparing NTs from AA- and OTA-treated rats with NTs from control rats. Heatmaps were used to graphically display the relative expression data after one-dimensional clustering of the genes. On the basis of 284 individual comparisons, a regression coefficient of 0.94 between the natural log ratios for Affymetrix RAE230A arrays and quantitative RT-PCR (TaqMan technology) data were previously obtained in this laboratory (data not shown). The latter supports the assumption that expression profiles generated with the Affymetrix platform, as presented here, can be used for semiquantitative comparisons of gene expression changes and subsequent data interpretation.
For functional analysis, significantly deregulated genes were characterized according to the biochemical role of its encoded protein, using information from databases, eg, NetAffx, SwissProt, Proteome, and PubMed. Depending on their increased or decreased expression, genes were further assigned to pathophysiological categories allowing the comparison of signaling pathways within the different types of preneoplastic lesions and NTs.
Statistical Analysis
Statistical analysis of histopathological and cell proliferation data were performed using GraphPad Prism 4.03. Significant differences in nuclear labeling indices or total number of lesions in treated and control rats were analyzed by one-way analysis of variance followed by Bonferroni’s test for multiple comparisons. Ranked nonneoplastic pathology data were analyzed using the nonparametric Kruskal-Wallis test followed by Dunn’s Multiple Comparison Test.
Results
Nonneoplastic Pathology
OTA. Eker rats treated with OTA for 3 and 6 months presented with significantly increased apoptosis, karyomegally, and peritubular fibrosis in the inner cortex (Figure 1, A–C; supplemental Table S1, http://ajp.amjpathol.org). The severity of apoptosis and karyomegally was higher in 3-months-treated males than in females, but similar in 6-months-treated rats of both sexes. At the 6-months time point, significantly increased necrosis was observed within the inner cortex of both sexes and cytoplasmic vacuolization in males. In addition, 6-months OTA-treated males and females exhibited a marked (but not significantly increased) severity and higher incidence of protein casts, tubular dilatation, and regenerating tubules than the respective controls.
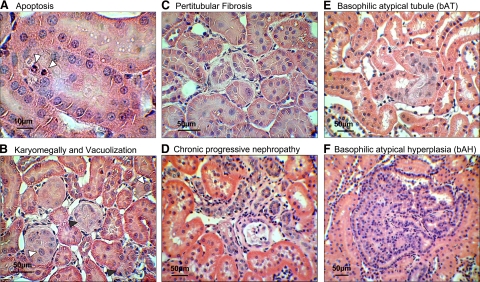
Figure 1.
Representative preneoplastic lesions as observed in H&E stained renal sections of OTA-treated rats apoptosis (white arrowheads) (A); karyomegally (white arrowheads) and cytoplasmic vacuolization (black arrowheads) in OTA-treated rats (B), peritubular fibrosis in OTA-treated rats (C), representative chronic progressive nephropathy observed in all treatment and control groups (D), bAT (E), and bAH (F).
AA. Treatment with AA resulted in weaker pathology compared with OTA-treated rats (supplemental Table S1, http://ajp.amjpathol.org). Although effects were not significant, 6 months of treatment with AA consistently appeared to result in more pronounced and higher incidences of pathological changes, protein casts, dilated tubules, and regeneration, than in the respective control groups of both sexes. Pathological changes were primarily observed in the corticomedullary section and in some cases in the medulla and the papilla.
A time-dependent increase in chronic progressive nephropathy was observed in treated and control groups and was characterized by thickening of the glomerular and proximal tubule basement membrane, increased regeneration and infiltrating mononuclear cells (Figure 1D).
Cell Proliferation
Site-specific cell proliferation of proximal tubules, distal tubules or collecting ducts, was assessed via BrdU immunostaining in deparaffinized renal sections (Figure 2, A and B).
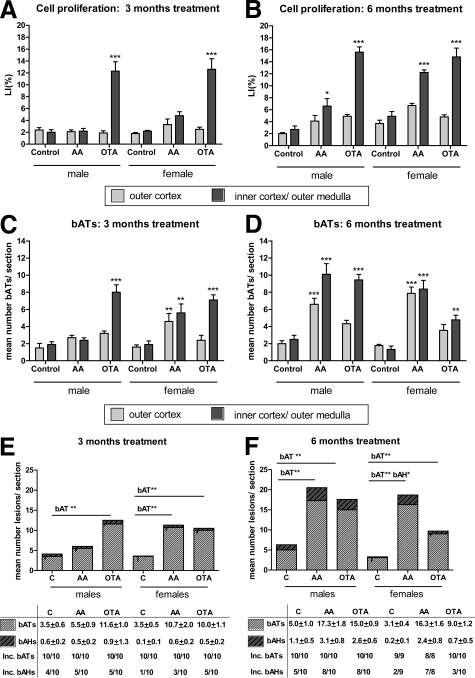
Figure 2.
A and B: Mean numbers ± SEM of BrdU S-phase labeling indices (LI%) of Eker rats treated with AA or OTA. LI%, determined for proximal tubules within randomly chosen fields of the outer (light gray) or inner cortex (dark gray) after 3 months (A) and 6 months (B) of treatment. C and D: Mean numbers ± SEM of bATs in AA- and OTA-treated and control male and female Eker rats after 3 (C) and 6 months (D) of treatment. Light gray bars, outer cortex; dark gray bars, inner cortex. E and F: Mean total numbers ± SEM and incidences (inc.) of bATs and bAHs in carcinogen treated and control rats of both sexes after 3- (E) and 6-months (F) of treatment. Striped light gray bars, bATs; striped dark gray bars, bAHs. A–F: Significant differences compared with the respective controls were tested using a one-way ANOVA followed by Bonferroni’s posttest for multiple comparisons and are indicated as ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
In general, female rats at the 6-months time point demonstrated an approximately 2- to 3-fold higher basal cell proliferation rate than males at all sites, whereas this was not apparent at the 3-months time point.
OTA. Three months of OTA treatment significantly increased cell proliferation rates 6.2- and 5.7-fold above controls in the proximal tubules of the inner cortex of male and female rats, respectively (Figure 2A). In contrast, higher cell proliferation was observed following 6 months of OTA treatment in the inner cortex of male rats (5.8-fold) compared with females (3.0-fold) (Figure 2B). No significant changes were observed in the outer cortex of OTA-treated rats.
AA. Three months of AA treatment did not change cell proliferation rates in the proximal tubules of the inner or the outer cortex in both male and female rats (Figure 2A). However, at the 6-months time point, a 2.4- and 2.5-fold increase in cell proliferation in proximal tubules of the inner cortex was observed in both males and females, respectively (Figure 2B).
When restricting cell proliferation analysis to proximal tubules with a nonpathological phenotype, mean cell proliferation rates were not significantly different in the outer or inner cortex of control and treated rats of both sexes (data not shown). Similarly, cell proliferation was not increased in distal tubules or collecting ducts of either sex, irrespective of the treatment (data not shown).
(Pre-)neoplastic Pathology
Preneoplastic (atypical tubule and atypical hyperplasia) and neoplastic (adenoma and carcinoma) lesions observed in kidneys of male and female Eker rats after 3 or 6 months of treatment were primarily of the basophilic phenotype, i.e., bAT (Figure 1E) and bAH (Figure 1F). Other lesion types, eg, oncocytic tubules and cysts, were rare and thus not evaluated further.
All treatment and control groups presented with a 100% incidence of bATs. However, a time-, compound-, and segment-dependent increase in total numbers of bATs was observable in treated males and females, when compared with the respective controls at both time points (Figure 2, C and D). Furthermore, compound-specific induction of bATs in the outer or inner cortex/outer medulla correlated well with a compound and segment-specific induction of cell proliferation (Figure 2, A–D).
OTA. Including all renal segments, a 3-months OTA treatment induced a 3.3- and a 2.8-fold increase in total bAT number in male and female rats, respectively (Figure 2E). A similar increase in bAT number was also observed at the 6-months time point, when compared with the respective controls, suggesting continued OTA-induced bAT formation in all groups (Figure 2F). In males, total numbers of bAH increased approximately 3-fold over time, whereas total numbers of bAH in OTA-treated females remained at similar levels at both time points, indicating a higher formation of preneoplastic lesions in male rats. This was confirmed by the 80% bAH incidence in male rats compared with the 30% incidence in females (Figure 2F).
AA. A significant 3.0- and 5.6-fold increase of the number of bATs/rat was observed in female rats at the 3- and 6-months time points, whereas male Eker rats showed a significant 3.5-fold increase of bAT number at the 6-months time point only (Figure 2F). Similarly, both male and female rats presented with higher total numbers of bAH following 3- and 6-months AA treatment when compared with the corresponding controls. However, the bAH/rat ratio was significantly higher at the 6-months time point only in female rats. Sporadic adenomas or carcinomas could be observed in all dose groups. However, total numbers or incidences were not significantly increased in AA- or OTA-treated rats when compared with corresponding control rats (data not shown).
Gene Expression Profiling
As preneoplastic lesions exhibited a similar basophilic phenotype in both sexes, gene expression profile analyses were restricted to preneoplastic lesions of male Eker rats. Laser-microdissected preneoplastic lesions (bATs and bAHs) and NTs of AA- and OTA-treated and control males were analyzed using microarrays to investigate gene expression profiles induced by AA and OTA as well as to differentiate pathways specific for the progression stage of the preneoplastic lesions.
Preneoplastic Lesions
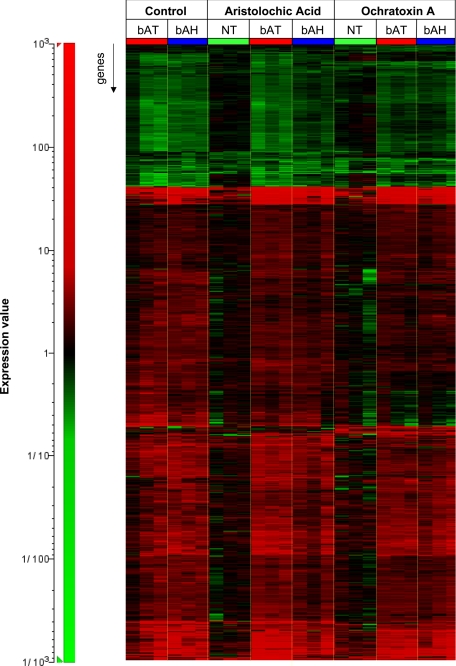
Gene expression profiling of microdissected lesions resulted in the differential expression of 570 annotated nonredundant genes when compared with tubules with normal morphology of control rats (NT(C)). Although different total numbers of genes appeared to be significantly differentially expressed in various sample types (Table 1), visualization of expression profiles of the union of selected genes revealed a qualitatively similar expression profile in all preneoplastic lesions, irrespective of treatment or lesion type (Figure 3).
Table 1.
Numbers and Categories of Significantly Differentially Expressed Genes in Preneoplastic Lesions
| End points | % of all significantly deregulated annotated genes per group
|
Description | |||||
|---|---|---|---|---|---|---|---|
| bAT | bAT | bAT | bAH | bAH | bAH | ||
| C | AA | OTA | C | AA | OTA | ||
| Reduced carcinogen uptake and biotransformation | 0 | 3.9 | 2.3 | 6.5 | 2.7 | 2.4 | Decreased expression of genes encoding solute carriers including organic anion and organic cation transporters. Decreased expression of genes involved in phase I and phase II biotransformation |
| Oxidative stress | 2.9 | 2.7 | 4.7 | 1.9 | 1.4 | 2.4 | Increased expression of ARE target genes and other genes known to be involved in oxidative stress responses. Decreased expression of genes mediating the cellular antioxidant defense |
| DNA damage response | 4.4 | 0.9 | 1.6 | 1.1 | 4.1 | 0 | Increased expression of genes encoding p53 target genes and genes coding for proteins involved in DNA damage repair |
| Apoptosis | 4.3 | 2.4 | 1.6 | 3.4 | 1.4 | 1.2 | Increased expression of proapoptotic genes. Decreased expression of antiapoptotic genes |
| Tumor and metastasis suppression | 2.9 | 3.0 | 4.7 | 2.3 | 2.7 | 3.5 | Increased expression of genes coding for tumor suppressor genes. Increased expression of tissue inhibitor of metalloproteinases or decreased expression of genes facilitating metastasis |
| Cell cycle progression | 2.9 | 5.1 | 5.5 | 3.1 | 5.5 | 3.5 | Increased expression of genes involved in DNA replication cell cycle progression, mitotic spindle/cytokineses or nucleosome formation |
| Cell growth/survival/proliferation | 10.1 | 8.3 | 8.6 | 6.9 | 6.8 | 10.6 | Increased expression of genes encoding growth and survival factors, (IGF)-PI3K-AKT and the mTOR/S6K pathway components, antiapoptotic genes, oncogenes. Decreased expression of tumor suppressor genes |
| Tumorigenesis | 1.4 | 0.9 | 1.6 | 1.1 | 2.7 | 1.2 | Increased or decreased expression of genes with unclear biochemical function, however, for which a similar direction of expression has been reported previously in different types of cancer |
| Cytoskeleton rearrangement | 7.2 | 7.1 | 7.8 | 10.7 | 6.8 | 7.1 | Increased expression of genes coding for Rho-GTPases, other proteins involved in positive regulation of actin polymerization, actin remodeling, stress fiber formation, or microtubule dynamics |
| Enhanced cell adhesion | 1.4 | 1.2 | 0.8 | 0.8 | 4.1 | 2.4 | Increased expression of genes coding for cell adhesion molecules |
| Angiogenesis | 2.9 | 4.5 | 5.5 | 1.9 | 4.1 | 4.7 | Increased expression of genes of the VEGF pathway and functioning in growth and survival of endothelial cells, vascular development and remodeling |
| Metastasis | 1.4 | 2.7 | 2.3 | 3.4 | 2.7 | 1.2 | Increased expression of genes coding for serin proteinases involved in ECM degradation. Decreased expression of genes coding for cell adhesion molecules or gap junction proteins |
| Dedifferentiation | 0 | 2.6 | 1.6 | 1.2 | 0 | 3.5 | Decreased expression of genes mediating the physiological homeostasis of the cell and/or organ |
| Stromatogenesis | 2.9 | 4.5 | 6.3 | 7.7 | 5.5 | 12.9 | Increased expression of genes encoding ECM components and components involved in stroma-cell interaction like integrins or integrin binding proteins |
| Immune response | 10.1 | 15.5 | 15.6 | 19.2 | 15.1 | 15.3 | Increased expression of genes encoding components of the acute phase response, inflammation, innate or adaptive immunity, complement system, TNF/cytokine pathway, or increased expression genes coding for proteins involved in antigen presentation |
| Lysosomal degradation | 1.4 | 2.7 | 2.3 | 1.9 | 1.4 | 2.4 | Increased expression of genes coding for lysosomal enzymes and proteins of the lysosomal membrane |
| Energy provision | 5.8 | 4.5 | 2.3 | 2.7 | 4.1 | 1.2 | Increased expression of genes coding for proteins supporting uptake of glucose, lipids and amino acids into the cell, as well as for proteins supporting glycogenolysis, glycolysis, gluconeogenesis, citrate cycle, and glutaminolysis |
| Precursor provision | 5.8 | 8.0 | 8.6 | 8.8 | 5.5 | 5.9 | Increased expression of genes coding for proteins involved in fatty acid synthesis, lipid storage, cholesterol uptake, cholesterol metabolism and phospholipid/glycolipid synthesis. Decreased expression of genes involved in β-oxidation fatty acids and amino acid degradation |
| Protein synthesis and trafficking | 4.3 | 1.2 | 3.1 | 3.4 | 2.7 | 3.5 | Increased expression of genes involved in protein sorting, vesicular transport or endo-/exocytosis. Increased expression of genes involved in translation or protein folding in the endoplasmatic reticulum |
| Protein degradation | 4.3 | 3.3 | 0 | 0.4 | 0 | 0 | Increased expression of genes coding for components of the ubiquitin-dependent protein catabolism |
| Neuronal differentiation | 2.9 | 3.3 | 3.1 | 2.7 | 5.5 | 2.4 | Increased expression of genes involved in neuronal signal transmission or neuronal differentiation |
| Cellular stress | 4.3 | 2.7 | 1.6 | 3.1 | 8.2 | 3.5 | Increased expression of genes involved in the NF-κB, or HIF pathways or other stress related pathways involved in hypoxia, acidosis or tubular damage. Decreased expression of genes encoding inhibitors of these pathways and end points |
| Osmotic stress | 7.2 | 1.5 | 2.3 | 1.1 | 1.4 | 1.2 | Increased expression of genes involved in water and sodium absorption, decreased expression of genes mediating cell volume homeostasis. Increased expression of genes involved in hyperosmotic response |
| Unknown end point | 10.1 | 7.7 | 6.3 | 5.0 | 5.5 | 8.2 | Genes with known biochemical function, but whose increased or decreased expression could not be given a specific meaning, due to the lack of information from literature |
ARE, antioxidant-responsive element; IGF-PI3K-AKT, insulin growth factor- phosphoinositol-triphosphate kinase-AKT; mTOR/S6K, mammalian target of rapamycin-ribosomal S6 kinase; VEGF, vascular endothelial growth factor; ECM, extracellular matrix; TNF, tumour necrosis factor; NF-κB, nuclear transcription factor B.
Figure 3.
Heat map comparing gene expression changes of microdissected bATs and bAHs of AA-, OTA-, and vehicle-treated Eker rats and microdissected healthy tubule from AA- and OTA-treated rats. Expression profiles were compared with microdissected NT of control rats. Gene expression ratios are indicated by the color scale: red, increased expression; green, decreased expression.
Tubules with Normal Morphology
Gene expression profiles of microdissected NTs of 6-months AA- and OTA-treated rats were compared with normal tubules of the respective control rats NT(C) to evaluate possible effects of both compounds on pathologically unaffected tubules. Only seven and six genes presented with significantly increased or decreased expression in NT of AA- and OTA-treated males, respectively (supplemental Table S2, http://ajp.amjpathol.org). However, none of those genes could be correlated with known AA- or OTA-induced mechanisms.
Functional Analysis of Genes with Significantly Changed Expression
Functional analysis of differentially expressed genes in preneoplastic lesions from treated and control rats identified a number of pathophysiologically relevant endpoints. Table 1 specifies the criteria used to assign genes with increased or decreased expression to these categories. The most prominent categories, containing the majority of differentially expressed genes, were similar in all lesion types. Importantly, all preneoplastic lesions of control and treated animals demonstrated a broadly decreased expression of genes encoding transporters and metabolic enzymes involved in the uptake and biotransformation of carcinogens, among them enzymes and transporters previously shown to be involved in AA- and OTA-mediated toxicity and carcinogenicity (ie, OATP1, OAT1, CYP1A1, NQO1, NQO2),36,37,38 (Table 1; supplemental Table S2, http://ajp.amjpathol.org).
Moreover, many differentially expressed genes of these categories could be directly associated with activated AKT, TORC1, and/or TORC2 pathways (supplemental Table S2, http://ajp.amjpathol.org and below) and may result from a functional loss of the TSC2 gene in preneoplastic lesions, ie, genes involved in cell cycle progression (increased expression of CDC2, CCNB1, CCNB2, AURKB; decreased expression of NUP50); cell growth, survival, and proliferation (increased expression of CTGF, POSTN, ITGB4, PINK, IGFBP1, and PIK3AP1; decreased expression of FOXOA1); protein synthesis (increased expression of RPS15A); increased expression of hypoxia-inducible factor-1α (HIF1α) target genes involved in angiogenesis (PAI1, CXCR4) and glucose uptake and glycolysis (PFKM, PFKC, ENO2, PGAM2, and GLUT2); and cytoskeleton rearrangement (increased expression of genes coding for Rac/Rho/Cdc42 signal transduction proteins, eg, RHOQ, RAC2, PAK3, IQGAP1, CDC42SE1, CDC42EP1, ELMO2, DOCK6, RAC2, and PLEKHG2; increased expression of protein kinase Cα substrates MARCKS and AKAP12). PubMed IDs for references, suggesting a direct or indirect involvement of the genes presented here in either TORC1 and/or TORC2-dependent pathways, are given in supplemental Table S2 (see http://ajp.amjpathol.org).
Moreover, a possible correlation between functional loss of TSC2 in preneoplastic lesions and active c-myc oncogene is indicated by the increased expression of C-MYC itself and its target genes EMP1 and EMP3.
TORC1 and TORC2 Pathway Activation in Preneoplastic Lesions
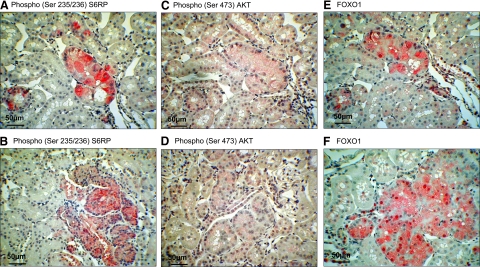
The observed changes in gene expression profiles point to a concomitant activation of AKT, TORC1, and/or TORC2 pathways. To prove this, immunostaining of phosphorylated ribosomal protein S6 at Ser235/236 (pS6RP) was used as an indicator of TORC1 activity.7 Staining of AKT phosphorylated at Ser473 (pAKT) provided confirmation of active TORC2 since active TORC2 was shown to directly phosphorylate AKT at Ser473.15
Comparable pS6RP staining was observed within bATs and bAHs of AA, OTA, and control male and female Eker rats (Figure 4, A and B). Sporadic preneoplastic tubules were negative for pS6RP staining in the carcinogen-treated and the control group (a maximum of six bATs in the 6-month AA treatment group). Positive pS6RP staining was also present in adenomas, carcinomas, and oncocytic cysts of all rats examined. pS6RP-positive immunostaining of preneoplastic lesions and tumors may thus be interpreted as corroborative evidence for activation of the TORC1/S6K/S6RP pathway.
Figure 4.
Proof of principle staining for concurrent TORC1 and TORC2 activation in preneoplastic lesions. Serial sectioned bAT with positive pS6RP (A), pAKT (C) and cytoplasmatic accumulation of FOXO1 (E). Representative images of bAH immunopositive for pS6RP (B), p-AKT (D), and FOXO1 (F). Additional images of pS6RP, p-AKT and FOXO1 are shown in Supplemental Figure 2 available at http://ajp.amjpathol.org.
pAKT immunostaining of bAT and bAH and tumors resulted in a weak but specific cytoplasmatic and sporadic nuclear staining in treated and control male rats (Figure 4, C and D). However, as pAKT staining appeared faint, additional immunostaining of AKT target FOXO1 was used. FOXO1 shuttles from the nucleus to cytoplasm for degradation when phosphorylated by active AKT.39 FOXO1 and FOXO3 phosphorylation was strongly reduced in embryonic fibroblasts derived from mice deficient in SIN1, which are unable to form a functional TORC2 complex.12 Accordingly, cytoplasmatic localization of FOXO1 can be indicative for a TORC2-dependent AKT activation. Tubules with NT presented primarily with nuclear FOXO1 staining. In contrast, strong cytoplasmic and nuclear staining in bATs and bAHs was detected, as expected for deregulated FOXO1 activation and/or half-life (Figure 4, E and F). Thus, the FOXO1 staining pattern further supports activation of the TORC2-AKT pathway in preneoplastic renal lesions. Additional images of pS6RP, pAKT, and FOXO1 stainings are provided in supplemental Figure S2 (see http://ajp.amjpathol.org).
Discussion
Nephrotoxicity and Accompanying Cell Proliferation Is Critical for the Formation of Preneoplastic Lesions but not for Lesion Progression in AA- and OTA-Treated Eker Rats
Three and six months of treatment of male and female Eker rats with OTA resulted in tubular degeneration and apoptosis accompanied with an increased cell proliferation and the occurrence of karyomegally and peritubular fibrosis in the inner cortex (Figure 1, A–C; Table S1, see http://ajp.amjpathol.org). Comparable findings were described in F344 rats treated with the same dose regimen,28,29,40 thus confirming comparable sensitivity of Eker rats to OTA nephrotoxicity.
Unlike OTA, no significant nephrotoxic effects were observed in AA-treated male or female Eker rats. However, at the 6-months time point, both sexes appeared to develop more pronounced cytoplasmatic vacuolization, protein casts, dilated tubules, regeneration, and chronic progressive nephropathy in the corticomedullary section, when compared with the respective control rats (supplemental Table S1, see http://ajp.amjpathol.org). These findings agree with reports on nephrotoxic effects in male Wistar rats that were orally treated with single doses of AA (10 to 100 mg/kg body weight).41 Similar nephrotoxic effects were observed in female Sprague-Dawley rats 3 or 6 months after treatment with a single oral dose of 50 mg/kg/day AAI.42
BrdU staining of S-phase nuclei revealed that AA- and OTA-induced cell proliferation correlated well with the segment-specific and time-dependent increases of nephrotoxic damage in both sexes, suggesting tubular regeneration of damaged areas. Indeed, by distinguishing different nephron segments and segments with normal or pathologically altered morphology, above data demonstrated that AA- and OTA-induced cell proliferation is restricted to pathologically altered proximal tubules.
Numerous studies show that nephrotoxcity and regenerative cell proliferation can be directly associated with renal cancer. Specifically, increased cell proliferation may favor tumor development, eg, by the fixation of mutations as well as by survival and promotion of initiated cells.3,43,44 It was therefore hypothesized that AA- or OTA-induced cell proliferation corresponds to increased numbers and/or progression rates of preneoplastic lesions in a compound-, sex-, and tubular segment-specific manner. Indeed, the 2-fold higher cell proliferation in the inner cortex of OTA-treated males compared with females at the 6-months time point corresponded well with the 1.7-fold higher increase in the number of bATs in males than in females (Figure 2, A–D).
The critical role of compound-induced cytotoxicity and accompanying cell proliferation for the onset of preneoplastic lesion formation is corroborated by the findings in the AA treatment groups. The 2-fold higher cell proliferation in female than in male rats after 3 months of AA treatment corresponded to the 2-fold higher number of bAT lesions in females at the same time point. Moreover, a similar sex-dependent trend for cell proliferation and bAT development, although not as pronounced, was also observed at the 6-months time point (Figure 2). However, a significant increase of cell proliferation was only detected in the inner but not in the outer renal cortex of AA-treated rats, whereas bATs were found in both areas of the cortex. This discrepancy suggests that a low cell turnover within the outer cortex may already allow the fixation of AA-induced mutations and thus formation of preneoplastic lesions. Thus, the results presented here indicate that nephrotoxicity and accompanying regenerative cell proliferation is an augmenting but not the sole contributor to renal carcinogenesis in AA- and OTA-treated Eker rats.
Decreasing ratios of bATs/rat to bAHs/rat were suggested to be indicative for lesion progression.45 The results presented here show that higher numbers of bATs are also accompanied by higher numbers of bAHs, thereby resulting in similar bAT to bAH ratios in all dose groups examined. The latter may indicate that AA or OTA exposure has only a limited influence on the progression of preneoplastic lesions. Taken together, these findings suggest that compound-dependent regenerative cell proliferation most likely supports the fixation of AA- and OTA-induced mutations, which may originate from AA-induced DNA adducts46 or OTA-induced oxidative stress and subsequent DNA damage.47,48 However, subsequent to the initial formation of preneoplastic lesions, compound treatment does not appear to influence lesion progression. These findings therefore corroborate the above assumption that regenerative cell proliferation is critically involved in the development of kidney cancer. In Eker rats, already carrying a first hit mutation within the TSC2 tumor suppressor, regenerative cell proliferation may contribute to a facilitated fixation of the second hit mutation and hence to a much earlier development of preneoplastic lesions than in wild-type strains.
Adaptation to Prolonged Carcinogen Treatment
Gene expression analysis of preneoplastic lesions demonstrated that bATs and bAHs of control, AA-, and OTA-treated male rats are qualitatively indistinguishable (Figure 3), thus corroborating the above observation that once initiated in a compound- and sex-dependent manner, clonal expansion of preneoplastic lesions is compound independent. The general insensitivity of preneoplastic lesions toward AA and OTA treatment may be explained by broadly decreased expression of transporters and metabolic enzymes in preneoplastic lesions. This would also suggest a dedifferentiation of specialized cell functions in favor of increased cell proliferation during lesion progression. Importantly, some of these transporters and metabolic enzymes were previously demonstrated to be involved in AA- and OTA-mediated toxicity and carcinogenicity.36,37,38,49 Decreased expression of these essential transporters and metabolic enzymes could therefore result in a reduced toxin uptake or bioactivation. A similar phenomenon is already known in the form of multidrug resistance where cancer cells have the ability to become resistant to chemotherapeutics.50 An additional explanation for the general insensitivity of preneoplastic lesions toward AA and OTA treatment may be that a temporarily reduced blood supply in fast-growing preneoplastic foci could reduce the amount of carcinogen reaching the cells, at least until angiogenesis is initiated.
Surprisingly, only marginal gene expression changes were observed in microdissected NTs following 6-months AA and OTA treatment (Figure 3). This finding stands in contrast to previous findings where 14 days of AA and OTA treatment of Eker and corresponding wild-type rats resulted in substantial gene expression changes in the renal cortex, eg, genes involved in response to oxidative stress and DNA damage.34 An explanation for this discrepancy may be that recurring cycles of AA- or OTA-induced damage and subsequent regeneration facilitate the positive selection of tubule cells toward carcinogen-insensitive cells with no obvious phenotypical abnormalities. However, to date it cannot be excluded that an increased extrarenal detoxification and/or excretion of both compounds may also contribute to the observed carcinogen adaptation following chronic administration.
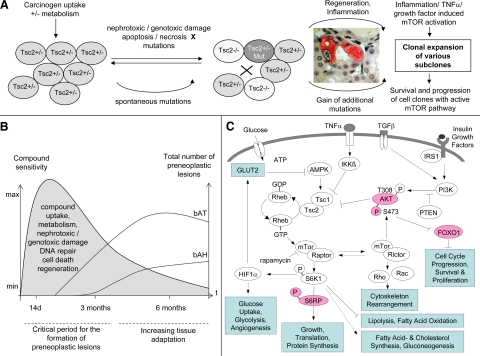
In summary, prolonged compound exposure may add little to the formation of additional preneoplastic lesions. This suggests the existence of a critical, albeit compound-specific period of exposure for genotoxic or nongenotoxic carcinogens, where normal renal tissue is not yet adapted (Figure 5B). In this critical period, normal renal tissue cells are still sensitive toward carcinogen treatment and appear incapable of sufficiently repairing compound-induced damage despite active regenerative cell proliferation, thus providing the possibility for fixation of mutations and thereby formation of preneoplastic lesions (Figure 5A). In Eker rats, this critical period of exposure could be sufficient for a broad range of compounds to induce the formation of preneoplastic lesions, because only one additional (spontaneous or compound induced) mutation would be necessary to receive a complete loss of tumor suppression. In wild-type rats, the critical period might be too short for some compounds to induce the fixation of both mutational hits, which could explain why only a limited number of nephrotoxins caused tumors in the 2-year rodent bioassay.
Figure 5.
A: Schematic overview over the postulated processes (incl. activated mTOR, cell clone survival and expansion) involved in the formation of preneoplastic lesions. B: Postulated time curve of tissue adaptation versus formation of preneoplastic lesions. C: Suggested mechanistic involvement of TORC1- and TORC2-dependent mTOR pathways in renal carcinogenesis. Blue, pathways and processes implicated by gene expression analysis. Red, protein expression or phosphorylation verified by immunostaining.
Importantly, this hypothesis would also suggest that the incidence and number of veritable tumors is primarily driven by the number of preneoplastic lesions formed during a critical period of exposure. This interpretation is supported by a study from Wolf et al27 who reported an indistinguishable number of preneoplastic lesions and tumors in Eker rats treated with 500 ppm of sodium barbital in feed for 4.5 months and sacrificed at 12 months and Eker rats treated with 500 ppm of sodium barbital in feed for the whole 12 months.
The Balance of TORC1 and TORC2 Pathways May Determine Preneoplastic Lesion Progression
Because carcinogen treatment may mainly affect early tumor formation in a compound- and sex-specific manner, similar gene expression profiles in preneoplastic lesions of AA, OTA, and control Eker rats may point to a central pathway involved in lesion progression. Functional analysis of differentially expressed genes in bATs and bAHs as well as immunohistochemical staining of TORC1 downstream target pS6RP and TORC2 downstream targets pAKT and FOXO1 in bATs and bAHs of control, AA-, and OTA-treated Eker rats may suggest that both downstream pathways of TORC1 and TORC2 were activated. The latter may result from a functional loss of the TSC2 gene in preneoplastic lesions either via loss of heterozygosity20 or additional mutations up- or downstream of the TSC2/TSC1 complex, eg, in the c-myc oncogene (Figure 5A). Eker rat preneoplastic lesion analysis demonstrated an increased expression of several HIF1α target genes and numerous additional genes involved in downstream responses of HIF1α, eg, angiogenesis and energy provision. The latter findings correspond well with a potential modulation of several cell growth regulators by activated TORC1; for instance, stabilization of the transcription factor HIF1α by TORC151 would thereby provide sufficient oxygen and energy supply for proliferating cells, eg, via increased angiogenesis and glycolysis.52 Under physiological conditions, activated S6K leads to phosphorylation and degradation of insulin receptor substrate proteins53,54 and consequently to reduced downstream phosphatidylinositol 3-kinase-AKT-TSC1/2 activation in mammalian cells. Because intact S6K-dependent negative feedback inhibition and limited oncogenic phosphatidylinositol 3-kinase-AKT signaling was still present in growth-limited benign hamartomas,55 continued and uncontrolled activation of both S6K and AKT could be a critical trigger in the progression of benign to rapidly growing malignant lesions.
The concomitant activation of TORC1 and TORC2 (Figures 4 and 5C) and therefore likely lack of feedback inhibition of mTOR-AKT signaling appears important for the survival and clonal expansion of preneoplastic lesions. Facilitated AKT activation due to TORC2-dependent Ser473 phosphorylation56 would not only further enhance the AKT-TSC2-TORC1 pathway but also influence other targets of the oncogenic AKT leading to reduced apoptosis and increased cell cycle progression (Table 1 and supplemental Table S2, see http://ajp.amjpathol.org). Moreover, preneoplastic lesion gene expression profiles support the previously shown function of TORC2 as an important regulator of the cytoskeleton through members of the Rho small GTPase family and phosphorylation of protein kinase Cα,13,14 which were discussed to play a role in cell proliferation, transformation, and metastasis.57 TORC2-dependent AKT phosphorylation and increased expression of members of the Rho small GTPase family, however, also suggest that TORC2 may be important for the initial formation of preneoplastic lesions. Indeed, Kenerson et al58 demonstrated that rapamycin inhibited renal preneoplastic progression to veritable renal tumors in Eker rats but not the formation of earliest preneoplastic lesions, suggesting that the balance between rapamycin-sensitive TORC1 and the insensitive TORC2 may be critical for preneoplastic lesion survival and clonal expansion. Rapamycin treatment may only inhibit the TORC1/HIF1α-dependent supply of energy and oxygen, which is essential for rapid clonal expansion of larger preneoplastic renal lesions to veritable tumors. In contrast, TORC2/AKT-dependent cell cycle progression and apoptosis inhibition may be important for the survival and proliferation of initiated cells and earliest preneoplastic lesions.
Overall Summary and Future Perspectives
The findings presented here suggest that AA- and OTA-specific nephrotoxic damage correlates well with the occurrence of regenerative cell proliferation and the formation of early preneoplastic lesions but not with their progression. In Eker rats, bAT to bAH progression is rather the result of compound-independent clonal expansion, most likely driven by a deregulated TORC1 and TORC2 pathway. The observed adaptation to carcinogen treatment also implies the existence of a specific time window where healthy tubules are still sensitive toward carcinogen treatment. Thus, the number of preneoplastic lesions formed during this critical period can determine the incidence and number of veritable tumors at later stages. In TSC2 mutant Eker rats, this critical period may be sufficient for a broad range of nephrotoxins and carcinogens to induce preneoplastic renal lesions. In wild-type strains, tissue adaptation and time-dependent differences in sensitivity toward nephrotoxins and carcinogens could be an additional factor that needs to be considered when predicting the carcinogenic potential of test compounds. Further research should clarify whether the suggested time-restricted sensitivity toward chemical exposure could also allow to dramatically reduce the period of exposure in the rodent bioassay while maintaining its predictive capacity.
Supplementary Material
Acknowledgments
We thank Tanja Lampertsdörfer and Gudrun von Scheven for help with the animal experiment, Alexandra Heussner and Evelyn O'Brien for help with the sacrifice, Margot Thiel and Kerstin Lotz for microarray hybridization, and Paul Pfluger and Erin Grant (University of Cincinnati, Cincinnati, OH) for critically reading the manuscript and language editing.
Footnotes
Address reprint requests to Daniel R. Dietrich, Human and Environmental Toxicology, Faculty of Biology, University of Konstanz, Jacob-Burckhardtstrasse 25, 78457 Konstanz, Germany. E-mail: Daniel.Dietrich@uni-konstanz.de.
Supported by Federal Ministry of Education and Research (0313024).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Lock EA, Hard GC. Chemically induced renal tubule tumors in the laboratory rat and mouse: review of the NCI/NTP database and categorization of renal carcinogens based on mechanistic information. Crit Rev Toxicol. 2004;34:211–299. doi: 10.1080/10408440490265210. [DOI] [PubMed] [Google Scholar]
- Hamilton JM. Renal carcinogenesis. Adv Cancer Res. 1975;22:1–56. doi: 10.1016/s0065-230x(08)60175-x. [DOI] [PubMed] [Google Scholar]
- Dietrich DR, Swenberg JA. Preneoplastic lesions in rodent kidney induced spontaneously or by non-genotoxic agents: predictive nature and comparison to lesions induced by genotoxic carcinogens. Mutat Res. 1991;248:239–260. doi: 10.1016/0027-5107(91)90060-2. [DOI] [PubMed] [Google Scholar]
- Bannasch P, Zerban H. Pathogenesis of renal cell adenomas and carcinomas in animal models. Contrib Nephrol. 1999;128:99–125. doi: 10.1159/000059975. [DOI] [PubMed] [Google Scholar]
- Hanna SC, Heathcote SA, Kim WY. mTOR pathway in renal cell carcinoma. Expert Rev Anticancer Ther. 2008;8:283–292. doi: 10.1586/14737140.8.2.283. [DOI] [PubMed] [Google Scholar]
- Pantuck AJ, Thomas G, Belldegrun AS, Figlin RA. Mammalian target of rapamycin inhibitors in renal cell carcinoma: current status and future applications. Semin Oncol. 2006;33:607–613. doi: 10.1053/j.seminoncol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson J, Short MP, Kwiatkowski DJ, Henske EP. Tuberous sclerosis-associated renal cell carcinoma: clinical, pathological, and genetic features. Am J Pathol. 1996;149:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Young AN, Master VA, Paner GP, Wang MD, Amin MB. Renal epithelial neoplasms: diagnostic applications of gene expression profiling. Adv Anat Pathol. 2008;15:28–38. doi: 10.1097/PAP.0b013e3181594720. [DOI] [PubMed] [Google Scholar]
- Furge KA, Dykema K, Petillo D, Westphal M, Zhang Z, Kort EJ, Teh BT. Combining differential expression, chromosomal and pathway analyses for the molecular characterization of renal cell carcinoma. Can Urol Assoc J. 2007;1:S21–S27. doi: 10.5489/cuaj.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Teh BT, Kanayama HO. Elucidation of the molecular signatures of renal cell carcinoma by gene expression profiling. J Med Invest. 2006;53:9–19. doi: 10.2152/jmi.53.9. [DOI] [PubMed] [Google Scholar]
- Stemmer K, Ellinger-Ziegelbauer H, Lotz K, Ahr HJ, Dietrich DR. Establishment of a protocol for the gene expression analysis of laser microdissected rat kidney samples with affymetrix genechips. Toxicol Appl Pharmacol. 2006;217:134–142. doi: 10.1016/j.taap.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Xiao GH, Everitt JI, Jin F, Walker CL. Allelic loss at the tuberous sclerosis 2 locus in spontaneous tumors in the Eker rat. Mol Carcinog. 1995;14:28–36. doi: 10.1002/mc.2940140107. [DOI] [PubMed] [Google Scholar]
- Hino O. Multistep renal carcinogenesis in the Eker (Tsc 2 gene mutant) rat model. Curr Mol Med. 2004;4:807–811. doi: 10.2174/1566524043359692. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Whiteley HE, Everitt JI. Preneoplastic and neoplastic lesions of rat hereditary renal cell tumors express markers of proximal and distal nephron. Vet Pathol. 1995;32:379–386. doi: 10.1177/030098589503200406. [DOI] [PubMed] [Google Scholar]
- Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, O'Toole T, Gibbons J, Belldegrun AS, Figlin RA. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- Klatte T, Pantuck AJ. Molecular biology of renal cortical tumors. Urol Clin N Am. 2008;35:573–580; vi. doi: 10.1016/j.ucl.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Walker C, Goldsworthy TL, Wolf DC, Everitt J. Predisposition to renal cell carcinoma due to alteration of a cancer susceptibility gene. Science. 1992;255:1693–1695. doi: 10.1126/science.1553556. [DOI] [PubMed] [Google Scholar]
- Morton LD, Youssef AF, Lloyd E, Kiorpes AL, Goldsworthy TL, Fort FL. Evaluation of carcinogenic responses in the Eker rat following short-term exposure to selected nephrotoxins and carcinogens. Toxicol Pathol. 2002;30:559–564. doi: 10.1080/01926230290105794. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Goldsworthy TL, Janszen DB, Harden R, Donner EM, David CS, Everitt JI. Promotion by sodium barbital induces early development but does not increase the multiplicity of hereditary renal tumors in Eker rats. Carcinogenesis. 2000;21:1553–1558. [PubMed] [Google Scholar]
- Boorman GA, McDonald MR, Imoto S, Persing R. Renal lesions induced by ochratoxin A exposure in the F344 rat. Toxicol Pathol. 1992;20:236–245. doi: 10.1177/019262339202000210. [DOI] [PubMed] [Google Scholar]
- NTP Toxicology and carcinogenesis studies of ochratoxin A (CAS no. 303-47-9) in F344/N rats (gavage studies). Natl Toxicol Program Tech Rep Ser. 1989;358:1–142. [PubMed] [Google Scholar]
- Mengs U, Lang W, Poch J-A. The carcinogenic action of aristolochic acid in rats. Arch Toxicol. 1982;51:107–119. [Google Scholar]
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem JL. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborova M, vom Brocke J, Simoes ML, Lord GM, Nortier JL, Hollstein M, Phillips DH, Schmeiser HH. Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis. 2007;28:2253–2261. doi: 10.1093/carcin/bgm082. [DOI] [PubMed] [Google Scholar]
- Fuchs R, Peraica M. Ochratoxin A in human kidney diseases. Food Addit Contam. 2005;22(Suppl 1):53–57. doi: 10.1080/02652030500309368. [DOI] [PubMed] [Google Scholar]
- Stemmer K, Ellinger-Ziegelbauer H, Ahr HJ, Dietrich DR. Carcinogen-specific gene expression profiles in short-term treated Eker and wild-type rats indicative of pathways involved in renal tumorigenesis. Cancer Res. 2007;67:4052–4068. doi: 10.1158/0008-5472.CAN-06-3587. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H, Stuart B, Wahle B, Bomann W, Ahr HJ. Comparison of the expression profiles induced by genotoxic and nongenotoxic carcinogens in rat liver. Mutat Res. 2005;575:61–84. doi: 10.1016/j.mrfmmm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Eckhardt U, Schroeder A, Stieger B, Hochli M, Landmann L, Tynes R, Meier PJ, Hagenbuch B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am J Physiol. 1999;276:G1037–G1042. doi: 10.1152/ajpgi.1999.276.4.G1037. [DOI] [PubMed] [Google Scholar]
- Bow DA, Perry JL, Simon JD, Pritchard JB. The impact of plasma protein binding on the renal transport of organic anions. J Pharmacol Exp Ther. 2006;316:349–355. doi: 10.1124/jpet.105.093070. [DOI] [PubMed] [Google Scholar]
- Stiborova M, Frei E, Arlt VM, Schmeiser HH. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat Res. 2008;658:55–67. doi: 10.1016/j.mrrev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- Rached E, Hard GC, Blumbach K, Weber K, Draheim R, Lutz WK, Ozden S, Steger U, Dekant W, Mally A. Ochratoxin A: 13-week oral toxicity and cell proliferation in male F344/n rats. Toxicol Sci. 2007;97:288–298. doi: 10.1093/toxsci/kfm042. [DOI] [PubMed] [Google Scholar]
- Mengs U, Stotzem CD. Renal toxicity of aristolochic acid in rats as an example of nephrotoxicity testing in routine toxicology. Arch Toxicol. 1993;67:307–311. doi: 10.1007/BF01973700. [DOI] [PubMed] [Google Scholar]
- Cui M, Liu ZH, Qiu Q, Li H, Li LS. Tumour induction in rats following exposure to short-term high dose aristolochic acid I. Mutagenesis. 2005;20:45–49. doi: 10.1093/mutage/gei007. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Ellwein LB. Genetic errors, cell proliferation, and carcinogenesis. Cancer Res. 1991;51:6493–6505. [PubMed] [Google Scholar]
- Barrett JC, Wiseman RW. Cellular and molecular mechanisms of multistep carcinogenesis: relevance to carcinogen risk assessment. Environ Health Perspect. 1987;76:65–70. doi: 10.1289/ehp.877665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich DR, Rasonyi T. Preneoplastic lesions in kidney and carcinogenesis by non-genotoxic compounds. Arch Toxicol Suppl. 1995;17:536–546. doi: 10.1007/978-3-642-79451-3_47. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 2002;17:265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- Kamp HG, Eisenbrand G, Janzowski C, Kiossev J, Latendresse JR, Schlatter J, Turesky RJ. Ochratoxin A induces oxidative DNA damage in liver and kidney after oral dosing to rats. Mol Nutr Food Res. 2005;49:1160–1167. doi: 10.1002/mnfr.200500124. [DOI] [PubMed] [Google Scholar]
- Marin-Kuan M, Nestler S, Verguet C, Bezencon C, Piguet D, Mansourian R, Holzwarth J, Grigorov M, Delatour T, Mantle P, Cavin C, Schilter B. A toxicogenomics approach to identify new plausible epigenetic mechanisms of ochratoxin a carcinogenicity in rat. Toxicol Sci. 2006;89:120–134. doi: 10.1093/toxsci/kfj017. [DOI] [PubMed] [Google Scholar]
- Stiborova M, Frei E, Sopko B, Sopkova K, Markova V, Lankova M, Kumstyrova T, Wiessler M, Schmeiser HH. Human cytosolic enzymes involved in the metabolic activation of carcinogenic aristolochic acid: evidence for reductive activation by human NAD(P)H:quinone oxidoreductase. Carcinogenesis. 2003;24:1695–1703. doi: 10.1093/carcin/bgg119. [DOI] [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases in transformation and metastasis. Adv Cancer Res. 2002;84:57–80. doi: 10.1016/s0065-230x(02)84003-9. [DOI] [PubMed] [Google Scholar]
- Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.