Abstract
Numerous past studies have suggested a critical role of the paracrine effect between tumor vascular endothelial growth factor (VEGF)-C and lymphatic FLT-4 in solid tumor-associated lymphangiogenesis. In contrast, the pathophysiological role of tumor cell-associated FLT-4 in tumor progression remains to be elucidated. Here, we investigated this role using a tumor implantation model. SAS cells, an oral squamous carcinoma cell line expressing both VEGF-C and FLT-4 but neither FLK-1/KDR nor VEGF-D were adopted for experiments. Stable transformants of dominant-negative (dn) SAS cells were established in which the cytoplasmic domain-deleted FLT-4 was exogenously overexpressed, which can lead to inactivation of endogenous FLT-4 through competitive antagonism and is associated with down-activation of endogenous FLT-4-related intracellular signals. In vitro and in vivo proliferation assays showed lower proliferative activity of dn-SAS cells. An immunohistochemical study revealed that the tumor lymphangiogenesis was significantly suppressed, and the level of human VEGF-C mRNA was significantly lower in dn-SAS cell-derived tumor tissues. Moreover, in vitro studies demonstrated that the significant suppression of VEGF-C and VEGF-A expression was evident in dn-SAS cells or wild-type SAS cells treated with either the FLT-4 kinase inhibitor MAZ51 or the inhibitor of FLT-4-related signals. These findings together suggested that the VEGF-C/FLT-4 autocrine loop in tumor cells was a potential enhancer system to promote cancer progression, and FLT-4 in tumor tissue might become an effective target for cancer therapy.
Many previous studies have demonstrated that tumor-associated angiogenesis/lymphangiogenesis plays a crucial role in tumor progression, and angiogenic/lymphangiogenic activity is frequently correlated with regional lymph node metastasis, distant metastasis, and the prognosis of patients with malignant neoplasm.1,2,3 It is well-known that tumor cell-derived vascular endothelial growth factor (VEGF)-A and VEGF-C are key growth factors for the promotion of angiogenesis/lymphangiogenesis in malignant tissue.4,5,6 VEGF receptor (VEGFR)-1 (FLT-1), VEGFR-2 (FLK-1/KDR), and VEGFR-3 (FLT-4) are receptors for VEGF families. Generally, VEGFR-1 and -2 are well-known as the receptors primarily expressed in blood endothelial cells in the vascular system, and the VEGF-A/VEGFR-2 paracrine interaction between tumor cells and blood endothelial cells is one of the most important systems for tumor-associated angiogenesis.4,7 In contrast, a current report demonstrated that VEGF-A was a lymphangiogenic factor and contributed to lymphangiogenesis in tumor tissue,8 suggesting a broad role of VEGF-A in tumor-associated induction of neovessels. FLT-4 is also well-known as a receptor primarily expressed in lymphatic endothelial cells and occasionally in newly formed blood endothelial cells, and the VEGF-C/FLT-4 paracrine interaction between tumor cells and lymphatic endothelial cells is one of the most important systems for tumor-associated lymphangiogenesis.3
According to a number of reports, although VEGF-A and VEGF-C are immunohistochemically not detected or weakly positive in normal epithelial cells, strongly positive reactions of these factors are frequently observed as a result of genetic transformation in various types of cancer cells.9,10 Frequently, their expressions are clinicopathologically correlated with clinical statuses such as regional lymph node metastasis and distant metastasis.3,10,11,12,13,14 In contrast, some recent studies have demonstrated that FLT-4 is also expressed not only in endothelial cells but also in a wide variety of malignant cells, including prostatic cancer, head and neck squamous cell carcinoma, endometrioid adenocarcinoma, malignant mesothelioma, leukemia and non-small cell lung carcinoma.13,14,15,16,17,18,19 Furthermore, FLT-4 expression in tumor cells has been reported to be a possible predictive factor to determine the clinical approach because it correlates with lymph node metastasis or poor prognosis in patients with prostatic cancer, endometrial carcinoma, oral squamous cell carcinoma, and non-small cell lung carcinoma.13,15,16,19 These studies suggest that FLT-4 in tumor cells contributes to the promotion of tumor progression. The underlying biological functions, however, have not yet been well characterized. There are a few traditional studies indicating the functions of FLT-4 in tumor cells, in which FLT-4 is reported to be an enhancer of the proliferative and antiapoptotic activity of leukemia cells and methothelioma cells in vitro.17,18 Additionally, a current report demonstrated that the VEGF-C/FLT-4 axis in lung adenocarcinoma cells played a role in promoting the activities of invasion and metastasis via FLT-4-associated src/p38 mitogen-activated protein kinase (MAPK)/c/EBP signal-dependent up-regulation of contactin-1 in vitro and in vivo.19 Therefore, the VEGF-C/FLT-4 autocrine system in tumor cells seems to be widely involved in tumor progression, including growth, invasion, and metastasis, and further unknown functions may exist.
The protein kinase C (PKC)-p42/44 MAPK and phosphatidylinositol-3 kinase (PI3K)-Akt, generally well accepted pathways related to cell growth, antiapoptosis, or migration, were reported to be the main downstream signals of FLT-4 in lymphatic endothelial cells.20 By contrast, our previous studies demonstrated that the gene expressions of some angiogenic/lymphangiogenic growth factors, including hepatocyte growth factor (HGF),21 VEGF-A,22 and VEGF-C (unpublished data), were stimulated by the p42/44 MAPK pathway in fibroblasts and vascular smooth muscle cells. In addition, recent studies have revealed that up-regulation of VEGF-A or VEGF-C in response to stimuli of various growth factors was mediated by PI3K or p42/44 MAPK in a variety of cell lines, including malignancy.23,24,25 Therefore, we hypothesized that the VEGF-C/FLT-4 autocrine system in tumor cells is involved not only in tumor cell proliferation, the maintenance of tumor cell viability, and the invasive activity of tumor cells, but also in tumor-associated angiogenic/lymphangiogenic potential through the modulation of expression profiles of angiogenesis/lymphangiogenesis-related growth factors via FLT-4-associated signals.
To validate our working hypotheses using a tumor implantation model, we established stable transformants forcibly and highly expressing a dominant-negative (dn) inhibitor: namely, cytoplasmic domain-deleted FLT-4 that is expressed on the cell membrane and dominant negatively inhibits endogenous FLT-4 autophosphorylation (Supplemental Fig. S1, see http://ajp.amjpathol.org). This inhibitory method makes possible a sustained blockade of the cells’ own Flt-4 activity without affecting that in the surrounding lymphatic endothelial cells in the tumor tissue. Using this animal model, we herein found the possible novel role of tumor cell-associated FLT-4 in tumor progression.
Materials and Methods
Cells and Reagents
The cancer cell lines SAS, HSC-2, TF, and KN (oral squamous cell carcinoma); A549 (lung adenocarcinoma); H157, QG56, and EBC-1 (lung squamous cell carcinoma); and HSG and ACC (adenoid cystic carcinoma of salivary gland) were maintained with RPMI 1640 supplemented with 100 units/ml penicillin/streptomycin and 10% fetal bovine serum. Human fibroblast (MRC5), mouse fibroblast (NIH3T3), and simian kidney fibroblast (Cos7) were purchased from American Type Culture Correction and maintained with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Human umbilical vein endothelial cells were isolated and maintained as described previously.26 MAZ51, a VEGFR-3 inhibitor, was purchased from Calbiochem (San Diego, CA). The PKC inhibitor bisindolylmaleimide I (Sigma-Aldrich Japan, Tokyo, Japan), the PI3K inhibitor wortmannin (Sigma-Aldrich Japan), the MEK1/2 inhibitor U0126 (Promega K.K., Tokyo, Japan), the p38 MAPK inhibitor SB203580 (Calbiochem), and the Akts inhibitor Akt inhibitor V (Calbiochem) were used for the inhibition of each signal transduction. Recombinant human VEGF-C was purchased from R&D Systems (Minneapolis, MN). Nonimmune goat IgG, anti-p42/44, and anti-phospho-p42/44 ERK rabbit monoclonal antibodies were purchased from Sigma-Aldrich Japan. Anti-human Flt-4 for recognizing the extracellular domain of FLT-4 and anti-human VEGF-C goat polyclonal antibodies were purchased from R&D Systems. Anti-human Flt-4 rabbit polyclonal antibody for recognizing the intracellular domain of FLT-4 was purchased from Millipore (Bedford, MA). Anti-Akt, anti-phospho-Akt, anti-p38 MAPK, and anti-phospho-p38 MAPK rabbit polyclonal antibodies, as well as anti-phospho-tyrosine mouse monoclonal antibody, were purchased from Cell Signaling Technology (Beverly, MA). Anti-murine lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) rabbit peptide antibody was produced in our laboratory as described previously,27 and anti-human/mouse von Willebrand factor (vWF) rabbit monoclonal antibody was purchased from BD Pharmingen (Franklin Lakes, NJ).
RT-PCR and Real-Time RT-PCR
Total cellular RNA was extracted from culture cells or implanted tumor tissues with the ISOGEN system (Wako Pure Chemical, Osaka, Japan), according to the manufacturer’s instructions, and treated with RNase-free DNase I (Behringer Roche Applied Science Japan, Tokyo, Japan). Subsequently, aliquots (25 ng) of total RNA were reverse-transcribed and used for PCR templates. Appropriate amplification of target genes was done in a T-gradient thermal cycler, (Biometra, Gottingen, Germany). PCR products were electrophoresed in agarose gel and stained with ethidium bromide. Real-time RT-PCR was performed for quantification of gene expression levels. Amplification of target genes was monitored in real time, and gene expression levels were quantified using Sequence Detection System model 7000 (Applied Biosystems, Tokyo, Japan), according to the manufacturer’s instructions for TaqMan methods. The oligonucleotide sequences of PCR primers and TaqMan probes are listed in Table 1.
Table 1.
Primer and Probe Sequences
| Primer name | Forward |
|---|---|
| RT-PCR | |
| Human FLT-4 (extra, AT:60, CN:30)* | 5′-TCAATGAGGAGTTCTGCCAGC-3′ |
| Human FLT-4 (intra, AT:68, CN:30) | 5′-CCAGCATCGTGTGGTACAAAGA-3′ |
| Human HGF (AT:60, CN:35)* | 5′-TTACGAGTGGCACATCTCTATA-3′ |
| Human VEGF-D (AT:60, CN:35)* | 5′-GTGGCTGTTGCAATGAAGAGA-3′ |
| Human Ang-1 (AT:60, CN:35)* | 5′-AATATGCCAGAACCCAAAAAGGT-3′ |
| Human Ang-2 (AT:60, CN:35)* | 5′-AGATCAAGGCCTACTGTGACAT-3′ |
| Human IGF-I (AT:60, CN:35)* | 5′-GCTGTTGATCTTTTATCAATAATG-3′ |
| Human IGF-II (AT:60, CN:35)* | 5′-ATGCTGGTGCTTCTCACCTTC-3′ |
| Human GAPDH (AT:60, CN:16)* | 5′-ACCAGGGCTGCTTTTAACTC-3′ |
| Human VEGF-A (AT:60, CN:30) | 5′-ACCATGCCAAGTGGTCCCAG-3′ |
| Human VEGF-C (AT:60, CN:30) | 5′-CTTCTTTAAACCTCCATGTGTGTC-3′ |
| Human VEGF-D (AT:60, CN:35) | 5′-CCAAACAGCTCTTTGAGATATCA-3′ |
| Human FLT-1 (AT:60, CN:35) | 5′-GCCCGGGATATTTATAAGAACC-3′ |
| Human FLK-1 (AT:60, CN:35) | 5′-TCATCTGTTACAGCTTCCAAGT-3′ |
| Human PDGF-A (AT:60, CN:35) | 5′-TGCTCCTCGGCTGCGGATAC-3′ |
| Human PDGF-B (AT:60, CN:35) | 5′-ACCAACGCCAACTTCCTGGTG-3′ |
| Human PDGF-C (AT:60, CN:35) | 5′-ATGAGCCTCTTCGGGCTTCTC-3′ |
| Human PDGFR-α (AT:60, CN:30) | 5′-TCTACAATAAGATCAAGAGTGG-3′ |
| Human PDGFR-β (AT:60, CN:35) | 5′-AAGCTGGTCAAGATCTGTGACT-3′ |
| Human HGF (AT:60, CN:35) | 5′-TTACGAGTGGCACATCTCTATA-3′ |
| Human c-MET (AT:60, CN:30) | 5′-GTCAATGACTTCTTCAACAAGAT-3′ |
| Human FGF-2 (AT:60, CN:35) | 5′-CCACTTCAAGGACCCCAAGC-3′ |
| Human FGFR-1 (AT:60, CN:35) | 5′-CGCTGGTTGAAAAATGGCAAAG-3′ |
| Human IGF-I (AT:60, CN:35) | 5′-TGCTTCCGGAGCTGTGATCT-3′ |
| Human IGF-II (AT:60, CN:35) | 5′-ATCCTTGATACAACAGCTGACC-3′ |
| Human IGFI-R (AT:60, CN:35) | 5′-GGGACGCTCTCCATGTTCTC-3′ |
| Human EGF (AT:60, CN:35) | 5′-GTCTGTGATTGAAATGGCCAATC-3′ |
| Human EGFR (AT:60, CN:30) | 5′-CTGATGGATGAAGAAGACATGGA-3′ |
| Human Ang-1 (AT:60, CN:35) | 5′-AATATGCCAGAACCCAAAAAGGT-3′ |
| Human Ang-2 (AT:60, CN:35) | 5′-AGATCAAGGCCTACTGTGACAT-3′ |
| Human Tie-2 (AT:60, CN:35) | 5′-GCCTCCAAAGATGATCACAGG-3′ |
| Human GAPDH (AT:60, CN:16) | 5′-ATCACTGCCACCCAGAAGACT-3′ |
| Real-time PCR with TaqMan probes | |
| Human VEGF-A* | 5′-TACGTACCCACACACAGCGC-3′ |
| Human VEGF-C | 5′-AGCCTGAAACAGCATACCAGGT-3′ |
| Human PDGF-B* | 5′-TGCATTCTTCGCTGCCATT-3′ |
| Human GAPDH | 5′-TTCGTCATGGGTGTGAACCAT-3′† |
| 5′-CTGTCGGAAATCTTCAAGTCA-3′† | |
| Mouse VEGF-A‡ | 5′-CCAACAGTGATGTCTGGTCCTATG-3′ |
| Mouse VEGF-C‡ | 5′-GTCATGGGTGTGAACCATGAG-3′ |
Oligonucleotide sequences of primer sets for RT-PCR, and those of TaqMan probes and primer sets for real-time RT-PCR. These primer sets indicated in the table were specific or available for a reaction to the indicated species of targets.
Primer set can not react to the corresponding mouse target gene,
These alternate primers were used to detect change in expression during blockade of VEGF-C/FLT-4 autocrine system in SAS cells in vivo,
Primer set can not react to the corresponding human target gene. F, forward primer; R, reverse primer; CN, cycle number; AT, annealing temperature.
Table 1.
Continued
| Reverse | Taqman probe |
|---|---|
| 5′-GTGGTTCCTCCAGGATGAAGA-3′ | |
| 5′-CTCCCCGGGGTCCATGATGAT-3′ | |
| 5′-TGACTGTGGTACCTTATATAGTT-3′ | |
| 5′-TGCACTCAAAGCAACTGCAGTT-3′ | |
| 5′-CAGTTGTCATTATCAGCATCTTTA-3′ | |
| 5′-CAAATACATTTGTCGTTGTCTCC-3′ | |
| 5′-GGTACAAATGCCACAGATGGA-3′ | |
| 5′-CCAGGTGTCATATTGGAAGAAC-3′ | |
| 5′-TCGCCCCACTTGATTTTGGA-3′ | |
| 5′-CTTTCTTTGGTCTGCATTCACA-3′ | |
| 5′-GAATGAACTTGTCTGTAAACATCCA-3′ | |
| 5′-TGCACTCAAAGCAACTGCAGTT-3′ | |
| 5′-GGTTTGATTCTTTCCAGGCTC-3′ | |
| 5′-CTGCTGTGTTGTCATAATGGAA-3′ | |
| 5′-TCGTAAATGACCGTCCTGGTC-3′ | |
| 5′-ACCGTCCGAATGGTCACCCGA-3′ | |
| 5′-CTATCCTCCTGTGCTCCCTCT-3′ | |
| 5′-TCTCTTGATGAAGGTGGAACT-3′ | |
| 5′-GCATGATCTCATAGATCTCGTC-3′ | |
| 5′-TGACTGTGGTACCTTATATAGTT-3′ | |
| 5′-ACCACCTGCATGATGAAGCGACC-3′ | |
| 5′-TTCGTTTCAGTGCCACATACCA-3′ | |
| 5′-GTACACCTTACACATGAACTCC-3′ | |
| 5′-GGTACAAATGCCACAGATGGA-3′ | |
| 5′-CCAGGTGTCATATTGGAAGAAC-3′ | |
| 5′-ACTCTCGGTCTCTGAGGCCAC-3′ | |
| 5′-CTGACACCATGATTTCAGCCA-3′ | |
| 5′-GCTGGACAGTGTTGAGATACTC-3′ | |
| 5′-GAAATCAGCACCGTGTAAGATC-3′ | |
| 5′-TTCAAGTTGGAAGGACCACATG-3′ | |
| 5′-GGGAGCCTTCCCATTGTCTTT-3′ | |
| 5′-ACCAGGAAATGAGCTTGACAA-3′ | |
| 5′-GCAAGCCGTAAAACTTCTGCA-3′ | 5′-FAM-AGTCACTCTCAGCGGCCATCGCTG-TAMRA-3′ |
| 5′-ATCTTCCTGAGCCAGGCATCT-3′† | 5′-FAM-ATCTCAAGCACCAGCGGACCTCG-TAMRA-3′ |
| 5′-TGGCTATAAGCAGCATCTTCCC-3′† | |
| 5′-TCTCCCTCCGTTTTCTGGATTT-3′ | 5′-FAM-CTCACATAGGGTGCAGCAACCAGCG-TAMRA-3′ |
| 5′-CATGTCACAGTAGGCCTTGATCTC-3′ | 5′-FAM-CCAGTGGCATCTACACACTGACCTTCCC-TAMRA-3′ |
| 5′-AGAGCTCCGCGCACGTC-3′ | 5′-FAM-CTTAGGAGGCACCCCCTACTGCGGC-TAMRA-3′ |
| 5′-ACTGTGGTCATGAGTCCTTCCA-3′ | 5′-VIC-CAAGATCATCAGCAATGCCTCCTGCA-TAMRA-3′ |
Construction of Plasmid Vector and Plasmid Templates
The PCR primers incorporating Hind-III and Xho-I sites for amplification of human dn-flt-4 are as follows: dn-flt-4, 5′-AAAAGCTTATGCAGCGGG-GCGCCGCGCTG-3′ (forward) and 5′-AACTCGAGCTACCACTGGCTGGCATCGTAG-3′ (reverse). A PCR amplicon using cDNA from human umbilical vein endothelial cells was inserted into a mammalian expression plasmid vector, pCEP4, according to general subcloning methods. The primer sequences to construct plasmid templates for real-time PCR standard are as follows: human and mouse vegf-a, 5′-CCATGCCAAGTGGTCCCAGG-3′ (forward) and 5′-TCTTTCTTTGGTCTGCAT-3′ (reverse), and human and mouse vegf-c, 5′-GAAATTACAGTGCCTCTCTC-3′ (forward) and 5′-CTAGTTCTTTGTGGGTCCAC-3′ (reverse). Each PCR amplicon using cDNA from MRC5 or NIH3T3 was inserted into a plasmid with a pCRII TA cloning kit (Invitrogen, Carlsbad, CA), according to the manufacturer’s instruction. The sequence of each insert was examined using the CEQ 2000 Sequence Detection System (Beckman Coulter, Fullerton, CA), and complete matching compared with those reported in GenBank (accession nos. NM_182925, NM_001025366, NM_001025250, NM_005429, and NM_009506 for dn-flt-4, human vegf-a, mouse vegf-a, human vegf-c, and mouse vegf-c, respectively) was confirmed.
Establishment of Stable Transformant
Constructed dn-flt-4-inserted pCEP-4 (pCEP4-dn-flt-4) and empty pCEP4 (pCEP4-emp) were transfected into SAS cells using LipofectAMINE 2000 reagent (LF2000; Invitrogen), according to the manufacturer’s instruction. Forty-eight hours after transfection, the culture medium was replaced with medium containing 100 μg/ml hygromysin (Promega, Madison, WI) (RPMI-hygro). At that concentration, wild SAS was completely killed. The cells were then maintained with RPMI-hygro until the selected cells had grown appropriately. Next, the selected cells were spread onto 96-multiwell plates for single-cell culture and were maintained with RPMI-hygro until they reached confluence. Single-cell-derived confluent cells were continuously maintained in RPMI-hygro in larger shares. Expression of dn-FLT-4 protein was confirmed by immunoblotting and flow cytometry.
Flow Cytometry
Dn-FLT-4 expression on the cell surface was confirmed using flow cytometry. Cultured stable clones (emp-SAS1 and dn-SAS4) were trypsinized and washed three times with PBS, and single-cell suspensions were generated. Cells (1 × 106) were incubated with anti-human FLT-4 mouse monoclonal antibody for 30 minutes, and subsequently incubated with FITC-labeled anti-mouse secondary antibody. Samples were analyzed in a FACScan flow cytometry (BD Biosciences, San Jose, CA) using the program CellQuest (BD Biosciences). Emp-SAS1 was used as control.
Animals
Male BALB/c nu/nu mice (5 weeks old) were from Kyudo (Tosu, Saga, Japan). All animal experiments were done under approved protocols and in accordance with recommendations for the proper care and use of laboratory animals by the Committee for Animal, Recombinant DNA, and Infectious Pathogen Experiments at Kyushu University and according to the Law (no. 105) and Notification (no. 6) of the Japanese Government.
Tumor Implantation Model
With mice under sufficient anesthesia by an i.p. injection of sodium pentobarbital, 1 × 106 tumor cells were intradermaly injected into the abdominal region. Tumor volumes were measured 7, 14, 17, 21, 24, and 28 days after implantation. Tumor volumes were estimated by the formula V = π/6× a2× b, where a was the short axis and b the long.28
Sample Preparation for Immunoblotting
Cells were lysed with 200 μl of cell lysis buffer (Promega) containing a mixture of protease inhibitors (1.5 mmol/L pepstatin, 0.01 M aprotinin, and 500 nmol/L phenylmethylsulfonyl fluoride), and the supernatant of the lysed cells was recovered. The amount of protein was determined using the Protein Assay kit (Bio-Rad, Hercules, CA). An aliquot of 20 μg of proteins was subjected to SDS-polyacrylamide gel electrophoresis and subsequent immunoblotting to detect intracellular signaling molecules or dn-FLT-4. To examine the phosphorylation level of endogenous FLT-4 in SAS cells, samples were prepared using immunoprecipitation. An aliquot of 500 μg of proteins was used. Nonspecific proteins bound to protein G-Sepharose beads and nonimmune IgG were eliminated by 3-hour exposure of the aliquot to protein G-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) and subsequently to nonimmune goat IgG, and the aliquot was recovered. For immunoprecipitation, the recovered aliquot was coincubated with protein G-Sepharose beads binding 3 μg of anti-human FLT-4 antibody for recognizing the intracellular domain. The beads were pelleted and heat-treated in 20 μl of mercaptoethanol sample buffer, after which the samples were subjected to SDS-polyacrylamide gel electrophoresis and subsequent immunoblotting.
Immunoblotting
Proteins of the prepared samples for immunoblotting described above were separated by SDS-polyacrylamide gel electrophoresis under reducing conditions and were then transferred to a polyvinylidene difluoride membrane. An hour after being blocked with PBS containing 5% nonfat milk and 0.1% Tween 20, the membrane was incubated overnight with each primary antibody-diluted PBS solution, containing 5% BSA and 0.1% Tween 20, at 4°C. The dilution rate was determined according to the manufacturer’s instructions. After several washings with PBS containing 0.1% Tween 20, the membrane was treated for 2 hours with an appropriate horseradish peroxidase-labeled secondary antibody, HistoFine (Dako, Glostrup, Denmark), at room temperature. The target proteins were visualized by a luminal chemiluminescent reagent, LumiGLO (Cell Signaling Technology). After visualization, the membrane was further washed and incubated with stripping solution (Nacalai Tesche, Kyoto, Japan) and subjected to reimmunoblotting (reprobing) for detecting the appropriate loading control.
Immunohistochemistry
Angiogenesis and lymphangiogenesis in implanted tumor tissue were immunohistochemically evaluated using anti-vWF and anti-LYVE-1 antibodies, respectively. Twenty-eight days after implantation, mice were sacrificed, and tumor masses were harvested. Formalin-fixed and paraffin-embedded sections were then prepared. The immunohistochemical procedure has been described previously.27,29 The lymphatic vessels were identified as LYVE-1-positive vessels. MacSCOPE software was used to measure the total numbers and areas of the vessels, with the apparent luminal areas framed by LYVE-1-positive lymphatic endothelial cells in the viable tumors. The blood vessels were identified as vWF-positive vessels, and their numbers and areas were evaluated with the same methods used to evaluate lymphatic vessels. The vessel number was expressed as that per unit of viable tumor area, and the vessel area was expressed as that per a vessel in the viable tumors. To compare the intensity of VEGF-C expression in emp-SAS1 cells with that in dn-SAS4 cells, using implanted tumor sections, an immunohistochemical experiment was done simultaneously and on a like-for-like basis.
Enzyme-Linked Immunosorbent Assay
VEGF-C, VEGF-A, and platelet-derived growth factor (PDGF)-BB contents in the culture medium were determined using Quantikine Immunoassay systems for human VEGF-C, for human VEGF165, and for human PDGF-BB, respectively, according to the manufacturer’s instructions (R&D Systems). For the enzyme-linked immunosorbent assay (ELISA), 105 tumor cells were disseminated and grown to subconfluence in 6-well culture plates, and the medium was replaced with serum-deprived medium and cultured. Twenty-four hours later, the medium was replaced with fresh medium with or without appropriate treatments for 12 or 24 hours and harvested as samples.
Statistical Analysis
All data were expressed as means ± SE and were analyzed by one-way analysis of variance with Fisher’s adjustment. Statistical significance was determined using the log-rank test, and P < 0.05 was considered statistically significant.
Results
Screening of Gene Expression of Angiogenesis/Lymphangiogenesis-Related Growth Factors and Cognate Receptors in Various Human Carcinoma Cell Lines
First, we examined the spontaneous expression profile of angiogenic/lymphangiogenic growth factors and cognate receptors in a wide variety of human carcinoma cell lines in vitro. Results assessed with RT-PCR are shown in Supplemental Table S1 (see http://ajp.amjpathol.org). Interestingly, carcinoma cell lines frequently possessed PDGF-AA/platelet-derived growth factor receptor (PDGFR)-α, VEGF-C/FLT-4, and/or epidermal growth factor/epidermal growth factor receptor (EGF/EGFR) autocrine systems. To clarify the role of the VEGF-C/FLT-4 system in tumor cells, we used SAS cells, in which both VEGF-C and FLT-4 but neither VEGF-D nor FLK-1 was expressed. These expression profiles in SAS cells were suitable for excluding the influences of the autocrine effects of VEGF-C/FLK-1 and VEGF-D/FLT-4.
Stable and Forced Expression of dn-FLT-4 Induces dn Inhibition of Endogenous FLT-4 Activity Associated with Down-Activation of FLT-4-related Downstream Signals and Reduced Proliferative Activity in SAS Cells
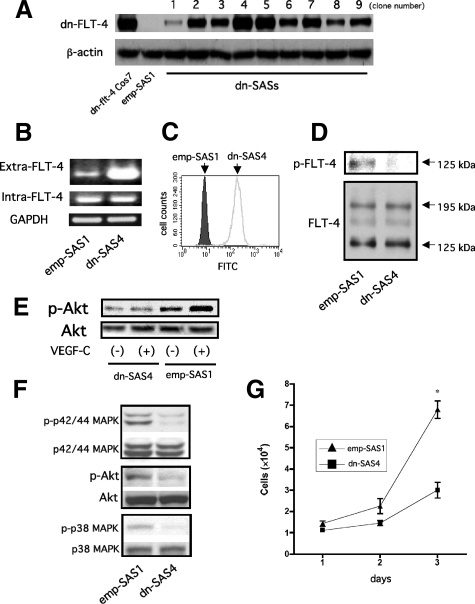
To achieve a sustained blockade of FLT-4 activity in SAS cells without directly affecting FLT-4 activity in lymphatic endothelial cells in the tumor implantation model, we established stable transformants expressing dn-FLT-4 (dn-SASs, nine clones) that is composed of extracellular and transmembrane domains (Supplemental Fig. S1A, see http://ajp.amjpathol.org) and control stable transformants (emp SASs, seven clones) were simultaneously established. We confirmed the stable expression of dn-FLT-4 in all nine clones by immunoblotting (Figure 1A). Next, we confirmed the expression levels of endogenous full-length FLT-4 and exogenous dn-FLT-4 mRNAs in one of the nine clones, dn-SAS4 cells, which showed the highest expression of dn-FLT-4 among the nine. One clone randomly selected from emp-SASs was used as a control (emp-SAS1). RT-PCR revealed that the mRNA level of dn-FLT-4 was markedly higher than that of endogenous FLT-4 (Figure 1B). In addition, flow cytometry revealed that a high level of dn-FLT-4 was expressed on the cell surface of dn-SAS4 (Figure 1C). We next examined whether or not the stable overexpression of dn-FLT-4 biochemically was able to lead to dn inhibition of endogenous FLT-4 activity. For immunoprecipitation, anti-human FLT-4 polyclonal antibody, which recognizes the cytoplasmic domain of FLT-4, was used. This antibody made it possible to precipitate endogenous full-length FLT-4 but not dn-FLT-4 in dn-SAS4. As shown in Figure 1D, phosphorylated FLT-4, which is detected as a 125-kDa band by immunoblotting under reducing condition, was almost completely blocked in dn-SAS4 cells. In addition, Akt, one of the FLT-4-related signaling molecules, was activated in response to VEGF-C stimulation in emp-SAS1 cells, whereas Akt activation in response to VEGF-C was not observed in dn-SAS4 cells (Figure 1E). These findings suggest that dn-FLT-4 is functional as a dn inhibitor. Next, we examined the effect of dn-FLT-4 on the spontaneous activation of the FLT-4-related intracellular signals p42/44 MAPK, PI3K-Akt, and p38 MAPK. As shown in Figure 1F, the phosphorylated levels of p42/44 MAPK, Akt, and p38 MAPK in dn-SAS4 cells were lower than those in emp-SAS1 cells. The reliability of the results was repeatedly confirmed using the same clone and several other clones (data not shown). Several recent studies have demonstrated that the VEGF-C/FLT-4 autocrine system in tumor cells contributes to the promotion of cell proliferation and viability in vitro.17,18 Therefore, we examined the effect of dn-FLT-4 on proliferative activity in vitro. Consistent with the previous study, reduced proliferative activity in dn-SAS4 cells was observed compared with that in emp-SAS1 cells (Figure 1G). Taking findings indicated in Figure 1 together, the VEGF-C/FLT-4 autocrine system is biologically functional, and p42/44 MAPK, Akt, and p38 MAPK pathways and proliferative activity are spontaneously activated via the VEGF-C/FLT-4 autocrine system in SAS cells.
Figure 1.
Spontaneous activation of FLT-4-related signals occurs partially via the VEGF-C/FLT-4 autocrine system in tumor cells. A: Expression of dn-FLT-4 in established stable transformants. Twenty-four hours after cultivation, cell lysates of established stable clones (dn-SASs, nine clones) were harvested and subjected to immunoblotting using an anti-human FLT-4 polyclonal antibody for the extracellular domain of FLT-4 (upper panel). Subsequently, the blot was stripped and reprobed with an anti-β-actin antibody as a loading control (lower panel). Cell lysates of transiently transfected pCEP-4-dn-flt-4 Cos7 cells (dn-flt-4-Cos7) were used as a positive control. Cell lysates from randomly selected emp-SAS cells (emp-SAS1) were used as a negative control. B: The dominant mRNA expression of dn-FLT-4 compared with that of endogenous FLT-4. Semiquantitative RT-PCR was performed with primer sets designed within the mRNA sequence of the extracellular domain (Extra-FLT-4) or cytoplasmic domain (Intra-FLT-4). The primer sequences and PCR conditions are listed in Table 1. C: Expression of dn-FLT-4 on the cell surface. Flow cytometry analysis was performed as described in Materials and Methods. D: The dn inhibition of endogenous FLT-4 activity by dnFLT-4 in dn-SAS cells. After 24-hour cultivation of emp-SAS1 cells or dn-SAS4 cells in serum-deprived medium, the cells were harvested, and each cell lysate was subjected to immunoprecipitation using anti-human FLT-4 polyclonal antibody for the cytoplasmic domain of FLT-4 and subsequently to immunoblotting using anti-phospho-tyrosine monoclonal antibody (upper panel). Phosphorylated 125-kDa FLT-4 was detected in emp-SAS1 but not dn-SAS4 cells (upper panel, arrow). Next, the blot was stripped and reprobed with anti-human FLT-4 antibody for the extracellular domain of FLT-4 as a loading control (lower panel). Latent FLT-4 and active FLT-4 were detected as 195-kDa and 125-kDa bands, respectively (lower panel, arrows). E: Activation of Akt by VEGF-C in emp-SAS but not in dn-SAS cells. After 24-hour cultivation of emp-SAS1 cells or dn-SAS4 cells in serum-deprived medium, the media were replaced with serum-deprived media, and the cells were stimulated with recombinant VEGF-C (100 ng/ml). After 15 minutes, cell lysate was harvested and subjected to immunoblotting for phosphorylated Akt (p-Akt, upper panel) and subsequently to reprobing for total Akt (lower panel). F: Suppression of FLT-4-related signal transductions in dn-SAS cells. Each harvested cell lysate in D was subjected to immunoblotting using antibody for phospho-p42/44 MAPK (p-p42/44 MAPK), p-Akt, or phospho-p38 MAPK (p-p 38 MAPK). Subsequent reprobing with an antibody for each total protein was performed. G: Blockade of FLT-4 activity in SAS cells suppressed tumor growth in vitro. Cells (1 × 104) of dn-SAS4 or emp-SAS1 cells were disseminated and grown on a culture plate, and trypan blue-negative (viable) cells were counted every other day until day 3. Three independent experiments were performed (∗P < 0.0001, n = 3, each group).
Sustained Blockade of the VEGF-C/FLT-4 Autocrine System in SAS Cells Reduces Tumor Growth Activity in Vivo
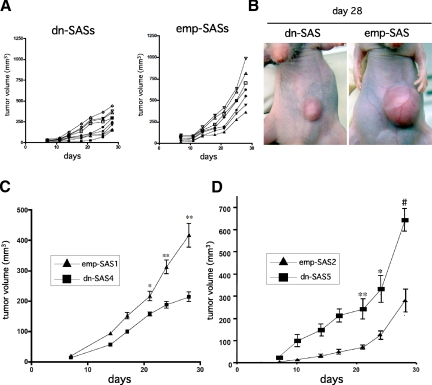
Next, to investigate a role of Flt-4 expressed in tumor cells on the tumor progression in vivo, we established a tumor implantation model using dn-SAS cells. We first examined time-dependent tumor growth with all established stable clones. Tumor growth of the nine clones expressing dn-FLT-4 (dn-SASs) showed a suppressive tendency compared with that of the seven control clones (emp-SASs) (Figure 2A). Representative tumors were indicated in Figure 2B. To statistically clarify the effect of dn-FLT-4 in tumor growth, dn-SAS4 (n = 10) and emp-SAS1 (n = 9) cells were used for a tumor implantation model. As a result, we confirmed a significant reduction of tumor volumes of dn-SAS4 cells compared with those of emp-SAS1 cells (Figure 2C). To eliminate unanticipated cloning artifacts, the similar experiments were performed using clone 5 and clone 7 shown in Figure 1A (dn-SAS5 and dn-SAS7, respectively) and another randomly selected control clone (epm-SAS2), and similar results were obtained (Figure 2D; Supplemental Fig. S2A, see http://ajp.amjpathol.org). These findings demonstrate that Flt-4 expressed in tumor cells promotes not only cell proliferative activity in vitro but also tumor growth in vivo.
Figure 2.
The VEGF-C/FLT-4 autocrine system in tumor cells is involved in promoting tumor growth in vivo. A–D: Blockade of FLT-4 activity in SAS cells suppressed tumor growth in vivo. A: The volumes of dn-SAS cell-derived tumors (nine clones, n = 1, each) and emp-SAS cell-derived tumors (seven clones, n = 1, each) were determined as described in Materials and Methods. B: Pictures of the smallest tumor mass among dn-SASs (left panel) and the largest mass among emp-SASs (right panel) were shown. C: Growth curves of implanted dn-SAS4 cell (n = 10)- and emp-SAS1cell (n = 9)-derived tumors are indicated (day 21, ∗P < 0.005 versus dn-SAS4; days 24 and 28, ∗∗P < 0.001 versus dn-SAS4). D: Growth curves of implanted dn-SAS5 cell (n = 6)- and emp-SAS2 cell (n = 6)-derived tumors are indicated (day 21, ∗P < 0.005 versus dn-SAS5; day 24, ∗∗P < 0.01 versus dn-SAS5; and day 28, #P < 0.001 versus dn-SAS5).
Sustained Blockade of the VEGF-C/FLT-4 Autocrine System in SAS Cells Inhibits Tumor-Associated Lymphangiogenesis in Implanted Tumor Tissue
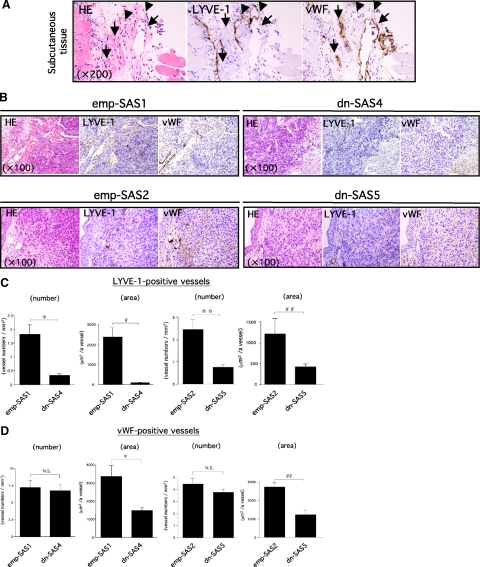
Several recent reports have demonstrated that angiogenic/lymphangiogenic growth factors such as VEGF-A and VEGF-C are positively regulated by p42/44 MAPK and/or PI3K signal transduction in normal and malignant cells.21,22,23,24,25 Thus, we hypothesized that the inactive FLT-4-mediated down-activation of p42/44 MAPK and Akt in dn-SAS4 cells (Figure 1F) was implicated not only in tumor growth but also in angiogenesis/lymphangiogenesis in implanted tumor tissue. We consequently performed an immunohistochemical study for blood and lymphatic vessels using serial sections of implanted tumor tissues. We first confirmed the specificity of antibodies for an immunohistochemical reaction. As shown in Figure 3A, the vWF-positive vessels in the peritumoral s.c. tissue showed no reaction to anti-LYVE-1 antibody. By contrast, LYVE-1-positive vessels showed no reaction to anti-vWF antibody. Therefore, these antibodies are extremely useful for visually separating these vessels. Immunohistochemical studies using these antibodies revealed that the number and area of LYVE-1-positive lymphatic vessels in viable tumor tissue were significantly lower in dn-SAS4 cell-derived tumors (n = 10) than those in emp-SAS1 cell-derived tumors (n = 9) (Figure 3, B and C). In contrast, the number of vWF-positive blood vessels was not significantly different (Figure 3, B and D). In addition, similar results were obtained using dn-SAS5 (Figure 3, B–D) and dn-SAS7 (Supplemental Fig. S2B, see http://ajp.amjpathol.org). These histological findings suggest that the VEGF-C/FLT-4 autocrine system in tumor cells contributes to the promotion of tumor-associated lymphangiogenesis.
Figure 3.
The VEGF-C/FLT-4 autocrine system contributes to tumor-associated lymphangiogenesis. A–D: Decrease in number and area of LYVE-1-positive lymphatic vessels in tumors derived from dn-SAS cells compared with emp-SAS cells. Serial sections of tumor tissues derived from emp-SAS1 cells (B, upper left series) and dn-SAS4 cells (B, upper right series) were subjected to H&E staining and to immunohistochemical staining for mouse LIVE-1 or mouse vWF. The same immunohistochemical staining was performed with serial sections of tumors derived from emp-SAS2 cells (B, lower left series) and dn-SAS5 cells (B, lower right series). Representative photographs were indicated. Specific reactions of anti-LYVE-1 antibody to lymphatic endothelial cells (A, arrowheads) and of anti-vWF antibody to blood endothelial cells (A, arrows) were confirmed in the peritumoral s.c. tissue. The number and area of LYVE-1-positive lymphatic vessels (C) and vWF-positive blood vessels (D) were analyzed as described in Materials and Methods (∗P < 0.001, #P < 0.0001, ∗∗P < 0.005, and ##P < 0.05).
The VEGF-C/FLT-4 Autocrine System Enhances Endogenous VEGF-C and VEGF-A Gene Expressions and Protein Secretions
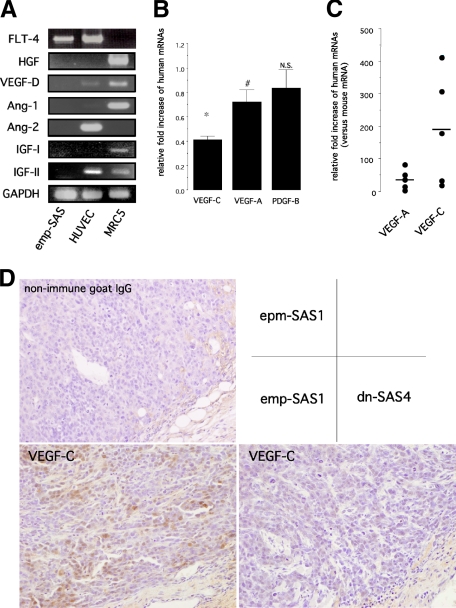
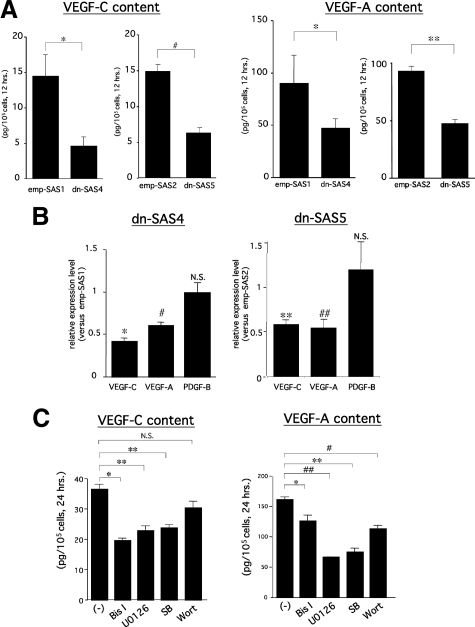
Numerous recent studies have demonstrated that several growth factors, including VEGF-C, possessed direct or indirect prolymphangiogenic activity.5,6,8,24,30,31,32,33,34,35 To clarify the mechanisms underlying the inhibition of tumor-associated lymphangiogenesis observed in dn-SAS cell-derived tumor tissues, we examined the expression levels of prolymphangiogenic factors reported previously. In our preliminary screening of growth factors expressed in cultured wild-type SAS cells, angiopoietin-1, angiopoietin-2, EGF, hepatocyte growth factor (HGF), insulin-like growth factor-I, insulin-like growth factor (IGF)-II, and fibroblast growth factor (FGF)-2 were not detectable by RT-PCR (Supplemental Table S1, see http://ajp.amjpathol.org). These undetectable factors under culture conditions were also undetectable in emp-SAS1 cell-derived tumor tissues (Figure 4A). In contrast, real-time RT-PCR revealed that levels of human VEGF-C and human VEGF-A mRNAs, but not human PDGF-B mRNA, were significantly reduced in dn-SAS4 cell-derived tumor tissues compared with those in emp-SAS1 cell-derived tumor tissues (Figure 4B). The reduction rate of VEGF-A was significant but small (approximately 25% reduction); by contrast, the reduction rate of VEGF-C was significant and large (approximately 60% reduction). Consistent with these results, the reduction of VEGF-C protein expressions in tumor cells was immunohistochemically apparent in dn-SAS4 cell-derived tumor tissues (Figure 4D). We further examined expression levels of SAS cell-derived human VEGF-C and VEGF-A mRNAs and endogenous mouse VEGF-C and VEGF-A mRNAs in tumor tissues. As shown in Figure 4C, each human mRNA level was dramatically higher than corresponding mouse mRNAs in emp-SAS1 cell-derived tumor tissues, suggesting that the host VEGF-C and -A hardly affect tumor angiogenesis/lymphangiogenesis in our animal model. Therefore, SAS cell-derived VEGF-A and -C, particularly VEGF-C, are potent factors to induce lymphangiogenesis in SAS cell-derived tumor tissue, and FLT-4 activity in SAS cells mainly contributes to induction of VEGF-C expression. To gain more evidence for the relationship between FLT-4 activity and expression levels of VEGF-C and VEGF-A in SAS cells, in vitro examinations were performed. An ELISA demonstrated that secretions of VEGF-C and VEGF-A proteins were significantly inhibited in dn-SAS4 cells compared with those in emp-SAS1 cells (Figure 5A), and PDGF-BB secretion was undetectable (data not shown). In addition, real-time RT-PCR revealed that levels of VEGF-C and VEGF-A mRNAs, but not PDGF-B mRNA, were significantly reduced in dn-SAS4 cells in vitro (Figure 5B). Similar results were obtained using dn-SAS5 cells (Figure 5, A and B) and dn-SAS7 cells (Supplemental Fig. S2C, see http://ajp.amjpathol.org). Next, to clarify the relationship between the down-regulation of VEGF-A and VEGF-C and the down-activation of FLT-4-associated signals in dn-SAS4 cells, we examined the crucial intracellular signals regulating the spontaneous expression of VEGF-A and VEGF-C in wild-type SAS cells. As a result, treatment with an MEK inhibitor (U0126), PKC inhibitor (Bis I), or p38 MAPK inhibitor (SB), but not a PI3K inhibitor (wortmannin), significantly inhibited both spontaneous VEGF-C protein secretions (Figure 5C) and the mRNA expressions (Supplemental Fig. S3, see http://ajp.amjpathol.org). In contrast, spontaneous VEGF-A secretion and the mRNA expression was significantly inhibited under treatment with each of the four inhibitors (Figure 5C; Supplemental Fig. S3, see http://ajp.amjpathol.org). Interestingly, the expression levels of both factors did not change in SAS cells treated with an Akt inhibitor (Supplemental Fig. S3, see http://ajp.amjpathol.org), suggesting that PI3K-dependent expression of VEGF-A is independent on the Akt signal, one of downstream signals of PI3K. These findings demonstrate that the FLT-4-associated signals PKC, p42/44 MAPK, and p38 MAPK are involved in both VEGF-A and VEGF-C expressions, that PI3K is involved in VEGF-A expression, and that PI3K-Akt is not involved in VEGF-C expression in SAS cells.
Figure 4.
Blockade of VEGF-C/FLT-4 autocrine system in tumor cells reduces expressions of VEGF-A and VEGF-C, especially VEGF-C, in vivo. A–C: Human VEGF-C, a key growth factor for lymphangiogenesis in implanted tumor tissue. Harvested dn-SAS4 cell- and emp-SAS cell-derived tumor tissues on day 28 were subjected to RT-PCR (A) or real-time RT-PCR (B and C) for the target genes indicated in the figures using the human-specific primer sets listed in Table 1. Human umbilical vein endothelial cells and MRC5 were used as positive control in A. In B, mRNA levels of human VEGF-A, human VEGF-C, and human PDGF-B in dn-SAS4 cell-derived tumor tissues were expressed as relative fold increase compared with those in emp-SAS1 cell-derived tumor tissues (∗P < 0.0001 and #P < 0.05; n = 5, each group). In C, the numbers of mRNA templates of human VEGF-A, mouse VEGF-A, human VEGF-C, and mouse VEGF-C were determined as described in Materials and Methods. The obtained data were expressed as relative levels of each human mRNA compared with the corresponding mouse mRNA. Horizontal bars show the mean score of relative expression level of each factor. D: Immunohistochemical study for human VEGF-C. Sections of implanted tumor tissues indicated in the figure were subjected to immunohistochemical staining for human VEGF-C. Nonimmune goat IgG was used as negative control. The pictures are representative results.
Figure 5.
Expression of VEGF-A and VEGF-C in tumor cells is reduced by blockade of VEGF-C/FLT-4 autocrine system or by inhibition of FLT-4-related signals in vitro. A and B: Decreased VEGF-A and VEGF-C expressions by blockade of VEGF-C/FLT-4 autocrine system in SAS cells in vitro. After 12-hour cultivation of dn-SAS4 cells and emp-SAS1 cells, the harvested media were subjected to ELISA (A), and the cells were then subjected to real-time RT-PCR (B) as described in Materials and Methods. The same experiments were performed using dn-SAS5 cells and emp-SAS2 cells (A and B). VEGF-C or VEGF-A content in culture medium was expressed as the amount of protein secreted from 105 cells for 12 hours (A, ∗P < 0.05, #P < 0.005, and ∗∗P < 0.001; n = 3, each group). mRNAs of VEGF-A, VEGF-C, and PDGF-B in dn-SAS4 cells or dn-SAS5 cells were expressed as relative fold increase compared with those in emp-SAS1 cells or emp-SAS2 cells, respectively (B, ∗P < 0.0005, #P < 0.0001, ∗∗P < 0.05, and ##P < 0.001; n = 3, each group). C: The effect of various intracellular signaling inhibitors on spontaneous expression of VEGF-A or VEGF-C in wild-type SAS cells. After 24-hour cultivation of wild-type SAS cells in serum-deprived medium containing each inhibitor indicated in the figure, the harvested media were subjected to ELISA. The working concentrations of inhibitors were as follows: 10 μmol/L U0126, 100 nmol/L wortmannin (Wort), 10 μmol/L bisindolylmaleimide I (Bis I), and 10 μmol/L SB203580 (SB). VEGF-C content (left panel) and VEGF-A content (right panel) in culture medium were expressed as the amount of protein secreted from 105 cells for 24 hours (∗P < 0.05, ∗∗P < 0.01, #P < 0.001, and ##P < 0.0005; n = 6, each group). hrs., hours.
FLT-4 Activity Positively Regulates Gene Expression and Protein Secretion of VEGF-A and VEGF-C in Oral Cancer Cells
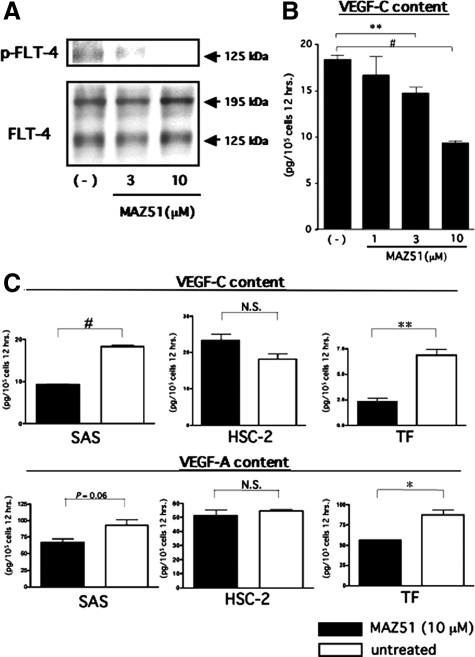
Next, for further validation of the FLT-4-dependent function as a positive regulator for spontaneous expressions of VEGF-C in SAS cells, we examined the dose-dependent effect of an inhibitor of FLT-4, MAZ51, on the expression level of VEGF-C in wild-type SAS cells. Consistent with the previous reports,36,37 the spontaneous phosphorylation level of FLT-4 in wild-type SAS cells was decreased by treatment with MAZ51 in a dose-dependent manner (Figure 6A), and the ELISA revealed a dose-dependent decrease of the VEGF-C content in culture media of wild-type SAS cells treated with MAZ51 (Figure 6B). We further examined the effect of MAZ51 treatment (10 μmol/L) on secretions of VEGF-C and VEGF-A using the oral squamoid cancer cell lines TF and HSC-2. Flt-4 is spontaneously expressed in TF but not in HSC-2 cells (Supplemental Table S1, see http://ajp.amjpathol.org). As a result, MAZ51 treatment induced significant reductions of both VEGF-A and VEGF-C secretions in TF but not in HSC-2 cells (Figure 6C). These findings suggest that FLT-4 activity in tumor cells plays an important role in the positive regulation of endogenous VEGF-C and VEGF-A. Regarding interpretation of the findings indicated in Figure 6, the specificity of MAZ51 for FLT-4 is important. According to the previous reports, 10 μmol/L MAZ51 almost completely inhibited FLT-4-tyrosin phosphorylation without affecting other tyrosine kinase receptors, at least IGF-IR, PDGFR, and EGFR.37 Although Flk-1/KDR has been reported to be weakly affected at this concentration,36 the oral squamoid cancer cell lines used in these experiments show no expression of Flk-1/KDR (Supplemental Table S1, see http://ajp.amjpathol.org). Additionally, HSC-2 cells without FLT-4 expression was used for excluding nonspecific effects of MAZ51. Therefore, the findings in Figures 5 and 6 suggest that autocrine activation of FLT-4 by VEGF-C in tumor cells positively regulates expressions of VEGF-C and VEGF-A via FLT-4-associated signal transductions, resulting in the formation of the VEGF-C/FLT-4 autocrine loop, which contributed to sustained high-level secretion of VEGF-C.
Figure 6.
Blockade of FLT-4 activity by MAZ51 reduces expression of VEGF-A and VEGF-C on oral squamoid cancer cells in vitro. A: MAZ51 inactivates FLT-4 in SAS cells. After 12-hour cultivation of wild-type SAS cells in serum-deprived medium with or without MAZ51 at the different concentrations indicated in the figure, each cell lysate was harvested and subjected to the experiments according to those described in Figure 1D. B and C: MAZ51 inhibits VEGF-A and VEGF-C secretions in oral squamoid cancer cells. After 12-hour cultivation of wild-type SAS cells in serum-deprived medium with or without MAZ51 at the different concentrations indicated in B, the harvested media were subjected to ELISA for VEGF-C (B). After 12-hour cultivation of wild-type SAS, TF, and HSC-2 cells in serum-deprived medium with or without MAZ51 (10 μmol/L), the harvested media were subjected to ELISA. VEGF-C content (B and C, upper graphs) and VEGF-A content (C, lower graphs) in culture medium were expressed as amounts of protein secreted from 105 cells for 12 hours (∗P < 0.01, ∗∗P < 0.005, and #P < 0.0001; n = 3, each group). hrs., hours.
Discussion
Using stable overexpression of dn-FLT-4, which exerts the dn inhibitory effect only for endogenous FLT-4 activity in tumor cells in vivo, we herein found direct evidence suggesting a role of tumor cell-derived FLT-4 in tumor progression. The body of our findings is that FLT-4 plays a precipitative role not only in tumor growth but also in tumor-associated neovascularization, especially in lymphangiogenesis via VEGF-C/FLT-4 autocrine system-mediated enhancement of secretion of VEGF-C (VEGF-C/FLT-4 autocrine loop).
Numerous previous reports have suggested that cytokine receptors expressed in tumor cells play an important role in tumor progression via autocrine/paracrine interaction with corresponding ligands. Through a screening of several angiogenesis/lymphangiogenesis-related factors in a variety of carcinoma cell lines, they were found frequently to possess autocrine systems of EGF/EGFR, PDGF-AA/PDGFR-α, and VEGF-C/FLT-4 (Supplemental Table S1, see http://ajp.amjpathol.org). Among these systems, active involvement of the EGF/EGFR autocrine/paracrine system in tumorigenesis has been clarified by numerous previous studies suggesting that the system promotes tumor growth, survival and invasive/metastatic activity, including promotion of tumor-associated angiogenesis.38 The role of the PDGF-AA/PDGFR-α autocrine system was also investigated in our previous study, which demonstrated that PDGF-AA expression played a precipitative role in tumor-associated angiogenesis via induction of VEGF-A by the PDGF-AA/PDGFR-α autocrine effect in non-small cell lung carcinoma, resulting in the promotion of tumor progression.29 There are some recent studies regarding the role of the VEGF-C/FLT-4 autocrine system in tumorigenesis.17,18,19 According to the in vitro studies, autocrine/paracrine activation of FLT-4 in tumor cells is involved in promoting tumor growth and cell viability.17,18 In addition, detailed examinations, including in vivo experiments, suggested that the VEGF-C/FLT-4 axis in lung adenocarcinoma cell lines was involved in promoting invasive/metastatic activity.19 Moreover, in recent clinicopathological studies, FLT-4 expression was confirmed in several malignant tumor cells,13,14,15,16,17,18,19 including oral squamous cell carcinoma, and some studies among them suggested that correlation between FLT-4 in tumor cells and the occurrence of regional lymph node metastasis or poor prognosis.13,15,16,19 On the basis of these backgrounds, our study herein demonstrated novel mechanisms underlying the active involvement of tumor cell-derived FLT-4 in tumor progression.
It is clear that the biological functions of FLT-4 in tumor cells are induced via FLT-4-associated signals. Regarding the signals, PKC-dependent p42/44 MAPK pathway(s) and PI3K-Akt pathway(s) were reported to be major signals in lymphatic endothelial cells.20 In addition, one study suggested that p38 MAPK was a critical downstream signal of FLT-4 in cancer cells.19 Here we demonstrated that spontaneous activation of these signals occurred partly via the VEGF-C/FLT-4 autocrine loop and that these signals were involved in positive regulation of VEGF-C and/or VEGF-A genes in the oral squamous carcinoma cell line SAS. In contrast, our hitherto existing evidences suggested that up-regulation of mRNAs of VEGF-A, VEGF-C, and HGF in response to FGF-2 depended strongly on Ras-independent p42/44 MAPK and/or on p38 MAPK in fibroblasts and vascular smooth muscle cells (21,22 and data not shown). These findings suggest that p42/44 and p38 MAPK pathway(s) are critical for the induction of VEGF-A or VEGF-C beyond cell types, and activation of these signal transductions via phenotypic transformation-dependent expression of growth factor receptors, including FLT-4, plays a role not only in the increased growth and invasive potentials of cancer cells but also in alteration of the tumor microenvironment such as tumor-associated neovascularization, thereby contributing to tumor progression.
We also demonstrate here that the number of vWF-positive blood vessels showed no significant change in implanted tumor tissue derived from dn-SAS cells compared with emp-SAS cells, although significantly reduced protein secretion and down-regulated gene expression of VEGF-A were confirmed in dn-SAS cells in vitro. This result was probably as a result of the difference between the in vivo and in vitro conditions. Briefly, SAS cells do not express HGF but abundantly express the cognate receptor c-MET (Supplemental Table S1, see http://ajp.amjpathol.org). The c-MET signal is known to be a positive regulator for the VEGF-A gene in a variety of cell types, including malignancy.39,40 In contrast, regarding the relationship between c-MET and VEGF-C, few studies have been reported. Consistent with the present situation, HGF could not stimulate the VEGF-C gene in SAS cells in our study (data not shown). Therefore, in vivo conditions may enhance VEGF-A but not VEGF-C secretion in SAS cells via the host HGF/tumor c-MET paracrine system. This hypothesis was supported by the examination of the in vivo expression level of human VEGF-A and VEGF-C (Figure 4B). c-MET has been reported to be linked with Ras-dependent/PKC-independent p42/44 MAPK and with PI3K-Akt signals in a variety of cell types.40,41 On the contrary, unlike c-MET-mediated up-regulation of VEGF-A, c-MET-mediated signaling pathways rarely lead to stimulation of the VEGF-C gene. It remains unclear why c-MET-dependent p42/44 MAPK activation cannot lead to VEGF-C up-regulation. PKC, however, may be a key intracellular signaling molecule related to VEGF-C gene expression. For example, a previous interesting report demonstrated that overexpression of Ras induced up-regulation of VEGF-A but not of VEGF-C in fibroblasts.23 In addition, our previous studies (21,22 and unpublished data) demonstrated that the FGF-2-dependent VEGF-C up-regulation was not affected by treatment with a Ras inhibitor, although up-regulation of VEGF-C in response to FGF-2 strongly depended on p42/44 MAPK. In our studies herein, the inhibition of PKC activity significantly reduced spontaneous VEGF-C expression in wild-type SAS cells; therefore, PKC activation may be essential for stimulation of the VEGF-C gene, and the finding that VEGF-A but not VEGF-C gene expression in dn-SAS4 cells dissociated between in vitro and in vivo in our study may be due to the different regulatory systems between VEGF-C and VEGF-A genes. Moreover, considering that the VEGF-C can activate the PKC-p42/44 MAPK pathway,20 PKC seems to be the main intracellular molecule to form the VEGF-C/Flt-4 autocrine loop in tumor cells.
We observed, herein, an interesting phenomenon regarding tumor angiogenesis. Unlike the blood vessel density, the area of vWF-positive blood vessels was significantly smaller in implanted tumor tissue derived from dn-SAS cells compared with emp-SAS cells. The underlying mechanism, however, remains unknown. Guessingly, the following may be involved in the interesting phenomenon: 1) the loss of blood vessel integrity-dependent reduction of blood flow may be involved, based on the tumor VEGF-C/blood endothelial Flt-4 paracrine system.42,43 2) Congestive degree in tumor may be involved, based on the tumor volume-dependent microcirculatory disturbance in the surrounding normal tissue.
Considering the purpose of this study, it is really useful to know about not only focal tumor conditions such as tumor microenvironment but also systemic conditions, especially lymphogenous metastatic status. We herein demonstrated only the former results, because the adopted cell line SAS has no potential to cause regional lymph node metastasis, at least in the setting of our present tumor implantation model. Moreover, although possible screening for almost all lymphangiogenesis-related factors were performed in the present study, we were not able to demonstrate strict and direct evidence that suppression of lymphangiogenesis by a blockade of FLT-4 was dependent on VEGF-C down-regulation in SAS cells. Therefore, further investigations with other adequate cell lines and/or animal models might be needed for scientific strengthening of our conclusion in the future.
Taking this study’s findings together with those previously reported, the VEGF-C/FLT-4 autocrine loop of tumor cells is a novel mechanism not only for promoting tumor growth but also for tumor-associated neovascularization, especially lymphangiogenesis, and likely contributes to advanced tumor progression in a wide variety of malignant tumors, including oral squamous cell carcinoma; thus, this system may become an effective target in therapy for malignant neoplasms.
Supplementary Material
Acknowledgments
We thank Hiroshi Fujii for his help with sectioning the tissue samples and Miss Chie Arimatsu for her help with animal experiments.
Footnotes
Address reprint requests to Mitsuho Onimaru, D.D.S., Ph.D., Division of Pathophysiological and Experimental Pathology, Department of Pathology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan. E-mail: onimaru@pathol1.med.kyushu-u.ac.jp.
Supported by a grant for the Promotion of Basic Scientific Research in Medical Frontiers by the Organization for Pharmaceutical Safety and Research and by a grant-in-aid (to K.S. and Y.Y.) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
M.M. and M.O. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Kazama S, Nagawa H. The expression pattern of vascular endothelial growth factor C and D in human esophageal normal mucosa, dysplasia and neoplasia. Hepatogastroenterology. 2004;51:1319–1322. [PubMed] [Google Scholar]
- O-charoenrat P, Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer. 2001;92:556–568. doi: 10.1002/1097-0142(20010801)92:3<556::aid-cncr1355>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Inoue Y, Matsuki R, Ishii K, Takahashi M, Abe M, Shirasuna K. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Int J Oncol. 2009;34:673–680. doi: 10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- Liao M, Wang H, Lin Z, Feng J, Zhu D. Vascular endothelial growth factor and other biological predictors related to the postoperative survival rate on non-small cell lung cancer. Lung Cancer. 2001;33:125–132. doi: 10.1016/s0169-5002(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Jennbacken K, Vallbo C, Wang W, Damber JE. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005;65:110–116. doi: 10.1002/pros.20276. [DOI] [PubMed] [Google Scholar]
- Lalla RV, Boisoneau DS, Spiro JD, Kreutzer DL. Expression of vascular endothelial growth factor receptors on tumor cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:882–888. doi: 10.1001/archotol.129.8.882. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H, Sato S, Mizunuma H, Smith SK. Expression of vascular endothelial growth factor (VEGF)-D and its receptor. VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9:1361–1369. [PubMed] [Google Scholar]
- Warburton G, Nikitakis NG, Roberson P, Marinos NJ, Wu T, Sauk JJ, Jr, Ord RA, Wahl SM. Histopathological and lymphangiogenic parameters in relation to lymph node metastasis in early stage oral squamous cell carcinoma. J Oral Maxillofac Surg. 2007;65:475–484. doi: 10.1016/j.joms.2005.12.074. [DOI] [PubMed] [Google Scholar]
- Masood R, Kundra A, Zhu S, Xia G, Scalia P, Smith DL, Gill PS. Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops. Int J Cancer. 2003;104:603–610. doi: 10.1002/ijc.10996. [DOI] [PubMed] [Google Scholar]
- Dias S, Choy M, Alitalo K, Rafii S. Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood. 2002;99:2179–2184. doi: 10.1182/blood.v99.6.2179. [DOI] [PubMed] [Google Scholar]
- Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, Hung MC, Kuo ML. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru M, Yonemitsu Y, Tanii M, Nakagawa K, Masaki I, Okano S, Ishibashi H, Shirasuna K, Hasegawa M, Sueishi K. Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated down-regulation in ischemic limbs. Circ Res. 2002;91:923–930. doi: 10.1161/01.res.0000043281.66969.32. [DOI] [PubMed] [Google Scholar]
- Tsutsumi N, Yonemitsu Y, Shikada Y, Onimaru M, Tanii M, Okano S, Kaneko K, Hasegawa M, Hashizume M, Maehara Y, Sueishi K. Essential role of PDGFRα-p70S6K signaling in mesenchymal cells during therapeutic and tumor angiogenesis in vivo: role of PDGFRα during angiogenesis. Circ Res. 2004;94:1186–1194. doi: 10.1161/01.RES.0000126925.66005.39. [DOI] [PubMed] [Google Scholar]
- Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K. Comparison of VEGF: VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhang D, Fallavollita L, Brodt P. Vascular endothelial growth factor C expression and lymph node metastasis are regulated by the type I insulin-like growth factor receptor. Cancer Res. 2003;63:1166–1171. [PubMed] [Google Scholar]
- Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, Takehara T, Inoue M, Hasegawa M, Kuwano H, Sueishi K. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol. 2006;26:2483–2489. doi: 10.1161/01.ATV.0000244684.23499.bf. [DOI] [PubMed] [Google Scholar]
- Nakano T, Nakashima Y, Yonemitsu Y, Sumiyoshi S, Chen YX, Akishima Y, Ishii T, Iida M, Sueishi K. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum Pathol. 2005;36:330–340. doi: 10.1016/j.humpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lazarov M, Kubo Y, Cai T, Dajee M, Tarutani M, Lin Q, Fang M, Tao S, Green CL, Khavari PA. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat Med. 2002;8:1105–1114. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, Sata S, Nakagawa K, Yoshino I, Maehara Y, Sueishi K. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65:7241–7248. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Kirkin V, Mazitschek R, Krishnan J, Steffen A, Waltenberger J, Pepper MS, Giannis A, Sleeman JP. Characterization of indolinones which preferentially inhibit VEGF-C- and VEGF-D-induced activation of VEGFR-3 rather than VEGFR-2. Eur J Biochem. 2001;268:5530–5540. doi: 10.1046/j.1432-1033.2001.02476.x. [DOI] [PubMed] [Google Scholar]
- Kirkin V, Thiele W, Baumann P, Mazitschek R, Rohde K, Fellbrich G, Weich H, Waltenberger J, Giannis A, Sleeman JP. MAZ51, an indolinone that inhibits endothelial cell and tumor cell growth in vitro, suppresses tumor growth in vivo. Int J Cancer. 2004;112:986–993. doi: 10.1002/ijc.20509. [DOI] [PubMed] [Google Scholar]
- De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung L, Chen Y, Huang X, Chan HM, Zhao P, Luk J, Vande Woude G, Wong J. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11:6190–6197. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- Tulasne D, Paumelle R, Leroy C, Reveneau S, Vandenbunder B, Fafeur V. Involvement of Ras-ERK signaling in multiple biological responses to HGF/SF. Ann NY Acad Sci. 2002;973:105–108. doi: 10.1111/j.1749-6632.2002.tb04615.x. [DOI] [PubMed] [Google Scholar]
- Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97:368–377. doi: 10.1038/sj.bjc.6603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 2000;96:546–553. [PubMed] [Google Scholar]
- Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, Nakagawa K, Hou X, Nagai Y, Hasegawa M, Sugimachi K, Sueishi K. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90:966–973. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.