Abstract
Decay-accelerating factor (DAF; CD55) is a membrane protein that regulates complement pathway activity at the level of C3. To test the hypothesis that DAF plays an essential role in limiting complement activation in the arterial wall and protecting from atherosclerosis, we crossed DAF gene targeted mice (daf-1−/−) with low-density lipoprotein-receptor deficient mice (Ldlr−/−). Daf-1−/−Ldlr−/− mice had more extensive en face Sudan IV staining of the thoracoabdominal aorta than Ldlr−/− mice, both following a 12-week period of low-fat diet or a high-fat diet. Aortic root lesions in daf-1−/−Ldlr−/− mice on a low-fat diet showed increased size and complexity. DAF deficiency increased deposition of C3d and C5b-9, indicating the importance of DAF for downstream complement regulation in the arterial wall. The acceleration of lesion development in the absence of DAF provides confirmation of the proinflammatory and proatherosclerotic potential of complement activation in the Ldlr−/− mouse model. Because upstream complement activation is potentially protective, this study underlines the importance of DAF in shielding the arterial wall from the atherogenic effects of complement.
Complement, a complex cascade of serine proteases, is well characterized as playing a pivotal role in inflammation and in bridging innate and adaptive immunity.1 Currently, complement is understood to be triggered by three proximal cascades, the classical, alternative, and mannose-binding lectin pathways, which converge on C3 at the central hub of the system. Cleavage of C3 leads to the generation of down-stream proinflammatory mediators, including the anaphylatoxins C3a and C5a and the membrane attack-complex C5b-9. Although the assembly and insertion of C5b-9 into cell membranes may lyse non-nucleated cells, sublytic levels can activate proliferation and/or proinflammatory gene expression.2
There is increasing interest in dissecting the possible roles of complement in atherosclerosis in vivo.3,4 Theoretically, many factors might activate complement in the arterial wall, including Igs, cholesterol crystals, enzymatically-modified low-density lipoprotein (LDL) and apoptotic cells.5,6,7,8,9,10,11,12 However, enzymatically-modified LDL is likely to be the most abundant stimulus for complement activation in atherosclerosis, and may act via the alternative pathway and also via direct binding of C1q and C-reactive protein.8,9,13,14,15 In addition, the classical and alternative pathways are capable of low grade “tick-over” activity.16,17
Previous experimental work has focused on the effects of natural or experimental deficiency of individual complement pathway components. Relevant studies are as follows: (1) rabbits with natural deficiency of C6 have been shown to be protected from diet-induced atherosclerosis18,19; (2) although C5 deficiency has been found to have no effect on lesion development in high fat diet-fed ApoE−/− mice,20 a recent study has shown protective effects of an anti-C5 antibody in ApoE−/− mice deficient in both Cd59a and Cd59b genes21; (3) C3 deficient mice crossed with Ldlr−/− single knock-out mice have been found to have increased aortic lipid deposition with impaired lesion development beyond the foam cell stage22; (4) crossing Factor B deficient mice with ApoE−/−Ldlr−/− double-knock-outs had no effect, arguing against an important role for the alternative pathway in that model23; and (5) more recently, we have reported that low fat diet-fed Ldlr−/− mice deficient in classical pathway activity through gene-targeting of C1q (C1qa−/−) show accelerated atherosclerosis with increased lesion complexity.24 The increased lesion size and complexity in low fat diet-fed C1qa−/−Ldlr−/− mice was associated with an increase in lesional apoptotic cells, consistent with previous studies that have demonstrated a direct role for C1q in apoptotic cell clearance, independent of terminal pathway activation.25,26 Recently, the role of the lectin pathway has also been shown to have atheroprotective functions in mice,27 in line with the involvement of mannose-binding lectin in apoptotic cell clearance and also with the association of mannose-binding lectin deficiency with accelerated atherosclerosis in humans.28,29
Complement activity is tightly regulated by a number of fluid-phase and membrane-bound inhibitors, including the two glycosylphosphatidylinositol-anchored membrane proteins decay-accelerating factor (DAF, CD55) and protectin (CD59). Although CD59 inhibits insertion of C9 into cell membranes and thus the development of C5b-9 membrane attack complexes, DAF binds to C3, thereby accelerating the decay of the two C3 convertases, C3Bb (alternative pathway) and C4b2a (classical and mannose-binding lectin pathways).30,31,32 Structurally, DAF is a multidomain protein comprising a proximal serine/threonine-rich region and four complement control protein (CCP) domains, of which CCP2 and CCP3 dissociate C3Bb and C4b2a oligomers into constituent proteins. The catalytic mechanism of DAF activity is not fully clear, but the crystal structure and substitution mutants identify Bb (Tyr338), DAF-CCP2 (Arg69, Arg96) and DAF-CCP3 (Phe148 Leu171) as key residues.33,34
The mouse has two DAF genes encoding glycosylphosphatidylinositol-anchored and trans-membrane forms, respectively, with the former being more representative of human DAF.35 Gene-targeting of the glycosylphosphatidylinositol-anchored form has led to the generation of a knock-out strain that is healthy but shows exaggerated inflammation in models of renal, autoimmune, and nervous system diseases.36,37,38,39 Recently, no protection or exacerbation of atherosclerosis was observed after crossing these mice with the ApoE−/− strain.40
Observations that the classical pathway exerts atheroprotective effects without terminal pathway activation suggest the importance of a strong complement regulatory system in the arterial wall.14,24 Consistent with this, we and others have recently published evidence that CD59 deficiency leads to an acceleration of atherosclerosis in Ldlr−/− and ApoE−/− mouse models, establishing the proatherogenic potential of the terminal complement pathway and highlighting the importance of CD59 in its regulation.21,40,41 In this article, we show that DAF also plays a role in the regulation of atherosclerosis in the Ldlr−/− model.
Materials and Methods
Reagents
Oil Red O (ceristain grade), dextrin, gelatin, Mayer’s Hematoxylin, L-glutamic acid, glycerol, sodium azide, calcium chloride, magnesium sulfate, and sodium phosphate were obtained from Merck Biosciences/BDH (Poole, UK). Buffered formal saline (4% w/w formaldehyde solution) was from Pioneer Research Chemicals (Colchester, Essex, UK). OCT compound was from CellPath (Newtown, Powys, UK). Other reagents were from Sigma-Aldrich (Poole, UK).
Antibodies
Primary antibodies included rat anti-mouse macrophages/monocyte MOMA-2 (Serotec, Oxford, UK), alkaline phosphatase-conjugated mouse anti-α actin (clone α1A4) (Sigma), rat monoclonal anti-mouse DAF (clone MD1, a kind gift from B.P. Morgan, Cardiff, UK), goat anti-mouse IgM (Abcam, Cambridge, UK), and rabbit anti-C5b-9 (Category no. 204903, Calbiochem, Merck Biosciences, Darmstadt, Germany). Secondary antibodies were biotinylated goat anti-rabbit IgG (DakoCytomation, Cambridgeshire, UK), biotinylated goat anti-mouse IgG (Dako, Ely, UK). Control antibodies were fluorescein-conjugated goat IgG (Santa Cruz Biotechnology, Inc., Heidelberg, Germany), biotinylated goat IgG (R&D Systems, Abingdon, UK), and anti-nuclear factor κB p65, biotin-conjugated anti-green fluorescent protein, rabbit anti-vitronectin, and polyclonal rabbit anti-clusterin (all from Santa Cruz Biotechnology).
Mice
DAF gene-targeted mice (daf-1−/−) were generated as described.36 Ldlr−/− mice were obtained from Jackson Laboratories (Bar Harbor, MA). Both daf-1−/− and Ldlr−/− mice were back-crossed for 10 generations on to the C57BL/6 background before intercrossing to form daf-1−/−Ldlr−/− double knockout mice. DAF genotypes were determined by PCR. C3 deficient Ldlr−/− (C3−/−Ldlr−/−) mice were made by crossing Ldlr−/− mice with a C3 gene-targeted strain.42 All mice in the study were female. Animals were housed in a specific pathogen-free environment and studied according to UK Home Office regulations. The experimental groups were gradually transferred onto low-fat or high-fat diets at 10 weeks of age, as described.41 All mice were studied at 22 weeks of age.
Lipoprotein, Cholesterol, and Triglyceride Analysis
Blood was withdrawn from the inferior vena cava after sacrifice and allowed to clot on ice. Serum was kept at 4°C for up to 24 hours before analysis. Lipoprotein profiles were analyzed on pooled sera by size-exclusion chromatography by using a SMART micro-FPLC system (Pharmacia, Stockholm, Sweden). Serum total cholesterol and triglycerides were measured enzymatically on each individual mouse by using Kit Infinity TR13421/2350-250 and Kit Infinity TR22421/2780-250, respectively (MediMark, Grenoble, France) according to the manufacturer’s instructions.
Quantification and Immunohistochemistry of Atherosclerotic Lesions
Mice were sacrificed by exsanguination under carbon dioxide. En face staining of thoracoabdominal aorta and quantification of the aortic root lesion area were conducted as described.24,41 Immunohistochemistry was performed by standard procedures on residual sections not required for analysis of lesion size, as described.24,41 Results of immunocytochemistry are presented as a percentage area fraction of the aortic root or as the percentage of lesional cells, as analyzed by Image ProPlus software above.
Confocal Microscopy Imaging
Cryosections were incubated with combinations of markers including the following: Cy3-labeled anti-α actin (clone α1A4) (Sigma); AlexaFluor 488-conjugated CD31 (Clone MEC13.3; BioLegend, Inc., San Diego, CA); AlexaFluor 568-labeled anti-CD68 (MCA1957A488, Serotec); fluorescein-conjugated goat IgG fraction to mouse complement C3 (Category no. 555000, MP Biomedicals, Solon, OH); biotinylated anti-mouse complement C3d (R&D Systems); fluorescein-streptavidin (Vector Laboratories, Peterborough, UK); and rat anti-mouse DAF (clone MD1) followed by biotinylated polyclonal rabbit anti-rat secondary and development with AlexaFluor 488- or AlexaFluor 568-conjugated streptavidin (Invitrogen, Paisley, UK). Nuclei in fluorescence sections were then counterstained with TOPRO-3 before mounting in 80% glycerol 20% PBS. Sections were examined by confocal or by standard fluorescence microscopy (for enumerating the percent of actin-positive cells and fibrous caps). The confocal used was a Zeiss LSM510 Meta (Zeiss, Welwyn Garden City, UK) using a standard trichannel set up using the Ar 488 nm line, the HeNe 543 nm line, and the HeNe 633 nm line, a 1 Airy Unit pinhole (adjusted for light wavelength) and three photomultipliers fed via green (505 to 530 nm bp), orange-red (560 to 615 nm bp), and far red (LP638 nm) filters. As advised by Zeiss, photomultiplier voltages and amplifier offset were adjusted online using fast scan to maximize image clarity without saturation, and gain was left at the manufacturer’s default. Photomultiplier voltages were typically green emission 600 V, orange-red emission 400 V, and far red emission 200 V.
Collagen Content in Atherosclerotic Lesions
Aortic root sections were stained with 1% Sirius red in saturated picric acid (Picrosirius Red) for 1 hour, as described. We adopted as closely as possible the methods of the laboratories of Libby and Hansson.43 Polarising microscopy was used to view these sections at ×4 magnification (Olympus BX50). Images were then captured with Olympus DP50 (Olympus, Southend-on-Sea, UK) digital microscopy camera. Thin collagen fibers (green) and thick collagen fibers (orange/red) were measured by using the National Institutes of Health open access generic image analysis software Image J (http://rsbweb.nih.gov/ij24). Images were first converted into three greyscale images (red, green, and blue). For red and green channels, lesions in each aortic root section were carefully drawn. After intensity thresholding each image, we measured the area fraction of threshold collagen (the relative amount of collagen area to selected lesion area) within each aortic root lesion, and expressed this as a percentage.
Enzyme-Linked Immunosorbent Assay for Detection of Mouse C3
Enzyme-linked immunosorbent assay plates were coated overnight with goat anti-mouse C3 (2 mg/ml; MP Biomedicals), which also reacts with C3b, or with goat anti-mouse C3d (2 mg/ml; R&D Systems). After blocking, the plates were incubated with wild-type, factor I-deficient or trypsin-treated wild-type sera as a source of intact C3, C3b, and C3d, respectively. Bound C3 was detected by adding biotinylated goat anti-mouse C3d (50 ng/ml) or biotinylated goat anti-mouse C3 (50 ng/ml). Plates were incubated with streptavidin-AP conjugated and the reaction visualized by subsequent addition of p-nitrophenyl phosphate substrate (Sigma Fast, Sigma-Aldrich Co., Gillingham, UK).
C3 Immunostaining in Renal Tissue
Kidney frozen sections from factor-I deficient mice were immunostained with fluorescein isothiocyanate-conjugated goat anti-mouse C3 or biotinylated-goat anti-mouse C3d. To confirm the affinity of goat anti-mouse C3d antibody, the kidney sections from factor-I deficient mice were treated in vitro with sera from mice with combined deficiency of factor H and C3 (as source of mouse factor I) for 30 minutes at 37°C and immunostained.
Quantification of C3 and C3d Deposition
Aortic roots were stained to quantify expression of both C3 and C3d. Sections for each cohort were immunostained and imaged contemporaneously, and quantified by confocal microscopy (Zeiss LSM510Meta). Green fluorescence staining was quantified by a minor modification of published methods.44,45 Using Image J (http://rsbweb.nih.gov/ij), the operator split the Red, Green, Blue image file for each section and then measured the intensity in the green channel taking care to restrict analysis to the lesion as a region of interest. Within each atherosclerotic lesion, we first obtained the area fraction for positive staining by measuring the total number of pixels and the fraction of those pixels that were non-zero. Using the histogram function on slides stained with the isotype control, a threshold was set below which fluorescence was deemed nonspecific. Then, for the specific positive pixels, we measured the absolute fluorescence intensity.44
Quantification of C5b-9 Deposition
Neoepitopes on C5b-9 were detected with a rabbit polyclonal antibody raised against human C5b-9 (Calbiochem, Merck Biosciences, Beeston, UK) that has previously been used by us and others to detect C5b-9 in mouse tissues.23,39 Aortic root sections were stained with anti-C5b-9 or control IgG, followed by biotinylated goat anti-rabbit IgG and the avidin-biotin complex-peroxidase system (Dako, Ely, UK), by using 3,3′-diaminobenzidine tetrahydrochloride as substrate. Anti-C5b-9 staining was scored blind on a 0, + (weak), ++ (moderate), and +++ (strong) semiquantitative scale.
Statistics
Typically, values for a given aortic root were the mean of five sections. Unless stated otherwise, data are expressed as mean ± SEM and were analyzed by two-tailed Student’s t-test. Statistical significance of all tests was defined as P < 0.05.
Results
Body Weight and Serum Lipids in daf-1−/−Ldr−/− Mice
The characteristics of the study groups are shown in Table 1. No major differences were observed between Ldr−/− and daf-1−/−Ldr−/− strains in body weight, total serum cholesterol, and triglyceride levels. Furthermore, there were no differences in the lipoprotein profiles of Ldlr−/− and daf-1−/−Ldlr−/− mice (not shown).
Table 1.
Body Weights and Total Serum Cholesterol and Triglycerides in 22-week-old Ldlr−/− and daf-1−/−Ldlr−/− Mice
| Ldlr−/− | daf-1−/−Ldlr−/− | |
|---|---|---|
| Low-fat diet | ||
| Number | 23 | 22 |
| Final body weight, g | 25.13 ± 0.68 | 23.90 ± 0.41 |
| Total cholesterol, mmol/L | 9.44 ± 1.58 | 9.21 ± 2.15 |
| Triglyceride, mmol/L | 1.76 ± 0.54 | 1.78 ± 0.46 |
| High-fat diet | ||
| Number | 18 | 17 |
| Final body weight, g | 25.22 ± 0.73 | 26.91 ± 0.45 |
| Total cholesterol, mmol/L | 23.04 ± 3.07 | 21.88 ± 5.33 |
| Triglyceride, mmol/L | 3.26 ± 0.73 | 3.36 ± 0.55 |
Values presented are mean ± SEM.
Effect of DAF Deficiency on Lesions in en face Preparations of Thoracoabdominal Aortae
Although lesions in en face preparations of thoracoabdominal aortae were barely detectable at 22 weeks in Ldlr−/− mice fed a low-fat diet, DAF deficiency led to significant enhancement (daf-1−/−Ldlr−/− 4.66 ± 0.63% versus Ldlr−/− 2.85 ± 0.34%, P < 0.03). A high-fat diet increased lesion areas in Ldlr−/− mice approximately threefold, with daf-1−/−Ldlr−/− mice still having significantly larger lesions (daf-1−/−Ldlr−/− 11.82 ± 0.89% versus Ldlr−/− 8.11 ± 1.04%, P < 0.03) (Figure 1A–E).
Figure 1.
DAF deficiency accelerates aortic lipid deposition: (A–D) 22-week-old mice were fed a low-fat (A and B) or a high-fat (C and D) diet from the age of 12 weeks. Mice were perfused in vivo with Sudan IV, after which aortae were excised and processed for en face staining as described in Methods. A and C depict Ldlr−/−mice and B and D depict daf-1−/−Ldlr−/− mice. Scale bar represents 1 cm. E: Comparison of lesion areas after image analysis of en face staining. Values are group means ± SEM.
Effect of DAF Deficiency on Atherosclerotic Lesion Area
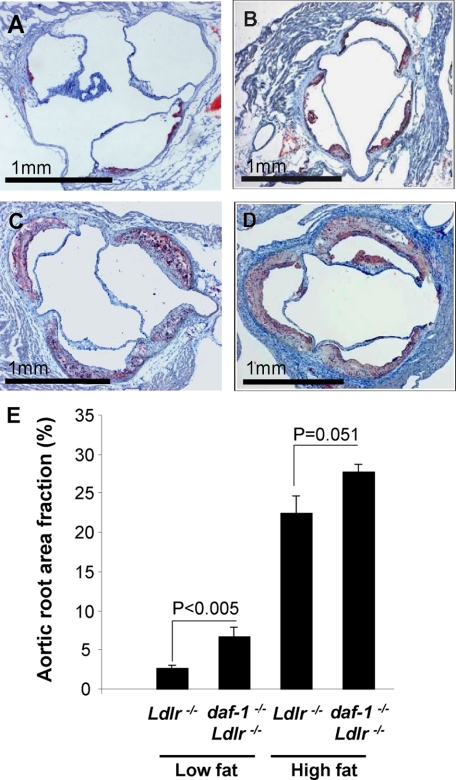
Increased atherosclerotic lesion formation in daf-1−/−Ldlr−/− mice fed a low-fat diet was also evident in the aortic roots, with larger lesions, either when expressed as absolute lesion area (daf-1−/−Ldlr−/− 76.16 ± 9.53 × 103 μm2 versus Ldlr−/− 29.42 ± 3.83 × 103 μm2; P < 0.001), or as a fraction of the aortic root (daf-1−/−Ldlr−/− 6.72 ± 1.11% versus Ldlr−/− 2.64 ± 0.35%; P < 0.005) (Figure 2, A, B, and E). Following a high-fat diet, aortic root lesions in Ldlr−/− mice were already so large as to take up around a third of the available lumen, and differences between Ldlr−/− mice and daf-1−/−Ldlr−/− mice did not reach statistical significance (lesion area fraction in daf-1−/−Ldlr−/− 27.73 ± 0.96% versus Ldlr−/− 22.44 ± 2.32%, P = 0.051) (Figure 2, C–E).
Figure 2.
DAF deficiency accelerates atherosclerotic lesion development in the aortic root: (A–D) photomicrographs of aortic roots in 22-week-old mice stained with Oil Red O and counterstained with hematoxylin after (A and B) a low-fat diet or (C and D) a high-fat diet from the age of 12 weeks. A and C depict Ldlr−/−mice and B and D depict daf-1−/−Ldlr−/− mice. Scale bars represent 1 mm. E: Comparison of lesion areas in Ldlr−/− and daf-1−/−Ldlr−/− mice, expressed as a percentage fraction of the aortic root area. Values are group means ± SEM.
Histological Analysis of Aortic Root Lesions
Aortic root lesions in low-fat diet-fed Ldlr−/− mice were composed almost entirely of macrophages, as detected by mAb MOMA-2, with poorly developed shoulders. In contrast, daf−/−Ldlr−/− mice fed a low-fat diet had lesions that had evolved from fatty streaks (Stary-Class II) to fibrous cap lesions (Stary Class IV), as judged by a significant increase in the α actin-positive vascular smooth muscle cells (VSMC) within lesions, well-defined shoulders and lipid cores (Figure 3, A–D). Lesions in the aortic roots of Ldlr−/− mice are well known to be rupture-resistant, and we did not observe ruptured plaques. Quantification of the lesion percentage taken up by VSMC showed an approximately sixfold increase in daf-1−/−Ldlr−/− mice (6.72 ± 2.18% in daf-1−/−Ldlr−/− versus 0.68 ± 0.01% in Ldlr−/−, P < 0.002) (Figure 3E). After a high-fat diet, the proportion of macrophages in relation to lesional cells dropped (Figure 3F), whereas the number of α-actin positive cells was increased in both strains (but more so in daf−/−Ldlr (15.69 ± 2.19 in Ldlr−/− and 33.40 ± 5.21 in daf-1−/−Ldlr−/−; P < 0.005)). In confirmation of the increased complexity of lesions in daf-1−/−Ldlr−/− mice, we observed increased percent of area fraction of thin collagen fibers (green Picrosirius Red staining) in the absence of DAF, both after a low-fat diet (2.19 ± 0.57 in daf-1−/−Ldlr−/− versus 0.38 ± 0.16 in Ldlr−/−; P < 0.001) and after a high-fat diet (7.74 ± 1.41 versus 1.06 ± 0.37; P < 0.0001). Similarly, the percent of area fraction of thick collagen fibers (orange-red Picrosirius Red staining) was also increased (4.42 ± 1.01 versus 3.70 ± 0.88 after a low-fat diet, P < 0.001; 10.99 ± 2.11 versus 4.30 ± 1.04 after a high-fat diet, P < 0.004). We observed diffuse lesional immunostaining of IgM and IgG, which was similar between strains (not shown).
Figure 3.
Increased α-actin staining reveals increased lesion complexity in daf −/−Ldlr −/− mice fed a low-fat diet: The photomicrographs are representative section images of aortic root lesions from (A and C) Ldlr−/− mice and (B and D) daf-1−/−Ldlr−/− mice fed (A and B) a low-fat or (C and D) high-fat diet, with VSMC stained with Cy3-conjugated anti-α actin (red). Nuclei are stained with TOPRO (far red and near infra-red original, blue pseudocoloured for visualization). L = lumen; Scale bars represent 50 mm. Quantification of (E) α actin staining and (F) macrophages identified by mAb MOMA-2, both expressed as percentage of lesional cells. The histogram shows a significant increase in VSMC in DAF deficient mice fed a low-fat diet. Values are group means ± SEM.
Distribution of DAF
Double immunofluorescence confocal staining of arterial tissue of Ldlr−/− mice showed clear expression of DAF by endothelial cells and macrophages, although DAF expression by endothelium over lesions appeared reduced (Figure 4, A–D). Furthermore, expression on macrophages appeared stronger on subendothelial macrophages than on macrophages nearer the necrotic core (Figure 4, E–H). DAF expression by VSMC was weak-absent (Figure 4, I–L). Furthermore, when these double-stained sections were considered together, there was no evidence for DAF expression within the acellular lipid core of lesions. There was also strong DAF staining in the adventitia, but this was not characterized further. No DAF staining was detected in aortic roots of daf-1−/−Ldlr−/− mice, verifying the specificity of the rat anti-mouse DAF mAb MD1 (not shown).
Figure 4.
Confocal microscopy showing localization of DAF in relation to endothelial cells, macrophages, and VSMC: Aortic root sections from high-fat-fed Ldlr−/− mice were analyzed by confocal microscopy to detect the presence of DAF on endothelial cells, macrophages, and VSMC. A: Rat anti-mouse DAF (clone MD1), followed by biotinylated polyclonal rabbit anti-rat secondary and development with AlexaFluor 568-conjugated streptavidin (pseudocoloured green). The filled green arrowhead shows strong DAF expression in the nonlesional area, whereas the open green arrowhead shows a weak DAF expression in the lesional area. B: AlexaFluor 488-conjugated anti-CD31 reacts with endothelial cells (pseudocoloured red). Filled red arrowhead shows maintained CD31 staining over lesion. C: TOPRO-3 nuclear dye (pseudocoloured blue) and D (merged image of A, B and C) where the filled yellow arrowhead points to colocalization of CD31 and DAF over the nonlesional area (yellow overlay = red/green combined), and the open red arrowhead points to positive CD31 (red) but weak DAF (green) expression over lesion. E: Rat anti-mouse DAF (clone MD1), followed by biotinylated polyclonal rabbit anti-rat secondary and development with AlexaFluor 488-conjugated streptavidin (green). The filled green arrows point to DAF expression in plaque and in adventitia. F: AlexaFluor 568-labeled anti-CD68 (macrophages, red), with filled red arrows showing CD68 expression by plaque and adventitia macrophages. G: TOPRO-3 as in C and H (merged image of E, F and G), where filled the yellow arrows point to colocalization of CD68 and DAF in some macrophages (yellow = red/green combined), both in the superficial plaque and in adventitia. I: DAF staining as in E. Filled green arrowheads show DAF expression in intima and adventitia. J: Cy3-conjugated anti-α actin to identify VSMC (red). K: TOPRO-3 as in C and L (merged image of I, J, and K), where the open block arrow shows little DAF colocalization with VSMC (if present, would be yellow = red/green combined). Scale bars represent 50 μm. L = lumen.
Validation of C3d Antibody for Use on Mouse Tissues
Before using the anti-C3d antibody to analyze atherosclerotic lesions, we validated its use for the detection of activated C3 in the mouse. First, the anti-C3d antibody failed to react with native C3 in wild-type plasma using a sandwich enzyme-linked immunosorbent assay, but reacted with wild-type serum activated by trypsin to generate C3d (Figure 5A). The anti-C3d antibody also failed to detect antigen in Factor I deficient serum, in which all circulating C3 has been shown by Western blotting to be C3b, and in which there is a defect in cleaving C3b to iC3b or C3d (Figure 5A).37 As previously reported,38 Factor I deficient mice have readily detectable mesangial deposition of C3b (Figure 5B). No staining was seen with the anti-C3d antibody (Figure 5C), demonstrating that the anti-C3d antibody does not react with C3 or C3b in tissue. After incubation of the sections with a source of murine factor I (serum from mice deficient in both C3 and factor H), which triggers the physiological cleavage of C3b to iC3b and C3d, mesangial C3 reactivity became apparent by using both the anti-C3 and anti-C3d antibody (Figure 5, D and E).
Figure 5.
Validation of polyclonal anti-C3d antibody for detecting activated C3. A: Enzyme-linked immunosorbent assays to detect C3 and C3d in sera from wild-type mice, factor I-deficient mice and sera from wild-type mice incubated with trypsin in vitro. The y axis represents optical density (OD) readings. The result is representative of two experiments; B–E: Using a polyclonal anti-C3 antibody, mesangial C3 staining is readily evident in the glomeruli of kidney sections from factor I-deficient mice (B). No staining is seen when an anti-C3d antibody is used (C) demonstrating that the anti-C3d antibody does not react with C3 in tissue. After the incubation of the sections with a source of murine factor I, mesangial C3 reactivity becomes apparent by using both the anti-C3 (D) and anti-C3d antibody (E).
Quantification of C3 and C3d Deposition
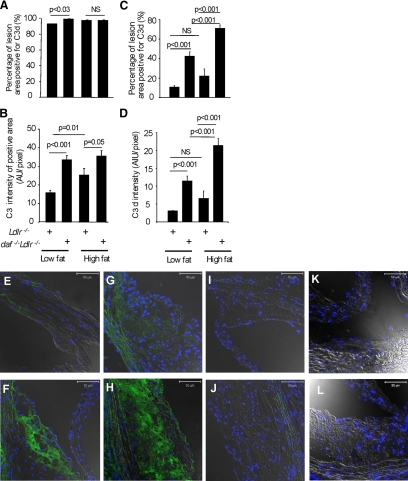
Staining of sections for C3 showed a diffuse distribution within lesions in both strains, although on the low-fat diet the area of staining was slightly greater in DAF deficient mice (P < 0.03) (Figure 6A). The intensity of C3 fluorescence staining was increased significantly by the high-fat-diet in Ldlr−/− mice, and was significantly greater in daf-1−/−Ldlr−/− mice compared with Ldlr−/− mice on either diet (Figure 6B). Applying the anti-C3d antibody to aortic root sections, positive staining was seen diffusely, implying complement activation products in both cellular and acellular regions of the plaques (Figure 6, E–L). Consistent with uncontrolled complement activation, quantitative immunofluorescence of C3d confirmed a significant fourfold increase in the area and intensity of staining for C3d in daf-1−/−Ldlr−/− mice on the low-fat diet (the percent of lesion positive for C3d 42.41 ± 4.01% versus 10.68 ± 1.13%, P < 0.001) and this increase was amplified by the high-fat diet (70.78 ± 3.55% versus 22.05 ± 6.91%, P < 0.001) (Figure 6, C and D). Notably, diet made no difference to lesional C3d in Ldlr−/− single knock-outs, testifying to the competence of C3 regulation in these animals (Figure 6, C and D). No C3 or C3d staining was seen in lesions of C3 deficient Ldlr−/− mice after a low-fat or high-fat diet, establishing specificity of staining.
Figure 6.
DAF deficiency significantly increases C3d deposition in atherosclerotic lesions: Histograms showing the percentage of lesion positive for C3 (A) and C3d (C), and intensity of positive C3 (B) and C3d (D) staining, where values are means ± SEM; (E–J) Photomicrographs of aortic root lesions of Ldlr−/− mice (E and G) and daf-1−/−Ldlr−/− mice (F and H) after a low-fat (E and F) or high-fat (G and H) diet were stained for confocal microscopy to visualize C3d. I and J: Corresponding IgG controls on section of low-fat (I) and high-fat (J) diet fed mouse. K and L: Absence of anti-C3d staining of aortic root sections of 22-week-old low-fat (K) and high-fat (L) diet high fed C3 deficient Ldlr−/− mice. Numbers studied were 16 Ldlr−/− mice and 11 daf-1−/−Ldlr−/− mice on a low-fat diet, and 15 −/− Ldlr−/− mice and 8 daf-1−/−Ldlr−/− mice on high-fat diet. Scale bars = 50 μm.
Semiquantitative Analysis of C5b-9 Deposition
Activation of the terminal pathway, as judged by deposition of C5b-9, was detected with diaminobenzidine immunocytochemical staining, and assessed on a semiquantitative scale. As shown in Figure 7, A–D, C5b-9 deposition was significantly greater in daf-1−/−Ldlr−/− mice than in Ldlr−/− mice, both on a low-fat diet (P < 0.002) and after a high-fat diet (P < 0.001).
Figure 7.
DAF deficiency significantly increases C5b-9 deposition in atherosclerotic lesions: Representatives photomicrographs of aortic root sections of a low-fat fed Ldlr−/− mouse stained with control rabbit IgG (A, score 0), a low-fat fed Ldlr−/− mouse (B, score +) stained with anti-C5b-9, and a low-fat fed daf-1−/−Ldlr−/− mouse (C, score +++) stained with anti-C5b-9. Scale bars represent 100 μm; L = lumen. D: The summary of semiquantitative scoring. Statistical analysis was performed with Mann-Whitney test.
In summary, therefore, deficiency of DAF significantly enhanced complement activation in Ldlr−/− mice, as judged both by C3d and C5b-9 deposition, and this was associated with increased atherosclerotic lesion formation.
Discussion
This study provides the first experimental evidence showing the importance of C3 regulation by DAF in protection from atherosclerosis. Thus, DAF deficiency led to an increase in lipid deposition in the aorta and an acceleration of lesion formation and VSMC content in the aortic root. As in our previous studies with C1q and CD59 deficiencies, these changes were most marked in mice fed a low-fat diet.24,41 Taking the three studies together, the data are consistent with a homeostatic role for the classical complement pathway in the arterial wall on a low-fat diet, which is overridden by the greater inflammatory milieu caused by high fat feeding.
The results of this study need to be seen alongside the previous failure to observe an effect of DAF deficiency on ApoE−/− mice.40 It should be noted that the Ldlr−/− and ApoE−/− mouse models are not totally interchangeable, with atherosclerosis in ApoE−/− being less dependent on diet.46 This may possibly have obscured seeing differences that in daf-1−/−Ldlr−/− mice were most prominent on the low-fat diet. Other factors that may have prevented observing a phenotype in daf-1−/−ApoE−/− mice include confounding effects of ApoE deficiency on macrophage and T lymphocyte function.47,48 A further consideration is that our study of daf-1−/−Ldlr−/− mice was restricted to female mice to reduce experimental variability. As male Ldlr−/− mice tend to have a more severe disease,49 we do not yet know whether there are gender differences in the effects we have observed.
A central part of this study has been the validation of the use of an anti-C3d antibody for analysis of C3 activation in mouse tissues. This antibody recognizes C3d but not intact C3 or C3b, the latter evident from the lack of staining of C3 or C3b in the glomeruli of Factor I deficient mice. We have not specifically examined reactivity of this antibody with iC3b. However, because this C3d-reactive antibody did not recognize native C3, we used it as a marker of C3 activation. Using this antibody, we found that there was detectable C3 activation in the aortic root lesions of Ldlr−/− mice fed a low-fat diet, and that this was markedly increased in the absence of DAF. Although DAF is primarily considered a cell surface protein, it is also found in soluble form, and this perhaps accounts for the increase in C3d in acellular as well as cellular regions of daf-1−/−Ldlr−/− lesions.50 Although high-fat feeding increased C3 activation in DAF deficient mice, no such effect was seen in Ldlr−/− single knock-outs, suggesting that DAF normally provides a robust control of C3 activation in response to hyperlipidemia. It is worth noting that the intensity of C3 staining also increased with high-fat feeding, suggesting a positive feedback loop whereby increased availability of C3 in the inflamed arterial wall (for example via local synthesis51) may increase C3 activation in the absence of DAF.
We also found that DAF deficiency leads to an obvious increase in C5b-9 deposition in the aortic root, both on the low-fat and high-fat diets. We interpret this observation as indicating the central importance of C3 in complement regulation and showing that increased terminal pathway activation in DAF deficient mice is sufficient to overwhelm the inhibitory capacity of CD59. In general, the phenotype of daf-1−/−Ldlr−/− mice was similar to that which we have previously reported for mice lacking CD59, apart from a greater prominence of VSMC in the aortic root lesions of high fat diet fed mice in the latter.41 The cause for this is not clear, but could relate to the capacity of CD59 to protect VSMC from the established pro-proliferative signaling effects of C5b-9.2,52 In this respect it is notable that VSMC showed only low level DAF expression, in contrast to a high level of CD59 expression seen previously.41
The mechanisms responsible for the increase in atherosclerosis in DAF deficient animals are likely at least in part to reflect increased C3 activation, and to include an increase in the recruitment and activation of monocytes via anaphylatoxins (C3a and C5a), and the effects on cell activation and proliferation of C5b-9.2,53 Clearly, an increased recruitment of monocytes could also be expected to amplify inflammation through the release of cytokines and other inflammatory mediators, but this has not been investigated further. Although we have failed to demonstrate a reduction in serum C3 in daf-1−/−Ldlr−/− mice (not shown), it is also possible that increased systemic complement activation may play a role. We are not aware of any interaction between DAF and the LDL receptor, but DAF does have other reported actions that might possibly have contributed to the effects we have observed, including acting as a signal transduction receptor on monocytes and T lymphocytes and influencing leukocyte adhesion.54,55,56,57,58,59
DAF and CD59 are just two of several regulators of complement activity, and establishing the relative roles in this model of other fluid phase (eg, Factor H60) and membrane-bound complement regulators now deserves further study, particularly with respect to their relative importance on different cell types (ie, macrophages versus VSMC). In this context, it should be noted that there is an additional membrane bound inhibitor of C3 activity in the mouse, designated complement receptor 1-related gene y.61 In view of the clear nonredundant role of DAF demonstrated by our data, determining the relative contributions of DAF and complement receptor 1-related gene y in arterial homeostasis will be of great interest. Further studies will also be needed to delineate the effects of agents found to be protective in other arterial disease models, such as C1 esterase inhibitor.62
Expression of complement proteins and complement regulators has been well documented in human atherosclerosis, underscoring the importance of understanding the details of complement biology in the arterial wall via analysis of mouse models.63 DAF is not expressed on normal VSMC but can be identified on VSMC as well as macrophages in advanced plaques, and is functionally competent as a complement regulator ex vivo.64,65 Furthermore, mRNA transcripts for DAF and other complement regulators appear to be proportionately less in plaques than transcripts for complement pathway proteins.51 This imbalance may be particularly important in allowing complement activation, in view of the limited capacity of the soluble regulator Factor H to penetrate into atherosclerotic lesions.60
In conclusion, we have presented data showing that DAF plays a central role in the regulation of complement activation in the arterial wall in mice, consistent with the emerging paradigm in which the proximal classical complement pathway has a critical homeostatic protective effect, while distal complement activity is atherogenic.
Footnotes
Address reprint requests to Dorian O. Haskard, Vascular Science Section, Imperial College, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK. E-mail: d.haskard@imperial.ac.uk.
Supported by the British Heart Foundation.
S.Y. and V.W.Y.L. contributed equally to this work.
References
- Walport MJ. Complement: first of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24:191–199. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- Oksjoki R, Kovanen PT, Meri S, Pentikainen MO. Function and regulation of the complement system in cardiovascular diseases. Front Biosci. 2007;12:4696–4708. doi: 10.2741/2419. [DOI] [PubMed] [Google Scholar]
- Haskard DO, Boyle JJ, Mason JC. The role of complement in atherosclerosis. Curr Opin Lipidol. 2008;19:478–482. doi: 10.1097/MOL.0b013e32830f4a06. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Holm J, Kral JG. Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand [A] 1984;92:429–435. doi: 10.1111/j.1699-0463.1984.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Vlaicu R, Rus HG, Niculescu F, Cristea A. Immunoglobulins and complement components in human aortic atherosclerotic intima. Atherosclerosis. 1985;55:35–50. doi: 10.1016/0021-9150(85)90164-9. [DOI] [PubMed] [Google Scholar]
- Seifert PS, Kazatchkine MD. Generation of complement anaphylatoxins and C5b-9 by crystalline cholesterol oxidation derivatives depends on hydroxyl group number and position. Mol Immunol. 1987;24:1303–1308. doi: 10.1016/0161-5890(87)90125-8. [DOI] [PubMed] [Google Scholar]
- Seifert PS, Hugo F, Tranum-Jensen J, Zahringer U, Muhly M, Bhakdi S. Isolation and characterization of a complement-activating lipid extracted from human atherosclerotic lesions. J Exp Med. 1990;172:547–557. doi: 10.1084/jem.172.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Dorweiler B, Kirchmann R, Torzewski J, Weise E, Tranum-Jensen J, Walev I, Wieland E. On the pathogenesis of atherosclerosis: enzymatic transformation of human low density lipoprotein to an atherogenic moiety. J Exp Med. 1995;182:1959–1971. doi: 10.1084/jem.182.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torzewski M, Klouche M, Hock J, Messner M, Dorweiler B, Torzewski J, Gabbert HE, Bhakdi S. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler Thromb Vasc Biol. 1998;18:369–378. doi: 10.1161/01.atv.18.3.369. [DOI] [PubMed] [Google Scholar]
- Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, Gingras AR, Mantovani A, Hack EC, Roos A. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Torzewski M, Paprotka K, Schmitt S, Barsoom H, Suriyaphol P, Han SR, Lackner KJ, Husmann M. Possible protective role for C-reactive protein in atherogenesis: complement activation by modified lipoproteins halts before detrimental terminal sequence. Circulation. 2004;109:1870–1876. doi: 10.1161/01.CIR.0000124228.08972.26. [DOI] [PubMed] [Google Scholar]
- Biro A, Thielens NM, Cervenak L, Prohaszka Z, Fust G, Arlaud GJ. Modified low density lipoproteins differentially bind and activate the C1 complex of complement. Mol Immunol. 2007;44:1169–1177. doi: 10.1016/j.molimm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Lachmann PJ, Hughes-Jones NC. Initiation of complement activation. Springer Semin Immunopathol. 1984;7:143–162. doi: 10.1007/BF01893018. [DOI] [PubMed] [Google Scholar]
- Manderson AP, Pickering MC, Botto M, Walport MJ, Parish CR. Continual low-level activation of the classical complement pathway. J Exp Med. 2001;194:747–756. doi: 10.1084/jem.194.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertinger P, Sørensen H. On the reduced atherogenic effect of cholesterol feeding in rabbits with congenital complement (C6) deficiency. Artery. 1977;1:177–184. [Google Scholar]
- Schmiedt W, Kinscherf R, Deigner HP, Kamencic H, Nauen O, Kilo J, Oelert H, Metz J, Bhakdi S. Complement C6 deficiency protects against diet-induced atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998;18:1790–1795. doi: 10.1161/01.atv.18.11.1790. [DOI] [PubMed] [Google Scholar]
- Patel S, Thelander EM, Hernandez M, Montenegro J, Hassing H, Burton C, Mundt S, Hermanowski-Vosatka A, Wright SD, Chao YS, Detmers PA. ApoE−/− mice develop atherosclerosis in the absence of complement component C5. Biochem Biophys Res Commun. 2001;286:164–170. doi: 10.1006/bbrc.2001.5276. [DOI] [PubMed] [Google Scholar]
- Wu G, Hu W, Shahsafaei A, Song W, Dobarro M, Sukhova GK, Bronson RR, Shi GP, Rother RP, Halperin JA, Qin X. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ Res. 2009;104:550–558. doi: 10.1161/CIRCRESAHA.108.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, Lichtman AH. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–3031. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- Persson L, Boren J, Robertson AK, Wallenius V, Hansson GK, Pekna M. Lack of complement factor C3, but not factor B, increases hyperlipidemia and atherosclerosis in Apolipoprotein E−/− low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2004;24:1062–1067. doi: 10.1161/01.ATV.0000127302.24266.40. [DOI] [PubMed] [Google Scholar]
- Bhatia V, Yun S, Leung V, Grimsditch CE, Benson GM, Botto M, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MC. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2001;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2004;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- Matthijsen RA, de Winther MP, Kuipers D, van dM I, Weber C, Herias MV, Gijbels MJ, Buurman WA. Macrophage-specific expression of mannose-binding lectin controls atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;119:2188–2195. doi: 10.1161/CIRCULATIONAHA.108.830661. [DOI] [PubMed] [Google Scholar]
- Madsen HO, Videm V, Svejgaard A, Svennevig JL, Garred P. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959–960. doi: 10.1016/S0140-6736(05)61513-9. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–3483. [PubMed] [Google Scholar]
- Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71:1–9. [PMC free article] [PubMed] [Google Scholar]
- Uhrinova S, Lin F, Ball G, Bromek K, Uhrin D, Medof ME, Barlow PN. Solution structure of a functionally active fragment of decay-accelerating factor. Proc Natl Acad Sci USA. 2003;100:4718–4723. doi: 10.1073/pnas.0730844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttner-Kondo L, Hourcade DE, Anderson VE, Muqim N, Mitchell L, Soares DC, Barlow PN, Medof ME. Structure-based mapping of DAF active site residues that accelerate the decay of C3 convertases. J Biol Chem. 2007;282:18552–18562. doi: 10.1074/jbc.M611650200. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Seldin MF, Gendler SJ. Molecular cloning and chromosomal localization of the mouse decay- accelerating factor genes: duplicated genes encode glycosylphosphatidylinositol-anchored and transmembrane forms. J Immunol. 1995;155:3079–3091. [PubMed] [Google Scholar]
- Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci USA. 1999;96:628–633. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogabe H, Nagaku M, Ishibashi Y, Wada T, Fujita T, Sun X, Miwa T, Madaio MP, Song W-P. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerlonephritis. J Immunol. 2001;167:2791–2797. doi: 10.4049/jimmunol.167.5.2791. [DOI] [PubMed] [Google Scholar]
- Miwa T, Maldonado MA, Zhou L, Sun X, Luo HY, Cai D, Werth VP, Madaio MP, Eisenberg RA, Song WC. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol. 2002;161:1077–1086. doi: 10.1016/S0002-9440(10)64268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP, Chamberlain-Banoub J, Neal JW, Song W, Mizuno M, Harris CL. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol. 2006;146:294–302. doi: 10.1111/j.1365-2249.2006.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G, Miwa T, Song WL, Lawson JA, Rader DJ, Zhang Y, Song WC. CD59 but not DAF deficiency accelerates atherosclerosis in female ApoE knockout mice. Mol Immunol. 2009;46:1702–1709. doi: 10.1016/j.molimm.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Leung VW, Botto M, Boyle JJ, Haskard DO. Accelerated atherosclerosis in low-density lipoprotein receptor-deficient mice lacking the membrane-bound complement regulator CD59. Arterioscler Thromb Vasc Biol. 2008;28:1714–1716. doi: 10.1161/ATVBAHA.108.169912. [DOI] [PubMed] [Google Scholar]
- Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikova O, Robertson AK, Wagsater D, Folco EJ, Hyry M, Myllyharju J, Eriksson P, Libby P, Hansson GK. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe−/− mice. Am J Pathol. 2009;174:693–700. doi: 10.2353/ajpath.2009.080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderlerer AR, Ali F, Johns M, Lidington EA, Leung V, Boyle JJ, Hamdulay SS, Evans PC, Haskard DO, Mason JC. KLF2-dependent, shear stress-induced expression of CD59: a novel cytoprotective mechanism against complement-mediated injury in the vasculature. J Biol Chem. 2008;283:14636–14644. doi: 10.1074/jbc.M800362200. [DOI] [PubMed] [Google Scholar]
- Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joven J, Rull A, Ferre N, Escola-Gil JC, Marsillach J, Coll B, Onso-Villaverde C, Aragones G, Claria J, Camps J. The results in rodent models of atherosclerosis are not interchangeable: the influence of diet and strain. Atherosclerosis. 2007;195:e85–e92. doi: 10.1016/j.atherosclerosis.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Pepe MG, Curtiss LK. Apolipoprotein E is a biologically active constituent of the normal immunoregulatory lipoprotein, LDL-In. J Immunol. 1986;136:3716–3723. [PubMed] [Google Scholar]
- Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. Absence of P-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miot S, Crespo S, Schifferli JA. Distinct forms of DAF in urine and blood. J Immunol Methods. 2002;260:43–53. doi: 10.1016/s0022-1759(01)00519-1. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1214–1219. doi: 10.1161/hq0701.092160. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Zwirner J, Werfel T, Wilken HC, Theile E, Gotze O. Anaphylatoxin C3a but not C3a(desArg) is a chemotaxin for the mouse macrophage cell line J774. Eur J Immunol. 1998;28:1570–1577. doi: 10.1002/(SICI)1521-4141(199805)28:05<1570::AID-IMMU1570>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Abe T, Fujita T. Decay-accelerating factor functions as a signal transducing molecule for human monocytes. J Immunol. 1992;149:1758–1762. [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor: interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- Kuraya M, Fujita T. Signal transduction via a protein associated with a glycosylphosphatidylinositol-anchored protein, decay-accelerating factor (DAF/CD55). Int Immunol. 1998;10:473–480. doi: 10.1093/intimm/10.4.473. [DOI] [PubMed] [Google Scholar]
- Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DW, Bruyninckx WJ, Louis NA, Lublin DM, Stahl GL, Parkos CA, Colgan SP. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksjoki R, Jarva H, Kovanen PT, Laine P, Meri S, Pentikainen MO. Association between complement factor H and proteoglycans in early human coronary atherosclerotic lesions: implications for local regulation of complement activation. Arterioscler Thromb Vasc Biol. 2003;23:630–636. doi: 10.1161/01.ATV.0000057808.91263.A4. [DOI] [PubMed] [Google Scholar]
- Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, Holers VM. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181:151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagdarsuren E, Bidzhekov K, Talab YD, Liehn EA, Hristov M, Matthijsen RA, Buurman WA, Zernecke A, Weber C. C1-esterase inhibitor protects against neointima formation after arterial injury in atherosclerosis-prone mice. Circulation. 2008;117:70–78. doi: 10.1161/CIRCULATIONAHA.107.715649. [DOI] [PubMed] [Google Scholar]
- Torzewski J, Bowyer DE, Waltenberger J, Fitzsimmons C. Processes in atherogenesis: complement activation. Atherosclerosis. 1997;132:131–138. doi: 10.1016/s0021-9150(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Seifert PS, Hansson GK. Decay-accelerating factor is expressed on vascular smooth muscle cells in human atherosclerotic lesions. J Clin Invest. 1989;84:597–604. doi: 10.1172/JCI114204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu F, Rus HG, Vlaicu R. Decay-accelerating factor regulates complement-mediated damage in the human atherosclerotic wall. Immunol Lett. 1990;26:17–23. doi: 10.1016/0165-2478(90)90170-u. [DOI] [PubMed] [Google Scholar]