Abstract
Linoleic acid-phospholipids stimulate high-density lipoprotein (HDL) net secretion from liver cells by blocking the endocytic recycling of apoA-I. Experiments were undertaken to determine whether apoA-I accumulation in the cell media is associated with membrane ATPase expression. Treatment of HepG2 cells with dilinoeoylphosphatidylcholine (DLPC) increased apoA-I secretion fourfold. DLPC also significantly reduced cell surface F1-ATPase expression and reduced cellular ATP binding cassette (ABC)A1 and ABCG1 protein levels by ∼50%. In addition, treatment of HepG2 cells with the ABC transporter inhibitor, glyburide, stimulated the apoA-I secretory effects of both DLPC and clofibrate. Pretreatment of HepG2 cells with compounds that increased ABC transport protein levels (TO901317, N-Acetyl-l-leucyl-l-leucyl-l-norleucinal, and resveratrol) blocked the DLPC-induced stimulation in apoA-I net secretion. Furthermore, whereas HepG2 cells normally secrete nascent preβ-HDL, DLPC treatment promoted secretion of α-migrating HDL particles. These data show that an linoleic acid-phospholipid induced stimulation in hepatic HDL secretion is related to the expression and function of membrane ATP metabolizing proteins.
High-density lipoprotein (HDL) is predominantly produced in the liver in humans and is formed by the synthesis and secretion of apolipoprotein components, followed by the lipidation of these proteins with specific lipids.1,2,3 HDL lipidation is believed to be regulated by the actions of the ATP-binding cassette (ABC) transporters, ABCA1 and ABCG1. ABCA1 and ABCG1 lipidate apolipoprotein (apo)A-I and convert nascent HDL particles to lipid-rich HDL.4,5,6 Through this process, the ABC transport proteins have been thought to play a central role in both the production and maturation of HDL.6 ABC transporter expression is regulated by the liver X receptor (LXR), and LXR agonists such as the oxysterols have been shown to increase the expression and lipid secretory activity of ABCA1.7 Recent work has shown that despite the activation of ABCA1, LXR agonists such as TO901317 actually inhibit the synthesis and secretion of HDL and apoA-I by liver-derived cells.8 This suggests that ABC transporter expression may not be linked to the production of HDL.
In addition to lipidating apoA-I, ABC transporters play other roles in HDL metabolism. ABCA1 has been shown to interact directly with apoA-I9,10 and to impact the endocytic uptake and resecretion of apoA-I.11,12,13,14 ABCA1-dependent endocytic uptake of apoA-I has been shown to promote the lysosomal degradation of apoA-I.12,13 Therapeutic compounds that are inhibitors of ABC transporters have been shown to modestly increase plasma HDL levels.15,16 Glyburide inhibits ABCA1 activity by blocking the ATPase activity of the protein.17 Glyburide has also been shown to block interactions between apoA-I and ABCA19,10 and to block apoA-I signaling through ABCA1.18 There is evidence to suggest that addition of an ABC transporter inhibitor, such as glyburide, to a fibrate therapy, may also increase the HDL raising potential of the fibrate.19
A different membrane ATPase, F1F0-ATP synthase (F1-ATPase), has also been shown to impact cellular HDL metabolism.20,21,22,23 Studies have shown that apoA-I can stimulate a plasma membrane bound F1-ATPase and promote the endocytic uptake of HDL through a specific plasma membrane G-protein coupled receptor, P2Y13.22 Inhibition of F1-ATPase with antibodies or selective inhibitors (IF1) blocks HDL endocytosis in hepatic cell culture and in vivo. Recent work suggests that niacin may act through this pathway and increase HDL secretion through reducing membrane F1-ATPase levels.24 Niacin reduces membrane F1-ATPase levels and inhibits the reuptake and recycling of apoA-I.
Linoleic acid (LA)-phospholipids are considerably more effective at stimulating hepatic apoA-I secretion and HDL production, than niacin and the fibrate drugs.25 Much like niacin, these compounds act through protein kinase C and mitogen-activated protein kinase pathways to activate a peroxisome-proliferator activator receptor (PPAR)α-dependent secretion of apoA-I. However, in contrast to the fibrate drugs, LA-phospholipids do not increase cellular apoA-I mRNA levels and instead increase apoA-I secretion by blocking the endocytic recycling of apoA-I.26 LA-phospholipids are therefore a novel class of HDL effectors that have a similar mechanism of action to niacin and are not metabolized by the cytochrome P450 enzymes.25,26,27
Experiments were undertaken to elucidate how an LA-phospholipid stimulation in apoA-I secretion may involve membrane ATPases. We show that increased apoA-I secretion is inversely related to cell membrane ATPase protein levels. Compounds that inhibit ATPase activity significantly stimulate apoA-I secretion and those that increase ABC transporter levels in HepG2 cells inhibit apoA-I secretion. The data suggests that hepatic apoA-I secretion is closely linked to the expression and function of membrane-bound ATP metabolizing proteins.
Materials and Methods
Chemicals
Phospholipids, soy phosphatidylinositol (PI) and dilinoeoylphosphatidylcholine (DLPC) were procured from Avanti Polar Lipids Inc., Alabaster, AL. TO901317, a synthetic LXRα agonist, was obtained from Cayman Chemical, Ann Arbor, MI. Resveratrol (trans-3,5,4′-trihydroxystilbene), ALLN (N-Acetyl-l-leucyl-l-leucyl-l-norleucinal), a calpain-I inhibitor, and glyburide an ATP-sensitive potassium channel blocker, linoleic acid sodium salt, and oligomycin (a mixture of oligomycins A, B and C; approx. 65% oligomycin A) were purchased from Sigma-Aldrich, Saint Louis, MO. Mouse monoclonal anti-human apoA-I (cat#H45402M), and horse radish peroxidase-conjugated goat anti-human apoA-I (cat#A-I K45252P) were obtained from Meridian Life Science, Saco, ME. The mouse monoclonal anti-human apoA-I (5F6 and 4H1) antibodies for Western blots were kindly provided by Dr. Ives Marcel. The goat polyclonal anti-human apoA-II antibody was from Chemicon International, Billerica, MA. β-actin (cat # 4967) and anti-rabbit IgG-HRP (cat # 7074) were obtained from Cell Signaling Technology, Danvers, MA. Mouse monoclonal anti-ABCA1 IgG (cat#G090) was purchased from ABM Inc., and affinity purified peroxidase linked goat anti-mouse antibody (cat#4741806) was purchased from Kirkegaard and Perry Laboratories. Affinity purified goat polyclonal anti-ABCG1 antibody (cat # 11150) and donkey anti-goat IgG-HRP (cat# sc-2020) were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Mouse monoclonal anti-β chain of F1F0-ATP synthase was purchased from Invitrogen, Carlsbad, CA. Unless otherwise stated, drugs and inhibitors were of analytical grade and were solubilized in dimethyl sulfoxide.
Cell Culture
HepG2 cells were cultured in normal glucose Dulbecco’s modified Eagle medium containing 10% fetal bovine serum and 1% penicillin/streptomycin. Almost confluent cells were subjected to stimulation with or without drugs for 24 hours under serum-starved conditions, as indicated.
Preparation of Phospholipid Vesicles
Phospholipid vesicles in PBS (1 mg/ml) were prepared by sonication as previously described.25 Briefly, phospholipids in chloroform were dried down under N2 gas and 1 ml of PBS was added by vortexing. The mix was then sonicated (Branson sonicator set at 100% duty cycle and 10% power) for 1 minute. The sonicated preparation was incubated for 30 minutes at 37°C in a water bath, and samples were resonicated for 5 minutes at 95% duty cycle and 10% power and filtered before use. Purity of all phospholipids was >99% (Avanti Polar Lipids) and was verified by high-performance liquid chromatography.
ApoA-I Enzyme-Linked Immunosorbent Assay
Protein in conditioned medium from each stimulation was analyzed by enzyme-linked immunosorbent assay (ELISA) on a 96 well plate according to manufacturer’s instructions, with minor modifications. Briefly, the Nunc Immuno-maxisorp 96 well plates were coated overnight with a mouse anti-human apoA-I monoclonal antibody. Samples and standards were incubated in the wells for 2 hours, followed by a 1-hour incubation with a horseradish peroxidase-linked goat anti-human apoA-I antibody. K-blue Max TMB substrate was added to each well and the reaction was stopped with a 1 M/L HCl solution; and the absorbance was recorded at 450 nm. The assay conditions were optimized to minimize any apoA-I conformation interference with the apoA-I ELISA.
Cholesterol Secretion Assay
Confluent HepG2 cells in 6 well plates were radio-labeled with 5μCi/ml [3H]-cholesterol in complete medium for 24 hours. Following incubation, cells were washed three times with pre-warmed serum free media and were treated with 12 μmol/L phospholipid vesicles in serum-free Dulbecco’s modified Eagle medium for 24 hours. Conditioned media was collected into tubes and the cells were lysed using 0.5 M/L NaOH. Pre-cleared media samples and cell lysates were mixed with the EcoLite scintillation liquid and were counted by TRI-CARB 2100TR Liquid Scintillation Analyzer. The cell lysates were quantified for total protein concentration using the bicinchoninic acid assay (as per manufacturer’s specifications).
Biotinylation and Isolation of Membrane Proteins
Plasma membrane proteins were biotinylated and isolated using a Cell Surface Labeling Accessory Pack (Pierce Chemical, Rockford, IL) according to manufacturer’s protocol. Briefly, cell monolayers were biotinylated with EZlink-sulfo-NHS-LC-biotin at 4°C for 30 minutes with gentle agitation. The cells were harvested after addition of Quenching solution and then washed three times with Tris-buffered saline (TBS) using centrifugation at 500 × g for 3 minutes each. Cells were then lysed using lysis buffer supplemented with protease inhibitors, followed by low-power sonication and centrifugation to disrupt the cells and were incubated on ice for 30 minutes. Cleared cell lysates were obtained for each sample as supernatant by centrifugation at 10,000 × g for 2 minutes at 4°C. Columns packed with Immobilized NeutrAvidin Gel were used to isolate labeled proteins. Finally SDS-polyacrylamide gel electrophoresis sample buffer supplemented with 50 mmol/L dithiothreitol was used to elute labeled proteins by centrifugation at 1000 × g for 2 minutes. Protein assay was performed by bicinchoninic acid method with minor modifications. Samples were first treated with sodium deoxicholate, followed by TCA precipitation. An equal amount of protein then separated on a 12% SDS-polyacrylamide electrophoresis gels, transferred to polyvinylidene difluoride membranes, analyzed by immunoblotting with ATP-synthase β antibody, and then visualized with the α-Innotech FluorChem HD Imager (Fisher Scientific).
Plasma Membrane Isolation by Ultracentrifugation
HepG2 cells were treated and plasma membranes were isolated as previously described.28,29 Briefly, cells were washed twice with 0.9% sodium chloride and scraped off in lysis buffer (10 mmol/L Tris [pH 7.4], 10 mmol/L sodium chloride, 1.5 mmol/L magnesium chloride, 0.05% sodium azide, 1 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L dithiothreitol). After homogenization by sonication, lysates were subjected to centrifugation at 1600 rpm for 2 minutes. Supernatants were then centrifuged at 100,000 × g for 30 minutes to prepare cytosolic and membrane fractions. Supernatant (cytosolic protein) and pellets (membrane protein) were then suspended in lysis buffer. Protein concentration was determined and an equal amount of protein was separated on 12% SDS-polyacrylamide gel electrophoresis and subjected to immunoblotting for ATP synthase and for peroxiredoxin-3 using specific antibodies.
Small Interfering RNA Knockdown of ABCA1
HepG2 cells were transiently transfected with All Stars Negative small-interfering (si)RNA or four different ABCA1-siRNA sequences (separately) from the Flexitube Gene Solution siRNA kit (Qiagen Inc., Mississauga, ON) by reverse transfection using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Complexes were prepared per manufacturer’s specifications with a Lipofectamine 2000-to-siRNA volume-to-mole ratio of 2:40 (μL to ρmol) in 200 μl of Opti-MEM I Reduced Serum Media (Invitrogen, Carlsbad, CA). HepG2 cells were trypsinized and seeded in 12-well plates at a density of 500,000 cells/well and then 200 μl of the transfection complexes were immediately added to the suspended cells. Transfection of the siRNA alone showed no adverse cytotoxic effects compared with nontransfected cells. Conditioned media was collected 48 hours post-transfection and an ELISA was performed to determine the amount of apoA-I. ABCA1 knockdown was confirmed by Western blot analysis.
Western Blot Analysis
After incubation with drugs for the indicated times and doses, cells were washed twice with ice-cold PBS-T on ice. Cells were lysed by adding buffer (NaF 1 mmol/L, NaCl 5 mmol/L, EDTA 1 mmol/L, NP40 1 mmol/L [Roche Diagnostics, Indianapolis, IN], HEPES 10 mmol/L, pepstatin A 1 mg/ml, leupeptin 1 mg/ml, aprotinin 1 mg/ml, Na3VO4 1 mmol/L, and phenylmethylsulfonyl fluoride 1 mmol/L) and total protein was extracted. Equal amounts of cell proteins were separated by SDS-12% polyacrylamide gel electrophoresis and were analyzed by Western blot with specific antibodies to ATP synthase, ABCA1, and ABCG1. Blots for the similar experiments were also subjected to β-actin for a loading control. Band intensity was analyzed with the α-Innotech FluorChem HD Imager.
Agarose Gel Immunoblots
Lipoprotein electrophoretic mobility was determined by apoA-I immunoblots of prepoured 0.5% agarose gels (Beckman, Paragon-Lipo). Conditioned media samples were loaded and the gel was electrophoresed with a barbital buffer in the Beckman Paragon Electrophoresis System at 100 V for 30 minutes, as previously described.30 Once complete, the gel was covered in 1× Tris-Glycine transfer buffer, and then layered with a presoaked nitrocellulose membrane. The gel and membrane were covered in plastic wrap and an even pressure of 3 kg was applied. After a 4 hour transfer, the membrane was blocked in 5% skim milk or bovine serum albumin for 1 hour at room temperature and incubated with the primary apoA-I antibodies (mix of 5F6 and 4H1, 1:5000 dilution) or apoA-II antibody, overnight at 4°C. The membrane was washed three times and then incubated with secondary antibody (1:10,000 dilutions) for 1 hour at room temperature. The membrane was incubated with SuperSignal West Femto for 3 minutes and then visualized using the α-Innotech FluorChem HD Imager.
Immunoanalysis of Nondenaturing Gradient Gel Electrophoresis
Conditioned media from HepG2 cells stimulated with DLPC were electrophoresed in triplicate on a 4% to 20% Tris-Glycine Novex gel (Invitrogen, Carlsbad, CA) under non-denaturing conditions for 19 hours at 100V alongside high molecular weight native markers (Amersham, Piscataway, NJ). The gel was then soaked in 0.1% SDS for 15 minutes to give the proteins a slight negative charge in order for unidirectional transfer onto a polyvinylidene difluoride membrane for 4 hours at 125V in Tris-glycine transfer buffer containing 20% methanol. The membrane was allowed to dry at which point the molecular weight markers were outlined and the membrane was cut in three. The apoA-I membrane was blocked with 5% milk/TBS-Tween (TBST) and then probed for apoA-I using a 1:2500 dilution of the monoclonal apoA-I antibodies (4H1 and 5F6) and a 1:20,000 dilution of the goat anti-mouse IgG linked HRP secondary antibody (KPL, Gaithersburg, MD) in 1% milk/TBST. ApoA-II was probed by blocking in 1% BSA/TBST followed by incubation with 1:1000 dilution of the goat anti-apolipoprotein A-II polyclonal antibody in 1% BSA/TBST and 1:10,000 dilution of the donkey anti-goat IgG-HRP secondary antibody in 1% BSA/TBST (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed using the West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) on the Fluorochem AlphaImager. Densitometry profiles were obtained using the 1D-Multi application of the AlphaEaseFC software.
Statistical Analysis
Values are shown as Mean ± SEM for at least 3 to 4 independent experiments as indicated, and P < 0.05 was considered significant. Differences between mean values were evaluated by one-way analysis of variance on ranks by a pairwise multiple comparison using the Student-Newman-Keuls posthoc test (SigmaStat; Systat Software, Inc., San Jose, CA).
Results
Effect of Glyburide on the Stimulation of apoA-I Secretion by Phospholipids
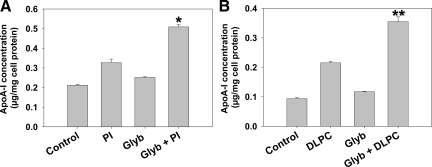
Linoleic acid-enriched phospholipids were shown to stimulate hepatic apoA-I secretion in both primary hepatocytes and HepG2 cells.25 To determine whether phospholipid-induced apoA-I secretion may involve ATP dependent reactions, experiments were undertaken with the ABC transporter inhibitor, glyburide. Glyburide (50 μmol/L) alone had a small stimulatory effect on apoA-I secretion (Figure 1A). However, PI and DLPC-induced apoA-I secretion was synergistically increased, by 60% and 75% respectively (Figure 1B) at 24 hours, when HepG2 cells were pre-incubated for 30 minutes with glyburide. Clofibrate (10 μmol/L) only had a mild stimulatory effect on apoA-I secretion, while clofibrate and glyburide showed significant increase in apoA-I secretion, at 33% over control (data not shown).
Figure 1.
Effect of glyburide on apoA-I secretion. HepG2 cells were pre-incubated with 50 μmol/L glyburide for 30 minutes and then incubated with soy phosphatidylinositol (PI, A) or dilinoleoylphosphatidylcholine (DLPC, B) (12 μmol/L) for 24 hours. ApoA-I was quantified in the media by ELISA. ApoA-I secretion is presented relative to total cell protein values and are expressed as mean ± SEM of four independent experiments; *P < 0.001 vs PI. **P < 0.001 vs DLPC.
Phospholipids Reduce Hepatic F1-ATPase Expression
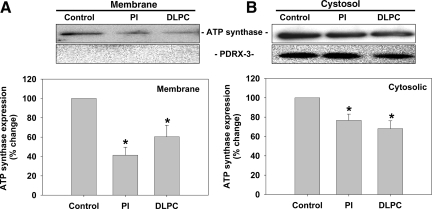
The effect of PI and DLPC on cellular F1-ATPase expression was determined in HepG2 cells. Cells were treated with PI or DLPC for 24 hours and then membrane and cytosolic proteins were fractionated and isolated centrifugally and probed for F1-ATPase. Membrane F1-ATPase was reduced ∼40% to 60% by PI and DLPC (Figure 2A). Cytosolic F1-ATPase levels were also significantly reduced (Figure 2B). Isolated membrane proteins did not contain mitochondrial proteins, as peroxiredoxin-3 was not detectible in the membrane proteins, but present in the cytosolic fraction (Figure 2A). Similar results were obtained after biotinylation and reisolation of plasma membranes, where membrane F1-ATPase levels were reduced by 18% after DLPC treatment (Supplementary Figure S1, see http://ajp.amjpathol.org). To determine whether inhibition of ATP synthase affects hepatic apoA-I secretion, cells were pretreated with oligomycin (5 μg/ml for 30 minutes) and then with PI or DLPC for 24 hours and apoA-I secretion was measured. Oligomycin blocked both basal and phospholipid-induced apoA-I secretion (Supplementary Figure S2, see http://ajp.amjpathol.org).
Figure 2.
Phospholipids inhibit F1-ATPase expression in HepG2 cells. HepG2 cells were incubated with PI and DLPC (12 μmol/L), or PBS as a control, for 24 hours. Membrane and cytosolic proteins were separated centrifugally and F1-ATPase expression was analyzed by Western blot. Blots were re-probed for peroxiredoxin-3 to determine purity of the membrane fraction. F1-ATPase protein expression in plasma membrane (A) and cytosolic fractions (B) are shown. Histograms representing densitometry analysis of F1-ATPase are shown and the values are expressed as mean ± SEM of three independent experiments. *P < 0.05 vs control.
Phospholipids Reduce Hepatic ABCA1 and ABCG1 Expression
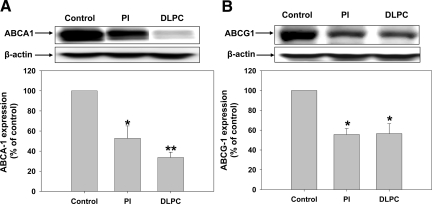
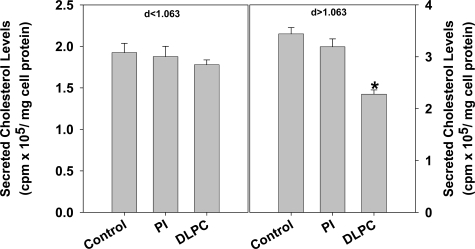
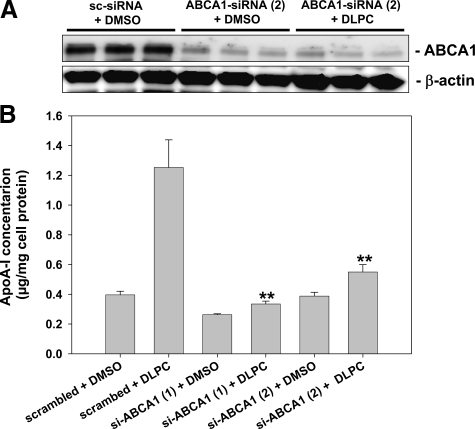
HepG2 cells were treated with PI or DLPC, and ABC transporter expression was measured. As shown in Figure 3, PI and DLPC reduced hepatic ABCA1 and ABCG1 protein expression. PI reduced ABCA1 and ABCG1 protein levels by 48% and 50% respectively, while DLPC reduced ABCA1 and ABCG1 × 74% and 50% respectively (Figure 3, A and B). To determine the effect of PI and DLPC on cellular cholesterol secretion, media cholesterol levels were measured after loading the HepG2 cells with 3H-cholesterol and treatment with PI or DLPC. Even though PI reduced ABCA1 and ABCG1 expression, the lipid had little effect on cellular cholesterol secretion. In contrast, DLPC significantly blocked cholesterol secretion (Figure 4). DLPC reduces the 3H-cholesterol levels in the d >1.063 lipoproteins (HDL), but had no effect on the cholesterol levels in the d <1.063 lipoproteins (Figure 4). To determine whether ABCA1 knockdown affects apoA-I secretion, HepG2 cells were treated with 2 different ABCA1-siRNA sequences and apoA-I secretion was measured. ABCA1 protein expression was reduced to below detection limits. Knockdown had minimal effect on basal apoA-I secretion but almost completely abolished DLPC-induced hepatic apoA-I secretion (Figure 5, A and B).
Figure 3.
Phospholipids inhibit ABCA1 and ABCG1 expression in HepG2 cells. HepG2 cells were incubated with PI, DLPC (12 μmol/L) or PBS as a control for 24 hours and ABC transporter expression was analyzed by Western blot. Histograms representing densitometry analysis of ABCA1 (A) and ABCG1 (B) are shown and the values are presented relative to total β-actin control and are expressed as mean ± SEM of at least four independent experiments. *P < 0.05, **P < 0.001 vs control.
Figure 4.
Effect of phospholipids on cholesterol secretion. HepG2 cells were incubated with 3H-cholesterol (5 μCi/ml) in complete media for 24 hours, washed, and then incubated for an additional 24 hours with PI or DLPC (12 μmol/L) in serum-free media. Media were analyzed to quantify media radioactivity and measure cholesterol levels in the d >1.063 lipoproteins (HDL) and d <1.063 lipoproteins. Radioactive cholesterol levels are presented as relative to total cell protein values and are expressed as average ± SEM of three independent experiments. *P < 0.001 vs control.
Figure 5.
ABCA1-siRNA blocks DLPC-induced hepatic apoA-I secretion. HepG2 cells were transfected with scrambled or ABCA1-siRNA as described and then treated with DLPC (12 μmol/L) for 24 hours. A: ABCA1 knockdown by siRNA was confirmed by Western blot analysis. B: ApoA-I was quantified in the media by ELISA. ApoA-I secretion is presented relative to total cell protein values and are expressed as mean ± SEM of four independent experiments; **P < 0.001 vs DLPC.
Phospholipids Affect HepG2 Cell HDL Speciation
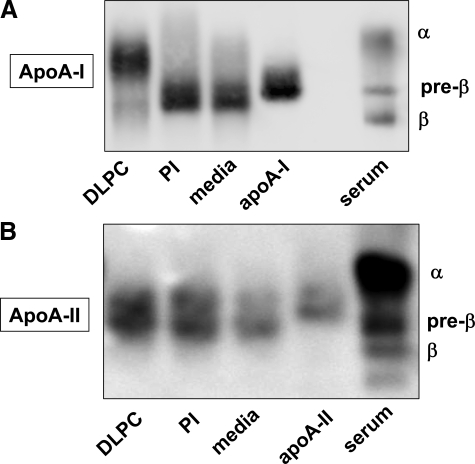
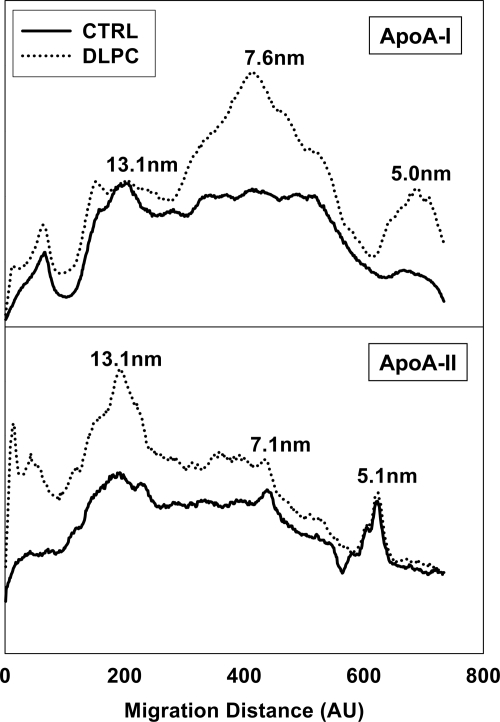
To determine the impact of ABC transporter inhibition by PI and DLPC on the kinds of HDL particles secreted by HepG2 cells, media HDL particles were characterized. Immunoblots of agarose gels showed that HepG2 cells primarily secrete HDL complexes that contain both apoA-I and apoA-II and exhibit a pre-β electrophoretic mobility (Figure 6, A and B). PI treatment increased the secretion of pre-β HDL particles, while DLPC treatment promoted the secretion of HDL complexes with an α-electrophoretic migration (Figure 6). DLPC promoted α-HDL secretion even at early (4 hours) time points (not shown). Non-denaturing gradient gels showed that, much as others have shown,8,31 HepG2 cells secrete HDL complexes ranging in size from 7 to 13 nm. Figure 7 illustrates densitometry profiles of immunoblots of conditioned PI or DLPC media samples that were electrophoresed on non-denaturing gradient gels and probed for apoA-I and apoA-II. DLPC stimulates the formation of 7.6 nm and 5.1 nm (lipid-poor) apoA-I particles (Figure 7, upper panel). PI has a similar effect on apoA-I secretion (not shown). DLPC also increases the HepG2 cell secretion of apoA-II, which is primarily found associated with larger apoA-I complexes, with a mean Stokes’ diameter of ∼13 nm (Figure 7, lower panel).
Figure 6.
Agarose electrophoresis of HepG2 cell media. HepG2 cells were incubated with PI and DLPC (12 μmol/L) for 24 hours and agarose gel electrophoresis was performed using the conditioned media. Agarose gels were immunoblotted for apoA-I (A) and apoA-II (B) to measure the electrophoretic mobility of HDL complexes secreted by HegG2 cells. The image shown is from one experiment and is representative of four independent experiments.
Figure 7.
Phospholipids affect HDL speciation. Non-denaturing gradient gel electrophoresis was performed on conditioned media from control and DLPC treated HepG2 cells and immunoblotted for apoA-I (upper panel) and apoA-II (lower panel). Densitometry profiles show that both apoA-I and apoA-II are associated with HDL complexes with Stoke’s diameters of 7 to 13 nm. The image shown is from one experiment and is representative of three independent experiments.
LXRα-Modulators Affect Phospholipid-Induced apoA-I Secretion
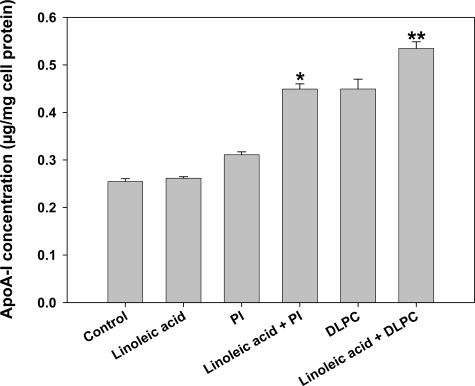
The nuclear receptor LXR has been shown to regulate the expression and activity of ABC transporters7 and therefore experiments were undertaken to test the effects of LXRα-antagonists/agonists on PI- and DLPC-stimulated apoA-I secretion from HepG2 cells. Since unsaturated fatty acids, such as linoleic acid, are known inhibitors of ABC transporter expression,32,33 we tested the effect of linoleic acid pretreatment on PI and DLPC induced apoA-I secretion in HepG2 cells. Linoleic acid (125 μmol/L) attenuated hepatic ABCA1 expression by ∼25% (Supplementary Figure S3, see http://ajp.amjpathol.org). Linoleic acid itself did not increase hepatic apoA-I secretion in HepG2 cells,25 however, linoleic acid augmented the effects of the PI and DLPC (Figure 8). When linoleic acid (125 μmol/L) was added to the HepG2 cells, the inductions in apoA-I secretion by PI and DLPC (12 μmol/L) were augmented by about 50% and 25% respectively.
Figure 8.
Effect of linoleic acid on apoA-I secretion. Aqueous vesicular mixtures containing 12 μmol/L of PI, DLPC and/or linoleic acid (LA) (125 μmol/L) were added to HepG2 cells and incubated for 24 hours. ApoA-I was quantified in the media by ELISA. ApoA-I secretion is presented relative to total cell protein values and are expressed as mean ± SEM of four independent experiments; *P < 0.001 vs PI, **P < 0.05 vs DLPC.
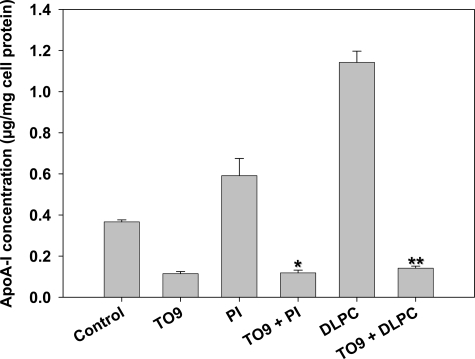
In contrast, the LXR agonist TO901317 (10 μmol/L) increased hepatic ABCA1 expression by 30% (Supplementary Figure S4, see http://ajp.amjpathol.org) and significantly down-regulated PI- and DLPC-induced apoA-I secretion from HepG2 cells (Figure 9). The LXR-agonist TO901317 was able to down-regulate basal apoA-I secretion, similar to that shown by Huuskonen and colleagues,8 and completely inhibited the stimulation of apoA-I secretion by PI and DLPC (Figure 9). At the dose used (10 μmol/L), TO901317 had no effect on cell viability or cellular protein (β-actin) levels. Resveratrol has also been shown to be an LXR agonist34 and, as with TO901317, resveratrol (10 μmol/L) inhibited PI- and DLPC-induced apoA-I secretion (Supplementary Figure S5, see http://ajp.amjpathol.org).
Figure 9.
Effect of the LXR agonist, TO901317, on apoA-I secretion. HepG2 cells were pre-incubated with 10 μmol/L of TO901317 for 30 minutes. and then with PI or DLPC (12 μmol/L) for 24 hours. ApoA-I was quantified in the media by ELISA. ApoA-I secretion is presented relative to total cell protein values and are expressed as mean ± SEM of three independent experiments; *P < 0.001 vs PI, **P < 0.001 vs DLPC.
Chemical Inhibition of ABCA1 Degradation Inhibits Phospholipid-Induced apoA-I Secretion
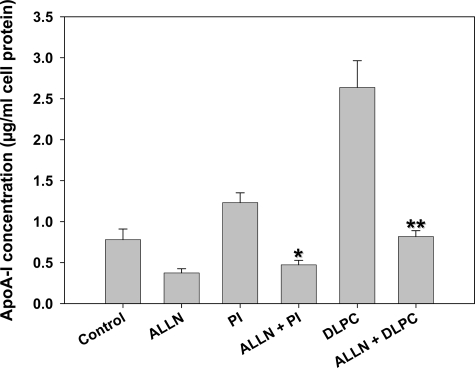
Cell surface ABCA1 levels have been shown to be increased by the calpain protease inhibitor, ALLN, through an inhibition of the cellular degradation of ABCA1.35 We therefore tested the effect of ALLN on PI- and DLPC-induced apoA-I secretion from HepG2 cells. Similar to that previously reported, ALLN (26 μmol/L) increased ABCA1 expression by 20% (Supplementary Figure S6, see http://ajp.amjpathol.org). ALLN (26 μmol/L) also reduced basal apoA-I secretion in HepG2 cells by >50% (Figure 10). ALLN completely blocked the PI- and DLPC-induced apoA-I secretion (Figure 10). ALLN had no effect on cell viability or cellular protein levels.
Figure 10.
Effect of the calpain inhibitor ALLN on apoA-I secretion. HepG2 cells were pre-incubated with 26 μmol/L of ALLN for 30 minutes. and then with PI or DLPC (12 μmol/L) for 24 hours. ApoA-I was quantified in the media by ELISA. ApoA-I secretion is presented relative to total cell protein values and are expressed as mean ± SEM of three independent experiments; *P < 0.001 vs PI, **P < 0.001 vs DLPC.
Discussion
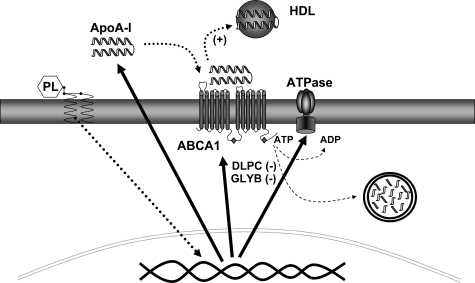
HDL synthesis and secretion does not appear to be regulated purely by the factors that control apoA-I synthesis. ApoA-I gene transcriptional stimulants, such as the PPARα agonist fibrate drugs, only modestly affect HDL secretion by hepatocytes25,36,37,38 and HDL levels in vivo.39 The most effective HDL therapeutic compound, niacin, appears to have little effect on apoA-I gene transcription, and instead regulates the recycling/reuptake of newly secreted apoA-I.24,40,41 While newly secreted apoB can also be taken up and degraded by hepatocytes, through a reuptake pathway, the importance of this pathway in the net secretion of apoB appears small.42 In contrast, apoA-I reuptake and degradation appears centrally important to HDL secretion and LA-phospholipid stimulants of HDL secretion appear to primarily act through this route.25,26,27 Much as niacin, these lipids do not increase apoA-I gene transcription but instead appear to impact the secretory process by regulating the retroendocytic degradation of apoA-I (Figure 11).26
Figure 11.
Linoleic acid-phospholipids increase apoA-I secretion and reduce the expression and activity of membrane ATPases. Linoleic acid-phospholipids act through mitogen-activated protein kinase and PPARα to promote apoA-I secretion. Induction of HDL secretion is associated with reduced levels of F1-ATPase and ABC transporters. Compounds that inhibit ATPase activity, DLPC, and glyburide (GLYB), stimulate apoA-I secretion. Compounds that increase membrane ATPase levels, inhibit apoA-I secretion. HDL secretion therefore appears closely linked to membrane ATPase protein expression and activity.
HDL retroendocytic pathways appear to involve several cell surface HDL binding proteins. A number of candidate HDL receptors have been identified in hepatocyte membranes, but the proteins shown to play the most active role in interactions with apoA-I are SR-BI and ABCA1.43 SR-BI has been shown to play a role in the uptake of HDL lipids in steroidogenic tissues and its importance in modulating the selective uptake of HDL cholesterol by the liver is well established.44 While SR-BI has been suggested to be important to the retroendocytic metabolism of HDL,45 siRNA knockdown studies have shown that SR-BI has little effect on the internalization of HDL.46 In contrast, other studies have shown that SR-BI over expression stimulates an HDL retroencocytic pathway that results in the uptake and resecretion of intact HDL particles.47 While PI had no effect on SR-BI expression, DLPC was shown to slightly reduce the expression of SR-BI in HepG2 cells (Supplementary Figure S7, see http://ajp.amjpathol.org). This suggests that SR-BI may be involved in regulating HDL secretion.
ABCA1 is also known to bind and interact with apoA-I48,49 and ABCA1 has been shown to play a central role in the internalization and intracellular transport of HDL.46 ABCA1 is believed to play a central role in regulating plasma HDL metabolism and mutations in the ABCA1 gene are known to be closely associated with low HDL levels in humans.50 Conversely, hepatocytes from ABCA1 knockout mice have been shown to have relatively normal apoA-I secretion.51,52 This appears in agreement with our ABCA1 siRNA result, which shows normal basal apoA-I secretion after a total blockage of ABCA1 expression (Figure 5). The fact that a blockage of ABCA1 expression has little effect on apoA-I secretion, suggests that ABCA1 is not solely responsible for the regulation of membrane retroendocytic pathways that control HDL secretion. The siRNA inhibition in ABCA1 expression, however, completely blocked a DLPC induction in apoA-I secretion. This shows that ABCA1 is a participant in the pathway that regulates the ability of the liver to stimulate HDL secretion. It may suggest that mutations that block expression of ABCA1 may be partly causative to low HDL levels, through an inhibition in the ability to up-regulate HDL secretion. The result may partly explain the low HDL levels seen in targeted ABCA1 knockout animals52 and patients with ABCA1 mutations that block expression.50
Western blot analysis showed that LA-phospholipids significantly decrease the cellular levels of ABCA1 and ABCG1 (Figure 3). LA-phospholipids therefore differ from classical PPARα agonists, ie, the fibrate drugs, which increase ABC transporter levels through PPARα-dependent LXR cross talk.53,54 LA-phospholipids may affect ABC transporter levels by enhancing the transport of unsaturated fatty acids into the cell. Unsaturated fatty acids have been shown to decrease ABCA1 and ABCG1 expression.32,33,55,56 Linoleic acid has been shown to directly reduce cellular ABCA1 protein levels through either an LXR/RXR promoter regulation33,55 or by phosphorylating and destabilizing ABCA1.56 Linoleic acid has no apoA-I secretory activity on its own, but its augmentation of the apoA-I secretory activities of PI and DLPC (Figure 8) appears to be related to effects on ABC transporter expression. Several studies suggest that ABCA1 plays a central role in the retroendocytic metabolism of HDL.11,12,13,14,46
The effect of linoleic acid on PI and DLPC-induced apoA-I secretion was similar to that observed with glyburide (Figures 1 and 8). Glyburide has no effect on the expression of ABC transporters but instead, is an inhibitor of the ATPase activity of ABCA1.17 Glyburide has been shown to block apoA-I binding to ABCA19,10 and to inhibit apoA-I/ABCA1 dependent cellular signaling.18 Glyburide has a small effect on HepG2 cells apoA-I secretion on its own, but synergistically affects the apoA-I secretory activities of PI and DLPC (Figure 1). This appears to be consistent with clinical studies that suggest glyburide may have a small HDL raising capacity.15,16 Glyburide also appears to stimulate the apoA-I secretion effects of a more classic PPARα agonist, clofibrate (data not shown). This supports the clinical findings, which suggest that adding a sulfonylurea to a fibrate therapy will increase patient HDL levels.19
In contrast to the effects of linoleic acid and glyburide, compounds that are known to increase cellular ABC transporter levels all inhibited apoA-I secretion (Figures 9 and 10). This was clearly evident with the LXR agonist TO901317 (Figure 9). TO901317 completely blocked the apoA-I secretory effects of both PI and DLPC, and also reduced the basal apoA-I secretion by ∼70%. This is consistent to the work of Huuskonen et al, which showed the same LXR agonist to inhibit the synthesis and secretion of apoA-I, through a transcriptional regulation involving hepatic nuclear factor-4.8 Resveratrol is different class of compound that has LXR agonist activity and raises ABCA1 levels34 and also has inhibitory effects on LA-phospholipid-induced apoA-I secretion. However, resveratrol is also a potent F1-ATPase inhibitor57 and its effects on apoA-I secretion were similar to that seen with oligomycin. ALLN is a calpain protease inhibitor, which has been shown to block degradative pathways and increase membrane ABCA1 levels.35 ALLN had almost identical effects on ABCA1 expression and apoA-I secretion to the LXR agonist (Figure 10). Taken together, the data shows that ABCA1 expression and activity are inversely related to LA-phospholipid-induced apoA-I secretion.
An LA-phospholipid-induced decrease in the expression and activity of ABC transporters would be expected to reduce cellular lipid secretion. This view was confirmed in experiments that showed reduced cellular cholesterol secretion in phospholipid treated cells (Figure 4). We show that DLPC blocks cellular cholesterol secretion and reduces cholesterol levels in the d >1.063 lipoproteins (HDL), but has no effect on cholesterol levels in the d <1.063 lipoproteins. However, even though the DLPC treated cells secrete less cholesterol, they appear to secrete more α-migrating HDL particles. HepG2 cells therefore appear able to secrete a lipid poor α-HDL. Both control and PI-treated HepG2 cells were shown to secrete nascent HDL particles with a pre-β electrophoretic mobility in agarose, while DLPC stimulated the secretion of α-migrating HDL particles containing both apoA-I and apoA-II (Figures 4 and 6). Immunoblots of non-denaturing gradient gels showed that apoA-II was primarily associated with larger apoA-I complexes having a mean Stokes’ diameter of >13 nm (Figure 6). This work therefore suggests that HepG2 cells can secrete both nascent and more complex HDL particles. Surprisingly, secretion of α-migrating HDL species is associated with a significant reduction in the expression of cellular ABCA1 and ABCG1 levels. This may suggest that HDL speciation may not require ABC transporters or that other intracellular or secreted factors may be stimulated by DLPC to promote the formation of a multiapoprotein α-migrating HDL complex.
This study shows that while ABCA1 transporters are involved in HDL secretion, they do not at alone in this process. ABC transporters may contribute as membrane ATPases. Linoleic acid has in fact been shown to inhibit other ATPase activities in the cell.32,55,58 Ecto-F1F0-ATP synthase (F1-ATPase) is a membrane ATPase that modulates an ADP-dependent, G-protein-retroendocytic uptake of HDL. A novel G-protein coupled receptor, P2Y13, has been recently shown to bind apoA-I and regulate its retroendocytic uptake.22 P2Y13 appears to be regulated by the coordinated efforts of F1-ATPase and adenylate kinase, which both play a role in modulating extracellular ADP levels.20,23,59 This pathway may be central to the HDL raising effects of niacin. By inhibiting F1-ATPase expression on the cell surface, niacin appears to block the reuptake of HDL and promote increased HDL secretion.24 LA-phospholipids also significantly decrease membrane F1-ATPase levels and may therefore act much like niacin to stimulate HDL secretion (Figure 11). Oligomycin inhibition of total cellular F1-ATPase, however, did not mimic the effects of DLPC and instead totally blocked apoA-I secretion. This may be expected as protein synthesis and secretion are known to be energy dependent and sensitive to oligomycin.60,61 It also may suggest that cellular apoA-I retroendocytic events may be uniquely sensitive to only the membrane, F1-ATPase activity.
We have shown that LA-phospholipids stimulate hepatic apoA-I secretion by blocking cell surface binding and degradation of apoA-I.26 LA-phospholipids appear to prevent membrane-recycling events by blocking ATPase ADP production on the cell surface and inhibiting the retroendocytic uptake of newly secreted HDL (Figure 11). Through this mechanism, DLPC appears to act similar to other structurally dissimilar HDL raising therapeutics, which may suggest that HDL secretion is fundamentally regulated by factors that modulate the endocytic recycling of apoA-I.
Supplementary Material
Acknowledgments
We thank Dr. Yves Marcel for the mouse monoclonal anti-human apoA-I antibody, Dr. Heidi McBride for the peroxiredoxin-3 antibody, and Dr. Vincent Soubannier for technical advice.
Footnotes
Address reprint requests to Daniel L. Sparks, Ph.D., The University of Ottawa Heart Institute, 40 Ruskin Street, Ottawa, Ontario, Canada, K1Y 4W7. E-mail: dsparks@ottawaheart.ca.
Supported by a grant from the Heart and Stroke Foundation of Canada (D.L.S.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Barter P, Kastelein J, Nunn A, Hobbs R. High density lipoproteins (HDLs) and atherosclerosis; the unanswered questions. Atherosclerosis. 2003;168:195–211. doi: 10.1016/s0021-9150(03)00006-6. [DOI] [PubMed] [Google Scholar]
- Brewer HB, Jr, Santamarina-Fojo S. New insights into the role of the adenosine triphosphate-binding cassette transporters in high-density lipoprotein metabolism and reverse cholesterol transport. Am J Cardiol. 2003;91:3E–11E. doi: 10.1016/s0002-9149(02)03382-9. [DOI] [PubMed] [Google Scholar]
- Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE. Cellular cholesterol efflux. Biochim Biophys Acta. 2001;1533:175–189. doi: 10.1016/s1388-1981(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Oram JF. HDL Apolipoproteins and ABCA1: partners in the removal of excess cellular cholesterol. Arterioscler Thromb Vasc Biol. 2003;23:720–727. doi: 10.1161/01.ATV.0000054662.44688.9A. [DOI] [PubMed] [Google Scholar]
- Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huuskonen J, Vishnu M, Chau P, Fielding PE, Fielding CJ. Liver X receptor inhibits the synthesis and secretion of apolipoprotein A1 by human liver-derived cells. Biochemistry. 2006;45:15068–15074. doi: 10.1021/bi061378y. [DOI] [PubMed] [Google Scholar]
- Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- Nieland TJ, Chroni A, Fitzgerald ML, Maliga Z, Zannis VI, Kirchhausen T, Krieger M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J Lipid Res. 2004;45:1256–1265. doi: 10.1194/jlr.M300358-JLR200. [DOI] [PubMed] [Google Scholar]
- Hassan HH, Denis M, Lee DY, Iatan I, Nyholt D, Ruel I, Krimbou L, Genest J. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J Lipid Res. 2007;48:2428–2442. doi: 10.1194/jlr.M700206-JLR200. [DOI] [PubMed] [Google Scholar]
- Denis M, Landry YD, Zha X. ATP-binding cassette A1-mediated lipidation of apolipoprotein A-I occurs at the plasma membrane and not in the endocytic compartments. J Biol Chem. 2008;283:16178–16186. doi: 10.1074/jbc.M709597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner LE, Panagotopulos SE, Johnson JD, Woollett LA, Hui DY, Witting SR, Maiorano JN, Davidson WS. An analysis of the role of a retroendocytosis pathway in ABCA1-mediated cholesterol efflux from macrophages. J Lipid Res. 2008;49:1322–1332. doi: 10.1194/jlr.M800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HH, Bailey D, Lee DY, Iatan I, Hafiane A, Ruel I, Krimbou L, Genest J. Quantitative analysis of ABCA1-dependent compartmentalization and trafficking of apolipoprotein A-I: implications for determining cellular kinetics of nascent high density lipoprotein biogenesis. J Biol Chem. 2008;283:11164–11175. doi: 10.1074/jbc.M707720200. [DOI] [PubMed] [Google Scholar]
- Singh T, Singh S, Bhullar GS. The effect of sulphonylurea therapy on serum total cholesterol and high density lipoprotein cholesterol. J Indian Med Assoc. 1992;90:259–261. [PubMed] [Google Scholar]
- Kerenyi Z, Samer H, James R, Yan Y, Stewart M. Combination therapy with rosiglitazone and glibenclamide compared with upward titration of glibenclamide alone in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;63:213–223. doi: 10.1016/j.diabres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kimura Y, Kioka N, Matsuo M, Ueda K. Purification and ATPase activity of human ABCA1. J Biol Chem. 2006;281:10760–10768. doi: 10.1074/jbc.M513783200. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Remaley AT, Feuerborn R, Wolinnska I, Engel T, von EA, Assmann G. Apolipoprotein A-I activates Cdc42 signaling through the ABCA1 transporter. J Lipid Res. 2006;47:794–803. doi: 10.1194/jlr.M500502-JLR200. [DOI] [PubMed] [Google Scholar]
- Smud R, Sermukslis B. Bezafibrate and fenofibrate in type II diabetics with hyperlipoproteinaemia. Curr Med Res Opin. 1987;10:612–624. doi: 10.1185/03007998709112415. [DOI] [PubMed] [Google Scholar]
- Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezon E, Champagne E, Pineau T, Georgeaud V, Walker JE, Terce F, Collet X, Perret B, Barbaras R. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- Martinez LO, Jacquet S, Terce F, Collet X, Perret B, Barbaras R. New insight on the molecular mechanisms of high-density lipoprotein cellular interactions. Cell Mol Life Sci. 2004;61:2343–2360. doi: 10.1007/s00018-004-4087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet S, Malaval C, Martinez LO, Sak K, Rolland C, Perez C, Nauze M, Champagne E, Terce F, Gachet C, Perret B, Collet X, Boeynaems JM, Barbaras R. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci. 2005;62:2508–2515. doi: 10.1007/s00018-005-5194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre AC, Vantourout P, Champagne E, Terce F, Rolland C, Perret B, Collet X, Barbaras R, Martinez LO. Cell surface adenylate kinase activity regulates the F(1)-ATPase/P2Y (13)-mediated HDL endocytosis pathway on human hepatocytes. Cell Mol Life Sci. 2006;63:2829–2837. doi: 10.1007/s00018-006-6325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Kamanna VS, Zhang MC, Kashyap ML. Niacin inhibits surface expression of ATP synthase {beta} chain in HepG2 cells: implications for raising HDL. J Lipid Res. 2008;49:1195–1201. doi: 10.1194/jlr.M700426-JLR200. [DOI] [PubMed] [Google Scholar]
- Pandey NR, Renwick J, Misquith A, Sokoll K, Sparks DL. Linoleic acid-enriched phospholipids act through peroxisome proliferator-activated receptors alpha to stimulate hepatic apolipoprotein A-I secretion. Biochemistry. 2008;47:1579–1587. doi: 10.1021/bi702148f. [DOI] [PubMed] [Google Scholar]
- Hopewell S, Pandey NR, Misquith A, Twomey E, Sparks DL. Phosphatidylinositol acts through mitogen-activated protein kinase to stimulate hepatic apolipoprotein A-I secretion. Metabolism. 2008;57:1677–1684. doi: 10.1016/j.metabol.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Pandey NR, Sparks DL. Phospholipids as cardiovascular therapeutics. Curr Opin Investig Drugs. 2008;9:281–285. [PubMed] [Google Scholar]
- Kauffmann HM, Keppler D, Kartenbeck J, Schrenk D. Induction of cMrp/cMoat gene expression by cisplatin, 2-acetylaminofluorene, or cycloheximide in rat hepatocytes. Hepatology. 1997;26:980–985. doi: 10.1002/hep.510260427. [DOI] [PubMed] [Google Scholar]
- Draber P, Draberova L, Heneberg P, Smid F, Farghali H, Draber P. Preformed STAT3 transducer complexes in human HepG2 cells and rat hepatocytes. Cell Signal. 2007;19:2400–2412. doi: 10.1016/j.cellsig.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Phillips MC. Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J Lipid Res. 1992;33:123–130. [PubMed] [Google Scholar]
- Chau P, Nakamura Y, Fielding CJ, Fielding PE. Mechanism of prebeta-HDL formation and activation. Biochemistry. 2006;45:3981–3987. doi: 10.1021/bi052535g. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oram JF. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem. 2002;277:5692–5697. doi: 10.1074/jbc.M109977200. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Miura S, von von Eckardstein A, Abe S, Fujii A, Matsuo Y, Rust S, Lorkowski S, Assmann G, Yamada T, Saku K. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191:11–21. doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Sevov M, Elfineh L, Cavelier LB. Resveratrol regulates the expression of LXR-alpha in human macrophages. Biochem Biophys Res Commun. 2006;348:1047–1054. doi: 10.1016/j.bbrc.2006.07.155. [DOI] [PubMed] [Google Scholar]
- Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- Vu-Dac N, Schoonjans K, Laine B, Fruchart JC, Auwerx J, Staels B. Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J Biol Chem. 1994;269:31012–31018. [PubMed] [Google Scholar]
- Kockx M, Princen HMG, Kooistra T. Studies on the role of PPAR in the fibrate-modulated gene expression of apolipoprotein A-I, plasminogen activator inhibitor 1, and fibrinogen in primary hepatocyte cultures from cynomolgus monkey. Ann NY Acad Sci. 1996;804:711–712. doi: 10.1111/j.1749-6632.1996.tb18676.x. [DOI] [PubMed] [Google Scholar]
- Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol. 2008;28:39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- Jin FY, Kamanna VS, Kashyap ML. Niacin decreases removal of high-density lipoprotein apolipoprotein A-I but not cholesterol ester by Hep G2 cells. Implication for reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 1997;17:2020–2028. doi: 10.1161/01.atv.17.10.2020. [DOI] [PubMed] [Google Scholar]
- Malik S, Kashyap ML. Niacin, lipids, and heart disease. Curr Cardiol Rep. 2003;5:470–476. doi: 10.1007/s11886-003-0109-x. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Brocia RW, Fisher EA. The unstirred water layer as a site of control of apolipoprotein B secretion. J Biol Chem. 1990;265:16741–16744. [PubMed] [Google Scholar]
- Hersberger M, von Eckardstein A. Modulation of high-density lipoprotein cholesterol metabolism and reverse cholesterol transport. Handb Exp Pharmacol. 2005;170:537–561. doi: 10.1007/3-540-27661-0_20. [DOI] [PubMed] [Google Scholar]
- Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276:25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- Cavelier C, Rohrer L, von Eckardstein A. ATP-Binding cassette transporter A1 modulates apolipoprotein A-I transcytosis through aortic endothelial cells. Circ Res. 2006;99:1060–1066. doi: 10.1161/01.RES.0000250567.17569.b3. [DOI] [PubMed] [Google Scholar]
- Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer C, Volf I, Pavelka M, Eckhardt ER, van der Westhuyzen DR, Schutz GJ, Stangl H. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J Biol Chem. 2006;281:11193–11204. doi: 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim Biophys Acta. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Singaraja RR, Brunham LR, Visscher H, Kastelein JJ, Hayden MR. Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler Thromb Vasc Biol. 2003;23:1322–1332. doi: 10.1161/01.ATV.0000078520.89539.77. [DOI] [PubMed] [Google Scholar]
- Maric J, Kiss RS, Franklin V, Marcel YL. Intracellular lipidation of newly synthesized apolipoprotein A-I in primary murine hepatocytes. J Biol Chem. 2005;280:39942–39949. doi: 10.1074/jbc.M507733200. [DOI] [PubMed] [Google Scholar]
- Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JZ, Tamasawa N, Murakami H, Matsui J, Yamato K, Suda T. Clofibrate, a peroxisome-proliferator, enhances reverse cholesterol transport through cytochrome P450 activation and oxysterol generation. Tohoku J Exp Med. 2003;201:251–259. doi: 10.1620/tjem.201.251. [DOI] [PubMed] [Google Scholar]
- Knight BL, Patel DD, Humphreys SM, Wiggins D, Gibbons GF. Inhibition of cholesterol absorption associated with a PPAR alpha-dependent increase in ABC binding cassette transporter A1 in mice. J Lipid Res. 2003;44:2049–2058. doi: 10.1194/jlr.M300042-JLR200. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, Langer C, Schachtrup C, Wiekowski J, Lorkowski S, Assmann G, von Eckardstein A. Polyunsaturated fatty acids and acetoacetate downregulate the expression of the ATP-binding cassette transporter A1. Diabetes. 2002;51:2922–2928. doi: 10.2337/diabetes.51.10.2922. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J Biol Chem. 2005;280:35896–35903. doi: 10.1074/jbc.M506210200. [DOI] [PubMed] [Google Scholar]
- Gledhill JR, Walker JE. Inhibition sites in F1-ATPase from bovine heart mitochondria. Biochem J. 2005;386:591–598. doi: 10.1042/BJ20041513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burth P, Younes-Ibrahim M, Santos MC, Castro-Faria Neto HC, de Castro Faria MV. Role of nonesterified unsaturated fatty acids in the pathophysiological processes of leptospiral infection. J Infect Dis. 2005;191:51–57. doi: 10.1086/426455. [DOI] [PubMed] [Google Scholar]
- Champagne E, Martinez LO, Collet X, Barbaras R. Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr Opin Lipidol. 2006;17:279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- McLeod LE, Proud CG. ATP depletion increases phosphorylation of elongation factor eEF2 in adult cardiomyocytes independently of inhibition of mTOR signalling. FEBS Lett. 2002;531:448–452. doi: 10.1016/s0014-5793(02)03582-2. [DOI] [PubMed] [Google Scholar]
- Stefanelli C, Bonavita F, Stanic I, Farruggia G, Falcieri E, Robuffo I, Pignatti C, Muscari C, Rossoni C, Guarnieri C, Caldarera CM. ATP depletion inhibits glucocorticoid-induced thymocyte apoptosis. Biochem J. 1997;322 (Pt 3):909–917. doi: 10.1042/bj3220909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.