Abstract
To determine if reproductive factors or exogenous estrogen are associated with risk of Parkinson’s disease (PD), we conducted a prospective study with 22 years of follow-up among post-menopausal participants in the Nurses’ Health Study. Relative risks (RRs) and 95% confidence intervals (CIs) of PD were estimated from a Cox proportional hazards model adjusting for potential confounders. Risk of PD was not significantly associated with any of the reproductive factors measured or exogenous estrogen use. Use of post-menopausal hormones, however, may modify the associations of smoking and caffeine intake with PD risk. The inverse relation between smoking and PD risk was attenuated among ever users of post-menopausal hormones (p for interaction = 0.05). Similar results were obtained for caffeine (p for interaction = 0.09). In exploratory analyses, women using progestin-only hormones were found to have an increased PD risk, but this result was based on a very small number of cases (n=4). In this large longitudinal study, we found no evidence of a beneficial effect of exogenous or endogenous estrogens on risk of PD. The use of post-menopausal hormone use may interact with other risk factors, but findings are preliminary and need confirmation in other populations.
Introduction
An increased risk of Parkinson’s disease (PD) in men compared to women is well established; men are approximately two times as likely as women to develop the disease1, 2. Experimental evidence supports a potential neuroprotective role for estrogens. Specifically, ovariectomized and male animals have decreased dopaminergic functioning compared to females with intact ovaries,3 and nigrostriatal dopaminergic damage following administration of neurotoxic agents is reduced in females compared to males4, 5. Additionally, administration of β-estradiol in vitro and in animal models of PD attenuates injury or reduces depletion of dopamine induced by neurotoxic agents6.
The association between estrogen use and risk of PD may be modified by other factors, specifically caffeine and smoking. Results of epidemiological studies suggest a reduced risk of PD associated with caffeine intake7 among women who do not use post-menopausal hormones (PMHs), but not among PMH users8, 9. This epidemiologic observation has been further supported by experimental evidence in ovariectomized mice in which caffeine confers neuroprotection with lower potency when estrogen (rather than placebo) is administered10. Similarly, there is consistently strong evidence that smoking is associated with a decreased risk of PD7 and exposure to smoking may increase circulating levels of estrogens11.
Epidemiologic evidence assessing the association between reproductive factors and hormone use and risk of PD has been inconsistent. In particular, use of PMHs has been associated with decreased risk in two studies12, 13, while no association has been found in others 9, 14–17. Further, the effect of PMHs is possibly dependent on type of menopause17. Some of these inconsistencies may be due to the retrospective exposure assessment and lack of consideration of smoking and other potential confounders in many of these studies.
To explore the potential role of endogenous and exogenous estrogen exposure and risk of PD, we conducted a prospective cohort study amongst participants in a large, longitudinal cohort, the Nurses’ Health Study (NHS). This prospective exposure assessment allows for detailed information on reproductive history and importantly, PMH use, which may vary over time. Because post-menopausal hormones may be a combination of progestin and estrogens, we have also utilized detailed information on preparation type to further explore this relationship. Progesterone has been shown to be neuroprotective and potentially modulate the activity of estrogen in PD animal models18, 19. Therefore, the specific type of hormone used may be relevant to assess the relationship with risk of PD.
Methods
Study population
The study population included participants in the Nurses’ Health Study. Briefly, the NHS cohort began in 1976 when 121,701 nurses aged 30–55 from one of eleven states returned mailed questionnaires regarding lifestyle factors, medical conditions and disease history20. The follow-up period lasted from the return date of the 1980 questionnaire or the date of the questionnaire in which a woman reported her periods had permanently ceased to the date of diagnosis of PD, death, date of diagnosis of stroke, loss to follow-up or the end of the study (June 30, 2002). Women with prevalent and incident stroke as well as prevalent PD were excluded from the analysis.
PD ascertainment
Case ascertainment for PD has been previously described21. Briefly, a question on lifetime occurrence of PD was first asked in 1994 and updated biennially. Participants who reported PD were asked permission to obtain from the treating neurologist (or internist or general practitioner) either a questionnaire confirming the diagnosis of PD or a copy of the medical records. A case is confirmed if the diagnosis is considered clinically definite or probable by the treating neurologist or internist, or if the medical record includes either a final diagnosis of PD made by a neurologist, or evidence at a neurological examination of at least 2 of the 3 cardinal signs (rest tremor, rigidity, and bradykinesia) in the absence of clinical features suggesting other diagnoses22 or non-responsiveness to levodopa treatment. The review of medical records was conducted by the investigators, blind to the exposure status. Overall, the diagnosis was confirmed by the treating neurologist in 87.4 % of the cases, by review of the medical records in 4.7 %, and by the treating internist without further support in the remaining 7.9 %.
In addition to self-reported PD, cases were also ascertained through examination of death certificates. Deaths are reported through next of kin, coworkers, postal authorities or the National Death Index and the follow-up for deaths in this cohort has been estimated to be 98%23. If PD was reported on the death certificate, permission was requested from the family to confirm disease in the same manner as previously described.
Exposure assessment
Menopause, menarche and parity
In 1976, women were asked their age of menarche and menopausal status. Further questions were asked of menopausal women, including age at menopause and type of menopause - natural, surgical, or radiation. Menopausal status was updated biennially. Parity, defined as pregnancy lasting at least 6 months, was assessed at baseline and biennially through 1984 and again in 1996. For variables that may change over time, these were assessed through the biennial questionnaires and exposure status updated. The accuracy of self-reported age at menopause, age at menarche and type of menopause has been validated in this cohort24.
Post-menopausal hormone use (PMHs)
A question on PMH use was asked biennially beginning in 1976. Specifically, at baseline, women were asked if following the cessation of menstruation, did they take female hormones and if so, for how long. Beginning in 1984, women were asked about specific types of PMH use allowing classification of estrogen only, progestin only or combination hormone use.
Oral contraceptive use
Oral contraceptive use was asked at baseline and biennially until 1984, at which point no women reported current use and therefore, after 1984, use was defined as ever/never. The validity of self-reported information regarding oral contraceptives has been validated in a complementary cohort, the Nurses’ Health Study II25.
Assessment of covariates
The 1980 survey included a food frequency questionnaire (FFQ) for the first time to assess participants’ dietary habits during the past year. Dietary surveys were updated roughly every four years thereafter. The reproducibility and validity of these FFQs have been assessed by comparison with detailed dietary records26.
Smoking history was first reported on the 1976 questionnaire and then updated biennially. Questions at baseline for current smokers included number of daily cigarettes and questions at baseline for ever smokers included average number of daily cigarettes. On subsequent questionnaires, current smoking information was updated biennially and updated pack-years of smoking thus calculated. Adult height was asked in 1976 and body weight was updated biennially; body mass index (BMI) was calculated by dividing height in kilograms by the square of height in meters. Total physical activity was calculated in metabolic equivalent tasks (METs)27.
Data Analysis
Cox proportional hazards models were used to calculate relative risks (RRs) and 95% confidence intervals (CIs) to assess the association between parity, PMH use, OC use, age at menopause and age at menarche and risk of PD adjusting for age in years and pack-years of smoking; multivariate analyses further controlled for alcohol consumption (average g/day), caffeine intake (average cumulative mg/day), physical activity (METS/wk) and BMI (kg/m2: <23, 23–25, 25–27, 28–30, 30+).
These multivariate analyses generated results similar to the age- and smoking-adjusted estimates and therefore the age- and smoking-adjusted estimates are presented. Further, sensitivity analyses were conducted including only cases with a clinical definite diagnosis of PD or with diagnoses by a neurologist or movement disorder specialist and did not materially change results. Potential interactions between PMH use and caffeine intake or cigarette smoking were examined by including a multiplicative interaction term in the smoking- and age-adjusted model and the statistical significance was evaluated using a likelihood ratio test. The interaction term was defined as a cross-product of either caffeine consumption (mg/day) or pack-years of smoking and use of PMH (ever vs. never).
Results
A total of 244 incident cases were identified during the follow-up period with an average age of diagnosis of 65.7 years. Baseline population characteristics according to type of menopause, use of PMHs and use of OCs upon entry are shown in Table 1.
Table 1.
Baseline characteristics according to post-menopausal hormone use, oral contraceptive use and type of menopause
| PMH use | OC use | Menopause type | |||||
|---|---|---|---|---|---|---|---|
| Never | Past | Current | Never | Ever | Natural | Surgical | |
| Age (yrs) | 51.0 | 52.7 | 50.8 | 52.3 | 49.3 | 52.8 | 48.6 |
| Current smoker (%) | 31.0 | 31.1 | 25.5 | 28.3 | 30.3 | 31.7 | 26.8 |
| Past smoker (%) | 37.3 | 40.4 | 40.0 | 37.5 | 41.1 | 40.2 | 38.1 |
| BMI (kg/m2) | 25.1 | 24.7 | 23.7 | 24.8 | 24.1 | 24.2 | 24.6 |
| Caffeine (mg/day) | 400.3 | 390.5 | 387.5 | 392.5 | 394.9 | 400.7 | 383.1 |
| Alcohol (g/day) | 6.2 | 6.6 | 6.7 | 6.1 | 7.1 | 6.6 | 6.2 |
| Age at menopause (yrs) | 47.6 | 45.3 | 47.0 | 47.5 | 47.0 | 49.9 | 43.9 |
Other than age, all estimates are standardized to the age distribution of the cohort.
Risk of PD, adjusted for age and smoking history, was not associated with PMH use, parity, age at menopause, OC use, number of reproductive years (years between menarche and menopause) or type of menopause (Table 2). Further, no increased PD risk was observed among women who underwent bilateral oophorectomy at a young age compared to women with natural menopause (RR=0.8, 95% CI=0.4, 1.4, for oophorectomy <38years), though this result was based on a small number of cases (n=12). There seemed to be a non-significant trend towards lower risk for older age at menopause amongst women with natural menopause. Every five years delay in age at menopause was associated with an 11% reduction in the risk of PD (RR=0.89 95% CI=0.72, 1.09, p for trend=0.27). There was also a significant positive association with OC use. Each additional five years of OC use was associated with a 20% increased risk of PD (RR=1.20, 95% CI=1.00, 1.43, p for trend=0.048).
Table 2.
Relative risk of PD associated with reproductive factors
| Person-years | No. Cases | RR (95% CI) | p - value | |
|---|---|---|---|---|
| PMH use | NA | |||
| Never | 556088 | 88 | REF | |
| Past | 292596 | 63 | 1.08 (0.78, 1.50) | |
| Current | 474192 | 89 | 1.18 (0.88, 1.59) | |
| Duration of PMH use | ||||
| Never | 556088 | 88 | REF | 0.79 |
| <5 | 386748 | 68 | 1.14 (0.83, 1.57) | |
| >=5 | 380039 | 84 | 1.14 (0.84, 1.54) | |
| Time since last PMH use | ||||
| Never | 556088 | 88 | REF | 0.23 |
| >24 months | 118059 | 33 | 1.26 (0.86, 1.89) | |
| <=24 months | 30111 | 6 | 1.15 (0.50, 2.64) | |
| Current | 407848 | 80 | 1.19 (0.87, 1.63) | |
| Type of PMH use | NA | |||
| Non-users | 432062 | 78 | REF | |
| Estrogen only | 258876 | 67 | 1.28 (0.93, 1.78) | |
| Progesterone only | 12782 | 4 | 3.41 (1.23, 9.47) | |
| Combination | 213966 | 36 | 0.97 (0.65, 1.44) | |
| Unknown | 208500 | 40 | 1.04 (0.71, 1.54) | |
| Age at menopause‡ | ||||
| < 45 | 85523 | 17 | REF | 0.27 |
| 45–49 | 206904 | 42 | 0.89 (0.51, 1.57) | |
| 50–54 | 374365 | 82 | 0.74 (0.44, 1.25) | |
| >=55 | 48397 | 11 | 0.60 (0.28, 1.28) | |
| Menopause type | NA | |||
| Natural | 729624 | 152 | REF | |
| Surgical, 0–1 ovaries | 220809 | 42 | 0.79 (0.69, 1.36) | |
| Surgical, 2 ovaries | 267442 | 34 | 0.97 (0.44, 1.44) | |
| OC use | ||||
| Never | 766291 | 164 | REF | 0.89 |
| Ever | 556586 | 76 | 1.02 (0.77, 1.36) | |
| Duration of OC use | ||||
| Never | 766291 | 164 | REF | 0.05 |
| < 5 | 371509 | 40 | 0.84 (0.59, 1.19) | |
| >= 5 | 185040 | 36 | 1.35 (0.93, 1.96) | |
| Number of children | ||||
| <2 | 169608 | 26 | REF | >0.99 |
| 2–3 | 698052 | 119 | 1.20 (0.78, 1.83) | |
| >3 | 422515 | 89 | 1.22(0. 78, 1.89) | |
| Age at menarche | ||||
| <12 | 293589 | 48 | REF | 0.67 |
| 12 | 347224 | 63 | 1.06 (0.73, 1.55) | |
| 13 | 402642 | 71 | 1.02 (0.70, 1.46) | |
| >13 | 268513 | 55 | 1.07 (0.73, 1.58) | |
Adjusted for smoking in pack-years.
amongst women with natural menopause
PMH use was, in general, not associated with PD risk (Table 2), although progestin alone use was associated with a 3-fold higher PD risk (RR=3.41, 95% CI = 1.23, 9.47; p=0.02). However, this finding should be interpreted cautiously as the analyses were based on a small number of cases. In subgroup analyses, estrogen only use tended to be associated with a higher PD risk among women with natural menopause (RR=1.67, 95% CI=1.08, 2.57; p=0.02). However, use of estrogen only or with progestin was not associated with PD risk in analyses amongst all post-menopausal women or amongst women with surgical menopause.
No statistically significant interaction between caffeine and post-menopausal hormone use as observed (Table 3). However, as in our previous studies, the inverse association between caffeine intake and PD risk seemed to be limited to never users of PMHs (p for trend=0.05), while no association was observed amongst ever users. Results were similar in analysis using baseline exposure information or when cases diagnosed in the first 4 years of follow-up were excluded. Likewise, current PMH use compared to never use tended to be related to a lower PD risk among individuals with low caffeine consumption (<100mg/day) (RR=0.68, 95% CI=0.31, 1.50), and to a higher risk amongst those with higher intakes (>100mg/day) (RR=1. 29, 95% CI=0.93, 1.80), but neither trend was statistically significant.
Table 3.
Relative risk of PD associated with smoking history and caffeine intake according to PMH use
| Hormone Use | ||
|---|---|---|
| Never (n=88) | Ever (n= 152) | |
|
Smoking history (median pack-years) |
||
| Never | 1.0 (Ref) | 1.0 (Ref) |
| Q1 (2) | 0.48 (0.19, 1.21) | 1.31 (0.81, 2.11) |
| Q2 (9) | 1.14 (0.61, 2.13) | 0.78 (0.44, 1.39) |
| Q3 (20) | 0.57 (0.26, 1.26) | 0.85 (0.49, 1.47) |
| Q4 (33) | 0.37 (0.15, 92) | 0.79 (0.44, 1.39) |
| Q5 (52) | 0.11 (0.03, 0.43) | 0.36 (0.17, 0.75) |
| p for trend | 0.0001 | 0.005 |
|
Caffeine intake (median mg/day) |
||
| Q1 (68) | 1.0 (Ref) | 1.0 (Ref) |
| Q2 (179) | 0.80 (0.44, 1.46) | 1.33 (0.84, 2.13) |
| Q3 (306) | 0.84 (0.46, 1.52) | 0.90 (0.54, 1.49) |
| Q4 (414) | 0.58 (0.29, 1.16) | 0.57 (0.31, 1.04) |
| Q5 (665) | 0.54 (0.25, 1.14) | 1.13 (0.66, 1.93) |
| p for trend | 0.05 | 0.60 |
Adjusted for age and smoking history (for caffeine analysis).
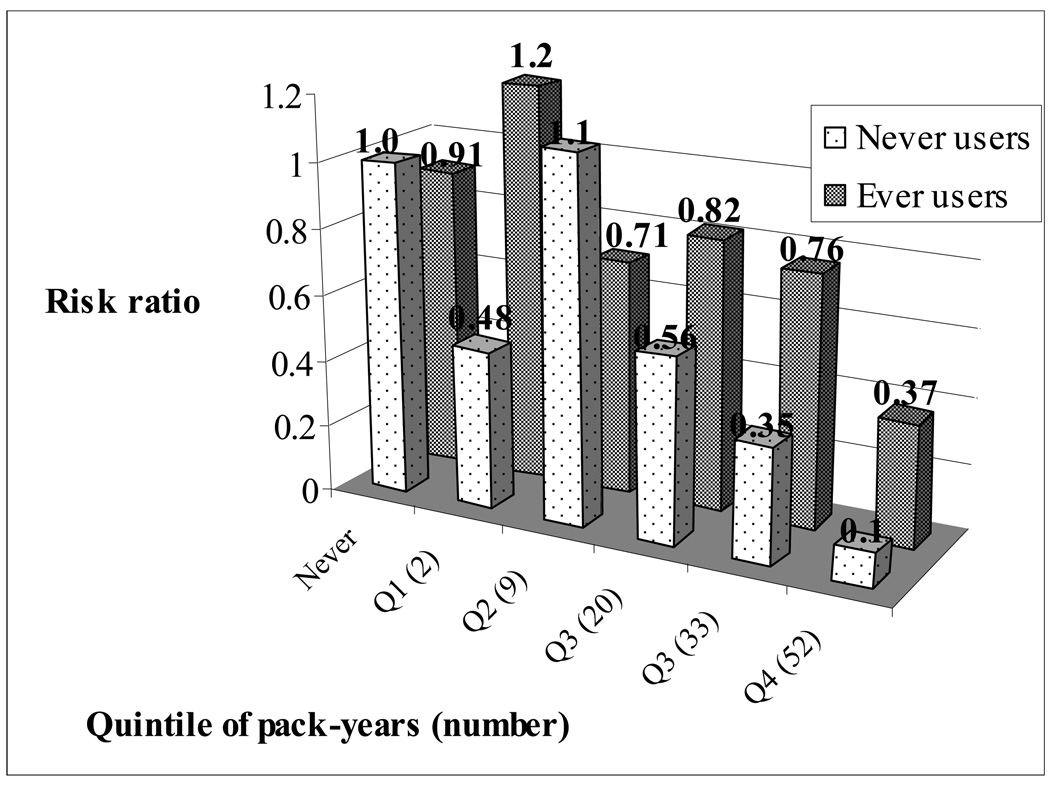
We observed a significant interaction between smoking and PMH use on PD risk (p=0.05, Table 3). Although the inverse relationship between pack-years of smoking and PD risk was observed in both ever and never users of PMH, the association appeared stronger among never users (Table 3). For each ten pack-year increase in smoking, the risk of PD was reduced by 28% amongst never users of PMHs (RR=0.72, 95% CI=0.61, 0.85, p=0.0001) and only 13% amongst ever users (RR=0.87, 95% CI=0.79, 0.96, p=0.005). These results remained significant in analysis using baseline exposure information or in the four year lag analysis. When considering the combined risk of smoking and PMH use, smoking in the highest three quintiles conferred more protection amongst never users of PMHs than ever users (Figure). Similarly, the association between PMH use and risk of PD varied by smoking status. Amongst ever smokers, current PMH use was related to a significantly increased risk of PD compared to never users (RR=1.75, 95% CI=1.11, 2.77; p=0.02), but not amongst never smokers.
Figure. Combined effect of PMHs and smoking (pack-years) on risk of PD adjusted for age.
Referent is never smokers who never used PMHs
Discussion
In this large longitudinal investigation, overall, we did not find any association between reproductive factors or use of exogenous estrogens and risk of PD.
Strengths of this study include the prospective design, the high follow-up rates, and use of repeated and validated assessment of reproductive factors and hormone use. In a sub-set of this cohort the mean levels of estradiol amongst postmenopausal women reporting current use of PMH were found to be well in excess of that for women not using PMHs28. Further, the validity of history of estrogen use and reproductive factors in our study is supported by the previous finding that these factors were strong predictors of ovarian and breast cancer in this cohort29, 30. Although the confirmation of PD diagnoses relied on reports from the treating neurologists or review of the medical records rather than on direct physical examination of all participants, and some diagnostic errors are inevitable, recent clincopathological studies have shown that the diagnosis of PD made by neurologists or movement disorder specialists has a high positive predictive value31, 32, and bias from this source is thus likely to be modest. Notably, results were not affected when analyses were restricted to cases diagnosed by a neurologist.
Also, we cannot exclude the possibility that an effect of hormones on PD disease would become manifest only at a very late age, although the fact that we found similar results for PD with onset before or after 65 years of age provides some evidence against this possibility. Additionally, although the timing of initiation of PMH use could be important for some disease outcomes33, 34, we were unable to address this question in relation with PD because the majority of women (80%) began using hormones around the time of menopause35, and therefore numbers were too small to explore a possible effect of late initiation of hormone use.
Finally, because reproductive factors and use of hormones correlate with other aspects of lifestyle, confounding is an important concern, and the availability of prospectively collected and validated information on strong risk factors, such as smoking and caffeine, is an important feature of our study. Nevertheless, as in all observational studies, residual confounding from unmeasured factors cannot be excluded.
Our findings are consistent with previous epidemiologic studies showing no association between PMH use and risk of PD14–17, but not with those showing a decreased risk associated with PMH use12, 13. Further, we could not confirm the observation of increased risk of PD associated with estrogen only or combination PMH use amongst women with hysterectomy reported in a recent nested case-control study17. We also did not confirm the recent finding of a possibly increased risk of parkinsonism associated with oophorectomy36. The number of women with oophorectomy in our study, however, was too small to exclude a moderate association. Even in the previous investigation, which was based on the follow-up of more than 2000 women with pre-menopausal oophorectomy, a significant increase in risk among oophorectomized women was found only in analyses including all cases of parkinsonism. Prior studies did not explore different types of PMHs, as in the current investigation. In sub-analyses exploring the relationship between types of post-menopausal hormones and risk of PD, a three-fold increased risk was observed with use of progestin-only hormones. However, this is based on a small number of cases and requires confirmation. Experimental evidence regarding the potential role of progestins in neuroprotection is inconsistent and although progesterone administration has been shown to be neuroprotective, common synthetic progestins found in post-menopausal hormones may not exert the same neuroprotective effect18, 37.
Our finding of no association between age at menopause and risk of PD is consistent with some studies15, 16, but not another in which older age at menopause was associated with decreased risk of PD17. We did not find an association between parity and risk of PD consistent with a recent investigation 17, in contrast with a case-control study showing increased risk of PD associated with increased duration of pregnancies16. The authors hypothesized that this could be explained by differences in estrogen metabolism and serum hormone binding protein (SHBG) amongst nulliparous compared to parous women. However, several studies of serum hormones have shown no differences related to parity associated with serum levels of estrogens38, 39 or SHBG amongst pre- and post-menopausal women38, 40. Lastly, we did not confirm the previously reported increased risk of PD associated with decreased years between menarche and menopause16.
We have previously reported significant interactions between caffeine intake and PMH use on risk of PD, in which the inverse relationship between caffeine and PD risk was only amongst never users of PMHs, while not amongst ever users. This interaction was still suggested in the current analysis, albeit appeared to be weaker than in our previous reports8, 9. This attenuation is consistent with the fact that most women in this cohort have ceased use of PMHs in recent years, and any effect of PMH use is likely diminishing over time. Supportively, this interaction was also suggested in animal experiments in which the neuroprotective effect of caffeine is reduced when estrogen is administered10.
The potential interaction with smoking is consistent with previous findings in this cohort9. Attenuation of the association between smoking and PD in the presence of estrogen suggests an interaction in which estrogen or its metabolites may block the activity of a protective pathway. Nicotine metabolism is increased in females compared to males and in the presence of administration or use of exogenous estrogens41. Thus, an increase in nicotine metabolism could result in decreased availability of a substrate important in a potential neuroprotective pathway. The mechanism by which estrogen could antagonize a putative protective effect of nicotine is, however, unclear.
The mechanism by which estrogen confers neuroprotection is unclear and likely complex. Several pathways have been suggested6, including anti-inflammatory properties42, anti-oxidant effects43, and regulating apoptotic pathways. The results of our study could be explained if either the reproductive factors investigated or use of post-menopausal hormones have at most modest effects on PD risk, or if their effects are modified by other behavioral, environmental, or genetic factors that remain to be identified. The suggestion in our data of interactions between post-menopausal hormones and smoking or caffeine is consistent with this possibility that seems worthy of further investigation in epidemiological and experimental studies.
In summary, the results of this large longitudinal study do not support a beneficial role for endogenous estrogen or use of post-menopausal hormones on risk of PD. Although risk of PD was higher among women using progestin only PMHs, this was a marginal finding based on small numbers.
Acknowledgments
This work was funded by grant NIH/NINDS R01 NS048517 and training grant T32 ES07069-26 and was in part supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, the National Institute of Health. The authors would like to thank Eilis O’Reilly for technical support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142(8):820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 2.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 3.Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond JDE. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci. 2000;20(23):8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DB, Ali SF, O'Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- 5.Tamas A, Lubics A, Lengvari I, Reglodi D. Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson's disease. Endocrine. 2006;29(2):275–288. doi: 10.1385/ENDO:29:2:275. [DOI] [PubMed] [Google Scholar]
- 6.Dluzen DE, McDermott JL. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol. 2000;29(5–6):387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- 7.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 8.Ascherio A, Weisskopf MG, O'Reilly EJ, et al. Coffee consumption, gender, and Parkinson's disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology. 2003;60(5):790–795. doi: 10.1212/01.wnl.0000046523.05125.87. [DOI] [PubMed] [Google Scholar]
- 10.Xu K, Xu Y, Brown-Jermyn D, et al. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci. 2006;26(2):535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windham GC, Mitchell P, Anderson M, Lasley BL. Cigarette smoking and effects on hormone function in premenopausal women. Environ Health Perspect. 2005;113(10):1285–1290. doi: 10.1289/ehp.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martignoni E, Nappi RE, Citterio A, et al. Reproductive life milestones in women with Parkinson's disease. Funct Neurol. 2003;18(4):211–217. [PubMed] [Google Scholar]
- 13.Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol. 2004;61(6):886–888. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- 14.Marder K, Tang MX, Alfaro B, et al. Postmenopausal estrogen use and Parkinson's disease with and without dementia. Neurology. 1998;50(4):1141–1143. doi: 10.1212/wnl.50.4.1141. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti MD, Maraganore DM, Bower JH, et al. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord. 2001;16(5):830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 16.Ragonese P, D'Amelio M, Salemi G, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology. 2004;62(11):2010–2014. doi: 10.1212/wnl.62.11.2010. [DOI] [PubMed] [Google Scholar]
- 17.Popat RA, Van Den Eeden SK, Tanner CM, et al. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology. 2005;65(3):383–390. doi: 10.1212/01.wnl.0000171344.87802.94. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143(1):205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 19.Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41(2):131–138. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 21.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 22.de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM. A population perspective on diagnostic criteria for Parkinson's disease. Neurology. 1997;48(5):1277–1281. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 23.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–325. doi: 10.1093/aje/126.2.319. [DOI] [PubMed] [Google Scholar]
- 25.Hunter DJ, Manson JE, Colditz GA, et al. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception. 1997;56(6):373–378. doi: 10.1016/s0010-7824(97)00172-8. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activity. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Tworoger SS, Missmer SA, Barbieri RL, Willett WC, Colditz GA, Hankinson SE. Plasma sex hormone concentrations and subsequent risk of breast cancer among women using postmenopausal hormones. J Natl Cancer Inst. 2005;97(8):595–602. doi: 10.1093/jnci/dji099. [DOI] [PubMed] [Google Scholar]
- 29.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 30.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. 2007;96(1):151–156. doi: 10.1038/sj.bjc.6603527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 32.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57(8):1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 33.Shantakumar S, Terry MB, Paykin A, et al. Age and menopausal effects of hormonal birth control and hormone replacement therapy in relation to breast cancer risk. Am J Epidemiol. 2007;165(10):1187–1198. doi: 10.1093/aje/kwm006. [DOI] [PubMed] [Google Scholar]
- 34.Koledova VV, Khalil RA. Sex hormone replacement therapy and modulation of vascular function in cardiovascular disease. Expert Rev Cardiovasc Ther. 2007;5(4):777–789. doi: 10.1586/14779072.5.4.777. [DOI] [PubMed] [Google Scholar]
- 35.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348(7):645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 36.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70(3):200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 37.Singh M. Mechanisms of progesterone-induced neuroprotection. Ann N Y Acad Sci. 2005;1052:145–151. doi: 10.1196/annals.1347.010. [DOI] [PubMed] [Google Scholar]
- 38.Madigan MP, Troisi R, Potischman N, Dorgan JF, Brinton LA, Hoover RN. Serum hormone levels in relation to reproductive and lifestyle factors in postmenopausal women (United States) Cancer Causes Control. 1998;9(2):199–207. doi: 10.1023/a:1008838412423. [DOI] [PubMed] [Google Scholar]
- 39.Chubak J, Tworoger SS, Yasui Y, Ulrich CM, Stanczyk FZ, McTiernan A. Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1296–1301. [PubMed] [Google Scholar]
- 40.Dorgan JF, Reichman ME, Judd JT, et al. Relationships of age and reproductive characteristics with plasma estrogens and androgens in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1995;4(4):381–386. [PubMed] [Google Scholar]
- 41.Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230(2):140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- 42.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 43.Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J Neurosci Res. 1998;54(5):707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]