Abstract
Alzheimer’s disease is characterized mainly by loss of neurons from the septal nucleus. In this study, neurons from the septal nucleus of the embryonic day 16 (E16) rat were grown in culture with a plane of astrocytes from the embryonic rat and in a defined medium in the absence of serum. Neurons were treated with beta-amyloid (Aβ: 0.1, 1, and 10 µM) on day in vitro (DIV) 1 and DIV 4 and fluorescent microscopy was used to measure survival and apoptosis following exposure of the treated cells on DIV 7. Reversal of neurotoxicity was studied using the potentially neuroprotective agents nerve growth factor (NGF, 100 ng/ml), basic fibroblast growth factor (bFGF, 5 ng/ml), insulin-like growth factors (IGF1 and IGF2, 10 ng/ml), and estrogen (10 nM), administered on DIV 4 and DIV 5, that is, subsequent to the Aβ (10 µM)-induced neurotoxicity. Aβ caused a significant decrease in survival at 10 µM, and a significant increase in apoptosis at 0.1 and 10 µM. IGF1, IGF2 and bFGF all caused a reversal of the Aβ-induced neurotoxic effect on survival while NGF and estrogen did not under these experimental conditions.
Keywords: beta-amyloid, neurotoxicity, apoptosis, septal, neuron
Introduction
Alzheimer’s disease is caused primarily by neurotoxic deposition of the protein beta-amyloid (Aβ) within specific areas of the brain including the septum [29]. These deposits are known as senile plaques and are thought to cause pathological neurofibrillary tangles. Aβ’s toxicity appears to be due to activation of the apoptotic (programmed cellular death) pathway within brain neurons [11], possibly a cascading result of enzymatic cleavage by caspase of the amyloid precursor protein (AβPP) [24].
One therapeutic approach to AD has been to find ways to decrease the deposition of Aβ by inhibiting the proteases that produce it. Such inhibitory mechanisms function by preventing protease accumulation, inflammation, and altering cholesterol homeostasis or amyloid aggregation by shifting ion content within the cells [15]. A second approach has been to replace the neurotrophins such as nerve growth factor (NGF) which are reduced or lost in AD. Neurotrophins are frequently neuroprotective and decreased levels are often associated with a number of brain disorders. Increasing the concentration of neurotrophins results in an increase in the survival of neurons at risk [19]. Other treatment pathways under investigation include other cellular growth factors and estrogens. Low levels of Aβ interact with the low affinity NGF receptor, p75, altering neurite development [36]. Nerve growth factor replacement therapy has been proposed for cognitive deficits due to a loss of cholinergic neurons of the basal forebrain [18]. In a mouse model of Down’s syndrome, AβPP increases led to a decrease in retrograde transport of NGF [28]. Estrogens also protect cholinergic function in the basal forebrain [5], have a role in the maintenance of learning and memory in the rat [32], and appear to be neuroprotective in humans when administered in hormone replacement therapy prior to or shortly after menopause [13, 40]. Insulin-like growth factor I (IGF1) and basic fibroblast growth factor (bFGF) have neuroprotective roles, upregulating gene expression in various protective pathways [42]. IGF1 acts via several pathways in the processing of the AβPP [1], and in prevention of vascular dysfunction [23]. Protection against stroke is conferred by bFGF in rats [39] and FGF protects against Aβ toxicity in endothelial cells [7]. Rat temporal cortex is protected against Aβ by IGF1 [2]. In fact, IGF1 has so many protective functions its decline appears to be related to senescence in the brain [37]. Increased insulin-like growth factor II (IGF2) also appears to slow the progression of Aβ induced neurodegeneration in mice [34].
Bilaminar cultures of neurons and astrocytes grown in apposition (about one mm apart) but not touching one another, allow for the study of effects of Aβ and various neuroprotective agents in the absence of serum and in the absence of the blood-brain barrier. The purposes of this study were two-fold: 1) to determine what effect Aβ has on survival and induction of apoptosis in septal neurons (particularly in response to varying concentrations of Aβ in this bilaminar model); and 2) to determine whether various agents which are neuroprotective might reverse the effects of Aβ on survival.
Materials and Methods
This study used a bilaminar model for the study of neurotoxic effects of Aβ and its reversal by various growth factors or hormones. Neurons and glial cells were grown in cell culture in close apposition to one another but not touching one another so that both direct and indirect (mediated by astrocytes) actions of Aβ and potential treatments might be studied in the absence of the blood-brain barrier.
Dissection and Cell Culture
Glial Plane
Astrocytes were cultured from embryonic day 16 (E16) Sprague-Dawley rat brains (Charles River; Wilmington, MA). Rats were euthanized by ether anesthesia. The care and use of, as well as all procedures involving, animals have been approved by Barry University's IACUC, in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals.
Astrocytes from the cerebral cortex were microdissected and cultured as described previously [3] and plated in 100 mm culture dishes (Corning; Corning, NY). Seven days before neuronal dissection, glial cells were transferred to 12 well cell culture cluster dishes. Twenty-four hours before neuronal dissection, medium was replaced with 1 ml N2.1 defined medium [3].
Neuronal Plane
Sprague-Dawley rat brains were dissected on E16 and were microdissected and cultured as described previously [3]. Neurons were plated on silane treated Poly-L-Lysine coated glass coverslips before treatment [17]. After 2 hours of incubation, coverslips were inverted over the 40–60% confluent glial plane within the 12 well culture dishes. Glial and neuronal planes were separated by about 1 mm by the paraplast feet on the coverslips. Cytosine arabinofuranoside (GIBCO, 10−5 M) was added after 1 day in vitro (DIV) to prevent glial proliferation. All cells were maintained in a humidified 37°C, 95% air/5% CO2 incubator (NUAIRE) until use.
Treatment of Experimental Groups
Dose Response
Cells were treated on DIV 1 and on DIV 4 with three concentrations of Aβ (25–35 fragment, 0.1 µM, 1 µM, and 10 µM, Biosource Camarillo, CA); this fragment is the biologically active part of the peptide [16, 20]. Controls were treated with sterile water only. Aβ was added to sterile-filtered water and allowed to aggregate at 4°C. On DIV 7 survival was visualized and quantified by fluorescence microscopy in random fields with 4.6 µg/mL propidium iodide (PI) and 15 µg/mL fluoresce diacetate (FDA) under a fluorescence microscope (Olympus BX-60) with a 450 nm excitation (Kapuscinski 1995).
Reversal
Cells were treated on DIV 1 and DIV 4 with Aβ (10 µM). On DIV 4 and DIV 5 cells were treated with growth factors (IGF1; 10 ng/ml, IGF2; 10 ng/ml, bFGF; 5 ng/ml, and NGF; 100ng/ml) (all from Upstate Biotechnology Incorporated, Lake Placid, NY) and estrogen (EST, Sigma: 10 nM). Each of these factors was diluted in DMEM and added to the media with the exception of EST, which was prepared in ethanol at a concentration of 25 mM and subsequently diluted 2.5 × 106 times in DMEM. On DIV 7 the cells received no treatment and survival was quantified by fluorescence microscopy with PI and FDA as described previously.
Apoptosis
Cells were treated as for the reversal experiment described previously. On DIV 7 silane-coated glass coverslips with adhered cells were placed in 12-well dishes and apoptosis was quantitated by ApopTag® Plus Fluorescein In Situ Apoptosis detection kit (Chemicon International Temecula, CA). Coverslips were placed on microscope slides and counterstained with 100 µL 46 µg/ml PI (Sigma St. Louis, MO) in HBSS. Cells were visualized under a fluorescence microscope (40X) with 450 nm excitation. Green and red fluorescent cells were counted in randomly selected fields from each slide.
Statistical Sampling and Analyses
All slides were assigned to treatment according to a randomized block design [43]; each slide was assumed to be independent of other slides, then assigned to treatment by lottery. Within a central area in each slide three to five randomly selected circular fields (area = 2.54 mm2 per field) were selected, and within each circular field the number of surviving cells was counted. The same method (field and area) was used to count dead cells. Cells stained red died by necrosis, while cells stained green died by apoptosis. The proportion of cells dead from apoptosis per field was calculated as (number of green cells)/(number of red cells + number of green cells).
SigmaStat® 3.0 [30] was used for all statistical tests, with appropriate interpretation [33, 43]. All tests used the α = .05 level for statistical significance, and we defined P = probability that there is no significant pattern present.
Results
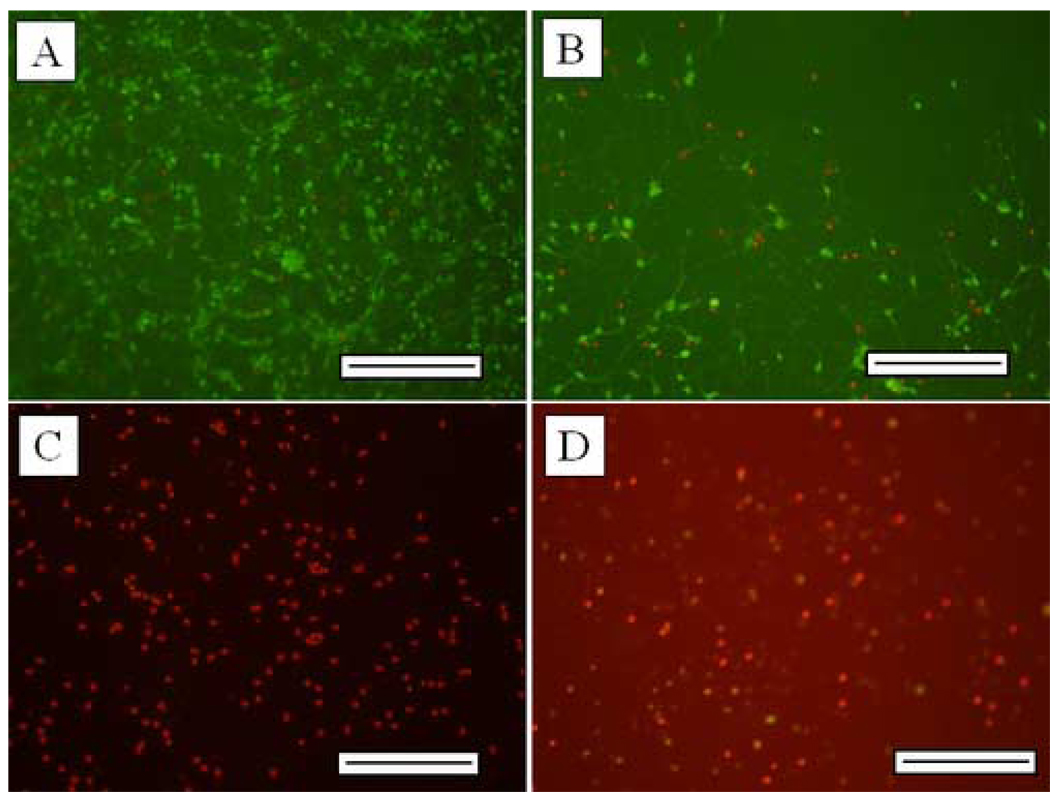
Figure 1 shows the photomicrographs of control and Aβ-treated neurons; survival of neurons is shown in figures 1A (control) and 1B (Aβ-treated), while apoptosis is shown in figures 1C (control) and 1D (Aβ-treated). These indicate an increase in dead cells (red nuclei) in the Aβ treatment relative to control, and an increase in apoptosis (green nuclei) in the Aβ treatment relative to control. We found significant effects due to Aβ in both percent survival of neurons exposed to Aβ, and in percent of dead cells killed by apoptosis.
Figure 1.
Photomicrographs of control and Aβ-treated neurons; survival of neurons is shown in figures 1A (control) and 1B (Aβ-treated), while apoptosis is shown in figures 1C (control) and 1D (Aβ-treated, see methods). Scale bar = 200 µm.
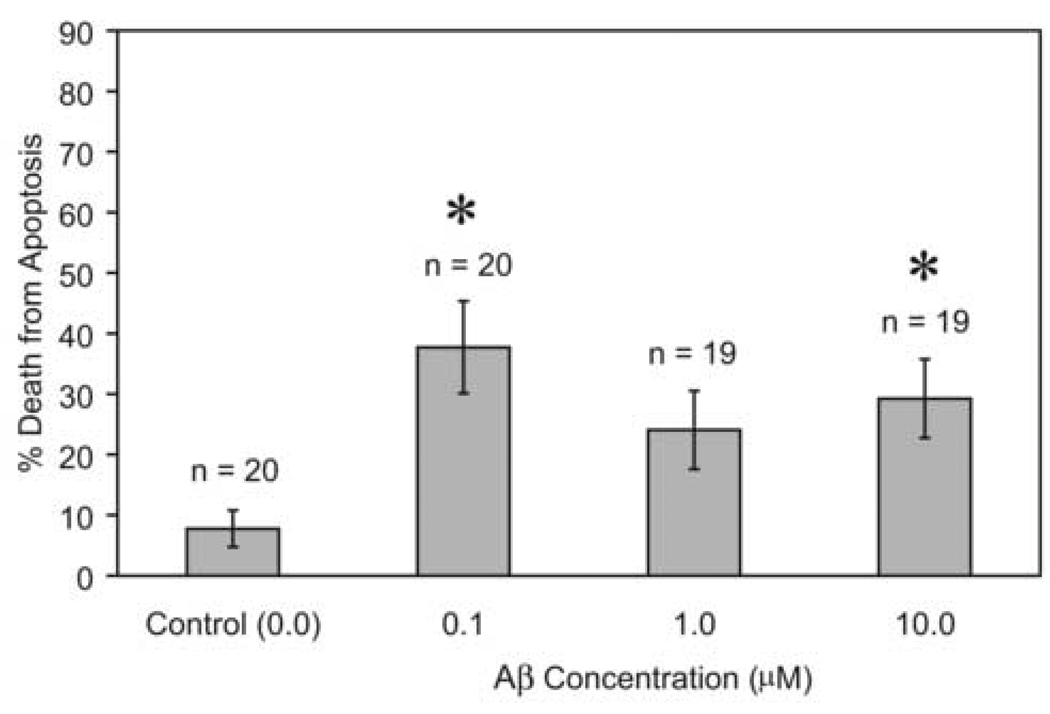
Figure 2 shows the mean percent survival of cells per microscopic field exposed to different concentrations of Aβ. One-way ANOVA indicated significant differences among treatment means (F = 3.086; d.f. = 3, 51; P = 0.035); the Holm-Sidak post-hoc test indicated the Aβ 10.0 µM treatment resulted in significantly reduced survival compared with control (t = 2.93; P = 0.005). The Aβ treatments for 0.1 µM (t = 1.94; P = 0.058) and 1.0 µM (t = 0.93; P = 0.356) did not result in significantly lower mean survival compared with control, while the 10 µM dose did result in significantly lower mean survival compared with control.
Figure 2.
Mean percent survival of cells per microscopic field (± 1 S.E.) exposed to different concentrations of Aβ; n = number of fields counted, and * indicates treatment mean percent significantly lower than control mean (no Aβ).
Figure 3 shows the mean percent of dead cells killed by apoptosis per microscopic field exposed to different concentrations of Aβ. One-way ANOVA indicated significant differences among treatment means (F = 4.315; d.f. = 3, 74; P = 0.007); the Holm-Sidak post-hoc test indicated the Aβ 0.1 µM treatment (t = 2.93; P = 0.005) and the 10.0 µM treatment (t = 2.93; P = 0.005) both resulted in significantly higher percent death by apoptosis compared with the control (0.0 µM Aβ). The Aβ treatment for 1.0 µM (t = 1.94; P = 0.058) did not result in significantly higher mean percent death by apoptosis compared with control, though its mean (24%) was over three times higher than the control mean (7.7%).
Figure 3.
Mean percent of dead cells killed by apoptosis per microscopic field (± 1 S.E.) exposed to different concentrations of Aβ; n = number of fields counted, and * indicates treatment mean percent significantly higher than control mean (no Aβ).
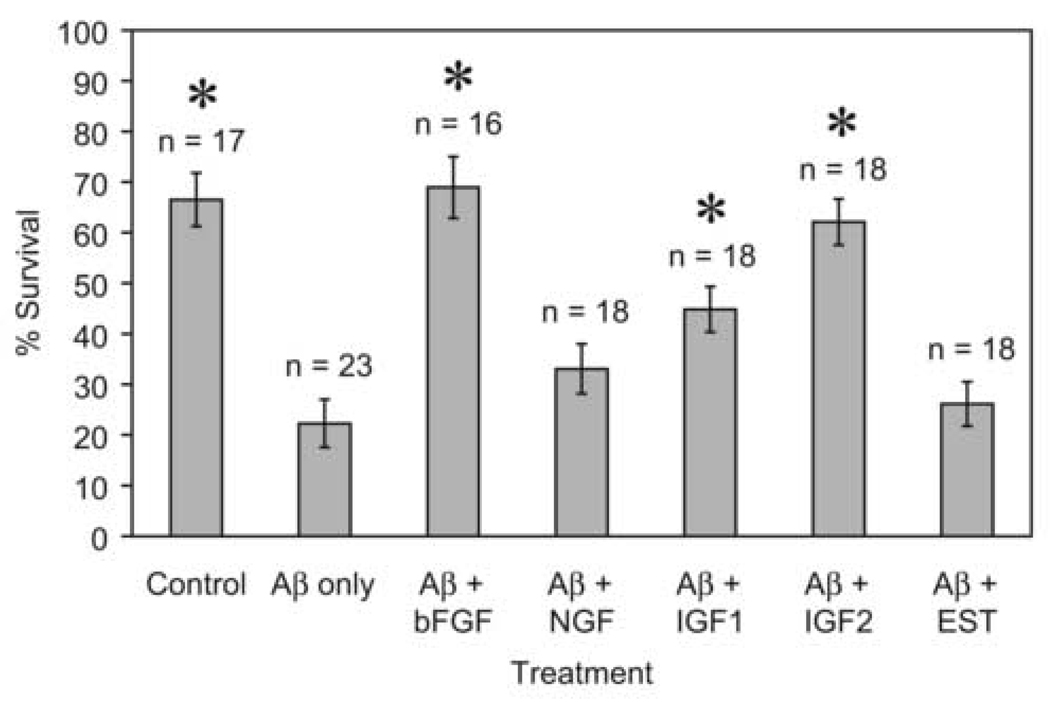
Figure 4 shows the mean percent survival of cells per microscopic field exposed to different combinations of Aβ (10 µM) with growth factors and estrogen. One-way ANOVA indicated significant differences among treatment means (F = 16.234; d.f. = 6, 121; P < 0.001); the Holm-Sidak post-hoc test indicated significantly higher mean survival compared with Aβ-only treatment for the control treatment (no Aβ, t = 6.57; P < 0.001), the Aβ+bFGF treatment (t = 6.81; P < 0.001), the Aβ+IGF1 treatment (t = 3.40; P = 0.001), and the Aβ+IGF2 treatment (t = 6.01; P < 0.001). The treatments for Aβ+NGF (t = 1.63; P = 0.101) and Aβ+EST (t = 0.58; P = 0.564) did not result in significantly increased mean survival compared with Aβ-only treatment.
Figure 4.
Mean percent survival of cells per microscopic field (± 1 S.E.) exposed to different combinations of Aβ (10 µM) and growth factors (bFGF = 5 ng/ml, NGF = 100 ng/ml, IGF1 and IGF2 = 10 ng/ml, EST= 10 nM); n = number of fields counted, and * indicates treatment mean percent significantly higher than mean for Aβ only.
Discussion
The important findings of this study were three-fold: 1) that Aβ significantly decreased the survival of septal neurons at 10 µM, 2) that Aβ significantly increased apoptosis at doses of 0.1 and 10 µM, and 3) that bFGF, IGF1, and IGF2 significantly reversed the apoptotic effect due to Aβ. Some of these effects may be indirectly mediated by the glial cells as in another study [3] we have shown that Aβ decreases survival of glial cells at 0.1, 1.0, and 10 µM concentrations under the same circumstances. The glial cell increase in apoptosis due to Aβ was reversed significantly by bFGF and IGF1. Differences between responsiveness of neurons and glial cells in our studies may be due to the shorter treatment of glial cells due to their propensity to divide. Astrocytes protect against the Aβ toxicity unless the astrocytes are themselves exposed to Aβ, in which case the protection is lost [27].
Studies in PC12 cells suggest that the increased cell death seen in AD may be due to apoptosis [12]. Neuroprotective factors appear to act by stabilizing Ca++ homeostasis [25] or by interrupting apoptosis pathways. Both bFGF and IGF1 increase the number of cells containing the Ca++-binding protein calbindin, while the other factors do not [31], a possible mechanism for their neuroprotection here. In addition, bFGF, but not NGF decreased Ca++ disturbances in hippocampal neurons [26]. Estrogen alters expression of Bcl proteins involved in apoptosis pathways [41].
Mammals have decreased IGF1 with age [38]. Insulin-like growth factor stimulates neuronal release and clearance of Aβ and IGF1 levels are altered in AD [6]. The dose of IGF1 which is neuroprotective in hippocampal neurons is 10uM, the dose used in these studies [9]. In the same study, IGF2 was effective but less potent. Effects of IGF1 in hippocampal neurons are particularly encouraging as they occur (as in this study) when IGF1 is administered subsequent to the Aβ insult as well as when the IGF1 and Aβ are administered simultaneously [8]. In mice transgenic for a mutant AβPP, IGF2 is over-expressed and may provide some protection to hippocampal neurons [35].
Although nerve growth factor supports the cholinergic neurons of the septum [18, 22], this treatment has limitations as a therapy. One approach to overcoming the broad effects of nerve growth factor due to its interaction with the low-affinity p-75 receptor has been to develop molecules which mimic individual domains within the NGF structure [21, 22]. These may be more effective than NGF itself. Estrogen is protective against Aβ in cell lines such as PC12 and human neuronal cells [4, 10] but was ineffective on the differentiated cells in this study. The estrogen and NGF may be acting by a similar pathway as both alter phosphotyrosine content in septal neurons over a similar time period [14]. It may be that this pathway is ineffective in reversal of Aβ effects although it is clearly effective in other respects.
Acknowledgements
We are grateful for the assistance and suggestions from Dr. Christophe Hengartner and Mr. Fabio Nascimento. Poincyane Assis-Nascimento was supported by NIH-MARC U*Grant T34GM08082, NIH-NIGMS RISE Grant, R25 GM 9244, and NIH MBRS SCORE Grant SO6 GM45455. Laura Mudd and Jeremy Montague were supported by NIH MBRS SCORE Grant SO6 GM45455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlerz L, Holback S, Multhaup G, Iverfeldt K. IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J Biol Chem. 2007;282:10203–10209. doi: 10.1074/jbc.M611183200. [DOI] [PubMed] [Google Scholar]
- 2.Aguado-Llera D, Arilla-Ferreiro E, Campos-Barros A, Puebla-Jimenez L, Barrios V. Protective effects of insulin-like growth factor-I on the somatostatinergic system in the temporal cortex of beta-amyloid-treated rats. J. Neurochem. 2005;92:607–615. doi: 10.1111/j.1471-4159.2004.02889.x. [DOI] [PubMed] [Google Scholar]
- 3.Assis-Nascimento P, Jarvis K, Montague JR, Mudd LM. Beta-amyloid toxicity in embryonic rat astrocytes. Neurochemical Research. 2007 doi: 10.1007/s11064-007-9335-8. [in press: revised manuscript accepted 16 March 2007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benvenuti S, Luciani P, Vannelli GB, Gelmini S, Franceschi E, Serio M, Peri A. Estrogen and selective estrogen receptor modulators exert neuroprotective effects and stimulate the expression of selective Alzheimer's disease indicator-1, a recently discovered antiapoptotic gene, in human neuroblast long-term cell cultures. J. Clin. Endocrinol. Metab. 2005;90:1775–1782. doi: 10.1210/jc.2004-0066. [DOI] [PubMed] [Google Scholar]
- 5.Bora SH, Liu Z, Kecojevic A, Merchenthaler I, Koliatsos VE. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Exp. Neurol. 2005;194:506–522. doi: 10.1016/j.expneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Carro E, Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer's disease. Eur. J. Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Donnini S, Cantara S, Morbidelli L, Giachetti A, Ziche M. FGF-2 overexpression opposes the beta amyloid toxic injuries to the vascular endothelium. Cell Death Differ. 2006;13:1088–1096. doi: 10.1038/sj.cdd.4401803. [DOI] [PubMed] [Google Scholar]
- 8.Dore S, Bastianetto S, Kar S, Quirion R. Protective and rescuing abilities of IGF-I and some putative free radical scavengers against beta-amyloid-inducing toxicity in neurons. Ann. N. Y. Acad. Sci. 1999;890:356–364. doi: 10.1111/j.1749-6632.1999.tb08015.x. [DOI] [PubMed] [Google Scholar]
- 9.Dore S, Kar S, Quirion R. Vol. 94. Washington, DC: 1997. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity, Proceedings of the National Academy of Sciences of the United States of America; pp. 4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H. Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J. Endocrinol. 2004;183:605–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- 11.Eckert A, Cotman CW, Zerfass R, Hennerici M, Muller WE. Lymphocytes as cell model to study apoptosis in Alzheimer's disease: vulnerability to programmed cell death appears to be altered. J. Neural Transm. 1998 Suppl. 54:259–267. doi: 10.1007/978-3-7091-7508-8_25. [DOI] [PubMed] [Google Scholar]
- 12.Eckert A, Marques CA, Keil U, Schussel K, Muller WE. Increased apoptotic cell death in sporadic and genetic Alzheimer's disease. Ann. N. Y. Acad. Sci. 2003;1010:604–609. doi: 10.1196/annals.1299.113. [DOI] [PubMed] [Google Scholar]
- 13.Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13:175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- 14.Green D, Jonusas A, Montague JM, Mudd LM. Tyrosine kinase activity of nerve growth factor and estrogen in embryonic septal neurons cultured from the rat. Neurochem. Res. 2002;27:1699–1705. doi: 10.1023/a:1021651530846. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J. Toward Alzheimer therapies based on genetic knowledge. Annu. Rev. Med. 2004;55:15–25. doi: 10.1146/annurev.med.55.091902.103607. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, el-Fakahany EE. beta-Amyloid 25–35 activates nitric oxide synthase in a neuronal clone. Neuroreport. 1993;4:760–762. doi: 10.1097/00001756-199306000-00041. [DOI] [PubMed] [Google Scholar]
- 17.Jousimaa J, Merenmies J, Rauvala H. Neurite outgrowth of neuroblastoma cells induced by proteins covalently coupled to glass coverslips. Eur. J. Cell Biol. 1984;35:55–61. [PubMed] [Google Scholar]
- 18.Lad SP, Neet KE, Mufson EJ. Nerve growth factor: structure, function and therapeutic implications for Alzheimer's disease. Curr. Drug Targets CNS Neurol. Disord. 2003;2:315–334. doi: 10.2174/1568007033482724. [DOI] [PubMed] [Google Scholar]
- 19.Lang UE, Jockers-Scherubl MC, Hellweg R. State of the art of the neurotrophin hypothesis in psychiatric disorders: implications and limitations. J. Neural Transm. 2004;111:387–411. doi: 10.1007/s00702-003-0100-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Suh YH, Kim S, Kim Y. Comparison of the structures of beta amyloid peptide (25–35) and substance P in trifluoroethanol/water solution. J Biomol Struct Dyn. 1999;17:381–391. doi: 10.1080/07391102.1999.10508369. [DOI] [PubMed] [Google Scholar]
- 21.Longo FM, Massa SM. Neuroprotective strategies in Alzheimer's disease. NeuroRx. 2004;1:117–127. doi: 10.1602/neurorx.1.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo FM, Massa SM. Neurotrophin-based strategies for neuroprotection. J. Alzheimers Dis. 2004;6:S13–S17. doi: 10.3233/jad-2004-6s606. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. J Neurosci. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu DC, Soriano S, Bredesen DE, Koo EH. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J. Neurochem. 2003;87:733–741. doi: 10.1046/j.1471-4159.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Scheff SW. Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J. Neurotrauma. 1994;11:3–33. doi: 10.1089/neu.1994.11.3. [DOI] [PubMed] [Google Scholar]
- 26.Mattson MP, Tomaselli KJ, Rydel RE. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 1993;621:35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- 27.Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F. Astrocyte modulation of in vitro beta-amyloid neurotoxicity. Glia. 2004;46:252–260. doi: 10.1002/glia.20005. [DOI] [PubMed] [Google Scholar]
- 28.Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Schliebs R. Basal forebrain cholinergic dysfunction in Alzheimer's Disease - interrelationship with beta-amyloid, Inflammation and neurotrophin signaling. Neurochem. Res. 2005;30:895–908. doi: 10.1007/s11064-005-6962-9. [DOI] [PubMed] [Google Scholar]
- 30.Richmond, CA: Systat Software Inc.; 2003. SigmaStat, SigmaStat advisory statistical software, version 3.0. [Google Scholar]
- 31.Silva A, Montague JR, Lopez TF, Mudd LM. Growth factor effects on survival and development of calbindin immunopositive cultured septal neurons. Brain Res. Bull. 2000;51:35–42. doi: 10.1016/s0361-9230(99)00188-4. [DOI] [PubMed] [Google Scholar]
- 32.Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am. J. Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 33.Sokal RR, Rohlf SJ. Biometry. New York: W.H. Freeman and Company; 1995. p. 887. [Google Scholar]
- 34.Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. Journal of Neuroscience. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J. Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Susen K, Blochl A. Low concentrations of aggregated beta-amyloid induce neurite formation via the neurotrophin receptor p75. J. Mol. Med. 2005;83:720–735. doi: 10.1007/s00109-005-0671-3. [DOI] [PubMed] [Google Scholar]
- 37.Trejo JL, Carro E, Garcia-Galloway E, Torres-Aleman I. Role of insulin-like growth factor I signaling in neurodegenerative diseases. J. Mol. Med. 2004;82:156–162. doi: 10.1007/s00109-003-0499-7. [DOI] [PubMed] [Google Scholar]
- 38.Trejo JL, Carro E, Lopez-Lopez C, Torres-Aleman I. Role of serum insulin-like growth factor I in mammalian brain aging. Growth Horm. IGF Res. 2004;14 Suppl A:S39–S43. doi: 10.1016/j.ghir.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx. 2005;2:120–128. doi: 10.1602/neurorx.2.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Wang R, Zhang YW, Zhang X. Estrogen, beta-amyloid metabolism/trafficking, and Alzheimer's disease. Ann N Y Acad Sci. 2006;1089:324–342. doi: 10.1196/annals.1386.036. [DOI] [PubMed] [Google Scholar]
- 41.Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J Neurosci. 2007;27:1422–1433. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida E, Atkinson TG, Chakravarthy B. Neuroprotective gene expression profiles in ischemic cortical cultures preconditioned with IGF-1 or bFGF. Brain Res. Mol. Brain Res. 2004;131:33–50. doi: 10.1016/j.molbrainres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Zar JH. Upper Saddle River, NJ: Prentice Hall; 1996. Biostatistical analysis; p. 662. + app pp. [Google Scholar]