Abstract
The aryl hydrocarbon receptor (AhR) was implicated as a mediator of xenobiotic toxicity over three decades ago. Although a complete picture continues to elude us, investigations by many laboratories during the ensuing period have revealed much about AhR biology in normal physiological processes, as well as the toxicities induced by the dioxins and related polychlorinated aromatic hydrocarbons. The findings are captured in numerous excellent reviews. This commentary attempts to inject a new perspective on some new as well as frequently overlooked observations in the context of established receptor properties. Specifically, we examine the impact of transient versus sustained receptor activation on AhR biology, and explore the potential role for cytochrome P450 expression in regulating AhR activity amongst various tissues. The growing recognition that AhR action functions through multiple mechanisms serves to further highlight the importance of limiting prolonged receptor activation.
Keywords: Ah receptor, Endogenous, Cytochrome P450
1. Mechanisms regulating sustained AhR activity
The eukaryotic Per-ARNT-Sim (PAS) domain protein family contains several members that function as sensors of extra-cellular signals and environmental stresses affecting growth and development [1]. Among these members, the aryl hydrocarbon receptor (AhR) regulates adaptive and toxic responses to a variety of chemical pollutants, including polycyclic aromatic hydrocarbons and polychlorinated dioxins, most notably 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The AhR is a soluble cytosolic ligand-activated transcription factor in a complex with the chaperone proteins hsp90 [2], and hsp23 [3] and an immunophilin-like protein [4–6]. Upon ligand activation, the AhR translocates into the nucleus, dissociates from the hsp proteins, and binds to DNA response elements (known as a xenobiotic responsive element or XRE) with the Ah receptor nuclear translocator (ARNT) protein, a heterodimerization partner and also a member of the PAS protein family [7]. The ARNT protein not only functions as a DNA-binding partner for the AhR, but appears to be required for the dissociation of the accessory chaperonins [7,8]. This process is illustrated as Scheme 1 (Fig. 1). AhR inducible genes include those encoding the xenobiotic metabolizing Phase I enzymes (cytochromes P450IA1, P450IA2, P450IB1), and Phase II enzymes (glutathione S-transferase Ya subunit, NAD(p)H:menadione oxidoreductase (NMO1), UDP-glucuronosyltransferase) [9–11]. The reader is referred to excellent reviews detailing our understanding of this transcriptional mechanism [1,12].
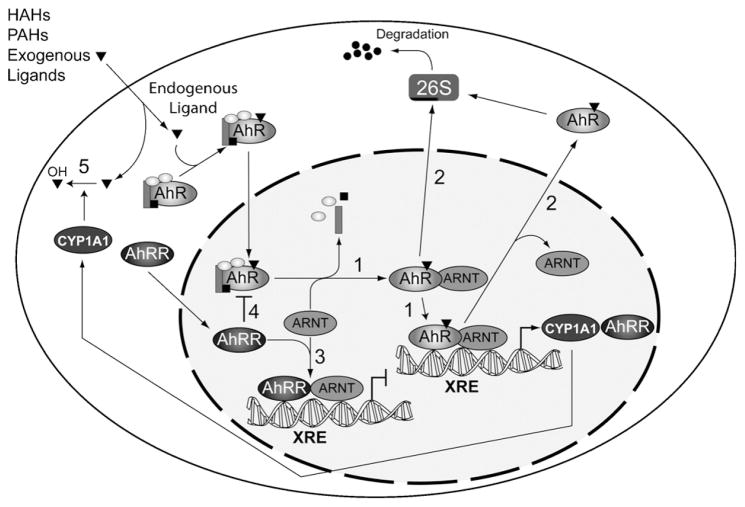
Fig. 1.
Multiple mechanisms to regulate aryl hydrocarbon receptor (AhR) activity. The diagram depicts the various mechanisms that have evolved to control AhR activity. (Scheme 1) Agonist-dependent dimerization of AhR with the AhR nuclear translocator (ARNT) concomitant with dissociation of the chaperones hsp90, p23 and immunopilin-like protein XAP2/ARA9/AIP; binding of AhR-ARNT complexes to xenobiotic response elements (XREs); and subsequent transcriptional activation. (Scheme 2) Proteolytic degradation of the activated AhR by the 26S proteosome. (Schemes 3 and 4) Repression of AhR activity by the AhR repressor protein (AhRR). (Scheme 5) Metabolic depletion of AhR agonists by cytochrome P4501A1 (CYP1A1) or related enzymes. Exogenous AhR ligands include halogenated aromatic hydrocarbons (HAHs) and polyaromatic hydrocarbons (PAHs).
Historically, studies on the AhR emphasized efforts to understand the molecular basis for TCDD toxicity, and insight into the mechanism of AhR signaling comes primarily from studies in mammalian cells exposed to xenobiotics, in particular TCDD. Recent studies using knockout mice generated by three independent laboratories demonstrated that TCDD toxicity is almost solely dependent upon a functional AhR [13–15]. By and large, these mice share many common features including resistance to TCDD toxicity, diminished reproductive fecundity, and smaller livers harboring fibrotic lesions. Hepatic defects include prolonged extramedullary hematopoiesis and portal hypercellularity with thickening and fibrosis. It is notable that as the AhR knockout mice age, hypertrophy and hyperplasia are detected in numerous organs including the heart, vasculature, gastric epithelium and skin [16,17]. Although phenotypic differences between the models were also noted, the basis for these remains unresolved [18]. Nevertheless, the knockout models demonstrate that the AhR plays a role in developmental processes and physiological homeostasis in the absence of stimulation by a deliberately administered exogenous agonist. Subsequent investigations using AhR hypomorphic and conditional knockout mice serve to reinforce the impression that the AhR plays key roles in normal physiological processes that are dissociable from TCDD-induced toxicity [19–21]. AhR expression in mice extends to numerous tissues in both embryos and adults. The receptor is detectable as early as day 10 of gestation in multiple tissues [14,22] consistent with a developmental role for the AhR. In adult animals, AhR expression is pronounced in several regions including female reproductive tissues, skin, bladder, lung and respiratory epithelium, and liver [23]. However, functional studies demonstrate that AhR expression extends to a host of other tissues.
AhR activation independent of agonist binding in mammalian systems has been proposed, but unequivocal evidence continues to elude researchers. Oesch-Bartlomowicz et al. proposed that the second messenger cAMP, could promote receptor nuclear translocation in a ligand-independent manner, but failed to demonstrate transcriptional activation [24]. Chang and Puga reported that AhR-dependent effects on cell proliferation could be dissociated from exogenous ligand binding [25]. However, neither study unequivocally established the absence of an endogenous agonist responsible for receptor activity. Deletion of the ligand-binding domain of the AhR generates a constitutively active receptor [25,26], but this mode of agonist-independent action may be unique to the mutation and not accurately recapitulate normal receptor biology. Studies using targeted mutations that disrupt ligand binding revealed that agonist-independent AhR activity comprised only 2% of that observed following agonist stimulation [27]. The physiological relevance of this paltry response is challenged and suggests that AhR signaling in mammalian systems is agonist dependent. Indeed, ligand-dependent receptor activation is well established, and several mechanisms capable of suppressing prolonged AhR signaling have been identified. These include rapid receptor degradation, the action of an AhR Repressor protein (AhRR), and agonist depletion through an enzymatic negative feedback loop (depicted as Schemes 2–5, respectively in Fig. 1). The evolution of multiple mechanisms designed to suppress sustained AhR activity implies that prolonged receptor signaling is physiologically deleterious. Sustained TCDD-induced AhR activation typically culminates in rapid protein ubiquitination that targets the receptor for proteosomal degradation (see Fig. 1, Scheme 2) [28–30]. The finding that ubiquitination appears to be triggered solely by the nuclear localization of the activated receptor [31–33] is striking because it implies that receptor degradation is a regulatory process fundamentally driven by receptor activity. Interestingly, transient, endogenous AhR signaling during cell cycle progression in rat 5 L hepatoma cells was not accompanied by a noticeable loss in AhR protein expression [34], suggesting that short-term receptor activation may not trigger the degradation pathway. The notion that receptor degradation is linked to prolonged receptor activity is further supported by the observation that AhR protein levels decline in hepatoma cells when cytochrome P4501A1 enzyme activity is inhibited, thus preventing agonist turn-over, resulting in sustained receptor activation [34]. Hence, rapid receptor degradation offers a mechanism for squelching prolonged receptor activation by removing the receptor under conditions of persistent agonist signaling.
AhR activity also appears to be regulated through the induction of the AhRR, depicted as Scheme 3 (Fig. 1). Like the AhR and ARNT, the AhRR is a member of the bHLH-PAS family and was originally proposed to function by both sequestering ARNT, and by forming a non-functional XRE-binding complex comprising the AhRR and ARNT, thereby functioning as a dominant negative protein capable of preventing AhR-mediated transcription [35,36]. Interestingly, the AhRR is constitutively expressed in some tissues such as heart and brain, whilst being inducible in other tissues such as the liver and lung [37]. Although it has been difficult to directly correlate increased AhRR expression with decreased AhR transcriptional activity, the finding that AhRR mRNA levels in AhR knockout mice are two to three orders of magnitude lower than in wild type mice underscores the notion that AhR controls the expression of this gene [37]. Complementary findings demonstrated increased AhRR mRNA expression in tissues of mice expressing the constitutively active AhR (CA-AhR) [26], consistent with the premise that AhR-mediated AhRR expression represents a negative feedback mechanism. The precise mechanism for AhRR action however, remains contentious. A recent study demonstrated that overexpression of ARNT failed to alleviate AhRR-mediated repression, implying that suppression of AhR activity by the AhRR is not a consequence of ARNT sequestration by the repressor [36]. Similarly, disrupting AhRR DNA binding did not restore AhR activity and even more surprising, when both mechanisms were blocked—through use of a DNA-binding defective AhRR in the presence of excess ARNT expression—transcriptional repression persisted. Since AhR overexpression can overcome AhRR repression [35], Evans et al. suggested that the AhR and AhRR compete for a limiting cofactor [36]. However, given that an AhRR truncation mutant lacking the C-terminal two-thirds of the protein—critical for PAS protein cofactor binding—still confers repressor activity, competition for a cofactor is questionable. A facile albeit highly speculative scenario is presented in Scheme 4 (Fig. 1), depicting AhRR activity as functionally interfering with AhR transformation, specifically receptor dissociation from the chaperone proteins inside the nucleus, rendering it competent to interact with nuclear partner proteins. This model for AhRR action reconciles existing data by being independent of ARNT binding, DNA binding, and cofactor sequestration. However, direct evidence for such a process is lacking.
The AhR is evolutionarily highly conserved [38,39]. Given that ancestral AhRs do not bind ligands, or at least not the xenobiotics associated with vertebrate receptor activation, it would appear that agonist binding is a more recent manifestation. Whether this is an adaptation for dealing with xenobiotic compounds or reflects a distinct physiological function in vertebrates is unclear. Exogenous AhR agonists are structurally diverse compounds that vary in their receptor-binding affinity as well as their metabolic stability. Potent exogenous agonists such as TCDD and related halogenated aromatic hydrocarbons (HAH) exhibit high binding affinity for the receptor (pM to nM range) and tend to be metabolically stable. Although AhR activation induces the expression of numerous XRE-containing genes that encode xenobiotic metabolizing enzymes, most notably cytochrome P4501A1, many high affinity agonists are poor substrates for these enzymes. The metabolic stability of these agonists sets the stage for continuous AhR activation, which typically culminates in toxic responses such as those observed following exposure to TCDD. Other exogenous AhR agonists include polyaromatic hydrocarbons (PAH), which exhibit low binding affinity (nM to μM range) and are metabolically labile. Although these low affinity agonists induce AhR-dependent gene expression, they do not typically elicit the toxic responses associated with exposure to high affinity agonists such as TCDD. Whilst receptor activation by these agonists appears to be sufficient for inducing some AhR-dependent gene expression, the rapid metabolic depletion of these agonists likely prevents sustained receptor activation and precludes the onset of toxic responses observed following exposure to TCDD.
The existence of an endogenous ligand(s) remains a central albeit unresolved question. In higher eukaryotes, evidence implicating the existence of endogenous AhR agonists includes cell culture studies demonstrating that AhR activity is markedly increased in the absence of CYP1 enzymatic activity, consistent with the idea that AhR-induced cytochrome P450 activity removes an endogenous agonist outlined in Scheme 5 (Fig. 1) [25,34]. Accordingly, Chang and Puga demonstrated that constitutive AhR nuclear localization and transcriptional activity were detected in the absence of a functional cytochrome P4501A1 enzyme [25]. Such sustained receptor activity was attributed to the accumulation of an endogenous AhR agonist that, under normal conditions, would be readily metabolized by P4501A1 activity. In this same study, the observed AhR transcriptional activity was diminished when functional P4501A1 enzymatic activity was expressed, thus conferring upon P4501A1 a role in down-regulating AhR activity by metabolically depleting receptor agonists through a negative feedback mechanism. Although a high-affinity endogenous AhR ligand has not been identified, numerous structurally diverse low affinity ligands exist [40]. These include tetrapyroles, tryptophan photoproducts, indole-containing compounds, sterols such as 7-ketocholes-terol and equilenin, and fatty acid metabolites. Whether they constitute physiologically relevant AhR agonists remains unclear, although a recent review parses this question and serves to highlight the gaps in current understanding [41]. However, it is entirely conceivable that several ligands exist, capable of uniquely regulating AhR activity in a tissue specific manner [42–44].
The premise that transient and sustained AhR activation affect the transcriptome differently, thereby culminating in distinct biological responses to receptor activation, highlights the importance of tightly regulating AhR signaling. Therefore, it is not surprising that multiple mechanisms exist for controlling both receptor activity and expression as discussed earlier (see Schemes 1–4). Whilst the biological consequences of transient AhR signaling can be distinguished from those of sustained signaling, less is known about the mechanisms that yield such disparate responses. It is likely that transient and sustained AhR signaling may induce distinct gene profiles. A recent study by Tijet et al. [45] used gene profile analysis to distinguish between genes that are regulated by physiological endogenous AhR signaling and those that are altered in response to TCDD. The results demonstrated that over 200 distinct genes were differentially expressed in the livers of Ahr−/− and wild type mice, suggesting that endogenous signaling through the AhR contributes to numerous physiological responses that include reproduction, growth and development. One of the genes identified encodes the proteinase inhibitor Serpina12, the mRNA for which was up-regulated 220 times in wild type mice as compared to hr−/− mice, presumably due to endogenous signaling cues. However, treatment of wild type mice with TCDD failed to induce the expression of this gene; in fact, its expression was downregulated by TCDD. This demonstrates that sustained receptor activation does not faithfully replicate receptor-mediated gene expression following transient receptor activation. Whether the observation with Serpina12 represents a direct or an indirect AhR response in unclear, but it is conceivable that prolonged receptor signaling increases the likelihood that less responsive XRE-regulated genes eventually recruit functional receptor complexes, thus ultimately modifying the transcriptome profile. Therefore, the duration of receptor signaling may influence both quantitative (i.e. amount of gene expression) and qualitative (i.e. gene expression spectrum) parameters thus tipping the balance from a homeostatic adaptive response to a toxic response. It is also formally possible that the agonist imparts unique properties. This is exemplified by the differential response to polycyclic aromatic hydrocarbons versus TCDD in ovarian Bax gene expression [46]. Suffice it to say that sustained AhR activity invokes a homeostatic disequilibrium underscored by a dramatically altered transcriptome.
Increasing evidence indicates that at least some of the biological responses that are deleteriously affected by TCDD actually require a certain amount of endogenous AhR signaling to proceed optimally. For example, AhR knockout mice exhibit defects in vascular development and rates of cell proliferation that are slower than found in wild type mice [47]. Likewise, hepatoma-derived cell lines devoid of the AhR exhibit a G1 cell cycle delay that can be alleviated upon reintroduction of a functional AhR [48,49]. Thus, in the absence of exogenous agonists, endogenous receptor activity appears to initiate or maintain biological processes required for proliferation. Yet, in the presence of a persistent exogenous agonist such as TCDD, such processes are either dampened or interrupted altogether. As a consequence, treatment with TCDD stalls proliferation and elicits a G1 arrest in multiple cell types, including hepatocytes [50,51], neuronal cells [52], thymocytes [53], and B cells [54]. The impact of AhR expression and activity on the rate of G1 passage is consistent with the idea that the receptor functions as a “throttle control” in G1 phase progression: AhR presence and transient endogenous activity promotes progression; yet when the receptor is absent or is provided with sustained agonist stimulation, movement through the cell cycle is stalled. The physiological implication is that the AhR plays a pivotal role in orderly passage through the G1 (growth) phase prior to commitment to the cell cycle culminating in cell division, whilst aberrant (i.e. sustained or lost) receptor activity can markedly disturb the process.
The premise that prolonged duration of AhR signaling is central to TCDD-mediated cell cycle arrest is strengthened by the observation that a similar G1 arrest occurs in cells treated with the cytochrome P4501A inhibitor 1-(1-propynyl)pyrene (1-PP) in the absence of TCDD. As a suicide substrate inhibitor of P4501A activity, 1-PP treatment presumably inhibits the metabolic depletion of endogenous AhR agonists, resulting in endogenous agonist accumulation and subsequent prolonged receptor signaling (Fig. 1, Scheme 5). This is evidenced by increased induction of AhR-regulated cytochrome P4501A1 protein expression as well as increased levels of the cell cycle inhibitor, p27Kip1 [34]. The observation that 1-PP treatment elicits a G1 arrest that is similar to that observed in cells treated with TCDD supports the notion that sustained receptor activation—regardless if it occurs via metabolically stable exogenous agonists or non-metabolized endogenous agonists—culminates in a maladaptive response not observed during transient, short-lived receptor signaling.
There are striking parallels between the receptor effects on hepatoma cell growth in culture and the observation that sustained AhR activity impairs G1/S progression during liver regeneration in vivo. Not only does sustained AhR activation produce a growth arrest in the regenerating liver following partial hepatectomy (PH), but akin to the transient CYP1A1 induction seen in the hepatoma cells, CYP1A1 is likewise transiently induced following liver resection in vehicle-treated mice, coincident with progression through G1/S phase [55]. Increases of P4501A1 protein are readily detectable by 36 h post-PH, peaking at levels similar to those observed in TCDD-treated mice. Analysis of CYP1A1 mRNA detected a pronounced (42-fold) increase in mRNA 24 h post-PH. In contrast to the effect of TCDD exposure, the transient activation of the AhR is insufficient for eliciting a G1 arrest in cycling hepatocytes in vivo. Whether transient AhR activation is required for normal cell cycle progression during liver regeneration remains unclear, but if the parallels between hepatoma cells and the normal liver persist, we anticipate that liver regeneration would be retarded under conditions where AhR activity is absent. The markedly altered liver phenotype in the AhR knock-out mouse due to vascular defects may confound assessment of the receptor’s role in PH-induced liver regeneration, but the conditional AhR knock-out model [21] should provide a suitable system in which to establish a role for the AhR in liver repair.
The significance of AhR activity in determining the fate of the ensuing biological response is further underscored in a study by Andersson et al. [26], which used mice that expressed a constitutively active AhR (CA-AhR). Expression of known AhR target genes including CYP1A1 was increased in all of the tissues that expressed the CA-AhR, indicating that the receptor mimicked the transcriptional action of the ligand-activated AhR. A striking finding emanating from this study concerns the marked incidence in gastric tumors observed in mice expressing the CA-AhR, which are not detected in wild-type mice. Furthermore, CA-AhR-expressing mice exposed to the tumor initiator N-nitrosodiethylamine (DEN) developed liver tumors with a 56% prevalence, far exceeding the 7% seen in wild-type mice [56]. In reconciling the tumorigenic potential of constitutive AhR activity with the inhibitory effects on cell cycle progression following agonist-induced sustained AhR activity, it is tempting to speculate that a CA-AhR may actually be suppressing normal growth in the stomach and liver thereby conferring a growth advantage upon any transformed cells in the tissue. In other words, given that sustained AhR signaling appears to target the G1/S phase checkpoint in cell cycle control, transformed cells that escape AhR-regulated checkpoint control manifest as tumors. These findings provide indirect support for the notion that the duration of AhR signaling, rather than the nature of the receptor agonist, determines the biological outcomes following receptor activation.
2. The role of other AhR-responsive P450s
The cytochrome P450 subfamilies CYP1 to CYP4 are responsible for most of the metabolism of foreign compounds, possess unique yet overlapping substrate specificities, and are often regulated by substrate-induced activation of gene transcription. Amongst these, several P450s are AhR target genes, including CYP1A1, 1A2, and 1B1, as well as CYP2S1 [57] and murine Cyp2a5 [58,59]. Conventional wisdom maintains that the AhR is a biosensor for compounds metabolized by these isozymes, and the receptor’s primary function is to induce expression of the CYP genes. Certainly from the standpoint of xenobiotic disposition this is a valid perspective. However, this may be putting the ‘cart before the horse’ as it applies to normal AhR function. The central premise in this commentary is that CYP activity serves to minimize prolonged AhR activity by removing endogenous receptor agonists in a negative feedback regulatory loop. In fact, this relationship between the AhR and the inducible cytochromes P450 seemingly mandates the existence of an endogenous ligand. However, given the structural diversity of both exogenous and putative endogenous AhR agonists, it is conceivable that metabolic depletion of various AhR agonists requires the induction of numerous and varied xenobiotic metabolizing enzymes, either instead of or in addition to cytochrome P4501A1. The AhR-inducible CYP1 and CYP2 enzymes exhibit broad tissue-specific expression, intimating the existence of multiple endogenous AhR agonists specific to distinct tissues or cell types.
Basal CYP1A1 expression is generally low or absent, but is readily inducible in most tissues examined. Although the identity of endogenous P4501A1 substrates remains unresolved, the enzyme contributes to 2-hydroxylation of 17β-estradiol and estrone in extrahepatic tissues including breast [60]. However, in the liver, these substrates are hydroxylated—at various positions—by several other cytochromes P450 as well, in keeping with known substrate overlap between P450s. In mammals, the CYP1A2 protein is constitutively expressed, primarily in the olfactory mucosa and liver. In the latter, CYP1A2 is also inducible through an AhR-mediated pathway. CYP1A2 carries out several known endogenous functions including uroporphyrinogen and melatonin oxidation and estradiol hydroxylation, particularly 4-hydroxylation [61]. In addition, CYP1A2 also metabolizes retinoids, arachidonic acid, and the sex steroids. CYP1B1 expression is extrahepatic, occurring primarily in steroid-responsive, mesodermal-derived tissues including the uterus, breast and prostate [62], and akin to CYP1A2, is involved in the metabolism of steroids, retinol and retinal, arachidonate, and melatonin. CYP2S1 expression is highest in epithelial tissues, primarily the intestinal tract, lung and trachea [63]. CYP2S1 metabolizes all-trans-retinoic acid to 4-hydroxy-retinoic acid and 5,6-epoxy-retinoic acid [64]. The mouse Cyp2a5—and orthologous rat CYP2A3 and the human CYP2A13 and CYP2A6—are present at high levels in the respiratory tract and lungs. Although no known endogenous substrate has been identified to date, Cyp2a5 expression is induced in the liver by certain pathogens and is frequently over expressed in liver tumors. Although Cyp2a5 is a documented AhR target gene, it is unclear whether the aforementioned increases are directly due to AhR activity. Clearly, AhR-responsive P450s are broadly expressed and serve to metabolize numerous compounds. However, retinols and estrogen are not AhR ligands, thus challenging the premise that P450 expression solely serves to regulate AhR activity. What remains unclear is whether these are the primary endogenous substrates, or whether the broad overlapping substrate specificity is masking the identity of additional substrates that indeed function as endogenous AhR agonists. Certainly, the observations that CYP1A1 expression is markedly increased in systems where P4501A1 activity is absent or suppressed are consistent with the idea that additional substrates exist. Lastly, it is noteworthy that the similarities in AhR-responsive CYP1 and CYP2 patterns of expression in adult mouse and human tissues and their transcriptional regulation, are highly conserved [65]. This is consistent with a physiologically important receptor-mediated mechanism affecting developmental and homeostatic processes, rather than merely responding to xenobiotics.
3. Multiple mechanisms of AhR action
The preceding discussion sought to illustrate that AhR signaling is tightly regulated, and that prolonged or persistent receptor activity can lead to deleterious consequences. A growing body of evidence is beginning to reveal a deeper level of complexity in AhR biology depicted by numerous, varied mechanisms. These are illustrated in Fig. 2. Upon DNA binding, the AhR/ARNT heterodimer forms the scaffold for multiple coactivator complexes associated with the receptor (Fig. 2, Scheme 1). Each complex imparts regulatory specificity upon the target gene transcriptional machinery, and its composition and mode of assembly may well vary between tissues, cells, and even amongst genes within a given cell. The reader is directed to a recent review [66] for a detailed description of AhR coactivators. It is worth noting that these coactivators (denoted as CoA in Fig. 2) include the chromatin remodeling protein Brg-1, the mediator TRAP-DRIP complex, and several established coactivators such as p160, p300/CPB, RIP140, TRIP230, and more recently GAC63 and BRCA1 [67,68]. How they assemble into a complex and impart transcriptional specificity is yet to be determined. We identified the retinoblastoma tumor suppressor protein (pRb) as a receptor-binding protein [69] and subsequently showed that AhR-mediated G1 phase cell cycle arrest required an interaction between the AhR and pRb [70]. Interestingly, two distinct mechanisms appear to be involved. First, pRb can function as a transcriptional coactivator in TCDD-mediated induction of CYP1A1 and possibly p27Kip1 [49], because targeted disruption of the AhR/pRb interaction dampens the induction response. Second, the AhR forms a quaternary repressor complex with pRb, E2F, and DP (the E2F-binding partner in transactivation, depicted in Fig. 2, Scheme 2) to suppress the transcription of S phase genes [71,72]. Subsequent studies using targeted point mutations designed to specifically disrupt AhR–DNA binding to the XRE whilst preserving the AhR/pRb interaction revealed that both mechanisms contributed to the G1 arrest response [73]. It is also noteworthy that the AhR/pRb interaction seems to be restricted to the hypophosphorylated “active” form of pRb [70,71]. Because hypophosphorylated pRb is confined to the G0 (quiescent) and G1 phase of the cell cycle, the AhR–pRb interaction—and functional consequences of this interaction—is likely to be cell cycle-dependent, implying that AhR activity may differ markedly between non-cycling and cycling cells within a given tissue or organ exposed to an agonist stimulus.
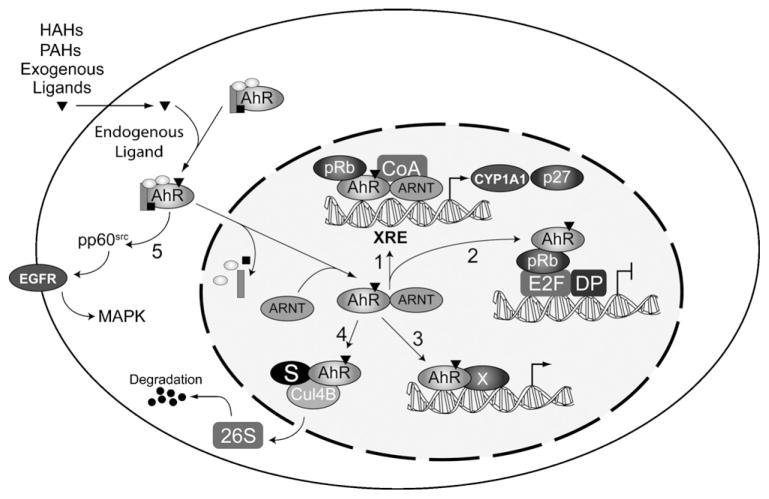
Fig. 2.
Multiple mechanisms involving aryl hydrocarbon receptor (AhR)-mediated cell signaling. The diagram depicts several mechanisms whereby AhR activity modifies cell signaling. Illustrated is the canonical xenobiotic response element (XRE)-bound AhR/ARNT complex with recruited coactivators (CoA; Scheme 1). AhR participation in transcriptional activation at non-XRE sites through indirect DNA-binding (Scheme 2), or direct DNA binding (Scheme 3). Additionally, two non-genomic modes of action involving AhR-mediated susbtrate (S) proteolysis involving cullin 4B (Scheme 4), and activation of epidermal growth factor receptor (EGFR; Scheme 5) are presented.
Incorporation of the AhR into the E2F complex reveals that the receptor can modulate gene expression involving regulatory elements distinct from the XRE, albeit through indirect DNA binding. This scenario is reinforced by a study showing that the ligand-activated AhR/ARNT dimer can interact directly with the unliganded estrogen receptor and promote formation of a transcriptionally active complex binding to estrogen response elements [74]. However, evidence is accumulating for non-consensus XRE sequences that appear to confer direct AhR DNA binding [75,76]. The composition of these AhR-containing complexes are not well defined (Fig. 2, Scheme 3 where ‘X’ denotes novel-binding partners), but Vogel et al. described an interaction between RelB (an NF-kB subunit) and the AhR modulating IL-8 gene expression through a new RelBAhRE cis-element [76]. A relationship between the NF-kB RelA (p65) subunit and AhR provides conflicting findings by variously demonstrating inhibition [77] or activation [78] of NF-kB activity. The discordance may be related to the target gene examined and the make-up of the DNA-bound complex. Whilst these observations are far from exhaustive, they do emphasize that the repertoire of AhR target genes may be far broader than previously suggested by the presence of XREs in the genome.
Probably more remarkable are two recent findings exposing AhR-mediated processes that are dissociated from direct transcriptional regulation. Ohtake et al. observed that upon agonist binding, the AhR could function as an adaptor in a cullin 4B E3 ubiquitin ligase complex and promote polyubiquitination and subsequent 26S proteosomal degradation of steroid receptors [79] (Fig. 2, Scheme 4). The possibility exists however, for agonist-dependent ubiquitin-mediated protein degradation being more widespread. Given that cullin 4B also contributes to cyclin E turn-over [80], it is tempting to speculate that the TCDD-dependent loss of CDK2-bound cyclin E in hepatectomized livers undergoing regeneration [55] may be due to the AhR promoting cyclin E ubiquitination and subsequent proteolysis. In a second example of non-genomic AhR signaling (Fig. 2, Scheme 5), Fritsche et al. demonstrated that a UVB-induced tryptophan photoproduct, 6-formylindolo[3,2-b]carbazole (FICZ), triggered EGF receptor (EGFR) activation in addition to CYP1A1 expression in irradiated skin [81]. Whilst the agonist properties of FICZ were previously documented (see Ref. [41] and references therein), the recent finding established a role for the AhR and FICZ in UVB-induced stress response in vivo. Specifically, FICZ-induced transformation of the cytosolic AhR complex in skin also triggered release of the tyrosine kinase pp60src and subsequent activation of EGFR-mediated MAPK cascade by an as yet unresolved mechanism. At this time, it is unclear whether this phenomenon is restricted to the skin, since FICZ is labile and has to date not been detected in the blood, but it seems entirely conceivable that other AhR ligands could activate EGFR signaling elsewhere in the body through this non-genomic process. The discovery of multiple mechanisms only serves to illustrate that biological consequences of prolonged receptor activation are likely to be many and varied.
4. Conclusion
Identification of new AhR roles and recent advances in previously identified mechanisms firmly establish the AhR as an important regulator of normal developmental and homeostatic processes. Our growing awareness of the complexity behind AhR biology is underscored by our inability to mechanistically resolve TCDD toxicity despite decades of research. Therefore, it stands to reason that the basis for TCDD toxicity may only be revealed once we understand the role played by the receptor in normal physiology. Hence, identification of physiologically relevant ligands will undoubtedly provide valuable insights relevant to both AhR toxicology and its place in normal cellular and tissue biology.
Acknowledgments
The authors wish to thank John Holmes for the artwork. This work was supported by grants from the National Institute of Environmental Health Sciences: R01ES007800, R01ES012018 and F32ES013588.
Abbreviations
- 1-PP
1-(1-propynyl)pyrene
- AhR
aryl hydrocarbon receptor
- AhRR
Ah receptor repressor protein
- ARNT
Ah receptor nuclear translocator
- bHLH
basic helix-loop-helix
- CA-AhR
constitutively active Ah receptor
- CDK
cyclin-dependent kinase
- CYP
cytochrome P450
- DEN
N-nitrosodiethylamine
- EGFR
epidermal growth factor receptor
- FICZ
6-formylindolo[3,2-b]carbazole
- HAH
halogenated aromatic hydrocarbons
- PAH
polyaromatic hydrocarbons
- PAS
Per-ARNT-Sim
- PH
partial hepatectomy
- pRB
retinoblastoma protein
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- XRE
xenobiotic response element
References
- 1.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annual Review of Pharmacology and Toxicology. 2000;40:519–61. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. The Journal of Biological Chemistry. 1988;263:13802–5. [PubMed] [Google Scholar]
- 3.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. The Journal of Biological Chemistry. 1999;274:13519–24. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 4.Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. The Journal of Biological Chemistry. 1997;272:11452–6. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 5.Ma Q, Whitlock JP., Jr A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. The Journal of Biological Chemistry. 1997;272:8878–84. [PubMed] [Google Scholar]
- 6.Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Molecular and Cellular Biology. 1998;18:978–88. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lees MJ, Whitelaw ML. Multiple roles of ligand in transforming the dioxin receptor to an active basic helix-loop-helix/PAS transcription factor complex with the nuclear protein ARNT. Molecular and Cellular Biology. 1999;19:5811–22. doi: 10.1128/mcb.19.8.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire J, Coumailleau P, Whitelaw ML, Gustafsson JA, Poellinger L. The basic helix-loop-helix/PAS factor Sim is associated with hsp90. Implications for regulation by interaction with partner factors. The Journal of Biological Chemistry. 1995;270:31353–7. doi: 10.1074/jbc.270.52.31353. [DOI] [PubMed] [Google Scholar]
- 9.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. The Journal of Biological Chemistry. 1991;266:4556–61. [PubMed] [Google Scholar]
- 10.Nebert DW. Drug-metabolizing enzymes, polymorphisms and interindividual response to environmental toxicants. Clinical Chemistry and Laboratory Medicine. 2000;38:857–61. doi: 10.1515/CCLM.2000.124. [DOI] [PubMed] [Google Scholar]
- 11.Jones PB, Galeazzi DR, Fisher JM, Whitlock JP., Jr Control of cytochrome P1-450 gene expression by dioxin. Science (New York NY) 1985;227:1499–502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock JP., Jr Induction of cytochrome P4501A1. Annual Review of Pharmacology and Toxicology. 1999;39:103–25. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–54. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science (New York NY) 1995;268:722–6. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Veterinary Pathology. 1997;34:605–14. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metabolism and Disposition The Biological Fate of Chemicals. 1998;26:1194–8. [PubMed] [Google Scholar]
- 18.Lahvis GP, Bradfield CA. Ahr null alleles: distinctive or different? Biochemical Pharmacology. 1998;56:781–7. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 19.Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10442–7. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic ARNT allele. The Journal of Biological Chemistry. 2004;279:16326–31. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- 21.Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17858–63. doi: 10.1073/pnas.0504757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors. I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Developmental Dynamics. 1995;204:133–43. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- 23.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, et al. Large-scale analysis of the human and mouse transcriptomes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4465–70. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, et al. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9218–23. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CY, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Molecular and Cellular Biology. 1998;18:525–35. doi: 10.1128/mcb.18.1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9990–5. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray IA, Reen RK, Leathery N, Ramadoss P, Bonati L, Gonzalez FJ, et al. Evidence that ligand binding is a key determinant of Ah receptor-mediated transcriptional activity. Archives of Biochemistry and Biophysics. 2005;442:59–71. doi: 10.1016/j.abb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Prokipcak RD, Okey AB. Downregulation of the Ah receptor in mouse hepatoma cells treated in culture with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Canadian Journal of Physiology and Pharmacology. 1991;69:1204–10. doi: 10.1139/y91-176. [DOI] [PubMed] [Google Scholar]
- 29.Reick M, Robertson RW, Pasco DS, Fagan JB. Down-regulation of nuclear aryl hydrocarbon receptor DNA-binding and transactivation functions: requirement for a labile or inducible factor. Molecular and Cellular Biology. 1994;14:5653–60. doi: 10.1128/mcb.14.9.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollenz RS. The aryl-hydrocarbon receptor, but not the aryl-hydrocarbon receptor nuclear translocator protein, is rapidly depleted in hepatic and nonhepatic culture cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Molecular Pharmacology. 1996;49:391–8. [PubMed] [Google Scholar]
- 31.Pollenz RS, Barbour ER. Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor-mediated gene regulation. Molecular and Cellular Biology. 2000;20:6095–104. doi: 10.1128/mcb.20.16.6095-6104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytoplasmic proteasome following nuclear export. The Journal of Biological Chemistry. 1999;274:28708–15. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 33.Roberts BJ, Whitelaw ML. Degradation of the basic helix-loop-helix/Per-ARNT-Sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. The Journal of Biological Chemistry. 1999;274:36351–6. doi: 10.1074/jbc.274.51.36351. [DOI] [PubMed] [Google Scholar]
- 34.Levine-Fridman A, Chen L, Elferink CJ. Cytochrome P4501A1 promotes G1 phase cell cycle progression by controlling aryl hydrocarbon receptor activity. Molecular Pharmacology. 2004;65:461–9. doi: 10.1124/mol.65.2.461. [DOI] [PubMed] [Google Scholar]
- 35.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes and Development. 1999;13:20–5. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans BR, Karchner SI, Allan LL, Pollenz RS, Tanguay RL, Jenny MJ, et al. Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: role of DNA binding and competition for AHR nuclear translocator. Molecular Pharmacology. 2008;73:387–98. doi: 10.1124/mol.107.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernshausen T, Jux B, Esser C, Abel J, Fritsche E. Tissue distribution and function of the aryl hydrocarbon receptor repressor (AhRR) in C57BL/6 and aryl hydrocarbon receptor deficient mice. Archives of Toxicology. 2006;80:206–11. doi: 10.1007/s00204-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 38.Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chemico-Biological Interactions. 2002;141:131–60. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 39.Hahn ME, Karchner SI, Evans BR, Franks DG, Merson RR, Lapseritis JM. Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: insights from comparative genomics. Journal of Experimental Zoology. 2006;305:693–706. doi: 10.1002/jez.a.323. [DOI] [PubMed] [Google Scholar]
- 40.Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, et al. Activation of the Ah receptor signaling pathway by prostaglandins. Journal of Biochemical and Molecular Toxicology. 2001;15:187–96. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chemical Research Toxicology. 2008;21:102–16. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. The Journal of Biological Chemistry. 2001;276:31475–8. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- 43.Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Molecular Pharmacology. 1997;52:590–9. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 44.Rannug A, Fritsche E. The aryl hydrocarbon receptor and light. Biological Chemistry. 2006;387:1149–57. doi: 10.1515/BC.2006.143. [DOI] [PubMed] [Google Scholar]
- 45.Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Molecular Pharmacology. 2006;69:140–53. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 46.Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nature Genetics. 2001;28:355–60. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 47.Ma Q, Whitlock JP., Jr The aromatic hydrocarbon receptor modulates the hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Molecular and Cellular Biology. 1996;16:2144–50. doi: 10.1128/mcb.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss C, Kolluri SK, Kiefer F, Gottlicher M. Complementation of Ah receptor deficiency in hepatoma cells: negative feedback regulation and cell cycle control by the Ah receptor. Experimental Cell Research. 1996;226:154–63. doi: 10.1006/excr.1996.0214. [DOI] [PubMed] [Google Scholar]
- 49.Kolluri SK, Weiss C, Koff A, Gottlicher M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes and Development. 1999;13:1742–53. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlicher M, Wiebel FJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes unbalanced growth in 5L rat hepatoma cells. Toxicology and Applied Pharmacology. 1991;111:496–503. doi: 10.1016/0041-008x(91)90253-b. [DOI] [PubMed] [Google Scholar]
- 51.Hushka DR, Greenlee WF. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits DNA synthesis in rat primary hepatocytes. Mutation Research. 1995;333:89–99. doi: 10.1016/0027-5107(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 52.Jin DQ, Jung JW, Lee YS, Kim JA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits cell proliferation through arylhydrocarbon receptor-mediated G1 arrest in SK-N-SH human neuronal cells. Neuroscience Letters. 2004;363:69–72. doi: 10.1016/j.neulet.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 53.Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, et al. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. Journal of Immunology. 2003;171:4582–91. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- 54.Morris DL, Karras JG, Holsapple MP. Direct effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on responses to lipopolysaccharide (LPS) by isolated murine B-cells. Immunopharmacology. 1993;26:105–12. doi: 10.1016/0162-3109(93)90002-8. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell KA, Lockhart CA, Huang G, Elferink CJ. Sustained aryl hydrocarbon receptor activity attenuates liver regeneration. Molecular Pharmacology. 2006;70:163–70. doi: 10.1124/mol.106.023465. [DOI] [PubMed] [Google Scholar]
- 56.Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Research. 2004;64:4707–10. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 57.Rivera SP, Saarikoski ST, Hankinson O. Identification of a novel dioxin-inducible cytochrome P450. Molecular Pharmacology. 2002;61:255–9. doi: 10.1124/mol.61.2.255. [DOI] [PubMed] [Google Scholar]
- 58.Arpiainen S, Raffalli-Mathieu F, Lang MA, Pelkonen O, Hakkola J. Regulation of the Cyp2a5 gene involves an aryl hydrocarbon receptor-dependent pathway. Molecular Pharmacology. 2005;67:1325–33. doi: 10.1124/mol.104.008078. [DOI] [PubMed] [Google Scholar]
- 59.Gokhale MS, Bunton TE, Zurlo J, Yager JD. Cytochrome P450 isoenzyme activities in cultured rat and mouse liver slices. Xenobiotica. 1997;27:341–55. doi: 10.1080/004982597240505. [DOI] [PubMed] [Google Scholar]
- 60.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–98. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 61.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 62.Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, et al. Cytochrome P450 CYP1B1 determines susceptibility to 7,12-dimethylbenz [a]anthracene-induced lymphomas. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1977–82. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saarikoski ST, Wikman HA, Smith G, Wolff CH, Husgafvel-Pursiainen K. Localization of cytochrome P450 CYP2S1 expression in human tissues by in situ hybridization and immunohistochemistry. Journal of Histochemistry and Cytochemistry. 2005;53:549–56. doi: 10.1369/jhc.4C6576.2005. [DOI] [PubMed] [Google Scholar]
- 64.Chen H, Howald WN, Juchau MR. Biosynthesis of all-trans-retinoic acid from all-trans-retinol: catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metabolism and Disposition The Biological Fate of Chemicals. 2000;28:315–22. [PubMed] [Google Scholar]
- 65.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Archives of Biochemistry and Biophysics. 2005;436:50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Archives of Biochemistry and Biophysics. 2005;433:379–86. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 67.Chen YH, Beischlag TV, Kim JH, Perdew GH, Stallcup MR. Role of GAC63 in transcriptional activation mediated by the aryl hydrocarbon receptor. Journal of Biological Chemistry. 2006;281:12242–7. doi: 10.1074/jbc.M512537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang HJ, Kim HJ, Cho CH, Hu Y, Li R, Bae I. BRCA1 transcriptional activity is enhanced by interactions between its AD1 domain and AhR. Cancer Chemotherapy and Pharmacology. 2008;62:965–75. doi: 10.1007/s00280-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ge NL, Elferink CJ. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. Journal of Biological Chemistry. 1998;273:22708–13. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 70.Elferink CJ, Ge NL, Levine A. Maximal aryl hydrocarbon receptor activity depends on an interaction with the retinoblastoma protein. Molecular Pharmacology. 2001;59:664–73. doi: 10.1124/mol.59.4.664. [DOI] [PubMed] [Google Scholar]
- 71.Puga A, Barnes SJ, Dalton TP, Chang C, Knudsen ES, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. The Journal of Biological Chemistry. 2000;275:2943–50. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 72.Marlowe JL, Knudsen ES, Schwemberger S, Puga A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. The Journal of Biological Chemistry. 2004;279:29013–22. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- 73.Huang G, Elferink CJ. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Molecular Pharmacology. 2005;67:88–96. doi: 10.1124/mol.104.002410. [DOI] [PubMed] [Google Scholar]
- 74.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–50. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 75.Sogawa K, Numayama-Tsuruta K, Takahashi T, Matsushita N, Miura C, Nikawa J, et al. A novel induction mechanism of the rat CYP1A2 gene mediated by Ah receptor-ARNT heterodimer. Biochemistry and Biophysics Research Communication. 2004;318:746–55. doi: 10.1016/j.bbrc.2004.04.090. [DOI] [PubMed] [Google Scholar]
- 76.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F, et al. A new partner of aryl hydrocarbon receptor-mediated transcription. Molecular Endocrinology. 2007;21:2941–55. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. Journal of Biological Chemistry. 1999;274:510–5. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 78.Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 79.Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–6. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- 80.Higa LA, Yang X, Zheng J, Banks D, Wu M, Ghosh P, et al. Involvement of CUL4 ubiquitin E3 ligases in regulating CDK inhibitors Dacapo/p27Kip1 and cyclin E degradation. Cell Cycle. 2006;5:71–7. doi: 10.4161/cc.5.1.2266. [DOI] [PubMed] [Google Scholar]
- 81.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8851–6. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]