Abstract
Fibromyalgia syndrome (FM) is characterized by pain and widespread hyperalgesia to mechanical, thermal, and electrical stimuli. Despite convincing evidence for central sensitization of nociceptive pain pathways, the role of peripheral tissue impulse input in the initiation and maintenance of FM is unclear. Therefore this randomized, double-blind, placebo-controlled trial of 22 normal female controls (NC) and 28 female FM subjects tested the effects of trapezius muscle (TrapM) tender point injections with 1% lidocaine on local pain thresholds as well as on remote heat hyperalgesia at the forearm. Prior to muscle injections shoulder pain was standardized by tonic mechanical muscle stimulation, resulting in local pain ratings of 4.0 ± 0.5 VAS units. Tonic muscle stimulation was interrupted for the TrapM injections but continued afterwards at the same level. NC as well as FM subjects experienced significant increases of TrapM pressure pain-thresholds from lidocaine but not placebo injections (p <.001). Additionally, heat-hyperalgesia of FM participants was significantly reduced at areas remote from the injection site (forearm) by lidocaine but not placebo (p = .02). Neither lidocaine nor saline injections significantly affected clinical FM pain ratings, a result most likely due to the very low dose of lidocaine (50 mg) used in this trial. Conclusion: Lidocaine injections increased local pain-thresholds and decreased remote secondary heat hyperalgesia in FM patients, emphasizing the important role of peripheral impulse input in maintaining central sensitization in this chronic pain syndrome; similar to other persistent pain conditions like irritable bowel syndrome and complex regional pain syndrome.
Keywords: Lidocaine, Hyperalgesia, Tonic, Fibromyalgia, Pressure, Chronic pain
1. Introduction
Fibromyalgia (FM) is a chronic pain syndrome that is defined by the presence of mechanical hyperalgesia and wide-spread pain, consistently felt in deep tissues [55]. It is related to central sensitization [8; 33] which may result from both peripheral and central mechanisms. Although several psychophysical and brain imaging studies provided support for central sensitization in FM [13; 26; 37–40; 46; 47; 54], less evidence exists for abnormalities of peripheral painful tissues and associated primary afferent neurons. Several FM studies described not only various abnormalities that could be associated with sensitization of deep tissue nociceptors [4–6; 10; 19; 27] but also reported associations of overall clinical pain intensity with the number of painful body areas [43] and ratings of local pains [49]. Thus, it is quite conceivable that pathophysiological changes in deep tissues of FM patients may result in increased responsiveness of neurons innervating these tissues, thereby providing tonic impulse input to the central nervous system. This input could induce and maintain central sensitization.
Ongoing afferent input from peripheral sources is known to dynamically maintain central sensitization and account for spontaneous pain, hyperalgesia, and allodynia [12; 28; 31]. Peripheral anesthetic blockade of critical somatic foci in complex regional pain syndrome (CRPS) effectively abolished both spontaneous and elicited pain as well as cold/mechano-allodynia within multiple body regions, including regions remotely distant from these critical foci [12]. A similar reversal occurred with sympathetic blocks in some CRPS patients [28; 31]. Similarly, the role of tonic impulse input for somatic pain has been tested in irritable bowel syndrome (IBS) patients by rectal administration of lidocaine gel [51]. This treatment normalized secondary heat hyperalgesia of IBS patients at the lower extremities who could not subjectively distinguish the lidocaine from placebo condition [51]. Importantly, these effects were not accompanied by systemic absorption of lidocaine because blood levels were below the limit of detection [51].
Because of accumulating evidence that tonic peripheral afferent activity is relevant for CRPS and IBS pain, we tested the hypothesis that lidocaine injections into the trapezius muscle (TrapM) would reduce local evoked muscle pain as well as secondary heat hyperalgesia in distal body areas, like the forearm of FM subjects. If central sensitization is maintained in spinal neurons by impulse input from muscle tissues, then it should be possible to normalize this enhanced central pain sensitivity and consequent heat hyperalgesia by reduction of muscle input. This rationale is based on evidence that sensitized spinal neurons receive input from multiple tissues, and is similar to that used in previous studies of CRPS [12; 28; 31] and IBS [51]. To avoid systemic analgesic effects related to drug absorption and the ability to subjectively distinguish lidocaine from saline placebo injections, only a very low dose of lidocaine (50 mg) was used. Therefore, we expected only a partial normalization of secondary heat hyperalgesia in the forearm and not necessarily an overall reduction of clinical pain. Hence, the present investigation was designed to test hyperalgesic mechanisms in FM based on results established in CRPS and IBS patients.
2. Materials and Methods
2.1 Study Participants
Normal control (NC) participants and FM subjects were recruited from the local community and FM support groups. Informed consent was obtained from all subjects and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The University of Florida Institutional Review Board approved the procedures and protocol for this study. Prior to testing, all subjects underwent a clinical examination and were excluded from the study if they had abnormal findings unrelated to FM. Use of analgesics, including non-steroidal anti-inflammatory drugs (NSAID) and acetaminophen, was not allowed during the study. All subjects were asked to discontinue analgesics for the duration of five drug half-lives before testing, except narcotics which had to be stopped at least two weeks prior to study entry. Low dose muscle relaxants and/or amitriptyline (≤ 10 mg/day) were permissible during the study for treatment of FM-related insomnia. Special care was taken to exclude participants from the study who previously had adverse events to lidocaine injections.
At 30 min before and 60 min after the injection of the study drug the participants’ heart rate and blood pressure were closely monitored. The study drug was always administered by the study physician (R.S.) who has extensive experience with the use of local anesthetics.
2.2 Experimental Design
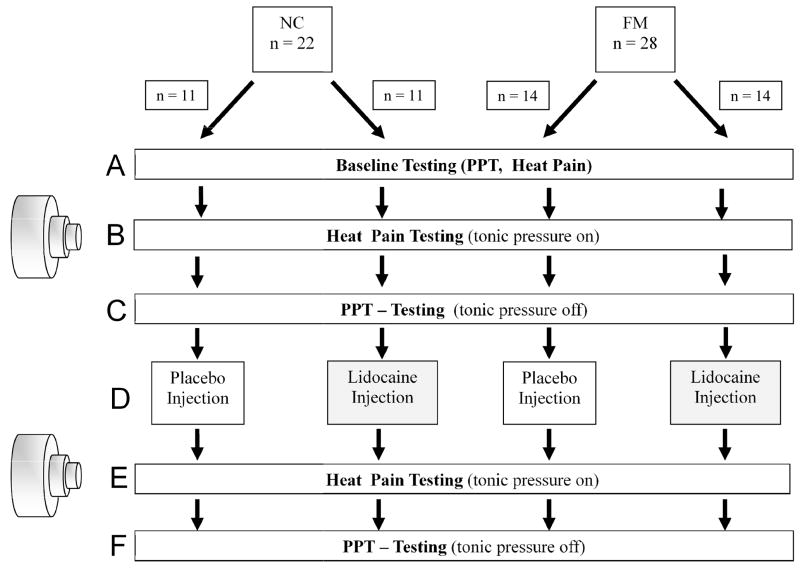
A parallel group, randomized, double-blind, placebo-controlled study design was used to evaluate the effects of a single lidocaine injection on primary and secondary hyperalgesia of NC and FM subjects (Figure 1). The injections site of the study drug was the TrapM of a randomly selected shoulder corresponding with a tender point (TP) according to the American College of Rheumatology FM Criteria [55]. The study design resulted in two groups each of NC [NC-placebo (NC-PL) and NC-lidocaine (NC-LI)] and FM [FM-placebo (FM-PL) and FM-lidocaine (FM-LI)] subjects. The NC and FM participants were placed on the examination table in the supine position. All participants were tested in the same order depicted in Figure 1. Although the shoulder used for TrapM TP stimulation was selected using a counter-balanced design, each individual received all test and stimulation procedures to the selected shoulder (pressure pain threshold [PPT] testing and tonic muscle stimulation) and ipsilateral forearm (heat testing). Shoulder injections were given into the same TrapM TP used for stimulation and testing.
Figure 1.
Flow diagram of study procedures. Two groups of NC and 2 groups of FM participants received either placebo or lidocaine injections into the TrapM TP. PPTs were tested at the shoulder (primary hyperalgesia) and 10 sec thermal ramps were applied to the forearms (secondary hyperalgesia) before and after the injections. The left side of this figure shows the pressure device used for tonic mechanical TrapM stimulation. Pressurized air was applied to the muscle stimulator resulting in expansion of a telescoping prong (diameter 1cm) for up to 4 cm. A calibrated force transducer mounted to the tip of the prong provided real-time information on an electronic display. This information was used to continuously adjust tonic pressure stimuli to the TrapM TP. TrapM = trapezius muscle; TP = tender point; PPT = pressure pain threshold; for detailed descriptions of sections; A–F = see text for more details (2.2).
Baseline testing (Figure 1A)
PPT testing of both TrapM TP was done. Testing of the TP contralateral to the injection site was also done as a condition check. Subsequently all subjects rated individually adjusted 10 sec heat ramp stimuli applied to the forearm ipsilateral to the side subsequently used for TrapM TP stimulation. Testing was done in counterbalanced fashion to avoid order effects.
Tonic TrapM TP stimulation and repeat heat ramp testing (Figure 1B)
While resting comfortably, all subjects had individually adjusted tonic pressure stimulation applied to a randomly selected TrapM TP to normalize shoulder pain at the TrapM across FM and NC subjects (4.0 ± 0.5 VAS units) (see 2.4.1). The application of pressure was only interrupted during the injection of the study drug. Otherwise tonic pressure was carefully maintained at the predetermined level throughout the experiment. The total duration of tonic TrapM TP stimulation was approximately 30 min for each subject. Shoulder pain ratings were obtained at the beginning, after 5 min, and at the end of tonic TrapM stimulation. After 5 min of tonic pressure stimulation, 10 sec sensitivity adjusted heat ramp pain ratings were obtained for the second time at the ipsilateral forearm (see 2.5.2).
PPT testing at the TrapM TPs (Figure 1C)
After suprathreshold heat pain testing at the forearm, tonic mechanical stimulation of the TrapM was interrupted for 1 min for repeat PPT testing.
Lidocaine or placebo injections (Figure 1D)
After PTT testing, either placebo or lidocaine was injected into the previously stimulated TrapM TP over 1 min. Slow injection of the study medication was undertaken to prevent lidocaine related side effects that could jeopardize allocation concealment. The study drug was always prepared by a study nurse who otherwise did not participate in the experiments. The investigators received the study drug in a syringe from the study nurse marked only with the participant’s identification number. Blinding of the investigators to the subjects’ diagnosis (NC vs FM), however, was not attempted because clinical pain ratings as well as sensitivity to heat and pressure stimuli made concealment of group membership improbable.
Heat ramp pain ratings at the forearm (Figure 1E)
5 min after the TrapM TP injections tonic shoulder muscle stimulation was resumed at the predetermined intensity and sensitivity adjusted heat ramp pain ratings were again obtained at the same forearm as before. A 5 min pause after the injection was used to allow the medications to reach maximal effectiveness.
PPT testing at the TrapM TP (Figure 1F)
After suprathreshold heat pain testing at the forearm, tonic mechanical stimulation at the TrapM was terminated and repeat PPT testing at the TrapM TP was performed.
2.2.1 Study Drugs
The study participants received 5 ml of either 1% lidocaine or 0.9% preservative-free saline injections into the previously stimulated TrapM TP. For this purpose the subjects were brought into the seated position and the study drug was slowly injected over 1 min by the study physician (R.S.) using a 27 g needle. The subjects were frequently asked to report any injection related sensation. If such sensations occurred the injection was interrupted and only resumed after resolution of all injection related symptoms. After the injection, a 5 min waiting period was observed by all subjects to allow the study drug to take full effect. During this time the subjects remained seated and vital signs were monitored. Afterwards they were again brought into the supine position and tonic shoulder stimulation resumed at the previously stimulated TrapM TP site. Special care was taken not to inject study medication into the skin because cutaneous anesthesia could have resulted in removing the blind from subjects and investigators. Study drug allocation (1% lidocaine or 0.9% saline) was determined for each subject using a computerized randomization scheme. At the end of the trial the participants were asked to estimate whether they had been injected with lidocaine, placebo, or were unable to tell whether they had been injected with either.
2.3 Ratings of Pain
2.3.1 Ratings of Experimental Pain
A 15 cm mechanical visual analogue scale (0 – 10) was used for ratings of experimental pain during mechanical and heat stimulation [29]. The scale was anchored on the left with “no pain at all” and on the right with “the most intense pain imaginable”.
2.3.2 Ratings of Somatic Pain
The same mechanical visual analogue scale (0 – 10) was also used for ratings of somatic pain of all study participants before and after the experimental protocol [30]. Although the NC subjects were required to be pain free at enrollment their somatic pain ratings were obtained before and after the testing session to capture possible new onset pains like back pain, headaches, etc.
2.4 Tonic Pressure Stimulation
Tonic pressure stimulation was applied to the TrapM halfway between the neck and acromion using a proprietary muscle stimulator with a telescoping plastic prong (see Figure 1). This location corresponded to one of the 18 tender point listed in the American College of Rheumatology (ACR) Criteria for FM [55]. The round telescoping prong (diameter: 1 cm) of the muscle stimulator could be advanced by pressurized air for up to 4 cm. Pressure applied to the muscle was measured using an electronic force transducer mounted to the tip of the prong that provided real-time pressure readings (in kg/cm2) via liquid crystal display.
The pressure stimulation site was selected in a counterbalanced fashion. The TrapM TP was marked with a marker pen for stimulation and injection. Constant mechanical pressure was applied to the TrapM (total time: approximately 30 min) except during PPT testing and drug injection. These brief intervals were considered useful to prevent tissue damage by the prong as well as to limit the duration of uninterrupted experimental pain. The intensity of mechanical stimuli was carefully maintained for each individual at a pre-determined pressure level (see 2.4.1) by adjusting the air pressure applied to the telescoping prong. This was particularly important after muscle injections, since analgesia or hyperalgesia were likely to affect the stimulus dependent pain ratings.
2.4.1. Adjustment of Tonic Pressure to Each Individual’s Pain Sensitivity
Pain sensations from fixed mechanical stimuli vary as a function of each subject’s peripheral and central sensitivity. Because this variability is frequently associated with “ceiling” or “floor” effects, we normalized individual pressure pains by applying the unique pressure necessary for TrapM pain ratings of 4.0 ± 0.5 VAS units. This manipulation provided a measure of mechanical pain sensitivity for each subject. To identify individual stimulus intensities associated with such moderate pressure pain ratings (4.0 ± 0.5 VAS units), each subject underwent several pressure pain trials (mean 2.5). Mechanical stimuli were started at 2 kg/cm2. If necessary, the pressure was raised or lowered during subsequent trials until subjects achieved target pressure pain ratings of 4.0 ± 0.5 VAS units. This pressure intensity was subsequently used for the tonic pressure stimulation at the TrapM TP.
2.5 Thermal probe
During the experiments a Peltier thermode with a contact surface of 3 × 3 cm (9 cm2) (TSA-2001, Medoc Advanced Medical Systems, Ramat Yishai, Israel) was used for the thermal stimuli. For heat pain testing the preheated probe was brought into firm contact with the skin of the volar forearm.
2.5.1 Heat Stimuli
Experimental pain was elicited by 10 sec sensitivity adjusted heat pulses to the volar surface of the forearm ispilateral to the stimulation and injection site (see 2.5.2). Three adjusted 10 sec heat pulses were applied to three different areas of the forearm separated by 10 cm, in counterbalanced order. At the end of each 10 sec heat stimulus the participants were immediately asked to rate the intensity of their experimental pain sensations using the VAS.
2.5.2 Adjustment of Heat Stimuli to Each Subject’s Pain Sensitivity
Similar to pressure pain, heat pain stimuli were adjusted to each individual’s pain sensitivity. Thus, to measure heat pain sensitivity, we determined the unique temperature for each subject during preliminary experiments that resulted in final heat pain ratings of 4.0 ± 0.5 VAS units during 10 sec stimuli. Stimulus intensities resulting in such pain ratings (4.0 ± 0.5 VAS units) were chosen to avoid ceiling or floor effects related to peripheral and central pain sensitivity of study subjects. To identify individual stimulus intensities associated with moderate heat pain ratings (4.0 ± 0.5 VAS units), each subject underwent several 10 sec heat pain trials. Stimulus trains were initially comprised of 10 sec 45°C heat stimuli. The temperature slowly increased from baseline to target temperature by 0.5°C/sec. After it reached target temperature it was maintained for 2 sec. If necessary, peak temperatures were raised or lowered during subsequent trials until subjects achieved maximal heat pain ratings of 4.0 ± 0.5 VAS units. This temperature was subsequently used for all heat pain experiments. Thus, similar to pressure pain stimuli, these heat pain trials provided a standard condition which was designed to be very similar within and between subject groups.
2.6 PPT Testing
All subjects were trained to attend to and rate mechanical pain stimuli applied to the TrapM. The test sites were located at the trapezius muscle TP and identical to those chosen for the muscle injection. The mechanical force transmitted to the muscle was tested with a calibrated mechanical pressure algometer (Somedic AB, Horby, Sweden). The rubber tip of the algometer was 1 cm in diameter. After the algometer was placed on the examined site pressure was gradually increased by 50 kPa/sec until pain threshold was reached. The subjects were instructed to push a hand-held button when the sensation changed from pressure to pain at the examination site. PPT testing was stopped at that moment and the results were automatically recorded. The average of three test results was used to calculate each subject’s PPT.
2.7 Tender Point Testing
Nine paired TPs as defined by the ACR Criteria [55] and two control points (at the center of the right forearm and the right thumbnail) were assessed by a trained investigator using a Wagner Dolorimeter (Force Measurement, Greenwich, CT). The rubber tip of the Dolorimeter was 1 cm in diameter. The Dolorimeter was placed on the examination site, and pressure was gradually increased by 1kg/sec. The subjects were instructed to report when the sensation at the examination site changed from pressure to pain. Pressure testing was stopped at that moment and the result recorded as positive (1) if maximal pressure was ≤ 4 kg. If no pain was elicited at ≥ 4 kg the test result was recorded as negative (0).
2.8 Statistical Analysis
Statistical analyses were conducted using SPSS 16.0 software (SPSS, Inc., Chicago, IL). All group results were averaged (SD). A series of mixed model ANOVAs for repeated measures was utilized to test experimental pain ratings for differences within and between groups (Alpha level = .05). A priori hypotheses were evaluated by simple contrasts (two-tailed). Chi-square analysis was used for testing of subjects’ estimates of study drug application (lidocaine or placebo).
3.0 Results
3.1 Study Population
We recruited 22 middle-aged healthy pain-free female subjects [mean age (SD): 44.7 (11.0) years] using advertisements posted throughout the University of Florida, Gainesville and 28 female FM subjects [44.6 (12.2) years]. All FM subjects fulfilled the 1990 ACR Criteria for FM [55]. NC and FM participants had 5.4 and 16.9 TP, respectively (p < .001). All participants were right handed except two females and included 41 Caucasian Non-Hispanics, four African-American and four Hispanic subjects.
3.2 Ratings of Somatic Pain in NC and FM Subjects
The healthy subjects reported no somatic pain before and after the muscle injections. In contrast, the FM-PL and FM-LI subjects’ average overall somatic pain scores were 3.3 (2.6) and 4.7 (2.7) VAS units before and 4.2 (2.3) and 4.6 (2.3) VAS units after the injections. A repeated measures ANOVA with time (2) as the within and treatment (2) as between subjects’ factors showed no significant effects for time (p > .05) or treatment (p > .05), indicating that neither injections with placebo nor injections with lidocaine into the TrapM TP significantly changed clinical FM pain.
3.3 Sensitivity Adjusted Heat Pain Stimuli
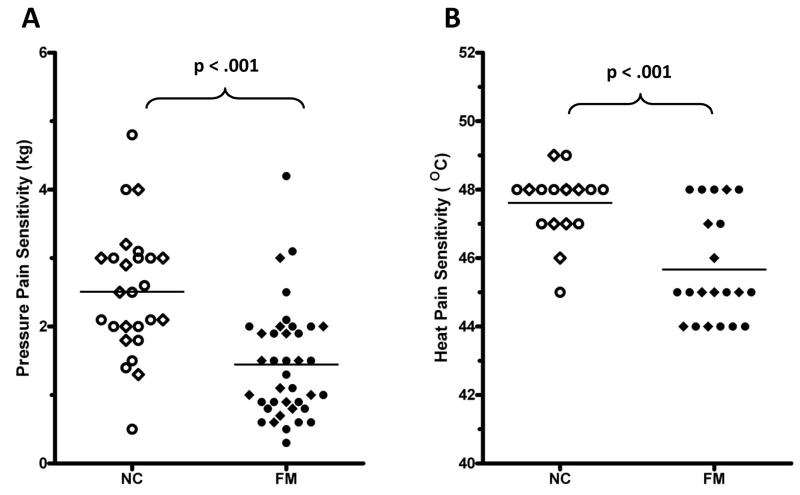
The intensity of 10 sec heat stimuli applied to the forearm was adjusted to each subject’s pain sensitivity as described in section 2.5.2. To achieve maximum heat pain ratings of 4.0 ± 0.5 VAS units in all participants, the average peak temperature of 10 sec heat pulses was significantly higher for the NC [47.6 (0.9) °C] than for FM subjects [45.9 (1.4) °C] (t(31) = 5.4; p < .001) (Figure 2B).
Figure 2.
Pressure pain (Panel A) and heat pain sensitivity (Panel B) of NC and FM subjects. All participants underwent prolonged sensitivity adjusted tonic pressure stimulation at the TrapM and 10 sec sensitivity adjusted heat stimulation at the forearm Average tonic pressure used to achieve shoulder pain ratings of 4.0 ± 0.5 VAS units was 2.3 kg for NC (n =16) and 1.1 kg for FM participants (n = 21) (p < .001) (Panel A). To achieve similar intensity levels of heat pain ratings at the forearm, stimulus temperatures of 48.7 °C and 47.4 °C were needed for NC (n = 22) and FM subjects (n = 28), respectively (p < .001) (Panel B). Open circles = NC-Placebo; open diamonds = NC-Lidocaine; filled circles = FM-Placebo; filled diamonds = FM-Lidocaine
3.4 Sensitivity Adjusted Tonic Pressure Stimuli at the Shoulder
Tonic pressure pain was adjusted to each subject’s pressure pain sensitivity as described in section 2.4.1. In order to achieve tonic pressure pain ratings of 4.0 ± 0.5 VAS units in all participants, the average pressure applied to the TrapM TP necessary for such tonic pain ratings was significantly higher for NC [2.32 (0.8) kg] than for FM subjects [1.1 (0.5) kg] (t(31) = 6.7; p < .001) (Figure 2A).
3.5 Effects of Lidocaine or Placebo Injections on Tonic Mechanical Shoulder Pain
3.5.1 Effect of Shoulder Injections on PPTs
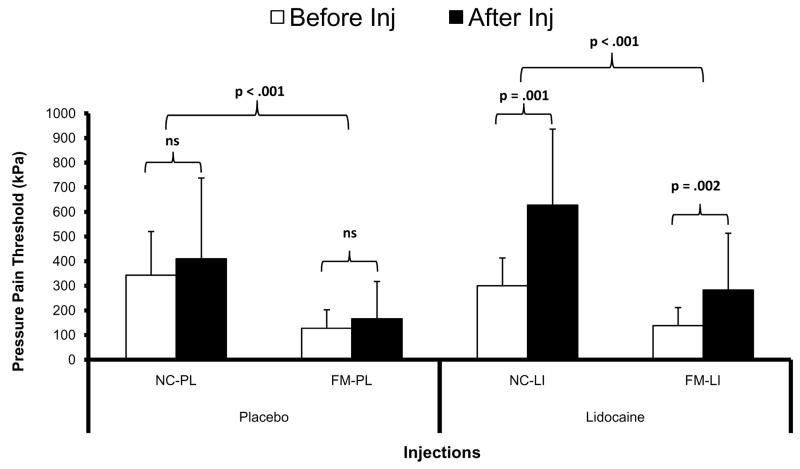
All study participants received either lidocaine or placebo injections into the same TrapM area where tonic mechanical stimuli were applied throughout the experiments. Tonic muscle stimulation was only briefly interrupted for the injection and for measuring PPT at the shoulder (Figure 1). Afterwards tonic pressure was resumed at the same predetermined intensity as before. After shoulder injections PPTs increased at the injection site for NC-LI and FM-LI subjects but not for NC-PL and FM-PL participants (Figure 3).
Figure 3.
Effects of lidocaine or placebo injections on pressure pain thresholds (PPT) at the TrapM. PPT [mean (SD)] were measured with an electronic algometer at baseline, before, and after shoulder injections with lidocaine or placebo. PPTs were significantly lower in FM subjects compared to NC (p < .001). However, only lidocaine but not normal saline placebo resulted in significant increases of PPTs in NC and FM participants (p = .001). TrapM = trapezius muscle
A repeated measures ANOVA with time (3) as the within subjects factor and treatment (2) and diagnostic group (2) as between subjects factors, showed significant main effects for time (F(1,62) = 31.0; p < .001) and diagnostic group (F(1,62) = 34.0; p < .001). There were significant interactions of time × treatment (F(1,62) =12.6; p = .001) and time × diagnostic group (F(1,62) = 4.1; p = .046) noted. These results indicate that lidocaine significantly increased PPTs at the TrapM injection site in NC-LI and FM-LI compared to placebo and that these effects were greater in NC than FM subjects.
In a second analysis the effects of shoulder injections on PPT at the opposite TrapM were explored. However, no significant PPT changes occurred at the opposite shoulder over time in any of the four groups with either lidocaine or saline injections. The lack of significant changes was further established by a repeated measure ANOVA with side (2) and time (2) as within subjects factors and treatment (2) and diagnostic group (2) as between subjects factor showed a significant main effect for side (F(1,62) = 27.4; p < .001). In addition, there was a significant side × time × treatment interaction noted (F(1,62) = 20.7; p < .001) which confirmed that PPT measured at the non-injected shoulder did not significantly change over time in contrast to the shoulder injected with lidocaine.
3.5.2 Tonic Pressure Pain Ratings
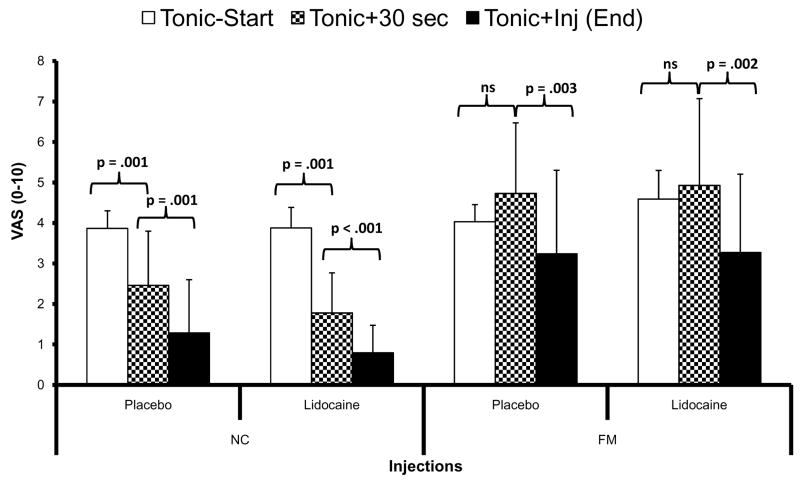
The natural history of tonic TrapM pain was determined over the first 30 sec of shoulder stimulation, followed by tonic pain ratings before and after muscle injections (Figure 4). As planned, the initial pain ratings of sensitivity adjusted tonic muscle stimuli (Tonic-start) were similar across groups and close to 4.0 VAS units (Figure 4). However, after 30 sec of constant tonic stimulation (Tonic+30 sec) the shoulder pain decreased in both NC groups but not in the FM participants. After the TrapM injections the tonic pressure pain ratings of both NC groups diminished further as did the mean tonic pain ratings of both FM groups (Figure 4). The statistical analysis shown below was performed to assess: a) possible interaction effects of needle insertion into the trapezius muscle on shoulder pain, as previously reported for dry needling of trigger points [17] and b) as a condition check to determine the time course of subjective pain ratings at the stimulated shoulder.
Figure 4.
Effects of muscle injections on sensitivity adjusted pressure pain ratings at the TrapM. Tonic pressure stimuli at the TrapM were adjusted to each individual’s mechanical pain sensitivity to achieve maximal pain ratings of 4.0 ± 0.5 VAS units. Initially, average (SD) pain ratings of sensitivity adjusted tonic shoulder stimuli of NC-PL and NC-LI were 3.9 (0.4) VAS units and 4.0 (0.4) VAS units, respectively (Tonic-start). Similarly, the tonic TrapM pain ratings of FM-PL and FM-LI was 3.9 (0.5) VAS units and 4.6 (0.7) VAS units, respectively. After 30 sec of tonic stimulation (Tonic+30 sec) the shoulder pain of NC-PL and NC-LI participants significantly decreased to 2.4 (1.2) and 1.8 (0.9) VAS units (all p = .001), respectively, but did not statistically change in FM subjects [FM-PL = 4.6 (1.6) and FM-LI = 5.3 (2.0) VAS units (all p > .05)] After lidocaine or placebo injections into TrapMs tonic pressure pain ratings decreased significantly in NC and FM subjects’ [NC-PL = 1.3 (1.3); NC-LI = 0.8 (0.7); FM-PL = 3.24 (2.1); FM-LI = 3.3 (1.9)] (all p < .004). ns = non-significant; TrapM = trapezius muscle.
For this purpose a mixed model ANOVA with time (3) as the within subjects’ factor and diagnostic group (2) as well as treatment (2) as between subjects’ factors was used which showed a significant main effect of time (F(2,70) = 32.1; p < .001) and diagnosis (F(1,35) = 26.8; p < .001) as well as a significant time × diagnostic group interaction effect (F(2,70) = 13.9; p = .001). There was no significant treatment × time × diagnostic group interaction noted (p > .05). The use of simple contrast indicated that the pressure pain ratings at the TrapM between Tonic-start and Tonic+30 sec were significantly different in NC (F(1,35) = 12.3; p = .001) but not in FM subjects (F(1,35) = .10; p > .05). After TrapM injections (Tonic+inj) pressure pain ratings declined significantly in NC as well as FM subjects (F(1,17) = 37.5; p < .001). This decrease of pain ratings, however, was independent of lidocaine or placebo application (F(1,17) = 0.10; p > .05). Whereas one likely explanation for this reduction of pressure pain in NC is habituation, this did not seem to occur in FM participants [36]. Their pressure pain ratings only declined after the muscle injections, although these effects were not different for lidocaine or placebo.
3.6 Effects of Shoulder Injections on Heat Pain at the Forearm
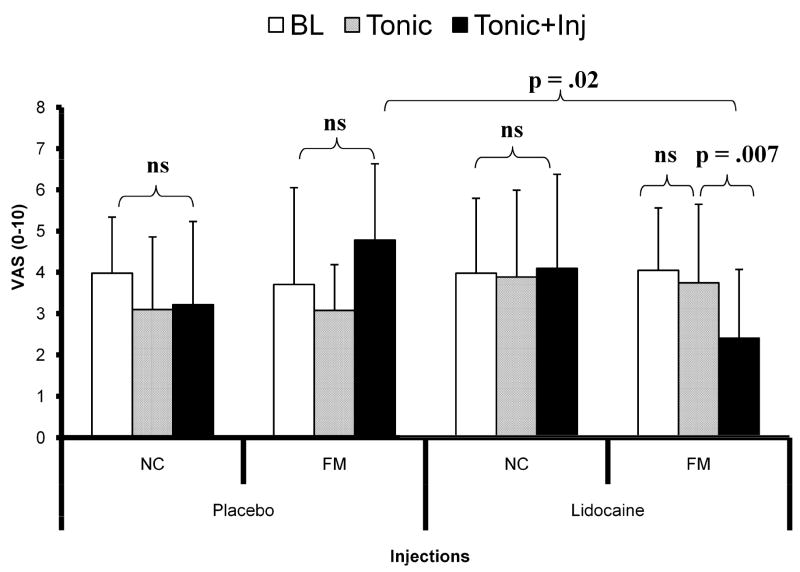
To test our main hypothesis, the effects of placebo or lidocaine injections on heat pain/hyperalgesia were examined at the forearm ipsilateral to the injections site during 10 sec sensitivity adjusted heat pulses (see Section 2.5.2). Heat pain ratings were obtained before and after shoulder injections with either lidocaine or placebo into the TrapM TP (see Figure 5). As planned the mean pain ratings of 10 sec heat pulses at the forearm were similar for all groups at baseline (see Table 2). Subsequently, no significant change in heat pain ratings was observed during tonic TrapM pressure and injection conditions in any group of subjects, except FM participants who were injected with lidocaine. In these subjects, lidocaine injections into the TrapM resulted in a significant reduction of heat hyperalgesia compared to the other two conditions (p = .007). Additionally, planned comparisons between both FM groups (FM-PL and FM-LI) showed significantly less heat hyperalgesia in the lidocaine group after the TrapM injections (p = .02) (Figure 5; Table 2).
Figure 5.
Heat pain ratings of NC and FM subjects at the forearm. For this experiment, 10 sec heat stimuli to the forearm were individually adjusted to achieve maximal pain ratings of 4.0 ± 0.5 VAS units in NC and FM participants at baseline. Average (SD) pain ratings of 10 sec heat pulses did not significantly change during all 3 experimental conditions (baseline, tonic TrapM stimulation, TrapM injection) in NC-PL, FM-PL and NC-LI subjects. Whereas forearm heat pain ratings of FM-LI participants did not change during tonic pressure pain stimulation, a significant decrease in heat hyperalgesia was observed after lidocaine injections (p = .007). Planned comparisons of medication effects between FM-LI and FM-PL showed significantly less heat hyperalgesia in the lidocaine group after the injection (p = .02). BL = baseline; Tonic = tonic shoulder stimulation; Tonic+Inj = tonic shoulder stimulation after TrapM injections with either lidocaine or placebo.
Table 2.
Effects of Lidocaine or Placebo Injections on Heat Pain at the Forearm
| Heat Pain Ratings VAS Units [Mean (SD)] | p - Value | |||
|---|---|---|---|---|
| Baseline | Tonic Stimulation | TrapM Injection | ||
| NC-Placebo | 4.0 (1.4); | 3.3 (2.2) | 3.2 (1.9) | ns |
| NC-Lidocaine | 4.0 (1.8) | 3.9 (1.3) | 4.1 (1.2) | ns |
| FM-Placebo | 3.7 (2.3) | 3.1 (1.8) | 4.8 (1.8) | ns |
| FM-Lidocaine | 4.1 (1.5) | 3.8 (1.7) | 2.4 (2.2) | .007 |
ns = non significant
A repeated measures ANOVA with time (3) as within subjects’ factor and diagnostic group (2) and treatment (2) as between subjects’ factors showed non-significant main effects for diagnostic group and treatment (p > .05). However, time × treatment (F(1,32) = 6.3; p = .017) and time × diagnosis (F(1,32) = 8.3; p = .007) interactions were significant. These findings indicate that heat hyperalgesia was not affected by placebo injections in NC-PL and FM-PL subjects, whereas lidocaine injections reduced heat hyperalgesia at the forearm in FM-LI subjects but not NC-LI. To decompose the significant time × diagnostic group interaction, an independent t-test of FM-LI and FM-PL heat pain ratings after shoulder injections was performed. This test showed significantly lower heat pain ratings of FM-LI compared to FM-PL subjects (t = 2.6; p = .02) (Figure 5).
3.7 Estimates of Lidocaine or Placebo Injections by Study Participants
At the end of the experiments the study participants were asked to estimate whether they received active study drug or placebo. The number of correct estimates was 11 out of 44 (25%) and the number of incorrect estimates was 12 out of 44 (27.3%). Twenty-one subjects (47.7%) remained undecided. A χ2 analysis indicated no significant difference between number of correct and incorrect estimates (χ2 = 0.18; p > .05).
4.0 Discussion
A single lidocaine injection into a TrapM TP of FM subjects resulted in decreased mechanical hyperalgesia at the shoulder as well as reduction of distal secondary heat hyperalgesia at the forearm. Heat hyperalgesia was present in FM participants because they required significantly lower stimulus intensities than NC subjects to achieve moderate heat pain ratings of 4.0 VAS units. Whereas lidocaine injections resulted in selective reductions of heat pain ratings of FM subjects which most likely reflects anti-hyperalgesic mechanisms, they had no analgesic effects on NC subjects. Furthermore, this effect was not dependent on expectations because drug allocation concealment was effectively maintained during this study, as shown by the subjects’ inability to correctly distinguish between lidocaine and saline injections. Similarly, the anti-hyperalgesic effects in FM participants were unlikely related to systemic lidocaine absorption because only a very small dose (50 mg) was injected into one muscle. Thus, these results show for the first time that reductions of impulse input from painful muscle tissue at least partially normalize distal heat hyperalgesia in FM patients, similar to local anesthetic blockade in other chronic pain syndromes, such as CRPS and IBS. Because this trial was designed as a “proof of concept” study, its major outcome measures were limited to primary muscle and secondary cutaneous hyperalgesia in FM. Tests using multiple local anesthetic injections into painful muscle areas may be required to determine significant effects of local anesthetic blockade on overall clinical pain.
Evidence for peripheral tissue and primary afferent abnormalities in FM
If tonic impulse input from muscles and other deep tissues is at least partly responsible for induction and maintenance of widespread FM pain, it is important to consider potential primary afferent mechanisms. The present study provides evidence that TrapM impulse input at least partly maintains secondary heat hyperalgesia of FM subjects at distant sites like the forearm. This finding argues against widespread sensitization of cutaneous nociceptors as an alternative FM mechanism. It is also supported by sensory testing of FM patients which showed abnormalities of cutaneous C-fiber pain specifically related to central abnormalities of temporal summation not sensitization of heat nociceptors [38].
Although our results support a role for impulse input from muscles in maintaining secondary heat hyperalgesia, no direct evidence for such input is presently available. However, several deep tissue abnormalities, including ragged red fibers and decreased microcirculation of muscles [4–6; 10; 11; 19; 27], provide indirect evidence for such mechanisms in FM, which may result in sensitization of intramuscular nociceptors, specifically ASIC 3 [35]. This type of sensitization may also depend on up-regulation of N-methyl-D-aspartate receptors and substance-P in deep tissues, as has been previously demonstrated in FM [5; 20].
The importance of peripheral impulse activity in dynamically maintaining central sensitization has been proposed for other chronic pain syndromes, like CRPS and IBS. Several studies of patients with these chronic pain syndromes reported normalization of secondary hyperalgesia/allodynia and ongoing clinical pain by local anesthetic blockade of critical local foci [12; 52; 53]. Similarly, local anesthetic blockade normalized widespread somatic hypersensitivity in animal models of IBS [57] and CRPS [24]. These results suggest common pathophysiological mechanisms across IBS, CRPS, and FM. Thus, understanding the peripheral mechanisms of one chronic pain condition, such as FM, may provide additional insights into the pathophysiology of IBS. This possibility is supported by the large overlap between FM and IBS symptoms, with up to 80% percent of FM also having IBS [56].
Future therapeutic strategies for FM
Not surprisingly, the normalization of heat hyperalgesia in our FM study was limited by the small dose injected into a single site. This limitation and the large variability in baseline clinical pain likely accounted for the observed lack of effects on overall clinical pain. Likewise, overall FM pain may be based on impulse input from multiple sites and therefore one might not expect effects on clinical pain after a single injection [49]. Overall, this study was not designed to develop or evaluate a treatment for FM, but rather test a highly specific mechanistic hypothesis about the contribution of muscle impulse input to secondary FM hyperalgesia.
Nevertheless, it is possible that multiple injections into painful muscle areas would effectively reduce ongoing FM pain. The feasibility of local anesthetics treatments was indirectly supported by observed pain reductions in CRPS and IBS patients [31; 52; 53]. If local anesthetic effects on overall clinical pain are indeed similar across these conditions, then one might predict long lasting effects in FM, similar to those observed for CRPS and IBS. Considerable evidence from studies of local anesthetics on normal and abnormal ion channels showed that injured nerves or nerve terminals were blocked for a much longer time than predicted by the pharmacokinetics of local anesthetics [9; 34].
Interactions between local and widespread pain: role of spatial and temporal summation
The effects of local injection into a single TP may have implications for understanding the mechanisms of widespread FM pain, because it is well known that small areas of local pain can have enhancing effects on overall pain sensitivity. This effect, however, depends on several factors including the duration of pain. In contrast to short lasting dental pains which do not enhance pain sensitivity of distal sites like the arms [14], chronic pain from myofascial temporomandibular disorder can profoundly increase pain sensitivity at remote areas [23]. Generalized hyperalgesia has also been described in patients with local pain syndromes like whiplash injury [7], IBS [25], back pain [21], and pelvic pain [3]. Some of these changes can be explained by increased recruitment of central neurons that become activated by nociceptive stimulation [16; 50] as well as by enhanced spatial summation [32]. Another local pain mechanism is spatial referral, i.e. tonic impulse input from local tissues can result in pain of remote areas and increased pain intensity [1; 2]. Thus, pain related to local tonic impulse input can summate with pain sensations from remote injuries resulting in wide-spread pain. Furthermore, it is possible that abnormal spatial summation mechanisms may also contribute to FM pain. Although spatial pain summation of FM patients appears to be normal during low-grade nociceptive input [47], it seems to become abnormally enhanced during more intense pain stimuli [18].
The effects of local anesthetics on hypersensitivity and pain of muscles seem to vary. Whereas injections of local anesthetics into trigger points of whiplash patients attenuated overall pain, mechanical hyperalgesia at remote sites was not affected [15]. In contrast, rectal application of lidocaine to IBS patients abolished rectal hyperalgesia and cutaneous pain sensitivity within converging lumbar dermatomes [52]. Thus, in some chronic pain conditions, such as FM, pain and generalized hypersensitivity may depend on impulse input from muscles and other deep tissues.
Abnormalities of temporal summation mechanisms are also clearly evident for FM. In contrast to healthy control subjects, FM patients have greater temporal summation of pain and more intense and protracted after-sensations in response to repeated heat pulses [41; 44–46; 48] or to mechanical stimulation of muscle [37]. Moreover, once temporal summation has occurred, only very low stimulation frequencies are required to maintain enhanced pain in FM [42]. These results are supported by animal studies, which showed that only low-frequency impulse input was required for maintenance of central sensitization once it had been established [22]. Thus local anesthetic blockade of this low-frequency input may be effective in normalizing widespread hyperalgesia that is so characteristic of FM.
Limitations
In NC and FM participants tonic TrapM pain was not only less intense after lidocaine but also after saline injections (Figure 4). This lack of a stronger effect from lidocaine on tonic muscle pain was puzzling and may be the result of several interacting variables, including local pain inhibitory mechanisms. In particular, we cannot exclude unspecific effects, including those from the needle insertion itself. Nevertheless, we do not consider these unspecific effects as a result of placebo analgesia, because placebo saline injections had neither effects on PPTs (at the shoulder) nor any effects on forearm heat hyperalgesia. The progressive decline of tonic shoulder pain in NC subjects despite continuous pressure stimulation suggests habituation (Figure 4). Decreased tonic pain only occurred after TrapM placebo and lidocaine injections in FM subjects. Nonetheless, the more selective and robust effects of lidocaine but not saline on PPTs in NC and FM subjects clearly established at least some anesthetic effects on local muscle pain.
Conclusions
Lidocaine injections into the TrapM increased muscle pressure pain thresholds at the shoulder (Figure 3). Most critically, lidocaine injections reduced distal heat hyperalgesia of FM subjects, supporting the role of tonic muscle impulse input in maintaining secondary hyperalgesia in this chronic pain syndrome. Similar hyperalgesic effects of tonic peripheral afferent activity have been previously described in other chronic pain syndromes, like CRPS and IBS. These results provide the most direct evidence to date for the important role of deep tissue impulse input in the pathogenesis of FM pain. Future studies will be necessary to show whether multiple or larger doses of local anesthetics can reduce not only secondary hyperalgesia but also clinical FM pain.
Table 1.
TrapM Pressure Pain Thresholds Before and After Injections
| Pressure Pain Threshold (kPa) Mean (SD) | p- Value | ||
|---|---|---|---|
| Before Injection | After Injection | ||
| NC-Placebo | 343.7 (177.3) | 409.9 (328.1) | ns |
| NC-Lidocaine | 300.2 (113.3) | 627.6 (309.0) | p < .001 |
| FM-Placebo | 127.7 (75.5) | 166.2 (151.9) | ns |
| FM-Lidocaine | 138.8 (73.5) | 283.3 (230.2) | p = .002 |
ns = non significant; kPa = kiloPascal
Acknowledgments
This work was supported by NIH grants NS-38767 and AR053541.
Footnotes
The authors have no financial or other relationships to report that might result in a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arendt-Nielsen L, Graven-Nielsen T. Muscle pain: sensory implications and interaction with motor control. Clin J Pain. 2008;24:291–8. doi: 10.1097/AJP.0b013e31815b608f. [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Svensson P. Referred muscle pain: basic and clinical findings. Clin J Pain. 2001;17:11–9. doi: 10.1097/00002508-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj P, Bajaj P, Madsen H, Moller M, Arendt-Nielsen L. Antenatal women with or without pelvic pain can be characterized by generalized or segmental hypoalgesia in late pregnancy. J Pain. 2002;3:451–60. doi: 10.1054/jpai.2002.128065. [DOI] [PubMed] [Google Scholar]
- 4.Bartels EM, Danneskiold-Samsoe B. Histological abnormalities in muscle from patients with certain types of fibrositis. Lancet. 1986;1:755–7. doi: 10.1016/s0140-6736(86)91779-4. [DOI] [PubMed] [Google Scholar]
- 5.Bengtsson A. The muscle in fibromyalgia. Rheumatology. 2002;41:721–4. doi: 10.1093/rheumatology/41.7.721. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson A, Henriksson KG, Larsson J. Muscle biopsy in primary fibromyalgia. Light-microscopical and histochemical findings. Scan J Rheumatol. 1986;15:1–6. doi: 10.3109/03009748609092661. [DOI] [PubMed] [Google Scholar]
- 7.Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Giani C, Zbinden AM, Radanov BP. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17:306–15. doi: 10.1097/00002508-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–9. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 9.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Drewes AM, Andreasen A, Schroder HD, Hogsaa B, Jennum P. Pathology of skeletal muscle in fibromyalgia: a histo-immuno- chemical and ultrastructural study. Br J Rheumatol. 1993;32:479–83. doi: 10.1093/rheumatology/32.6.479. [DOI] [PubMed] [Google Scholar]
- 11.Elvin A, Siosteen AK, Nilsson A, Kosek E. Decreased muscle blood flow in fibromyalgia patients during standardised muscle exercise: A contrast media enhanced colour doppler study. Eur J Pain. 2006;10:137–44. doi: 10.1016/j.ejpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–94. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 13.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 14.Hansson P, Ekblom A, Lindblom U, Marchettini P. Does acute intraoral pain alter cutaneous sensibility? J Neurol Neurosurg Psychiatry. 1988;51:1032–6. doi: 10.1136/jnnp.51.8.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Stefano GD, Radanov BP, Curatolo M. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med. 2004;5:366–76. doi: 10.1111/j.1526-4637.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoheisel U, Koch K, Mense S. Functional reorganization in the rat dorsal horn during an experimental myositis. Pain. 1994;59:111–8. doi: 10.1016/0304-3959(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 17.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73:256–63. doi: 10.1097/00002060-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Kalyan-Raman UP, Kalyan-Raman K, Yunus MB, Masi AT. Muscle pathology in primary fibromyalgia syndrome: a light microscopic, histochemical and ultrastructural study. J Rheumatol. 1984;11:808–13. [PubMed] [Google Scholar]
- 20.Kim SH, Jang TJ, Moon IS. Increased Expression of N-Methyl-D-Aspartate Receptor Subunit 2D in the Skin of Patients with Fibromyalgia. J Rheumatol. 2006;33:785–8. [PubMed] [Google Scholar]
- 21.Lapossy E, Maleitzke R, Hrycaj P, Mennet W, Muller W. The frequency of transition of chronic low back pain to fibromyalgia. Scand J Rheumatol. 1995;24:29–33. doi: 10.3109/03009749509095151. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 23.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–51. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 24.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992;598:271–8. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- 25.Okifuji A, Turk DC. Stress and psychophysiological dysregulation in patients with Fibromyalgia syndrome. Appl Psychophysiol Biofeedback. 2002;27:129–41. doi: 10.1023/a:1016243710507. [DOI] [PubMed] [Google Scholar]
- 26.Petzke F, Clauw DJ, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effect of two types of stimuli and ascending versus random modes of presentation. Arthritis Rheum. 2000;43:S173. [Google Scholar]
- 27.Pongratz DE, Spath M. Morphologic aspects of fibromyalgia. Z Rheumatol. 1998;57:47–51. doi: 10.1007/s003930050234. [DOI] [PubMed] [Google Scholar]
- 28.Price DD, Bennett GJ, Rafii A. Psychophysical observations on patients with neuropathic pain relieved by a sympathetic block. Pain. 1989;36:273–88. doi: 10.1016/0304-3959(89)90086-9. [DOI] [PubMed] [Google Scholar]
- 29.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Harkins SW. Combined use of visual analogue scales and experimental pain in providing standardized measurement of clinical pain. Clin J Pain. 1987;3:1–8. [Google Scholar]
- 31.Price DD, Long S, Wilsey B, Rafii A. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clin J Pain. 1998;14:216–26. doi: 10.1097/00002508-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–9. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 33.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon RL, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 34.Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- 35.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–12. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008;140:420–8. doi: 10.1016/j.pain.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Staud R. Temporal Summation of Pain from Mechanical Stimulation of Muscle. In: Gracely RH, Choy E, editors. Fibromyalgia: About Mechanisms. Lavaur, France: Pierre Fabre; 2005. pp. 52–57. [Google Scholar]
- 38.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber abnormalities of fibromyalgia patients are specifically related to temporal summaiton. Pain. 2008;139:315–25. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 40.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–89. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–89. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–96. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Staud R, Price DD, Robinson ME, Vierck CJ. Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–43. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staud R, Robinson ME, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–22. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 46.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 47.Staud R, Vierck CJ, Robinson ME, Price DD. Spatial summation of heat pain within and across dermatomes in fibromyalgia patients and pain-free subjects. Pain. 2004;111:342–50. doi: 10.1016/j.pain.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the NDMA receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal controls. J Pain. 2005;6:323–32. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- 49.Staud R, Vierck CJ, Robinson ME, Price DD. Overall fibromyalgia pain is predicted by ratings of local pain and pain related negative affect: possible role of peripheral tissues. Rheumatology. 2006;45:1409–15. doi: 10.1093/rheumatology/kel121. [DOI] [PubMed] [Google Scholar]
- 50.Ushida T, Willis WD. Changes in dorsal horn neuronal responses in an experimental wrist contracture model. J Orthop Sci. 2001;6:46–52. doi: 10.1007/s007760170024. [DOI] [PubMed] [Google Scholar]
- 51.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 52.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–30. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 53.Verne GN, Sen A, Price DD. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. Journal of Pain. 2005;6:493–6. doi: 10.1016/j.jpain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Vierck CJ., Jr Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–63. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 56.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–56. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain. 2008;139:218–24. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]