Abstract
Killing of Neisseria meningitidis can result from complement-mediated bactericidal activity (SBA) or opsonophagocytosis (OPA), or a combination of the two mechanisms. While SBA titers ≥1:4 confer protection, recent evidence suggests that this threshold titer may not be required. For example, the incidence of meningococcal disease declines between ages 1 and 4 years without evidence of acquisition of SBA titers ≥1:4. Meningococcal polysaccharide vaccination also elicited OPA and lowered the risk of disease in patients with late complement component deficiencies whose sera did not support SBA. Sera from healthy adults immunized with an outer membrane vesicle vaccine showed OPA killing of N. meningitidis with C6-depleted complement, and whole blood from complement-sufficient non-immunized adults with SBA titers <1:4 also frequently had killing activity. Collectively the data indicate that SBA titers <1:4 and/or vaccine-induced OPA can confer protection against meningococcal disease.
Keywords: Opsonic activity, complement-mediated killing, anti-meningococcal vaccination
1. Introduction
Following exposure to Neisseria meningitidis, the likelihood of acquiring invasive meningococcal disease depends on the virulence of the particular organism, host factors affecting innate susceptibility, and the presence or absence of serum antibodies (reviewed in [1]). Although the pathogen has evolved several mechanisms to evade killing by human complement [2], activation of the complement cascade is a critical host defense against developing meningococcal disease (reviewed in [3]). Binding of antibody or C-reactive protein engages C1q and activates the classical pathway, while binding of mannose-binding protein to the bacterial surface activates the lectin complement pathway [4]. The alternative pathway serves as an amplification loop for both the classical and lectin pathways, and increases C3 activation [2]. The resulting deposition of C3b on the bacterial surface acts as a ligand for opsonization. Subsequent cleavage of C5 leads to the assembly of the C5b-9 membrane attack complex (MAC), which inserts into the bacterial lipid bilayer and causes bacteriolysis. In the absence of specific anti-meningococcal antibodies most pathogenic strains of group B and C meningococci are relatively resistant to killing by the complement pathways. Serum antibodies elicit complement-mediated serum bactericidal activity (SBA) by the classical pathway. However, efficient killing of meningococci by the classical complement pathway may require a fully functional alternative pathway [5].
Observations of a high incidence of meningococcal disease in patients with late complement component deficiencies (LCCD), whose serum complement supports opsonic activity but not bacteriolysis because of an inability to form a MAC [6-13], underscore the importance of SBA in protection. To measure serum bactericidal activity, dilutions of test sera that have been heat-inactivated to eliminate internal complement activity are incubated with bacteria and an exogenous source of complement (typically non-immune human serum or infant rabbit serum). The bactericidal titer is defined by the dilution of test serum that results in a 50 percent decrease in CFU/ml of bacteria after incubation at 37 degrees for ∼1 hr. Currently, a SBA titer of ≥1:4 measured with human complement is the only widely accepted surrogate of vaccine efficacy. However, SBA is a relative term, and because of biologic variability in the assay some authorities prefer to use a higher threshold titer of ≥1:8 as a measure of protection (reviewed in [1, 13-15]). On the other hand, the assay appears to be a relatively insensitive measure of antibody functional activity and, as discussed below, titers <1:4 may be sufficient for protection. Whether persons can be protected by SBA titers <1:4 [14], or by opsonic activity in the absence of SBA [15], are important questions to address. New vaccines for prevention of meningococcal disease are in development. With the exception of a few areas of the world such as sub-Saharan Africa, the incidence of meningococcal disease is too low to perform randomized studies to determine the efficacy of these vaccines. Vaccine efficacy, therefore, is inferred from SBA responses [16-18]. New antigens that elicit antibodies that bind to the surface of bacteria, activate C3 deposition, have opsonic activity, or confer passive protection in animal models, may be promising vaccine candidates [19]. However, if they do not elicit SBA, they usually will not be included in a new vaccine because of the difficulty of establishing that the antigens contribute to protective immunity.
This paper reviews recent seroepidemiologic studies and experimental data that indicate that an SBA titer of ≥1:4, while highly specific for protection against meningococcal disease, is an insensitive indicator of protective immunity. Further, in some situations SBA titres <1:4 and/or OPA activity appear to be sufficient for protection. These conclusions have important implications with respect to our understanding of the mechanisms by which serum antibody confers protection against meningococcal disease.
2. Seroepidemiologic studies
2.1 SBA titers ≥1:4 are sufficient but not necessary for protection
In a seminal study, Goldschneider et al. investigated the ability of SBA ≥1:4 to confer protection against epidemic group C meningococcal disease in military recruits [20, 21]. Baseline sera were obtained during the first week of basic training. Based on studies of a randomized sample, an estimated 82% of the 14,000 recruits had SBA titers ≥1:4 against the epidemic group C strain. However, 51 of the 53 recruits who subsequently developed meningococcal disease had SBA titers <1:4. The authors concluded that a titer ≥1:4 conferred protection against disease but also pointed out that many of the estimated 2600 recruits with titers <1:4 were likely exposed to the epidemic strain and did not develop disease. Indeed more than half of a small group of recruits with SBA titers <1:4 who were demonstrated to have acquired throat carriage with the epidemic strain did not develop disease. Therefore, while titers ≥1:4 conferred protection, titers <1:4 did not necessarily indicate susceptibility.
Additional evidence supporting the importance of SBA titer ≥1:4 in protection came from observations reported in the Goldschneider paper that titers ≥1:4 were rarely detected in children 2 months to 2 years of age, the age group with the highest incidence of disease [20]. The prevalence of SBA titers ≥1:4 increased with increasing age, which was coincident with a dramatic decrease in the age-incidence of meningococcal disease (Figure 1, Panel A). Many other studies have confirmed a high incidence of meningococcal disease during the first year or two of life, which declines rapidly with increasing age [22-25]. However, the decline in group B disease between ages 1 and 10 years in a recent UK study occurred without a corresponding increase in the prevalence of group B serum bactericidal titers ≥1:4 (Figure 1, Panel B) [22]. The high incidence of disease in the UK children under one year of age indicated that the population was exposed to group B organisms. But the rapid decline in the incidence of disease after age 1 year without a corresponding increase in prevalence of SBA titers ≥1:4 indicated that alternative mechanisms were responsible for acquisition of protective group B immunity. The most likely mechanisms include an age-related maturation of the alternative complement pathway [26, 27], acquisition of SBA antibodies at serum dilutions <1:4 [28], opsonic activity [15], or a combination of two or all three mechanisms.
Figure 1.
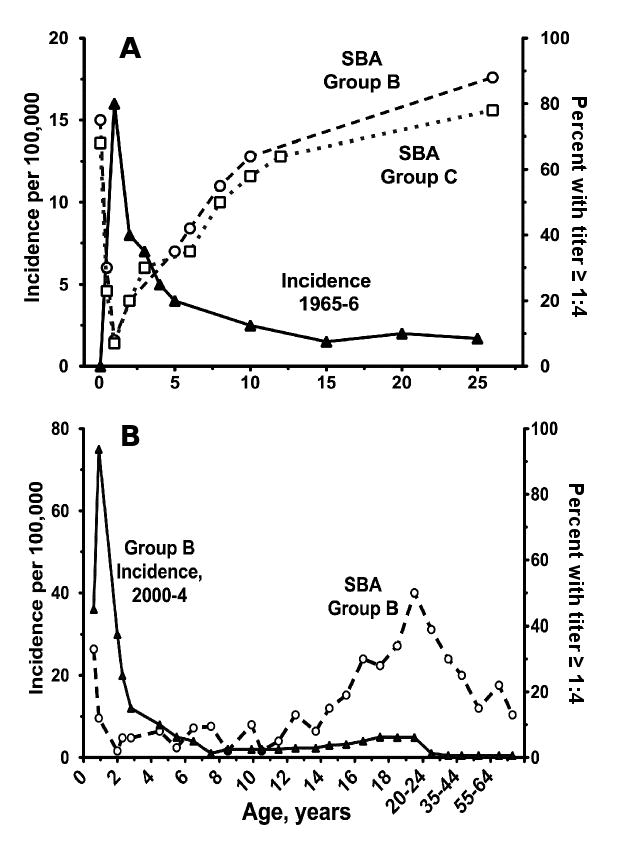
Age-incidence (black triangles) of meningococcal disease in relation to percent of population with SBA titers ≥1:4 (open circles [group B] or squares [group C]). Panel A, adapted from a figure published by Goldschneider et al. from a study in the U.S. in the 1960s [14] with permission from the publisher. Panel B, adapted from a figure published by Trotter et al. from a study in the U.K. in 2000-2004 [20] with permission from the publisher. Note that the respective X and Y axes of the figures in the two panels have different scales.
The UK study also found a lower prevalence of SBA titers in teenagers (10 to 30%) or young adults (∼50%, Figure 1, Panel B) [22] than reported in adults by the Goldschneider study [20] (80 to 90%, Figure 1, Panel A). Three large meningococcal surveys performed in the UK between 1999 and 2001 found that ∼1 in 20 to 25 teenagers carried group B strains in their throats [29, 30] with higher carriage rates in those who attended pubs/clubs, reported intimate kissing, or smoked cigarettes [31]. These data indicated that many teenagers in the UK with SBA titers <1:4 were exposed to group organisms, including strains from hypervirulent genetic lineages, but few developed disease.
Other recent studies from North America and Europe confirmed that SBA titers of ≥1:4 were relatively uncommon in young adults (depending on the strain, typically 10 to 25%) [32-36]. While SBA titers of ≥1:4 are less prevalent in the population than they were in the United States in the 1960s, there has not been a corresponding increase in the incidence of meningococcal disease (since 2000, rates of disease in the U.S. have been the lowest in the last 50 years, ∼0.33 per 100,000 per year1). Collectively, these observations are inconsistent with the hypothesis that serum bactericidal titers ≥1:4 are required for protection against meningococcal disease.
2.2 Estimates of population-based immunity based on SBA titers measured with rabbit complement suggest that SBA titers ≥1:4 with human complement are not required for protection
For many years it has been known that use of rabbit complement augmented serum bactericidal titers as compared with titers measured with human complement [37, 38]. Despite the higher titers, rabbit complement assays were developed and were used extensively [39-42] because it was easier to standardize the assay using commercially-available infant rabbit serum pools as complement sources than with locally obtained non-immune human sera. In post-licensure studies performed in the United Kingdom, SBA titers ≥1:8 with rabbit complement correlated with the efficacy of group C meningococcal conjugate vaccination [16-18].
One reason that SBA titers measured with rabbit complement are higher than titers measured with human complement may relate to recent observations that N. meningitidis binds complement factor H (fH) [43, 44]. Factor H is a down-regulating molecule in the complement pathway and binding of this molecule to the bacterial surface contributes to the ability of the organism to avoid complement-mediated killing by non-immune human serum [43, 44] or whole blood [45, 46]. Note that binding is specific for human fH [47]. Thus, when N. meningitidis was incubated with infant rabbit serum, rabbit fH was not bound to the bacteria, and C3 activation was not down-regulated[47]. As a result, there was extensive deposition of rabbit C3 on the bacterial surface in the absence of added antibody (Figure 2, Panel A). However, the rabbit C3 activation was not sufficient for eliciting complement-mediated bacteriolysis, which is why the infant rabbit serum appeared to be a suitable source of complement for performing bactericidal assays of heat-inactivated test sera. When we measured group C bactericidal titers of heat-inactivate sera from children immunized with meningococcal polysaccharide vaccine using rabbit complement, the addition of human fH markedly lowered the titers (Figure 2, Panel B). In a previous study, many sera with bactericidal titers between 1:8 and 1:64 measured with rabbit complement had titers <1:4 when assayed with human complement [38]. Thus, if vaccine efficacy correlates with group C serum bactericidal titers ≥1:8 measured with rabbit complement, then on a population basis, vaccine-induced serum bactericidal titers ≥1:4 measured with human complement are not required for protection.
Figure 2.
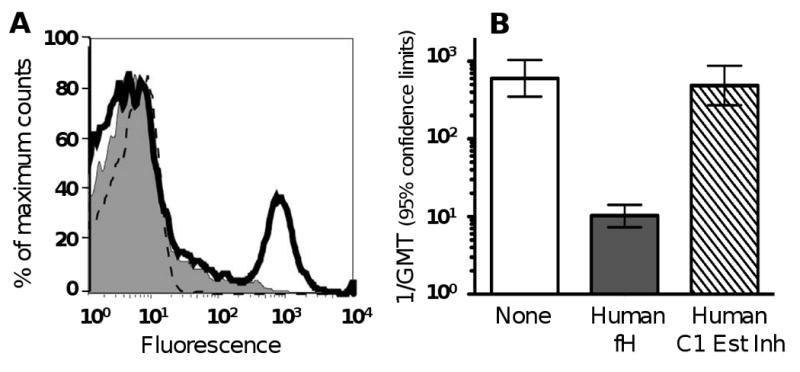
Effect of addition of human fH on down-regulation of rabbit C3 and human serum vaccinee bactericidal titers measured with rabbit complement against group C N. meningitidis strain 4243. Panel A. Live bacteria were incubated with 20% infant rabbit serum alone (open histograms shown as solid lines), or rabbit serum plus human fH, 25 μg/ml (shaded histograms). The control, no serum, is shown by the dotted lines. Rabbit C3 deposition was measured by flow cytometric detection. Panel B. Bactericidal titers of sera of 19 children, aged 4- to 5- years, obtained 1 month after meningococcal polysaccharide vaccination. Titers were measured with 20% rabbit complement alone (none), or with 25 μg/ml of human fH or, as a negative control, 25 μg/ml of human C1-esterase inhibitor. The differences between the respective geometric mean titers measured in human sera using rabbit complement in the absence or presence of human fH were significant (P<0.001). Adapted from published data [44] with permission of the publisher.
2.3 Outer membrane vesicle (OMV) vaccine efficacy studies suggest that SBA titers ≥1:4 are sufficient but not necessary to maintain protection against meningococcal group B disease
Table 1 summarizes the results of published randomized trials or observational studies of group B OMV vaccine efficacy. In general, the observed proportions of subjects developing SBA titers ≥1:4 four- to six-weeks after vaccination paralleled the observed estimates of vaccine efficacy. However, in several studies, notably in Iquique, Chile, and São Paolo, Brazil, the point estimates of efficacy in the older age groups were higher than the corresponding SBA response rates. Also, in most of the studies, the proportion of subjects with SBA titers ≥1:4 declined beginning several months after immunization (for example, in Norway, from 97% of subjects with titers ≥1:4 at 6 weeks after the second dose of vaccine, to 32% and 42% at 1 and 2 years, respectively). Thus, the overall vaccine efficacy rates reflected follow-up periods when increasing proportions of the immunized groups had SBA titers <1:4. The data from these studies, therefore, are consistent with the hypothesis that SBA titers ≥1:4 are sufficient for protection but are not necessarily needed for protection.
Table 1.
Summary of studies of group B meningococcal outer membrane vesicle vaccine efficacy
| Year of vaccination [ref] |
Vaccine strain & formulation |
No. of doses |
Location (population vaccinated) |
Age | 1 mo. Post SBA Titer ≥1:4 (%) |
Follow-up (mos) |
Efficacy (%) |
(95% confidence interval) |
|---|---|---|---|---|---|---|---|---|
| 1987-89 [72] | B:4:P1.15/Group C PS | 2 | Cuba (106,000) | 11-16 yrs | NRa | 16 | 83 | 42, 95 |
| 1989-90 [73, 74] | B:4:P1.15/Group C PS | 2 | Sao Paolo, Brazil (2.4 million) | 3-23 mos | 13 | 0 to 22 | -37 | -100, 73 |
| 24-47 mos | 43 | 0 to 22 | 47 | -72, 84 | ||||
| 4-7 yrs | 52 | 0 to 22 | 74 | 16, 92 | ||||
| 1990 [75] | B:4:P1.15/ Group C PS | 2 | Rio de Janeiro, Brazil (1.6 million) | 6-23 mos | NR | 0 to 12 | 41 | -96, 82 |
| 24-47 mos | NR | 0 to 12 | 14 | -165, 72 | ||||
| 4-9 yrs | NR | 0 to 12 | 71 | 34, 87 | ||||
| 1987-1989 [76] | B:15:P1.3/ Group C PS | 2 | Iquique, Chile (40,800) | 1-4 yrs | 12b | 30 | -39 | -100, 73 |
| 5-21 yrs | 35b | 30 | 70 | 14, 91 | ||||
| 1988-91 [15, 77] | B:15:P1.7,16 | 2 | Norway (171,800) | 14-16 yrs | 97 | 0.5 to 10 | 87 | 17,98c |
| 11 to 20 | 59 | -27,86c | ||||||
| 21 to 29 | 30 | -93,75c | ||||||
| 0.5 to 29 | 57 | 14, 79c | ||||||
| 2004-2006 [78] | B:4:P1.7-2,4 | 3 | New Zealand (905,507 person-yrs) | <1-19 yrs | 72-92d | 1 to 24 | 73 | 52 -85 |
| 2004-2007 [79] | B:4:P1.7-2,4 | 3 | New Zealand (160,870) | 6-59 mos | 75d | 0-12 | 82 | 34-95 |
| 12-24 | 33 | -147-90 | ||||||
The two Brazil studies were retrospective case-control studies, which used the same vaccine produced in Cuba. The study in New Zealand was prospective observational. The remaining studies were prospective, randomized trials. The vaccines tested were OMV with the exception of the one used in Chile, which was purified outer membrane proteins (OMP). C PS = group C polysaccharide vaccine was mixed with OMV or OMP (all vaccines shown except those used in Norway and New Zealand). All vaccines were adsorbed with Al(OH)3.
NR, not reported.
Percent with ≥4-fold titer increases between pre- and post-immunization sera as measured against strain used to prepare vaccine.
Asymptotic intervals based on the number of reported number of cases during each interval, assuming two large homogeneous of vaccinated or not vaccinated persons and calculated by StatXact® (Cytel, Cambridge MA, http://www.cytel.com/products/statxact/)
72% of infants immunized with 3 doses beginning at 6 to 10 weeks of age had titers ≥1:4; and 92% of older age groups [80].
3. Experimental studies
3.1 Whole blood from persons with SBA titers <1:4 can kill N. meningitidis
To investigate the hypothesis that bactericidal titers <1:4 and/or opsonic antibodies contribute to protective immunity against N. meningitidis, we collected blood samples from healthy adults using recombinant hirudin as the anticoagulant [4], which is a specific thrombin inhibitor not known to affect complement activation. Log-phase bacteria from different group B test strains were incubated with the fresh blood specimens and quantitative cultures were obtained at 0, 1, 2 and 4 hours. Figure 3 shows data from two representative donors with SBA titers <1:4 [28]. In blood from one of the donors (Donor 3), CFU/ml of each of the three test strains remained stable or increased by more than 10-fold during the four hours of incubation. In contrast, the blood from the second donor (Donor 4) killed all three strains (decreases in CFU/ml of >99% by 1-2 hours of incubation). Thus, although both donors had SBA titers <1:4, Donor 3 would appear to be at greater risk of developing invasive disease if exposed to one of these meningococcal strains than Donor 4 whose blood was highly effective in killing the bacteria by OPA and/or SBA.
Figure 3.
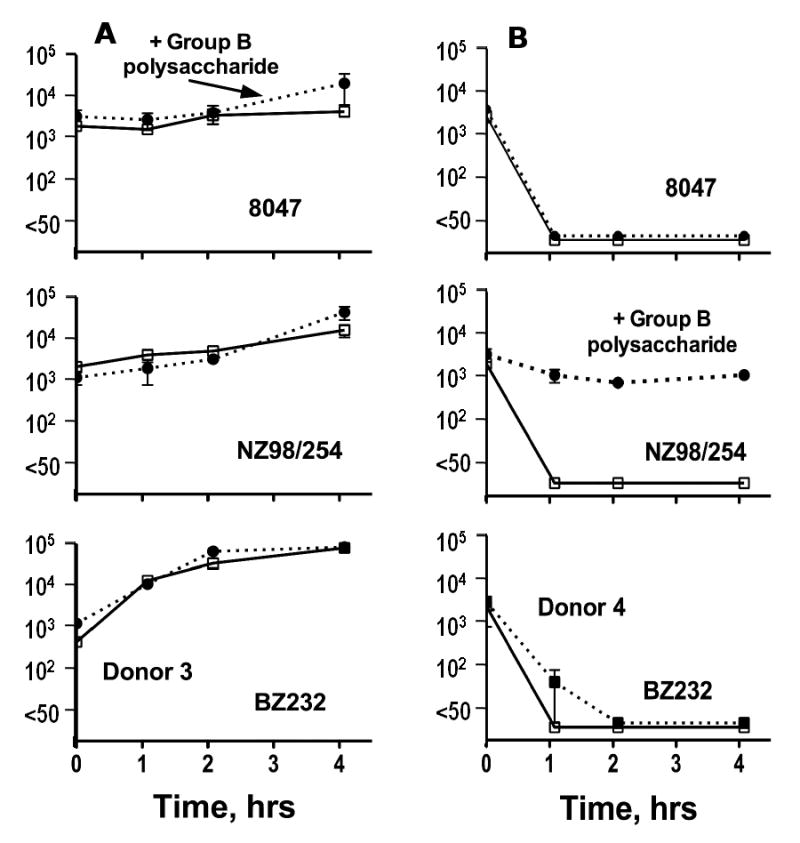
Survival of N. meningitidis group B bacteria in whole blood of two adults (Donor 3 (left) and Donor 4 (right)) with SBA titers <1:4. Data are for group B strains 8047, NZ98/254 and BZ232. Adapted from published data of the author [26] with permission of the publisher. Data on Y axes represent CFU/ml on a log10 axis. Error bars represent range in duplicate samples for each time point. Dashed lines represent data for blood containing 50 μg/ml of purified group B polysaccharide as an inhibitor.
We performed similar investigations of SBA and whole blood killing of N. meningitidis in blood samples from 15 healthy adults. SBA titers ≥1:4 were present in 7, 13 and 20 percent, respectively, against strains BZ232, NZ98/254 and 8047 [28]. The corresponding percentages of killing in the whole blood assay were 40, 47 and 87 percent. Depending on the stringency of the definition of whole blood killing and the strain tested, blood from 23 to 83 percent of subjects with SBA titers <1:4 had killing activity against N. meningitidis (Table 2). For some of the SBA-negative donors, the presence of leukocytes were required for whole blood killing activity since in parallel experiments no killing was observed with their plasma samples [28]. However, with other donors, plasma alone was sufficient for bacterial killing, which suggested that killing activity was present at serum or plasma dilutions <1:4. In summary, killing of meningococci by whole blood and/or plasma can be rapid (<1 hr), effective (≥99%) and was 4- to 6-fold more frequent than an SBA titer ≥1:4. Similar results were observed in earlier studies by other investigators using slightly different whole blood killing assays [48-51]. The data support the hypothesis that many adults with SBA titers <1:4 are immune from developing meningococcal disease.
Table 2.
Killing of N. meningitidis group B strains by whole blood from subjects with serum bactericidal titers <1:4
| Test strain | No. of subjects with SBA titers <1:4 | No. of blood samples with killing activity (percent; 95% CI) | |
|---|---|---|---|
| Definition 1a | Definition 2b | ||
| 8047 | 12 | 10 (83; 55-95) | 5 (42; 19-68) |
| NZ98/254 | 13 | 5 (38; 18-64) | 3 (23; 8-50) |
| BZ232 | 14 | 5 (36; 16-61) | 4 (29; 12-55) |
Previously published data of the author [26]. Reproduced with permission of the publisher.
≥1 log10 decrease in CFU/ml after two hours of incubation as compared with CFU/ml at time 0
≥2 log10 decrease CFU/ml after one hour incubation as compared with CFU/ml at time 0
3.2 Passive protective activity by antibody in animal models
The infant rat model has been used to measure the ability of passively administered antibodies or sera from humans enrolled in clinical vaccine trials to confer protection against meningococcal bacteremia [19, 36, 52-60]. While protective activity was observed by antibodies or immune serum that lacked bactericidal activity, interpretation of the protection must now take into consideration that rat fH does not bind to N. meningitidis [47]. Therefore, the passive protection occurred in the presence of unregulated alternative complement pathway activation in the rat blood, which would have rendered the organisms more susceptible to killing than in human blood with fH is bound to the bacterial surface. In the future, the use of animals treated with human fH [47], or transgenic animal models expressing human fH, may permit studies of the protective role of antibodies under conditions that more closely mimic innate human immunity and disease. Alternatively, a recently described passive protective assay using an ex vivo human blood meningococcal bacteremia model may provide a more sensitive measure of protective immunity than a SBA titer of 1:4 or greater [14].
4. Patients with late complement component (LCCD) deficiencies
4.1 Killing of N. meningitidis by PMNs in the absence of complement-mediated serum bactericidal activity
Patients with LCCD (i.e., C5, 6, 7 or 8) lack SBA because their complement cascade cannot form the membrane attack complex (MAC). Because these patients can activate C3 deposition on the bacteria by the classical and alternative pathways, opsonic function is intact [61]. A major argument against a protective role of opsonic activity against meningococcal disease stems from observations that LCCD patients are at ∼1000-fold increased risk of developing meningococcal disease than complement-sufficient persons (reviewed in [6, 7]). In contrast, there does not appear to be a similar increased risk of pneumococcal disease where protective activity is mediated only by activation of C3 and opsonophagocytosis. However, patients with hyposplenic function and normal complement activity also appear to be at increased risk for developing severe meningococcal disease [62-65], which supports an independent contribution of phagocytic activity in protection against meningococcal disease.
The strains causing meningococcal disease in LCCD patients often belong to capsular groups such as W-135, X or ungroupable [66, 67]. In industrialized countries, these strains rarely cause disease in complement-sufficient persons. Up to 41 percent of LCCD patients with one episode of meningococcal disease will develop recurrences (as compared with <1 percent of complement-sufficient persons), and prior episodes in LCCD patients did not appear to decrease the risk of subsequent episodes [6]. Thus, the immune responses of LCCD patients to natural exposures or to meningococcal disease appear to be poorly protective.
Despite these observations meningococcal vaccination may be of benefit to patients with LCCD since several studies reported that meningococcal polysaccharide vaccination elicited opsonophagocytic antibodies [61, 68-70]. A study from Russia also found that meningococcal A, C, Y and W-135 polysaccharide vaccination increased the average time to a subsequent episode from 5 years in unvaccinated patients to 8 years in vaccinated patients (P<0.01) (Figure 4) [13]. Stated another way, the average risk of acquiring disease was 0.098 episodes per individual per year among non-vaccinees vs. 0.027 episodes per individual per year in vaccinees, which corresponded to a vaccine efficacy of ∼72 percent. The conclusions of this study should be interpreted with caution since allocation to the vaccination or unvaccinated groups was not randomized, the sample sizes were small, and the analysis was retrospective. Nevertheless, the observations were consistent with the reports described above that meningococcal polysaccharide vaccination of LCCD patients elicited serum antibodies that killed N. meningitidis by opsonophagocytosis.
Figure 4.
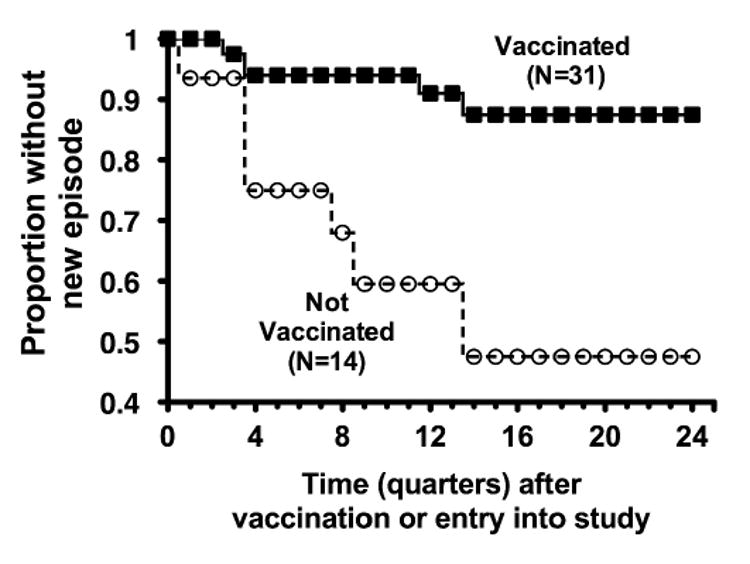
Analysis of time to onset of meningococcal disease in patients with late complement component deficiencies in relation to meningococcal polysaccharide (A, C, Y and W-135) vaccination. The observed differences between the two groups were significant (P<0.01, see text). Adapted from a figure previously published [12] with permission of the publisher.
5. Antibodies to non-capsular antigens can kill N. meningitidis by complement-mediated opsonophagocytosis
Although antibodies directed at non-capsular antigens elicit complement-mediated SBA, some authors questioned whether these antibodies independently activate opsonophagocytic killing [7, 61, 68]. To investigate this question, we developed an opsonophagocytic killing assay performed with human polymorphonuclear leukocytes (PMNs) and non-immune human serum as a complement source that had been depleted of C6 by using an anti-C6 adsorbent column [15]. The C6-depleted complement did not support serum bactericidal activity by an anti-PorA mAb in the absence of added PMNs (Figure 5, Panel A). When PMNs were added to the reaction, there was effective anti-PorA killing of the meningococci. We assayed the ability of sera from complement-sufficient adults to kill N. meningitidis by this opsonophagocytosis assay. The sera were heat-inactivated to remove internal complement activity. There were examples of immunized adults with SBA titers <1:4 measured with C6-sufficient complement whose sera killed N. meningitidis in the presence of human PMNs and C6-depleted complement (Figure 5, Panel B).
Figure 5.


Killing of strain NZ98/254 by antibody, PMNs and C6-depleted human serum (C6D) as a complement source. Panel A. Anti-PorA P1.4 mAb activity in the presence of 20% C6D and 40 human PMNs per CFU of bacteria (black triangles with solid line); or in the absence of PMNs (no PMNs, open circles with dashed line); or with PMNs, plus antibody without C6-depleted complement (X, showing data point at one anti-P1.4 mAb concentration). Panel B. An example of killing of meningococci in an opsonophagocytic assay by a 1:5 dilution of heat-inactivated post-immunization serum from an adult immunized with an OMV vaccine plus recombinant GNA 2132. Hatched bars; sera were assayed with human PMNs, and 20% C6D as the complement source. There was no bacterial killing by the sera when tested with C6-sufficient complement in the absence of added PMNs (white bars). Data represent results of four replicate experiments for each test serum. Adapted from previously published data of the author [27] with permission of the publisher.
We compared the SBA and OPA responses of adults who had been immunized with a meningococcal OMV vaccine prepared from group B strain H44/76, which was given alone or combined with a novel recombinant protein, called genome-derived neisserial antigen (GNA) 2132 [15]. When the pre- and post-immunization sera were assayed for SBA with an exogenous C6-sufficient human serum as the complement source, there were no significant differences in the respective proportion of subjects in each vaccine group who developed SBA titers ≥1:4. The results against strain H44/76, which was used to prepare the OMV vaccine, are shown in Figure 6 (Panel A). When the sera were assayed for their ability to kill the bacteria by opsonophagocytosis with a C6-depleted complement source (Figure 6, Panel B), a higher proportion of subjects given the OMV vaccine plus recombinant GNA 2132 developed serum opsonophagocytic killing activity than subjects given the OMV vaccine alone (P<0.02). Thus, vaccine-induced serum antibodies to non-capsular antigens can have opsonic activity with C6-depleted complement, and the data suggested that a combination vaccine of recombinant GNA 2132 and OMV was more effective at eliciting serum opsonic activity than the OMV vaccine alone.
Figure 6.
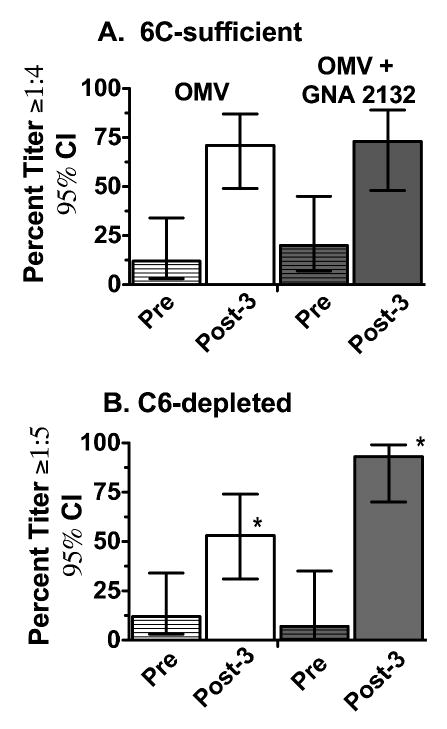
Serum bactericidal (C6-sufficient complement) and opsonophagocytic killing (C6-deficient complement) of sera from vaccinated adults measured against N. meningitidis group B strain H44/76, which was used to prepare the OMV vaccine. OMV vaccine group (N=17, white bars); combination OMV vaccine + recombinant GNA 2132 vaccine group (N=15, gray bars). Bars with hatches represent pre-immunization sera and bars without hatches represent sera obtained 1 month after a third dose of vaccine. Panel A. Complement-mediated bactericidal activity with C6-sufficient complement. Panel B. Opsonophagocytic killing of meningococci measured with C6-depleted complement and human PMNs. Comparisons of the respective percentages of subjects in the two groups with opsonophagocytic killing activity after vaccination, *P=0.02. Similar respective trends were observed for two other heterologous test strains but the differences were not statistically significant. Adapted from published data of the author [27] with permission of the publisher.
6. Surrogates, correlates and future directions
To assess protective meningococcal immunity, the results of SBA assays should be supplemented by additional serologic assays that may be more sensitive for predicting protection against meningococcal disease. These assays might include assessment of functional mechanisms responsible for protective immunity (i.e., surrogates of protection). These surrogates might include complement-mediated bactericidal activity of sera or plasma assayed at dilutions <1:4 [28, 46], serum OPA [15], or the ability of sera to confer passive protection in an ex vivo whole human blood model of meningococcal bacteremia [14, 28], or in an animal model of bacteremia in the presence of human fH [41]. For vaccines containing fHbp antigens, the ability of serum antibodies to inhibit binding of human fH to the bacteria as measured by flow cytometry [46], or to immobilized fHbp as measured by ELISA [71, 72], may also prove useful as a useful surrogate of protection since in the absence of bound fH, many strains of N. meningitidis were rapidly killed by non-immune human plasma or blood [46, 73].
A correlate of protection refers to the results of an assay that predicts protection against disease but which does not directly measure a functional activity directly related to the protective mechanism. For example, as described above, low SBA titers measured with rabbit complement predicted protective immunity of the UK population immunized with meningococcal group C conjugate vaccine [18, 48] but many of the sera with positive titers measured with rabbit complement would be expected to be negative if measured with human complement [38]. Conceivably the positive rabbit titers correlated with another mechanism of protection, for example, human SBA at titers <1:4 or OPA.
The ability of sera or antibodies to activate human C3b deposition on the surface of live, encapsulated bacteria correlated with passive protective activity against meningococcal bacteremia in an infant model [19, 57]. Although interpretation of these observations is limited by lack of binding of rat fH and unregulated complement activation [47], future studies should investigate whether the ability to elicit human C3b deposition predicts passive protection in animals treated with human fH, or predicts in vitro OPA or SBA that may be present in serum dilutions <1:4. Serum anticapsular antibody concentrations measured by antigen binding assays also are likely to correlate with protective immunity against meningococcal disease caused by group A, C, Y or W-135 strains, particularly if antibody avidity is measured in parallel and factored into the analysis [34, 74, 75].
In summary, while a SBA titer of ≥1:4 with human complement confers protection against developing meningococcal disease, the studies reviewed above indicate that this picture of protection is an incomplete story. By relying only on SBA titers ≥1:4 as a measure of protection, public health decisions on whether or not to license a new vaccine, the optimal age to administer the vaccine, the number of doses required, or the duration of protective immunity, will be based on imprecise inferences that underestimate protective immunity. A high priority should be development of more sensitive and reproducible serologic assays for assessing protection immunity against meningococcal disease.
Acknowledgments
I am grateful to the following colleagues who kindly reviewed the manuscript and offered helpful critical comments: Sanjay Ram (University of Massachusetts), Johan Holst (Norwegian Institute of Public Health, Oslo) and Jo Anne Welsch, Peter Beernink and Alexander Lucas (Children's Hospital Oakland Research Institute). This work was supported by Public Health Service grant R01 AI46464 from the National Institute of Allergy and Infectious Diseases, NIH. The work at Children's Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant number C06 RR-16226 from the National Center for Research Resources, NIH.
Footnotes
Conflict of interest. The author is principal investigator of laboratory research conducted on behalf of Children's Hospital Oakland Research Institute that is funded by grants from Novartis Vaccines and Diagnostics, and Sanofi Pasteur. He also holds a paid consultancy from Novartis and is an inventor on patents or patent applications in the area of meningococcal B vaccines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Granoff DM, Harrison L, Borrow R. Meningoccal Vaccines. In: Plotkin SA, Offit P, Orenstein WA, editors. Vaccines. 5th. Philadelphia: Saunders Elsevier; 2008. pp. 399–434. [Google Scholar]
- 2.Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007;15(5):233–40. doi: 10.1016/j.tim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Ram S, Volgel U. Role of complement in defense against meningococcal infections. In: Frosch M, Maiden MC, editors. Handbook of Meningococcal Disease: Meningococcal disease, pathogenicity and prevention. Weinheim, Germany: Wiley-VCV; 2006. pp. 273–93. [Google Scholar]
- 4.Sprong T, Brandtzaeg P, Fung M, Pharo AM, Hoiby EA, Michaelsen TE, et al. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood. 2003;102(10):3702–10. doi: 10.1182/blood-2003-03-0703. [DOI] [PubMed] [Google Scholar]
- 5.Söderström C, Braconier JH, Danielsson D, Sjöholm AG. Bactericidal activity for Neisseria meningitidis in properdin-deficient sera. J Infect Dis. 1987;156(1):107–12. doi: 10.1093/infdis/156.1.107. [DOI] [PubMed] [Google Scholar]
- 6.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4(3):359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12(3):295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 8.Fijen CA, Kuijper EJ, Hannema AJ, Sjöholm AG, van Putten JP. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups. Lancet. 1989;2(8663):585–8. doi: 10.1016/s0140-6736(89)90712-5. [DOI] [PubMed] [Google Scholar]
- 9.Fijen CA, Kuijper EJ, te Bulte MT, Daha MR, Dankert J. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin Infect Dis. 1999;28(1):98–105. doi: 10.1086/515075. [DOI] [PubMed] [Google Scholar]
- 10.Derkx HH, Kuijper EJ, Fijen CA, Jak M, Dankert J, van Deventer SJ. Inherited complement deficiency in children surviving fulminant meningococcal septic shock. Eur J Pediatr. 1995;154(9):735–8. doi: 10.1007/BF02276718. [DOI] [PubMed] [Google Scholar]
- 11.Platonov AE, Beloborodov VB, Gabrilovitch DI, Khabarova VV, Serebrovskaya LV. Immunological evaluation of late complement component-deficient individuals. Clin Immunol Immunopathol. 1992;64(2):98–105. doi: 10.1016/0090-1229(92)90186-r. [DOI] [PubMed] [Google Scholar]
- 12.Platonov AE, Beloborodov VB, Vershinina IV. Meningococcal disease in patients with late complement component deficiency: studies in the U.S.S.R. Medicine (Baltimore) 1993;72(6):374–92. doi: 10.1097/00005792-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Platonov AE, Vershinina IV, Kuijper EJ, Borrow R, Kayhty H. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine. 2003;21(2730):4437–47. doi: 10.1016/s0264-410x(03)00440-7. [DOI] [PubMed] [Google Scholar]
- 14.Plested J, Welsch JA, Granoff DM. An ex-vivo meningococcal human blood bacteremia model for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol. 2009;16 doi: 10.1128/CVI.00007-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plested JS, Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol. 2008;15(5):799–804. doi: 10.1128/CVI.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine. 2005;23(1718):2222–7. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 17.Balmer P, Borrow R. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev Vaccines. 2004;3(1):77–87. doi: 10.1586/14760584.3.1.77. [DOI] [PubMed] [Google Scholar]
- 18.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from post-licensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–6. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188(11):1730–40. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 20.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129(6):1327–48. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trotter C, Findlow J, Balmer P, Holland A, Barchha R, Hamer N, et al. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin Vaccine Immunol. 2007;14(7):863–8. doi: 10.1128/CVI.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51(9):717–22. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- 24.Rosenstein NE, Perkins BA, Stephens DS, Lefkowitz L, Cartter ML, Danila R, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis. 1999;180(6):1894–901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344(18):1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 26.de Paula PF, Barbosa JE, Junior PR, Ferriani VP, Latorre MR, Nudelman V, et al. Ontogeny of complement regulatory proteins - concentrations of factor h, factor I, c4b-binding protein, properdin and vitronectin in healthy children of different ages and in adults. Scand J Immunol. 2003;58(5):572–7. doi: 10.1046/j.1365-3083.2003.01326.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferriani VP, Barbosa JE, de Carvalho IF. Complement haemolytic activity (classical and alternative pathways), C3, C4 and factor B titres in healthy children. Acta Paediatr. 1999;88(10):1062–6. doi: 10.1080/08035259950168081. [DOI] [PubMed] [Google Scholar]
- 28.Welsch JA, Granoff D. Immunity to Neisseria meningitidis group B in adults despite lack of serum bactericidal activity. Clin Vaccine Immunol. 2007;14(12):1596–602. doi: 10.1128/CVI.00341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiden MC, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359(9320):1829–31. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 31.MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12(6):950–7. doi: 10.3201/eid1206.051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell LA, Ochnio JJ, Glover C, Lee AY, Ho MK, Bell A. Analysis of meningococcal serogroup C-specific antibody levels in British Columbian children and adolescents. J Infect Dis. 1996;173(4):1009–13. doi: 10.1093/infdis/173.4.1009. [DOI] [PubMed] [Google Scholar]
- 33.Jones GR, Williams JN, Christodoulides M, Jolley K, Heckels JE. Lack of immunity in university students before an outbreak of serogroup C meningococcal infection. J Infect Dis. 2000;181(3):1172–5. doi: 10.1086/315352. [DOI] [PubMed] [Google Scholar]
- 34.Granoff DM, Morgan A, Welsch JA. Immunogenicity of an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine in 2-year old children. Vaccine. 2005;23(34):4307–14. doi: 10.1016/j.vaccine.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 35.Amir J, Louie L, Granoff DM. Naturally-acquired immunity to Neisseria meningitidis group A. Vaccine. 2005;23(8):977–83. doi: 10.1016/j.vaccine.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 36.Welsch JA, Granoff D. Naturally acquired passive protective activity against Neisseria meningitidis Group C in the absence of serum bactericidal activity. Infect Immun. 2004;72(10):5903–9. doi: 10.1128/IAI.72.10.5903-5909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zollinger WD, Mandrell RE. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40(1):257–64. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol. 2001;8(3):616–23. doi: 10.1128/CDLI.8.3.616-623.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4(2):156–67. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jodar L, Cartwright K, Feavers IM. Standardisation and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A and C vaccines. Biologicals. 2000;28(3):193–7. doi: 10.1006/biol.2000.0253. [DOI] [PubMed] [Google Scholar]
- 41.Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69(3):1568–73. doi: 10.1128/IAI.69.3.1568-1573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159(10):907–13. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 43.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176(12):7566–75. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 44.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177(1):501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, et al. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun. 2009;77(1):292–9. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis. 2008;197(7):1053–61. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 47.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77(2):764–9. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Findlow J, Taylor S, Aase A, Horton R, Heyderman R, Southern J, et al. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect Immun. 2006;74(8):4557–65. doi: 10.1128/IAI.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ison CA, Anwar N, Cole MJ, Galassini R, Heyderman RS, Klein NJ, et al. Assessment of immune response to meningococcal disease: comparison of a whole-blood assay and the serum bactericidal assay. Microb Pathog. 1999;27(4):207–14. doi: 10.1006/mpat.1999.0296. [DOI] [PubMed] [Google Scholar]
- 50.Ison CA, Anwar N, Cole MJ, Pollard AJ, Morley SL, Fidler K, et al. Age dependence of in vitro survival of meningococci in whole blood during childhood. Pediatr Infect Dis J. 2003;22(10):868–73. doi: 10.1097/01.inf.0000091283.10199.dc. [DOI] [PubMed] [Google Scholar]
- 51.Ison CA, Heyderman RS, Klein NJ, Peakman M, Levin M. Whole blood model of meningococcal bacteraemia--a method for exploring host-bacterial interactions. Microb Pathog. 1995;18(2):97–107. doi: 10.1016/s0882-4010(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 52.Toropainen M, Saarinen L, van der Ley P, Kuipers B, Kayhty H. Murine monoclonal antibodies to PorA of Neisseria meningitidis show reduced protective activity in vivo against B:15:P1. 7,16 subtype variants in an infant rat infection model. Microb Pathog. 2001;30(3):139–48. doi: 10.1006/mpat.2000.0419. [DOI] [PubMed] [Google Scholar]
- 53.Toropainen M, Kayhty H, Saarinen L, Rosenqvist E, Hoiby EA, Wedege E, et al. The infant rat model adapted to evaluate human sera for protective immunity to group B meningococci. Vaccine. 1999;17(2021):2677–89. doi: 10.1016/s0264-410x(99)00049-3. [DOI] [PubMed] [Google Scholar]
- 54.Toropainen M, Saarinen L, Wedege E, Bolstad K, Makela PH, Kayhty H. Passive protection in the infant rat protection assay by sera taken before and after vaccination of teenagers with serogroup B meningococcal outer membrane vesicle vaccines. Vaccine. 2005;23(40):4821–33. doi: 10.1016/j.vaccine.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Toropainen M, Saarinen L, Wedege E, Bolstad K, Michaelsen TE, Aase A, et al. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect Immun. 2005;73(8):4694–703. doi: 10.1128/IAI.73.8.4694-4703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toropainen M, Saarinen L, Vidarsson G, Kayhty H. Protection by meningococcal outer membrane protein PorA-specific antibodies and a serogroup B capsular polysaccharide-specific antibody in complement-sufficient and C6-deficient infant rats. Infect Immun. 2006;74(5):2803–8. doi: 10.1128/IAI.74.5.2803-2808.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welsch JA, Rossi R, Comanducci M, Granoff DM. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol. 2004;172(9):5606–15. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 58.Granoff DM, Morgan A, Welsch JA. Persistence of group C anticapsular antibodies two to three years after immunization with an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J. 2005;24(2):132–6. doi: 10.1097/01.inf.0000151035.64356.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou VC, Koeberling O, Welsch JA, Granoff DM. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J Infect Dis. 2005;192(4):580–90. doi: 10.1086/432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vu DM, Welsch JA, Zuno-Mitchell P, Dela Cruz JV, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis. 2006;193(6):821–8. doi: 10.1086/500512. [DOI] [PubMed] [Google Scholar]
- 61.Andreoni J, Käyhty H, Densen P. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J Infect Dis. 1993;168(1):227–31. doi: 10.1093/infdis/168.1.227. [DOI] [PubMed] [Google Scholar]
- 62.Locker GJ, Wagner A, Peter A, Staudinger T, Marosi C, Rintelen C, et al. Lethal Waterhouse-Friderichsen syndrome in posttraumatic asplenia. J Trauma. 1995;39(4):784–6. doi: 10.1097/00005373-199510000-00035. [DOI] [PubMed] [Google Scholar]
- 63.Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7(12):657–60. doi: 10.1046/j.1198-743x.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 64.Castagnola E, Fioredda F. Prevention of life-threatening infections due to encapsulated bacteria in children with hyposplenia or asplenia: a brief review of current recommendations for practical purposes. Eur J Haematol. 2003;71(5):319–26. doi: 10.1034/j.1600-0609.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 65.Bilukha OO, Rosenstein N. Centers for Disease Control and Prevention. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR7):1–21. [PubMed] [Google Scholar]
- 66.Orren A, Caugant DA, Fijen CA, Dankert J, van Schalkwyk EJ, Poolman JT, et al. Characterization of strains of Neisseria meningitidis recovered from complement-sufficient and complement-deficient patients in the Western Cape Province, South Africa. J Clin Microbiol. 1994;32(9):2185–91. doi: 10.1128/jcm.32.9.2185-2191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fijen CA, Kuijper EJ, Tjia HG, Daha MR, Dankert J. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin Infect Dis. 1994;18(5):780–4. doi: 10.1093/clinids/18.5.780. [DOI] [PubMed] [Google Scholar]
- 68.Ross SC, Rosenthal PJ, Berberich HM, Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987;155(6):1266–75. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 69.Platonov AE, Vershinina IV, Käyhty H, Fijen CA, Würzner R, Kuijper EJ. Antibody-dependent killing of meningococci by human neutrophils in serum of late complement component-deficient patients. Int Arch Allergy Immunol. 2003;130(4):314–21. doi: 10.1159/000070219. [DOI] [PubMed] [Google Scholar]
- 70.Fijen CA, Kuijper EJ, Drogari-Apiranthitou M, Van Leeuwen Y, Daha MR, Dankert J. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine. Clin Exp Immunol. 1998;114(3):362–9. doi: 10.1046/j.1365-2249.1998.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beernink PT, Granoff DM. Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infect Immun. 2008;76(6):2568–75. doi: 10.1128/IAI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect Immun. 2008;76(9):4232–40. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, et al. Factor H-binding protein is important for meningococcal survival in human whole blood and serum, and in the presence of the antimicrobial peptide LL-37. Infect Immun. 2009;77(1):292–9. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Granoff DM, Harris SL. Protective activity of group C anticapsular antibodies elicited in two-year-olds by an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J. 2004;23(6):490–7. doi: 10.1097/01.inf.0000129686.12470.e6. [DOI] [PubMed] [Google Scholar]
- 75.Harris SL, King WJ, Ferris W, Granoff DM. Age-related disparity in functional activities of human group C serum anticapsular antibodies elicited by meningococcal polysaccharide vaccine. Infect Immun. 2003;71(1):275–86. doi: 10.1128/IAI.71.1.275-286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


