In response to various hypertrophic stimuli, the heart undergoes alterations in structure and function. One mechanism by which the heart adapts to physiologic and pathologic stimuli is an increase in size of individual cardiac myocytes and when those stimuli are removed, the size of the myocytes can decrease. Modulation of cardiac size thus requires a very careful balance between protein synthesis and protein degradation and any imbalance in these systems could be of potential damage to the heart. Increased protein synthesis in hypertrophied hearts is accompanied by “protein quality control” to eliminate aggregated unfolded proteins, but a failure of this monitoring system eventually leads to cardiac dysfunction 1. Heat shock proteins (HSP) have chaperone-like properties that can bind to unfolded proteins and prevent their denaturation and aggregation 2. In recent genome surveys of mice, rats, and humans, small molecular weight heat shock proteins (sHSPs ~15-30kDa) have been identified. CryAB is a member of the sHSPs that is expressed at high levels in cardiomyocytes 2. CryAB has chaperone-like properties and also binds to both desmin and cytoplasmic actin which helps to maintain cytoskeletal integrity. Previous studies have shown that a mutation in CryAB mimics a form of desmin-related cardiomyopathy 3. Overexpressing CryAB in cultured cardiomyocytes and in transgenic mouse hearts protects against ischemia -reperfusion (IR)-induced cell death 4,5; on the other hand, double knockout of the small heat shock proteins CryAB/SHSP2 induces abnormal cardiac growth and defective myocardial relaxation 6.
In this issue of Circulation Research, Kumarapeli et al 7 address and confirm the roles of CryAB in response to a different type of cardiac insult: pressure overload. Additionally, they used neonatal rat cardiac myocytes (NRCM) overexpressing CryAB to determine its effects on agonist stimulation. After 2 weeks of thoracic aortic constriction (TAC) in NTG mice, there were significantly increased levels of CryAB protein with concomitant increases in fetal genes. However, mice with cardiomyocyte-restricted overexpression (~5 fold) of CryAB (TG) displayed 15% less ventricular hypertrophy and attenuated fetal gene expression compared to NTG mice after 2 weeks of TAC. In addition, echocardiography and cardiac catheterization assessments demonstrated that cardiac function indicated by fractional shortening and dP/dtmax were preserved in TG mice after 2 weeks of TAC. Consistent with the intact heart data, CryAB overexpression in cultured NRCM attenuated NE, PE, or ISO-mediated cellular hypertrophy. On the other hand, mice with germ-line ablation of the CryAB/HSPB2 genes (KO) displayed concentric cardiac hypertrophy after 2 weeks of TAC with greater increases in fetal gene expression. Although LV systolic function and contractile function did not differ among NTG, TG, and KO mice at 10 weeks, LV diastolic function was compromised in the KO mice, which was hypothesized to be due to a decrease in phospholamban expression. Compared to NTG mice, KO mice displayed both systolic and diastolic dysfunction with greater increase in fetal genes at 18weeks of age under basal conditions. Thus, CryAB and HSPB2 are required to maintain normal cardiac function in both basal conditions as well as in response to a general stress 8.
Another issue addressed by this paper is the connection between CryAB and the calcineurin/NFAT pathway, long implicated in pathologic cardiac hypertrophy. Genetic manipulation of signaling pathways in mice and biochemical analyses have shown that calcium/calmodulin (Ca2+/CaM)-dependent signaling plays a pivotal role in pathological cardiac hypertrophy 9. An increase in cytoplasmic Ca2+ binds to CaM and activates calcineurin which can dephosphorylates the nuclear factors of activated T-cells (NFAT). Dephosphorylated NFATs migrate into the nucleus and promotes gene expression. Recent work delineates several negative regulators of calcineurin pathway. The z-disc-protein calsarcin-1 (CS1) prevents cardiomyocyte hypertrophy in response to several Gq-coupled agonists such as angiotensin-II, phenylephrine, and endothelin-1. Overexpression of CS1 results in suppression of cardiac hypertrophy in response to Gq agonist stimulation without impairment of contractile function. Deficiency of CS1 sensitizes mouse hearts for calcineurin signaling and thereby exacerbates pathological cardiac hypertrophy 10. However, calsarcin-deficient mice subjected to exercise exhibited no differential hypertrophic growth 11. Similar results were reported in hearts from forkhead box transcription factors, O subfamily (Foxo)3-null mice that exhibit increased modulatory calcineurin interacting protein (MCIP)1.4, a direct downstream target of the calcineurin/NFAT pathway, and a hypertrophic phenotype with normal systolic function at baseline 12. These results suggest that inhibition of the hypertrophic response is not necessarily associated with a decrease in cardiac function.
Both in vivo and in vitro models were used by Kumarapeli et al 7 to define the role of CryAB in the calcineurin/NFAT pathway. The MCIP1.4 isoform has been shown to be tightly controlled by an alternative promoter containing 15 NFAT binding sites in the intron located upstream of MCIP1 exon 4 13 and has been used as a direct downstream target of the calcineurin/NFAT pathway or a calcineurin-sensitive gene in cultured NRCMs. In KO mice, molecular analyses revealed that MCIP1.4 mRNA expression was significantly increased and western blotting showed that the NFAT nuclear to cytoplasmic ratio was increased compared to WT mice. These results demonstrate that the absence of CryAB sensitizes the heart to calcineurin signaling. Moreover, the combination of CryAB overexpression and cyclosporine A (CsA) treatment in NRCM did not have additive effects. Thus, the cardiac hypertrophic suppression by CryAB is likely through the same pathway as CsA. In contrast, the expression of MCIP1.4 was significantly reduced in TG mice. These results suggest that CryAB attenuates cardiac hypertrophy by inhibiting the calcineurin-NFAT signaling pathways.
This study addresses the critical role of CryAB in the early phase of the cardiac hypertrophic response to pressure overload. A schematic representation of the role of Hsp is illustrated in Figure 1. Given that TG mice attenuate hypertrophy, fetal gene expression, and maintain cardiac function, it is tempting to speculate that CryAB is a critical molecule to distinguish early hypertrophic responses from later, more pathological hypertrophic responses. Supporting this view, the heat shock transcription factor 1 (HSF1) and HSP genes, such as Hsp70 and Hsp27, were elevated after 8 weeks of exercise training 14 and in the early phase of pressure overload 15, but not after 5 weeks of pressure overload 14. In addition, recent work suggests that the IGF-1/PI3K pathway, the well established physiological cardiac hypertrophic pathway 16, is involved in HSF1 activation 17. For example, a member of this pathway, GSK-3β, is a negative regulator of HSF1 and phosphorylation of GSK3β by Akt activates HSF1 and Hsp70 17. Moreover, a recent study showed that increased levels of Hsp27 by lipopolysaccharide treatment in the myocardium was attenuated by the PI3K inhibitor, LY294002, and an increased phosphorylation of GSK3β was correlated with reduced cardiac infarct size 18. In the Kumarapeli et al 7 study, NTG mice had not yet shown significant decreases in the parameters of major LV function and the levels of CryAB protein were elevated at 2 weeks after TAC. These results imply that the heart is still in an adaptive phase of cardiac hypertrophy rather than maladaptive hypertrophy. Thus, it would be interesting to see whether CryAB levels change after long term pressure overload, or in a maladaptive phase. Further, it would be of interest to determine the exercise responsiveness of the hearts from the animals described in Kumarapeli et al 7.
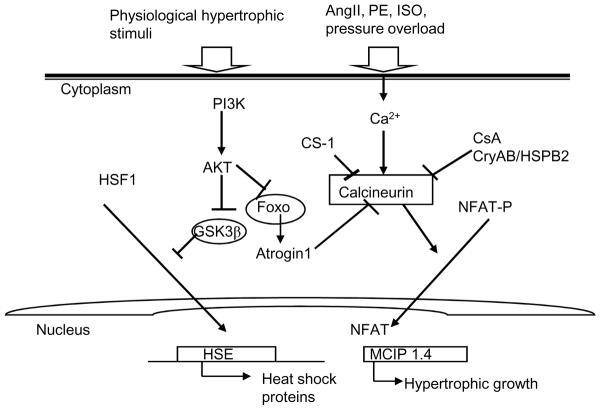
Figure 1.
A schematic representation of the role of Hsps in cardiac hypertrophy. Activation of hsp gene expression is highly conserved and requires the conversion of HSF1 monomers to form homotrimers, which bind to specific recognition motifs, termed heat shock elements (i.e. inverted repeats of nGAAn), located within promoter regions of target genes, and activates transcription of HSPs 19. Pressure overload or neurohormonal activation triggers calcineurin/NFAT signaling pathways. Activation of CryAB inhibits cardiac hypertrophy, at least in part, by blunting calcineurin/NFAT signaling pathways. CS-1, calsarcin-1; CsA, cyclosporine A; CryAB, alphaB crystalline; Foxo, Forkhead box transcription factor; GSK-3β, glycogen synthase kinase 3β; HSE, heat shock element; HSF1, heat shock transcription factor1; NFAT, nuclear factor of activated T-cells.
Acknowledgments
Sources of Funding This work was supported by NIH grant to L.A. Leinwand (HL050560-14).
Footnotes
Disclosures: None
References
- 1.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–28. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol. 2005;38:433–44. doi: 10.1016/j.yjmcc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K-i, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in {alpha}-B-crystallin desmin-related cardiomyopathy. Circulation. 2005;112:3451–61. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison LE, Hoover HE, Thuerauf DJ, Glembotski C, C Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92:203–11. doi: 10.1161/01.res.0000052989.83995.a5. [DOI] [PubMed] [Google Scholar]
- 5.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgenic overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apotosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 6.Kadono T, Zhang XQ, Srinivasan S, Ishida H, Barry WH, Benjamin IJ. CRYAB and HSPB2 deficiency increases myocyte mitochondrial permeability transition and mitochondrial calcium uptake. J Mol Cell Cardiol. 2006;40:783–9. doi: 10.1016/j.yjmcc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Kumarapeli ARK, Su H, Huang W, Tang M, Zheng H, Horak KM, Li M, Wang X. alphaB-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008:xxx–xxx. doi: 10.1161/CIRCRESAHA.108.180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinz I, Robbins J, Rajasekaran NS, Benjamin IJ, Ingwall JS. Unmasking different mechanical and energetic roles for the small heat shock proteins CryAB and HSPB2 using genetically modified mouse hearts. FASEB J. 2008;22:84–92. doi: 10.1096/fj.07-8130com. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins BJ, Dai Y-S, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–8. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 10.Frank D, Kuhn C, van Eickels M, Gehring D, Hanselmann C, Lippl S, Will R, Katus HA, Frey N. Calsarcin-1 protects against angiotensin-II induced cardiac hypertrophy. Circulation. 2007;116:2587–96. doi: 10.1161/CIRCULATIONAHA.107.711317. [DOI] [PubMed] [Google Scholar]
- 11.Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004;10:1336–43. doi: 10.1038/nm1132. [DOI] [PubMed] [Google Scholar]
- 12.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, LU G, MOrris DJ, Castrillon DH, Gerard RD, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–68. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–8. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto M, Minamino T, Toko H, Kayama Y, Zou Y, Sano M, Takaki E, Aoyagi T, Tojo K, Tajima N, Nakai A, Aburatani H, Komuro I. Upregulation of heat shock transcription factor 1 plays a critical role in adaptive cardiac hypertrophy. Circ Res. 2006;99:1411–8. doi: 10.1161/01.RES.0000252345.80198.97. [DOI] [PubMed] [Google Scholar]
- 15.Nishizawa J, Nakai A, Komeda M, Ban T, Nagata K. Increased preload directly induces the activation of heat shock transcription factor 1 in the left ventricular overloaded heart. Cardiovasc Res. 2002;55:341–8. doi: 10.1016/s0008-6363(02)00404-2. [DOI] [PubMed] [Google Scholar]
- 16.McMullen JR, Shioi T, Huang W-Y, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The iInsulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110{alpha}) pathway. J Biol Chem. 2004;279:4782–93. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 17.Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J Neurochem. 2000;75:2401–8. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- 18.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–53. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 19.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]