Abstract
Background
The association of particulate matter (PM) with cardiovascular morbidity and mortality is well documented. PM-induced ischemia is considered a potential mechanism linking PM to adverse cardiovascular outcomes.
Methods and Results
In a repeated-measures study including 5,979 observations on 48 patients aged 43–75 years, we investigated associations of ambient pollution with ST-segment level changes averaged over half-hour periods, measured in the modified V5 position by 24-hr Holter electrocardiogram monitoring. Each patient was observed up to 4 times within one year after a percutaneous intervention for myocardial infarction, acute coronary syndrome without infarction, or stable coronary artery disease without acute coronary syndrome. Elevation in fine particles (PM2.5) and black carbon (BC) levels predicted depression of half-hour averaged ST-segment levels. An interquartile increase in the previous 24-h mean BC level was associated with a 1.50-fold increased in risk of ST-segment depression ≥0.1 mm (95% CI: 1.19, 1.89) and a −0.031 mm (95% CI: −0.042, −0.019) decrease in half-hour averaged ST-segment level (continuous outcome). Effects were greatest within the first month after hospitalization, and for patients with myocardial infarction during hospitalization or with diabetes.
Conclusions
ST-segment depression is associated with increased exposure to PM2.5 and BC in cardiac patients. The risk of pollution-associated ST-segment depression may be greatest in those with myocardial injury in the first month after the cardiac event.
Keywords: air pollution, particles, cardiovascular diseases, myocardial infarction, ST-segment depression
Traffic exposure has been documented as a trigger for myocardial infarction,1 but electrophysiologic evidence linking elevated ambient pollution to either myocardial injury or ischemia is limited. A recent chamber study of 20 men with prior myocardial infarction and stable coronary artery disease demonstrated increased ST-segment depression in response to exercise during concomitant controlled exposure to dilute diesel exhaust.2 Associations of increased ambient particle mass with ST-segment depression have been demonstrated in two additional panel studies: one of 45 non-hospitalized adults from Helsinki, Finland with stable coronary heart disease;3 and another of 24 free-living elderly adults from Boston, Massachusetts.4 We hypothesized that pollution influenced ST-segment level in patients with documented coronary artery disease after hospitalization and percutaneous coronary intervention for either an acute coronary syndrome or chronic stable coronary artery disease with worsening symptoms. We further investigated whether myocardial injury increased vulnerability to pollution effects and whether patients were most at risk for pollution effects in the first month after hospitalization.
Methods
Study participants and design
Prior to discharge we recruited a panel of patients with documented coronary artery disease from the greater Boston area (within route 495) who had undergone percutaneous coronary intervention for an acute coronary syndrome (acute myocardial infarction or unstable angina pectoris) or for worsening stable coronary artery disease. We excluded those with atrial fibrillation and left bundle branch block(LBBB) because of the intent to evaluate heart rate variability as well as ST-segment depression as an outcome. Coronary artery bypass graft surgery within the last 3 months was an exclusion criterion because the non-specific ST and T wave changes post-operatively would preclude accurate interpretation of new ST and T wave changes. Patients with psychiatric illness or drug abuse problems were excluded because of compliance and reliability issues. Active cigarette smoking was an exclusion criterion at entry to the study, and no participant reported current smoking at the time of recruitment. While we did have 4 subjects who reported recidivist smoking, each reported smoking only at one visit, and only 1 reported more than 0–1 cigarettes per day at that visit (Table 1). The protocol included a home visit within 2 to 4 weeks after hospital discharge, followed by three additional follow-up visits at approximately 3-month intervals. At the first visit a baseline screening questionnaire was administered regarding medications, pulmonary and cardiac symptoms, and smoking history. Twenty-four-hour 3-lead Holter ECG monitoring (Marquette Seer Digital Recorder, Marquette Inc., Milwaukee, WI) was also performed with electrodes in modified V5 and aVF positions. For subsequent visits, participants were administered a brief questionnaire regarding cardiac and respiratory symptoms and medication use, and then received 24-hr Holter monitoring. The study design was reviewed and approved by the human subjects committees of the Brigham and Women’s Hospital and the Harvard School of Public Health. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Table 1.
Participant characteristics
| Characteristic | N (%)* |
|---|---|
| Sex, N (%) | |
| Female | 9 (19) |
| Male | 39 (81) |
| Age, years | |
| Median | 57 |
| Range | 43–75 |
| Race, N (%) | |
| Black | 1 (2) |
| White | 45 (94) |
| Asian and other | 2 (4) |
| Discharge diagnosis, N (%) | |
| Myocardial infarction (MI) | 19 (40) |
| Unstable angina pectoris | 19 (40) |
| Worsening stable coronary artery disease | 10 (20) |
| Visits, N (%) | |
| 1 | 47 (37) |
| 2 | 35 (27) |
| 3 | 25 (20) |
| 4 | 21 (16) |
| Total | 128 (100) |
| Smoking, N (%) | |
| Never | 17 (35) |
| Former | 27 (56) |
| Current | 4 (8) |
| Medication use, N (%) | |
| β blocker | 45 (94) |
| Calcium channel blocker | 8 (17) |
| Angiotensin-converting enzyme inhibitor | 27 (56) |
| Statin | 45 (94) |
| Aspirin | 48 (100) |
| Ever had myocardial infarction, N (%) | |
| Yes | 29 (60) |
| No | 19 (40) |
| Diabetes, N (%) | |
| Yes | 12 (25) |
| No | 36 (75) |
| Ischemia episodes after hospitalization, N (%)† | |
| Yes | 13 (27) |
| No | 35 (73) |
| ST-segment level, modified V5 lead, mm | |
| Median ‡ | −0.11 |
| Range | −3.65-2.85 |
Percentages may not add up to 100 because of rounding.
13 participants with 38 episodes
Based on 5,979 observations of half-hour averaged ST-segment levels on the 48 participants in analyses.
Processing of Holter recordings
As described in a previous study (4), the digital Holter recordings were downloaded to a MARS Ultra 60 playback system (Marquette Inc.) for analysis. ST-segments were evaluated for the average value for each half-hour interval and for ischemia episode. Recordings were visually scanned by an experienced analyst to censor artifacts. Custom algorithms were created to calculate the average value referenced to the P-R isoelectric values for each segment. Each participant obtained approximately 48 successful half-hour averaged ST-segments per visit during 24-hr Holter monitoring period for further data analyses. Separately, each ischemia episode was evaluated by using real-time ECG strips examined by an experienced analyst and physician blinded to air pollution status. A table of J-point values, ST-segment values, ST-segment slope, and heart rate was printed for each candidate episode beginning 10 min before each episode and ending 10 min after the resolution of each episode. The ST-segment value 60 msec after the J-point was used to define the ST-segment value. An episode of ischemia was defined as ≥1 mm horizontal or downsloping ST-segment depression compared to the resting baseline, lasting ≥1 minute, and separated by ≥ 5 minutes from other episodes.
Environmental data
Ambient PM2.5 and BC were collected at an ambient monitor site operated by the Harvard School of Public Health, which was a median distance of 17.6 kilometers from participant homes. Hourly measurements of carbon monoxide (CO), ozone (O3), nitrogen dioxide (NO2), and sulfur dioxide (SO2) were obtained from state monitoring sites in Boston, taking the mean of site values for each gas. There were five sites for NO2 and SO2 measurement; four sites were for CO measurement; and three sites for O3 measurement. Continuous PM2.5 was measured with a tapered element oscillating microbalance (TEOM; model 1400A; Rupprechtand Patashnick, Albany, NY). The TEOM sample filter is heated to 50°C, leading to season-specific temperature-related loss of semivolatile mass. Season-specific calibration factors were used to correct for the losses of mass.5 The calibration factors were obtained by regressing continuous PM2.5 concentrations averaged over 24-hr periods on the corresponding collocated integrated 24-hr Harvard Impactor (Air Diagnostics Environmental Inc., Harrison, ME, USA) low volume Teflon filter gravimetric measurements. BC was measured with a model AE-14 aethalometer (Magee Scientific Inc., Berkeley, CA). Hourly temperature was obtained from the National Weather Service First Order Station at Logan Airport.
Statistical analyses
We applied linear additive models6 to analyze the association between half-hour averaged ST-segment levels and air pollutants, including PM2.5, BC, CO, O3, NO2 and SO2 at previous 1-to 6-, 12-, 24 -, 48-, 72-hr means in single-pollutant models. The moving average was computed only if 75% of the data were present. Untransformed ST-segment levels were used for analyses because this outcome was normally distributed.7 Each regression model included fixed effects for participant, day of the week, and visit order. The models also adjusted for several smooth terms as fit by penalized cubic regression splines to reflect possible nonlinear effects of several continuous covariates. These terms included: visit date, hour of the day, and apparent temperature.8 The smooth term of hour of the day accounts for serial autocorrelation among measurements taken on the same day, above that explained by the subject-specific intercepts in the model. Autocorrelation plots of the residuals were checked to see whether this term sufficiently accounted for autocorrelation in the data, and results suggested it did. Apparent temperature was calculated as: −2.653 + (0.994×Ta) + (0.0153×Td2), where Ta is the air temperature and Td is the dew point. To eliminate concurvity or correlation/collinearity between the penalized splines of date and apparent temperature, apparent temperature was regressed against date, with the residuals from this model included in all final models. All results are scaled to the interquartile increase in pollutant level, for the appropriate cumulative average.
In secondary analyses, the additive mixed logistic regression models were used to estimate associations between the probability of ST-segment depression ≥ 0.1 mm and pollution. These analyses, which fall within the generalized additive mixed model (GAMM)9 framework, contained the same terms as the linear models, with the exception that the participant-specific terms were treated as random effects. Effect modification by medical diagnosis, visit (the first visit versus the rest of the visits), and daytime (0900-2200 hr) versus nighttime (2300-0800 hr) were assessed in separate linear additive models and additive mixed logistic regression models by including interaction terms between air pollution effects and each potential effect modifier. We also evaluated the sensitivity of the results to the potential effects of drop-out by estimating the effect modification by visit after excluding participants having only one visit. Finally, multi-pollutant as well as single pollutant analyses were performed when less tightly correlated pollutants potentially representing different sources or components could be jointly entered into the model. All statistical analyses were performed using R statistical software version 2.4.1. Estimates of the effects of air pollutants were scaled to interquartile range (IQR), the difference between the 25th and the 75th percentile, in levels for the appropriate hour mean of air pollutants.
Results
Forty-eight participants were recruited for the study and 128 visits with 5,979 half-hour observations were included in analyses. Of the 48 participants, 35 (Table 1) had more than one visit. The median age of the population was 57 years; 81% of the participants were male. Nineteen (40%) of the participants had a discharge diagnosis of myocardial infarction, thirteen (27%) had ischemic episodes during the 24-h Holter periods after hospitalization and twelve (25%) had diabetes. Most participants took beta-blockers and aspirin (Table 1). Ambient air pollution and temperature levels are summarized in Table 2. The mean levels for U.S. EPA criteria pollutants at 24-hr averaging time were all below accepted or proposed National Air Quality Standards (Table 2).10
Table 2.
Ambient air pollution and temperature levels during holter monitoring (5,979 valid measurements)
| 25th percentile | 50th percentile | 75th percentile | Maximum | |
|---|---|---|---|---|
| Ambient air pollution | ||||
| PM2.5 (µg/m3) | ||||
| 12-hr mean | 6.18 | 8.91 | 13.18 | 37.13 |
| 24-hr mean | 6.38 | 9.20 | 13.31 | 40.38 |
| BC (µg/m3) | ||||
| 12-hr mean | 0.49 | 0.75 | 1.04 | 3.50 |
| 24-hr mean | 0.54 | 0.79 | 1.01 | 2.44 |
| CO (ppm) | ||||
| 12-hr mean | 0.35 | 0.48 | 0.62 | 1.88 |
| 24-hr mean | 0.37 | 0.46 | 0.62 | 1.56 |
| O3 (ppb) | ||||
| 12-hr mean | 12.69 | 20.78 | 29.23 | 83.60 |
| 24-hr mean | 14.32 | 21.28 | 28.62 | 71.84 |
| NO2 (ppb) | ||||
| 12-hr mean | 17.30 | 21.40 | 25.77 | 46.04 |
| 24-hr mean | 18.02 | 21.40 | 24.94 | 44.45 |
| SO2 (ppb) | ||||
| 12-hr mean | 3.09 | 4.43 | 6.40 | 29.39 |
| 24-hr mean | 3.29 | 4.55 | 6.43 | 23.72 |
| Temperature (°C) | 0.75 | 7.90 | 18.97 | 39.49 |
| 1-hr mean |
Associations of Particulate Air Pollutants with ST-segment Depression
Ambient levels of particulate air pollutants rose early in the morning at 04:00 AM and were at their peak between 07:00 and 08:00 AM, while half-hour mean ST-segment levels fell in the morning at 05:00 AM and were at their lowest between 15:00 and 16:00 PM. Pearson correlations showed moderate correlations between PM2.5 and BC (r=0.56) and low correlations between PM2.5 and O3 (r=0.20), SO2 (r=0.25), or NO2 (0.38) among all 2 × 2 combinations of the 6 air pollutants.
Increases in the mean 1- to 24-hr PM2.5 and BC levels predicted depression of half-hour averaged ST-segment levels (Figure 1). For ST-segment depression as a continuous outcome, the cumulative effect was strongest at 24 hr and waned after 48 hr (Figure 1). This association persisted with adjustment for elevation in half-hour averaged heart rate, which was itself associated with a very small but significant ST-segment depression (Estimated effect = −0.004 mm for an IQR increase in mean half-hour heart rate; 95% CI = −0.005, −0.003). An interquartile increase in 12 - to 24 - hour PM2.5 and BC predicted a small but significant decrease in the mean half-hour heart rate (e.g., −1.03 beats per minute for 12-h BC (95% CI: −1.65, −0.41).
Figure 1.
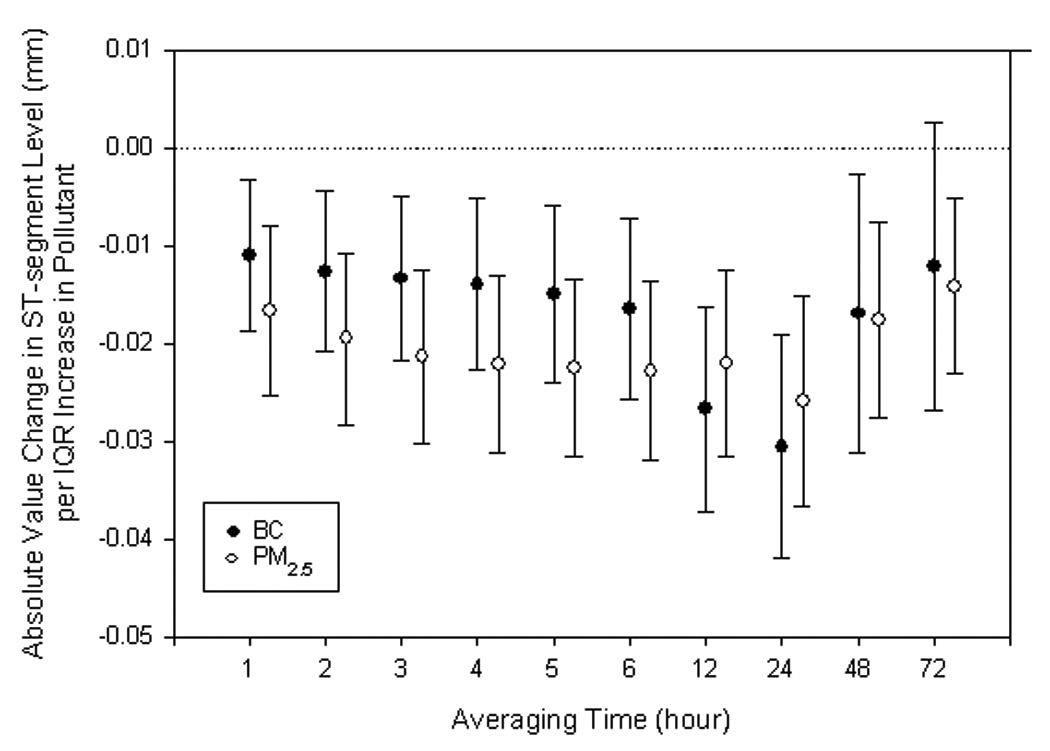
The effects of fine particles (PM2.5) and black carbon (BC) on half-hour averaged ST-segment level estimated by single-pollutant models, scaled to interquartile range (IQR) increase in levels for individual hour mean. Error bars indicate 95% confidence intervals.
Logistic regression analyses showed that increases in PM2.5 and BC were also associated with an increased risk of ST-segment depression ≥ 0.1 mm (Table 3). Cumulative effects were greatest at 48 hours. The estimated risk increased by 1.43-fold per IQR increase in the mean 48-hr PM2.5 (95% CI: 1.19, 1.74) and 1.72-fold per IQR increase in the mean 48-hr BC (95% CI: 1.28, 2.31) (Figure 2).
Table 3.
Ambient pollution as a predictor of ST-segment level.
| Air pollutant | Estimated ST-segment change in mm, for continuous outcome*† |
Estimated relative risk, for ST-segment depression ≥ 0.1 mm*‡ |
||
|---|---|---|---|---|
| 12-hr mean (95% CI) | 24-hr mean (95% CI) | 12-hr (95% CI) | 24-hr (95% CI) | |
| Single-pollutant model | ||||
| PM2.5 (µg/m3) | −0.022 (−0.032, −0.012) | −0.026 (−0.037, −0.015) | 1.02 (0.86, 1.21) | 1.22 (0.99, 1.50) |
| BC (µg/m3) | −0.027 (−0.037, −0.016) | −0.031 (−0.042, −0.019) | 1.13 (0.94, 1.37) | 1.50 (1.19, 1.89) |
| CO (ppm) | 0.013 (0.003, 0.024) | 0.007 (−0.004, 0.019) | 0.70 (0.58, 0.84) | 0.84 (0.68, 1.03) |
| O3 (ppb) | 0.001 (−0.012, 0.014) | 0.004 (−0.01, 0.019) | 0.88 (0.70, 1.09) | 0.59 (0.45, 0.77) |
| NO2 (ppb) | −0.01 (−0.022, 0.002) | −0.029 (−0.041, −0.017) | 1.06 (0.87, 1.29) | 1.51 (1.23, 1.85) |
| SO2 (ppb) | −0.032 (−0.042, −0.023) | −0.033 (−0.043, −0.023) | 1.23 (1.08, 1.41) | 1.41 (1.18, 1.69) |
| Two-pollutant models | ||||
| PM2.5 + NO2 | ||||
| PM2.5 (µg/m3) | −0.023 (−0.034, −0.012) | −0.017 (−0.029, −0.004) | 0.99(0.82, 1.21) | 1.00 (0.80, 1.25) |
| NO2 (ppb) | 0.003 (−0.01, 0.017) | −0.019 (−0.033, −0.006) | 1.06 (0.85, 1.34) | 1.51 (1.19, 1.92) |
| PM2.5 + SO2 | ||||
| PM2.5 (µg/m3) | −0.009 (−0.02, 0.001) | −0.014 (−0.025, −0.002) | 0.87 (0.71, 1.05) | 1.04 (0.83, 1.30) |
| SO2 (ppb) | −0.028 (−0.039, −0.018) | −0.028 (−0.039, −0.017) | 1.30 (1.12, 1.52) | 1.39 (1.14, 1.70) |
| BC + SO2 | ||||
| BC (µg/m3) | −0.013 (−0.024, −0.001) | −0.015 (−0.029, −0.002) | 0.97 (0.78, 1.21) | 1.30 (1.01, 1.68) |
| SO2 (ppb) | −0.027 (−0.038, −0.017) | −0.026 (−0.038, −0.015) | 1.24 (1.06, 1.45) | 1.28 (1.04, 1.57) |
| PM2.5 + BC | ||||
| PM2.5 (µg/m3) | −0.011 (−0.023, 0.001) | −0.012 (−0.026, 0.003) | 0.92 (0.74, 1.14) | 0.87 (0.65, 1.17) |
| BC (µg/m3) | −0.019 (−0.032, −0.006) | −0.022 (−0.038, −0.006) | 1.20 (0.94, 1.52) | 1.68 (1.22, 2.32) |
All effect estimates are scaled to the interquartile increase in pollutant level, for the appropriate cumulative average.
All models adjusting for indicators for participant, day of the week and order of the visit, smooth functions of visit date, hour of day, and hourly temperature.
All models included fixed effects for day of the week and order of the visit, random effects for participant, and smooth functions of visit date, hour of day and hourly temperature.
Figure 2.
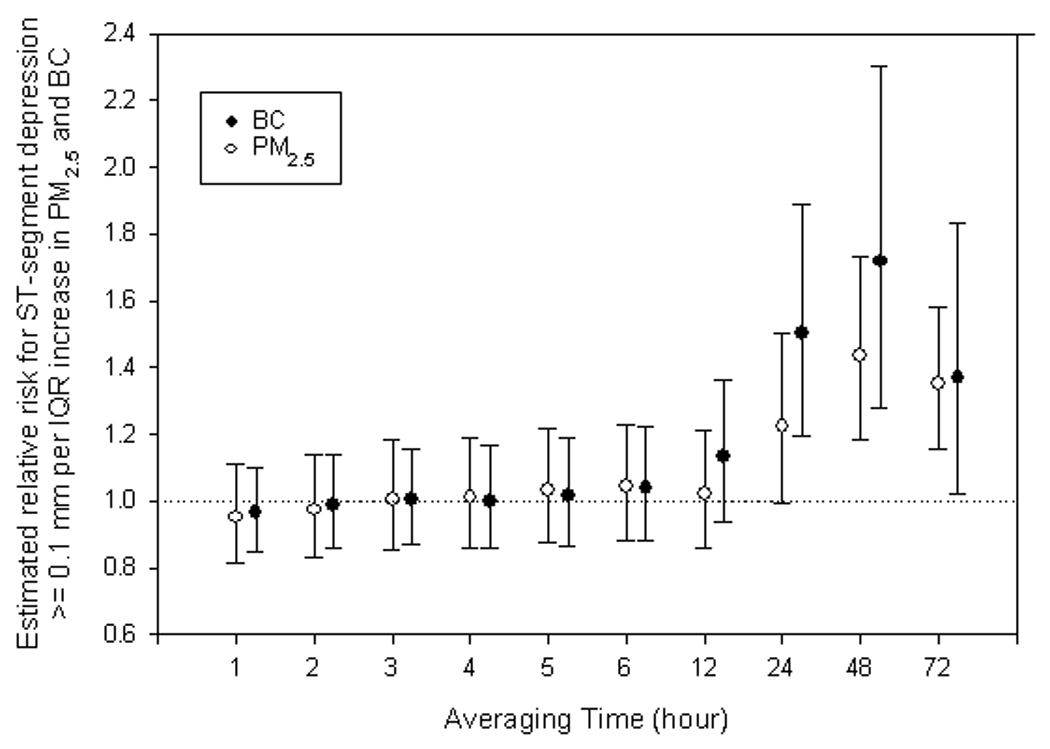
The effects of fine particles (PM2.5) and black carbon (BC) on half-hour averaged ST-segment depression ≥ 0.1 mm below a subject-visit setpoint, as estimated by additive mixed logistic regression models, scaled to interquartile range (IQR) increase in levels for individual hour mean. Error bars indicate 95% confidence intervals.
Associations of Gaseous Air Pollutants with ST-segment Depression
In single pollutant models, elevation in the mean 12-hr and 24-hr NO2 and SO2 levels also predicted depression of half-hour averaged ST-segment levels. No significant association of ST-segment depression was observed with CO and O3. The effect of the traffic marker BC11 predominated in models with both BC and PM2.5 (Table 3). Findings were similar for PM2.5 and NO2, a somewhat less specific marker for traffic. In models with PM2.5 and SO2, effects were divided between the two pollutant measures, neither of which is specific to a particular source (see discussion).12, 13
Effect Modification by Medical Condition and Time since Hospitalization
In models in which 12-h and 24-h average pollution predicted ST-segment level as a continuous outcome, we found effect modification of PM2.5 and BC effect by myocardial infarction discharge diagnosis, time since discharge (visit number), diabetes diagnosis, and diurnal pattern (Table 4). Participants with myocardial infarction discharge diagnosis showed a change of −0.042 mm in ST-segment associated with increased previous 12-hr mean PM2.5, whereas participants without myocardial infarction discharge diagnosis showed a change of −0.012 mm in ST-segment level (interaction P value = 0.002). We also found stronger effects of particulate pollution on participants at the first visit than at the rest of the visits (interaction P value < 0.001). Participants with diabetes (42% of whom had ever had a myocardial infarction; 75% of whom had coronary artery disease diagnoses without current myocardial infarction at the time of enrollment) showed higher response to increased levels of particulate pollution than those without diabetes (interaction P value < 0.001). Participants showed higher response to increased level of PM2.5 effect during daytime. There was no effect modification by personal smoking or ETS. With the exception of the comparison of daytime vs. nighttime, evidence for effect modification in the logistic models was not consistent between pollutants and between cumulative averages. Use of β blocker, calcium channel blocker, statin, or angiotensin-converting enzyme inhibitor medications did not modify the effects of air pollution on ST-segment depression.
Table 4.
Effect modification of association of ST-segment level with ambient pollution*
| PM2.5, µg/m3 | BC, µg/m3 | |||
|---|---|---|---|---|
| 12-hr mean (95% CI) | 24-hr mean (95% CI) | 12-hr mean (95% CI) | 24-hr mean (95% CI) | |
| Myocardial infarction | ||||
| Yes | −0.042 (−0.057, −0.026) | −0.027 (−0.043, −0.012) | −0.041(−0.055, −0.027) | −0.053 (−0.068, −0.038) |
| No | −0.012 (−0.023, 0.00) | −0.025 (−0.038, 0.011) | −0.011 (−0.025, 0.004) | −0.004 (−0.020, 0.012) |
| P value, interaction | 0.002 | 0.787 | 0.002† | <0.001 |
| Visit | ||||
| Visit 1 | −0.102 (−0.12, −0.085) | −0.127 (−0.148, −0.105) | −0.081 (−0.099, −0.064) | −0.111 (−0.132, −0.090) |
| Visits 2–4 | 0.006 (−0.005, 0.017) | 0.001 (−0.011, 0.013) | −0.001 (−0.013, 0.012) | −0.007 (−0.020, 0.005) |
| P value, interaction | <0.001 | <0.001 | <0.001 | <0.001 |
| Diabetes | ||||
| Yes | −0.097 (−0.119, −0.074) | −0.118 (−0.144, −0.091) | −0.034 (−0.061, −0.006) | −0.059 (−0.092, −0.026) |
| No | −0.009 (−0.019, 0.002) | −0.013 (−0.024, −0.002) | −0.032 (−0.044, 0.021) | −0.025 (−0.037, −0.013) |
| P value, interaction | <0.001 | <0.001 | 0.935 | 0.103 |
| Diurnal pattern | ||||
| Daytime (0900-2200 hr) | −0.032 (−0.043, −0.021) | −0.031 (−0.043, −0.020) | −0.028 (−0.039, −0.017) | −0.022 (−0.032, −0.011) |
| Nighttime (2300-0800 hr) | −0.006 (−0.018, 0.006) | −0.018 (−0.030, −0.005) | −0.022 (−0.036, 0.007) | −0.020 (−0.032, −0.007) |
| P value, nteraction | <0.001*** | 0.233 | 0.349 | 0.964 |
All effect estimates are scaled to the interquartile increase in pollutant level, for the appropriate cumulative average. All models adjusting for indicators for participant, day of the week and order of the visit, smooth functions of visit date, hour of day, and hourly temperature.
Discussion
Our study results suggest that patients may be most vulnerable to air pollution ischemic effects in the month after hospitalization for evaluation and treatment of coronary artery disease. Moreover, myocardial injury (infarction) during hospitalization may put patients at greater risk of post-hospitalization pollution-associated ST-segment depression than acutecoronary syndrome or worsening stable coronary disease without myocardial injury. Three other studies found a relationship between elevated ambient pollution and ST-segment depression in the elderly, or in subjects with stable coronary artery disease, but did not study patients in the immediate post-hospitalization period.2–4 Consistent with other studies suggesting that diabetes is a risk factor for air pollution-associated cardiac morbidity,14 patients with diabetes were also at greater risk of developing pollution-related ST-segment depression than patients without diabetes.
Potential mechanisms through which ambient pollution may increase the risk of ischemia in vulnerable patients with coronary artery disease have been reviewed.15, 16 Mechanisms considered include impaired fibrinolytic activity, decreased myocardial oxygen supply related to either vasoconstriction or transient thrombus formation, possibly resulting from systemic or local inflammation,17, 18 oxidative stress,19 endothelial dysfunction,20, 21 and/or autonomic dysfunction.22 Patients with Type 2 diabetes are known to have chronic endothelial vascular dysfunction, chronic systemic inflammation, oxidative stress, and chronic autonomic dysfunction,23–25 all of which may increase the risk of acute pollution effects.
While the Aphea-II European study suggested that SO2 itself plays an independent role in triggering ischemia heart disease admissions,26 at the low levels found in Boston, SO2 is more likely to be a marker for exposure to pollution from a number of sources including diesel exhaust, home heating fuel oil, and regional power plant emissions.12, 13 BC is a more specific surrogate for diesel and non-diesel traffic particle exposure that is either local orregionally transported,11 and the multiple pollutant model including PM2.5 and BC suggested a strong traffic effect. We found no associations of either O3 or CO on ST-segment level. Carbon monoxide, known to have ischemic effects on the risk of arrhythmia at high levels,27 was likely encountered at levels too low for measurable effects in this study, and, given the local nature of CO levels that are above background, imprecision in exposure estimations may have also contributed to the null associations.28 Ozone, which has well documented and reproducible pulmonary effects,29 has not been as reproducibly related to cardiac effects in large epidemiologic studies, even in high O3 level cities like Mexico city.30
While the size of the half-hour pollution-associated decrements in ST-segment levels were small in continuous models, we also found pollution effects on the risk of ST-segment depression ≥ 0.1 mm. Palinkas and colleagues found changes of similar magnitude measured during stress testing predictive of subsequent increased risk of adverse cardiac events among patients with chest pain syndromes.31 This finding supports the likelihood that the more subtle pollution-related ST-segment depression represents ischemia.
Our study has several limitations. The magnitude of ST-segment depression in these patients was generally small; averaging over half-hour periods likely combined periods of greater and lesser levels of ST-segment depression. It is unknown whether the small, but statistically significant, ST-segment values represent transient myocardial ischemia or transient inflammatory responses to exacerbations in air pollution. Compared to the effect modification results for the continuous outcome analyses, the results for the logistic analyses were less stable and less consistent, perhaps due to loss of information incurred by dichotomizing the primary endpoint. The use of central site pollution measurements for these analyses may have resulted in some misclassification of exposure. This limitation may be more relevant for BC, which has more local traffic sources, than for PM2.5.32 Short-term exposures may be less well estimated than longer term averaged exposures because they may be more influenced by brief local personal exposures that differ from exposures measured at the central site. That may in part account for the somewhat stronger associations with longer (24-h) averaged exposures in the continuous models. It is also true that in the logistic models we cannot discount that the null findings at shorter lags may be due to exposure misclassification. Alternatively, with the logistic models, the evidence for an effect with longer or greater cumulative exposure may suggest that longer/greater cumulative exposure to pollution may be needed for a more extreme (though from a clinical point-of-view still modest) level of ST-segment depression. The sample size is relatively small, limiting the potential to evaluate interactions between participant characteristics and pollutant exposure. In addition, because of the selective nature of the population pool, the generalizability to other patient populations may be limited. However, there is no reason to believe that the internal comparisons within and between subjects are biased.
Our study suggests immediate as well as longer cumulative effects of air pollution on ST-segment depression. While peak cumulative effects of PM2.5 and BC on ST-segment level were at 6 to 48 hours, effects were also seen in relation to the prior 1-to 2-hour averaged pollution levels, which is consistent with the findings of Peters1 and Mills,2 that exposure to traffic can trigger myocardial infarction. However, it is possible that the longer term summary air pollution for 6 to 48 hours simply are more accurate measures of air pollution exposure than the single hour and thus correlate to endpoints better. The time course of effect may remain unclear but at the least we can see rapid effects after exposure to air pollution.
Of the more than 1 million patients who suffer a myocardial infarction (MI) in the United States each year, one quarter to one third of survivors will die within 12 months, and a significant proportion will experience reinfarction or sudden death over the ensuing years.33 The immediate post-MI period has been well demonstrated to be a period of increased risk of recurrent events, and electrical instability.34 In a double-blind, randomized, crossover study of 20 men with history of myocardial infarction exposed to exercise plus either diesel exhaustor filtered air, diesel pollution was demonstrated to worsen the effects of vigorous exercise on the risk of ST-segment depression.35, 36 Our study suggests that these effects of air pollution on increased risk of ST-segment depression and ischemia may be heightened in the immediate period after an acute coronary event, when risk of ischemia might be reduced by reduction in pollution exposure, including exposure to traffic.
Acknowledgments
Funding Sources
This work was supported in part by NIEHS (National Institute of Environment Health Sciences) P01 ES009825, NIEHS-00002, Environmental Protection Agency R832416-01-0, and National Science Council-095-SAF-I-564-602-TMS.
Footnotes
Disclosures
Drs. Coull and Schwartz have previously received a grant from the American Chemistry Council. Dr. Suh has previously received consultant money for evaluating pollution monitoring methods for the Sierra Club. Drs. Suh, Schwartz, Coull, and Zanobetti have previously received a research grant from the Electric Power Research Institute (EPRI). The other authors report no conflicts.
Clinical Perspective
Of the more than 1 million patients who suffer a myocardial infarction (MI) in the United States each year, one quarter to one third of survivors will die within 12 months, and a significant proportion will experience reinfarction or sudden death over the ensuing years. We evaluated the association of elevated air pollution with ST-segment depression in 48 patients followed after hospitalization for myocardial infarction, acute coronary syndrome without infarction, or stable coronary artery disease without acute coronary syndrome. An interquartile increase in the previous 24-h mean black carbon, a marker for traffic pollution, was associated with a 1.50-fold increased in risk of ST-segment depression ≥0.1 mm (95% CI: 1.19, 1.89) and a −0.031 mm (95% CI: −0.042, −0.019) decrease in half-hour averaged ST-segment level (continuous outcome). Effects were greatest within the first month after hospitalization and for patients with myocardial infarction during hospitalization or with diabetes. Our study suggests that the effects of air pollution on increased risk of ST-segment depression and ischemia may be heightened in the immediate period after an acute coronary event, when risk of ischemia might be reduced by reduction in pollution exposure, including exposure to traffic.
References
- 1.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 2.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 3.Pekkanen J, Peters A, Hoek G, Tiittanen P, Brunekreef B, de Hartog J, Heinrich J, Ibald-Mulli A, Kreyling WG, Lanki T, Timonen KL, Vanninen E. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation. 2002;106:933–938. doi: 10.1161/01.cir.0000027561.41736.3c. [DOI] [PubMed] [Google Scholar]
- 4.Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, Verrier RL, Nearing BD, Canner MJ, Suh H, Stone PH. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect. 2005;113:883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen G, Sioutas C, Koutrakis P, Reiss R, Lurmann FW, Roberts PT. Evaluation of the TEOM method for measurement of ambient particulate mass in urban areas. J Air Waste Manage Assoc. 1997;47:682–689. doi: 10.1080/10473289.1997.10463923. [DOI] [PubMed] [Google Scholar]
- 6.Hastie T, Tibshirani R. Generalized additive models. London: Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro SW, MB An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 8.Schwartz J. Nonparametric smoothing in the analysis of air polution and respiratory illness. Canadian J Stat. 1994;22:471–487. [Google Scholar]
- 9.Dominici F, McDermott A, Zeger SL, Samet JM. On the use of generalized additive models in time-series studies of air pollution and health. Am J Epidem. 2002;156:193–203. doi: 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- 10.National Ambient Air Quality Standards (NAAQS) Vol 2007. U.S. Environmental Protection Agency; 2007. [Google Scholar]
- 11.Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, Nearing B, Verrier R, Stone P, MacCallum G, Speizer FE, Gold DR. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J Air Waste Mgt Assoc. 2000;50:1184–1198. doi: 10.1080/10473289.2000.10464165. [DOI] [PubMed] [Google Scholar]
- 13.Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect. 2001;109:1053–1061. doi: 10.1289/ehp.011091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanobetti A, Schwartz J. Are diabetics more susceptible to the health effects of airborne particles? Am J Respir Crit Care Med. 2001;164:831–833. doi: 10.1164/ajrccm.164.5.2012039. [DOI] [PubMed] [Google Scholar]
- 15.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 16.Mittleman MA, Verrier RL. Air pollution: small particles, big problems? Epidemiology. 2003;14:512–513. doi: 10.1097/01.ede.0000081995.47783.74. [DOI] [PubMed] [Google Scholar]
- 17.Ruckerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, Heinrich J, Marder V, Frampton M, Wichmann HE, Peters A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 18.Dubowsky S, Gold DR, Schwartz J, Coull B, Suh H. Air pollution and inflammatory markers in blood. Epidemiology. 2004;14:S23. [Google Scholar]
- 19.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 21.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 22.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 23.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 24.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 25.Jacob G, Costa F, Biaggioni I. Spectrum of autonomic cardiovascular neuropathy in diabetes. Diabetes Care. 2003;26:2174–2180. doi: 10.2337/diacare.26.7.2174. [DOI] [PubMed] [Google Scholar]
- 26.Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, Rossi G, Zmirou D, Ballester F, Boumghar A, Anderson HR, Wojtyniak B, Paldy A, Braunstein R, Pekkanen J, Schindler C, Schwartz J. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Effects of carbon monoxide on myocardial ischemia. Environ Health Perspect. 1991;91:89–132. doi: 10.1289/ehp.919189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold DR, Damokosh AI, Pope CA, 3rd, Dockery DW, McDonnell WF, Serrano P, Retama A, Castillejos M. Particulate and ozone pollutant effects on the respiratory function of children in southwest Mexico City. Epidemiology. 1999;10:8–16. [PubMed] [Google Scholar]
- 30.Borja-Aburto VH, Castillejos M, Gold DR, Bierwinski S, Loomis D. Mortality and ambient fine particles in Southwest Mexico City, 1993–1995. Environ Health Perspect. 1998;106:849–855. doi: 10.1289/ehp.106-1533229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palinkas A, Toth E, Amyot R, Rigo F, Venneri L, Picano E. The value of ECG and echocardiography during stress testing for identifying systemic endothelial dysfunction and epicardial artery stenosis. Eur Heart J. 2002;23:1587–1595. doi: 10.1053/euhj.2002.3170. [DOI] [PubMed] [Google Scholar]
- 32.Rojas-Bracho L, Suh HH, Koutrakis P. Relationships among personal, indoor, and outdoor fine and coarse particle concentrations for individuals with COPD. J Expo Anal Environ Epidem. 2000;10:294–306. doi: 10.1038/sj.jea.7500092. [DOI] [PubMed] [Google Scholar]
- 33.American Heart Association, Heart Disease and STroke Statistics. Dallas, TX: American Heart Association; 2005. [Google Scholar]
- 34.Gheorghiade M, Fonarow GC. Management of post-myocardial infarction patients with left ventricular systolic dysfunction. Am J Med. 2007;120:109–120. doi: 10.1016/j.amjmed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Miller K, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, JD K. Long-term exposure to air pollution and incidence of cardiovascular events in women. New England Journal of Medicine. 2007;356:511–513. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 36.Mittleman MA. Air pollution, exercise, and cardiovascular risk. N Engl J Med. 2007;357:1147–1149. doi: 10.1056/NEJMe078139. [DOI] [PubMed] [Google Scholar]


