Abstract
Pauci-immune focal necrotizing glomerulonephritis (FNGN) is a severe inflammatory disease associated with autoantibodies to neutrophil cytoplasmic antigens (ANCA). Here we characterize autoantibodies to lysosomal membrane protein-2 (LAMP-2) and show that they are a new ANCA subtype present in almost all individuals with FNGN. Consequently, its prevalence is nearly twice that of the classical ANCAs that recognize myeloperoxidase or proteinase-3. Furthermore, antibodies to LAMP-2 cause pauci-immune FNGN when injected into rats, and a monoclonal antibody to human LAMP-2 (H4B4) induces apoptosis of human microvascular endothelium in vitro. The autoantibodies in individuals with pauci-immune FNGN commonly recognize a human LAMP-2 epitope (designated P41–49) with 100% homology to the bacterial adhesin FimH, with which they cross-react. Rats immunized with FimH develop pauci-immune FNGN and also develop antibodies to rat and human LAMP-2. Finally, we show that infections with fimbriated pathogens are common before the onset of FNGN. Thus, FimH-triggered autoimmunity to LAMP-2 provides a previously undescribed clinically relevant molecular mechanism for the development of pauci-immune FNGN.
Pauci-immune crescentic FNGN is an acute inflammatory disease that results in rapid, irreversible kidney failure, typically in the context of a systemic small vessel vasculitis, such as microscopic polyangiitis or Wegener’s granulomatosis1,2. Injury is caused by neutrophils and macrophages that localize in glomerular capillaries without detectable immune deposits. Despite this, pauci-immune FNGN is thought to be an autoimmune disease because of its almost invariable association with ANCA. ANCA were originally described in 1982 (ref. 3) and have proved invaluable for diagnosis and monitoring of disease activity4.
The pathogenicity of ANCA has been examined extensively ever since the soluble lysosomal enzymes myeloperoxidase and proteinase-3 were identified as targets2,5. Antibodies to these enzymes activate primed neutrophils and cause neutrophil-dependent endothelial injury in vitro2, and administration of antibodies to myeloperoxidase provokes FNGN in rodents6, especially when injected together with lipopolysaccharide (LPS)7; antibodies to proteinase-3 are less effective8,9. However, individuals with FNGN have other autoantibodies as well—for example, autoantibodies to the extracellular domain of human LAMP-2 (ref. 10)—but their role in pathogenesis role is unknown.
LAMP-2 is a heavily glycosylated type 1 membrane protein11. In humans, three splice variants are known (LAMP-2A, LAMP-2B and LAMP-2C), all with identical extracellular domains12. LAMP-2 has established roles in adhesion13 and cellular homeostasis, including autophagocytosis14 and antigen presentation15. In neutrophils, LAMP-2 is integrated into the membranes of myeloperoxidase- and proteinase-3–containing intracellular vesicles, and thus autoantibodies to human LAMP-2 give positive results in fluorescence ANCA assays. In contrast to myeloperoxidase and proteinase-3, human LAMP-2 shuttles between lysosomes and the cell membrane16, is abundant on neutrophil and endothelial cell surfaces and is thus directly accessible to circulating antibodies.
It is not known what provokes autoantibody synthesis in pauci-immune FNGN, but bacterial infections may be involved, as first postulated by Wegener in his original 1936 report of granulomatous ‘septic’ vasculitis17, an idea subsequently confirmed by others18,19. Three explanations have been invoked20,21: infection-induced systemic activation of granulocytes, induction of autoantibodies by bacterial superantigens and molecular mimicry between microbial antigens and host proteins. However, there are no known sequence homologies between bacterial proteins and myeloperoxidase and proteinase-3, except for a complex homology with a complementary sequence of proteinase-3 (ref. 22). Similar analyses have not been applied to human LAMP-2.
Here we establish that autoantibodies to human LAMP-2 are highly prevalent in pauci-immune FNGN and provide evidence of their pathogenicity by showing that they activate neutrophils and kill human blood microvascular endothelium in vitro and cause pauci-immune FNGN when administered to rodents. Unexpectedly, auto-antibodies to LAMP-2 in individuals with FNGN commonly recognize an epitope with considerable homology to the bacterial adhesin FimH and cross-react with it. We therefore determined whether exposure to FimH could induce antibodies to human LAMP-2 and initiate pauci-immune FNGN through molecular mimicry. The results lead us to propose a previously undescribed molecular mechanism both for the induction and development of injury in this human disease.
RESULTS
Autoantibodies to human LAMP-2 are common in FNGN
We established the prevalence of autoantibodies to hLAMP-2 in sera from 84 individuals with biopsy-proven active pauci-immune FNGN, either at presentation (n = 62) or during relapse (n = 22). ANCA were detectable by standard immunofluorescence assays in 80 of them (95%), and ELISA for the canonical ANCA were positive in 70 of them (83%); myeloperoxidase-specific ANCA were found in 38 people, and proteinase-3–specific ANCA were found in 39 people, including seven with antibodies to both antigens. Using a specific ELISA, we detected antibodies to human LAMP-2 in 78 of the 84 (93%) sera (Fig. 1a), and we validated the results by western blotting and indirect immunofluorescence on the O-glycosylation deficient CHO cell line ldlD cells stably expressing human LAMP-2 on their surface (Supplementary Fig. 1a online). Notably, the human LAMP-2 ELISA was negative in all but six of the individuals when they were in remission after immunosuppressive therapy. Assays for human LAMP-2 were also negative in 53 healthy controls and in all but 1 of 30 ANCA-negative subjects with other types of glomerulonephritis. We also found human LAMP-2–specific antibodies in eight ANCA-positive individuals with FNGN and antibodies to myeloperoxidase in other contexts, including Goodpasture’s disease and systemic lupus erythematosus, whereas assays were only positive in 1 of 20 individuals with localized vasculitis without renal disease, supporting the association of autoantibodies to human LAMP-2 with active inflammation in individuals with pauci-immune FNGN.
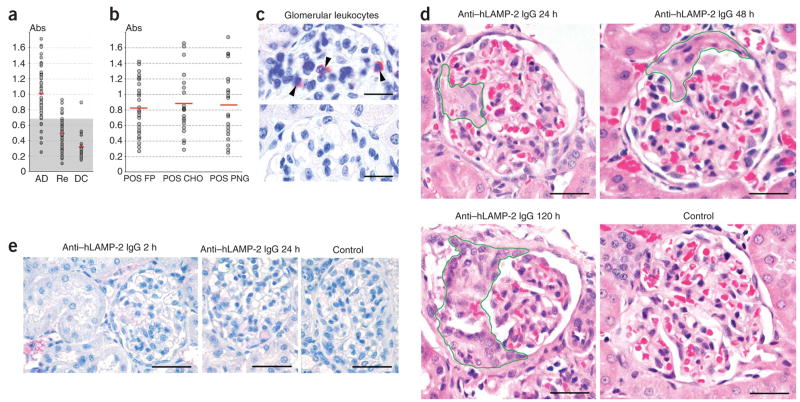
Figure 1.
Antibodies to human LAMP-2 (hLAMP-2) in pauci-immune FNGN. (a) Absorbance values of a specific ELISA to assay autoantibodies to LAMP-2 measured in sera from individuals with pauci-immune FNGN and active disease (AD), from the same individuals after treatment when in remission (Re) or from ANCA-negative disease controls (DC) with other types of glomerulonephritis. The shaded area indicates the 99.5% confidence interval for the normal range. (b) Absorbance values of sera from 32 individuals with autoantibodies to LAMP-2 assayed in three different ELISAs with the following substrates: purified unglycosylated, E. coli–expressed human LAMP-2 fusion protein (POS FP); glycosylated mammalian expressed human LAMP-2 (POS CHO); and PNGaseF-treated mammalian-expressed human LAMP-2 (POS PNG). The mean value for each ELISA is indicated as is the upper limit of normal. (c) Glomerular CD45 positive leukocytes (arrowheads) in WKY rats injected 24 h earlier with 10 mg of rabbit antibodies to LAMP-2 (anti-hLAMP2 IgG, top) or 10 mg normal rabbit IgG (bottom). Scale bar, 25 μm. (d) Morphology of representative glomeruli from WKY rats injected intravenously with IgG to rat LAMP-2. Segmental necrosis is present after 24 h, cellular crescents after 48 h and segmental scarring with fibrocellular crescents and adhesions after 120 h. Scale bars, 25 μm. (e) Direct immunoperoxidase staining of representative glomeruli from WKY rats injected with rabbit IgG to LAMP-2. Small quantities of deposited IgG is seen in blood vessels and occasional circulating leukocytes after 2 h but not at later time points. Scale bar, 50 μm.
Our standard ELISA used recombinant unglycosylated human LAMP-2 expressed in E. coli as substrate, so we developed additional ELISAs to test whether autoantibodies from subjects with FNGN also recognized glycosylated mammalian human LAMP-2. These ELISAs used as substrate either glycosylated human LAMP-2 purified from culture supernatants of CHO DG44 cells expressing a soluble extra-cellular domain or glycosylated human LAMP-2 that had been digested with the N-glycanase PGNaseF to remove N-glycans. The results were compared with those from the standard ELISA with unglycosylated human LAMP-2 prepared in E. coli (characterized in Supplementary Fig. 1b). The autoantibodies bound equally well to glycosylated and unglycosylated human LAMP-2, indicating that the epitopes they recognize are not occluded by glycosylation (Fig. 1b). The results were confirmed by indirect immunofluorescence that showed the autoantibodies bound to human LAMP-2 expressed on the surface of ldlD cells before and after removal of O- and N-glycans (data not shown). Thus, autoantibodies from subjects with FNGN bind epitopes on the protein backbone that remain accessible in fully glycosylated mammalian human LAMP-2 in the plasma membrane.
Injection of IgG antibodies to human LAMP-2 causes FNGN
To determine whether antibodies to LAMP-2 cause injury in vivo, we injected 15 WKY rats intravenously with human LAMP-2–specific rabbit IgG that cross-reacts with rat LAMP-2. All rats developed sustained hematuria quantified by Combur-Test (Roche) according to the manufacturer’s instructions: negative at baseline and at 2 h (n = 2); negative to trace at 24 h (n = 4); 1+ to 2+ at 48 h (n = 5) and 2+ to 3+ at 120 h (n = 4). The urine protein:creatinine ratio increased 25-fold from 0.017 ± 0.019 at baseline to 0.305 ± 0.098 and 0.416 ± 0.14 at 24 h and 120 h, respectively. The treated rats developed severe renal injury with leukocyte infiltration (Fig. 1c). Rats culled after 24 h had focal capillary necrosis in a mean of 22.2% of glomeruli (range 17–25%). Rats culled later had crescents resulting in a mean of 21% of glomeruli after 48 h (range 16–24%) and 18.5% after 120 h (range 6–20%; Fig. 1d). Injected antibodies were rapidly cleared from the circulation, and only minimal deposition of rabbit IgG was detectable in kidneys of rats killed 2 h after injection, whereas there was none at later time points (Fig. 1e and Supplementary Fig. 1c). Normal control rats (n = 8) and rats injected with normal rabbit IgG (n = 4) did not develop hematuria, proteinuria (data not shown) or morphological injury (Fig. 1d, control). Thus, antibodies to LAMP-2 are pathogenic and can cause pauci-immune FNGN.
Antibodies to LAMP-2 activate neutrophils and endothelium
Because ANCAs specific for myeloperoxidase and proteinase-3 activate primed human neutrophils in vitro23,24, we tested whether antibodies to human LAMP-2 do the same. Incubation with H4B4, a monoclonal antibody to human LAMP-2, induced significantly more neutrophil ‘shape change’25 than a monoclonal antibody to CD4; H4B4 (10 μg ml−1) resulted in 20.3% shape changed of neutrophils (range 17.0–22.3%) versus 8.5% (7.1–9.3%) with CD4 (P < 0.05). The results with 20 μg ml−1 of the antibodies were 43.0% (36.9–49.2%) versus 12.5% (10.2–14.1%) respectively (P 0.05). The results for untreated neutrophils was 8.5% (7.1–9.3%). A monoclonal antibody to proteinase-3 (1F11) also induced neutrophil shape change but to a significantly lesser degree (mean 16.5%, range 13.4–21.1% for 10 μg ml−1 and mean 30.6%, range 21.3–36.5% for 20 μg ml−1 ; each with P < 0.05 compared to H4B4). These results were confirmed by staining for actin condensation26 (Fig. 2a–d). H4B4 also induced neutrophil degranulation (Supplementary Fig. 1d). Incubation with 10 μg ml−1 H4B4, 1F11 or CD4 released 83% (range 80–85%), 57% (40–90%) or 10% (0–21%) myeloperoxidase, respectively (expressed as a percentage of myeloperoxidase released by treatment with 10 ng ml−1 tumor necrosis factor-α (TNF-α)). H4B4 and 1F11 treatments were both significantly different from control treatment (P < 0.05).
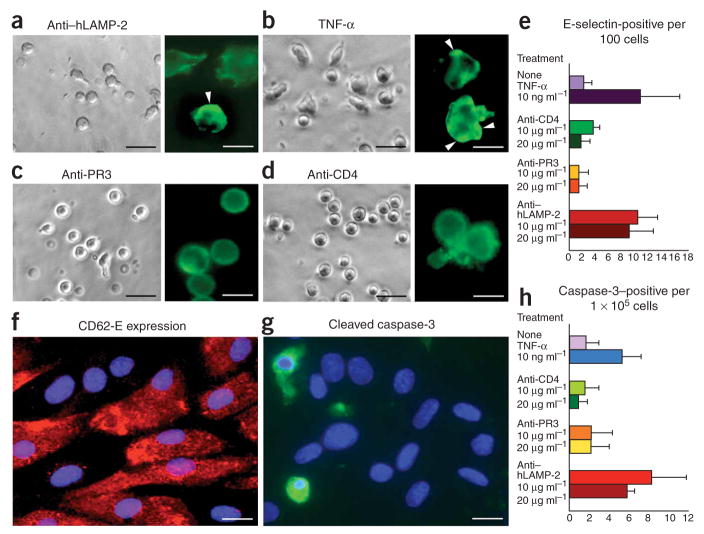
Figure 2.
Antibodies to hLAMP-2 activate neutrophils and kill human microvascular endothelium. (a–d) Purified human neutrophils were incubated with 10 μg ml−1 H4B4, a monoclonal antibody to human LAMP-2 (a); 2 ng ml−1 TNF-α (b), 10 μg ml−1 1F11, a monoclonal antibody to proteinase-3 (c) or 10 μg ml−1 of monoclonal antibody to CD4 (d). H4B4 and TNF induced significantly more shape change than the other two treatments, and the insets confirm that the shape change is associated with actin condensation (insets in a–d; scale bars, 50 μm). When the same antibodies were incubated with purified human dermal microvascular blood endothelial cells (BECs), H4B4 significantly increased CD62E (E-selectin) expression (P < 0.01) whereas 1F11 and CD4 had no effect (e, f). H4B4 also significantly increased the number of cells expressing activated caspase-3 (P < 0.05), a marker of apoptosis (g, h). Scale bars, 50 μm. Values in e and h represent means ± s.d.
We then incubated the same monoclonal antibodies with purified human dermal microvascular blood endothelium27, the principal target of injury in pauci-immune FNGN (Fig. 2e–h). H4B4 significantly increased the number of cells expressing CD62E (E-selectin) (P <0.01) (Fig. 2e,f). It also induced apoptosis, as determined by the proportion of endothelial cells expressing cleaved caspase-3 (Fig. 2g,h), and significantly reduced their number after 20 h of culture (464 ± 195 cells and 366 ± 76 cells per ten high-power fields for 10 μg ml−1 and 20 μg ml−1, respectively) compared to treatment with the antibodies to CD4 (623 ± 89 cells and 680 ± 78 cells for 10 μg ml−1 and 20 μg ml−1, respectively) and proteinase-3 (615 ± 220 cells and 600 ± 10 cells for 10 μg ml−1 and 20 μg ml−1, respectively) (P < 0.05). Thus, antibodies to human LAMP-2 kill microvascular blood endothelium directly without the need for neutrophils or an external source of antigen.
Autoantibodies to LAMP-2 recognize two major epitopes
Using our previously validated SPOTscan analysis28, we characterized the epitopes recognized by autoantibodies from individuals with pauci-immune FNGN and identified two common human LAMP-2 epitopes, P41–49 (HGTVTYNGS) and P331–341 (QGKYSTAQDCS). These were recognized by autoantibodies from nine (82%) and seven (64%) of eleven randomly selected subjects, respectively, and five sera recognized both epitopes (Fig. 3a). Sequential alanine substitution confirmed the specificity of both interactions and identified the single tyrosine residue in each epitope as crucial for strong antibody binding (data not shown). Subsequent post-treatment sera from five of the subjects with pauci-immune FNGN in which antibodies to LAMP-2 were no longer present (LAMP-2–negative sera) did not recognize either epitope, nor did sera from five healthy controls (data not shown).
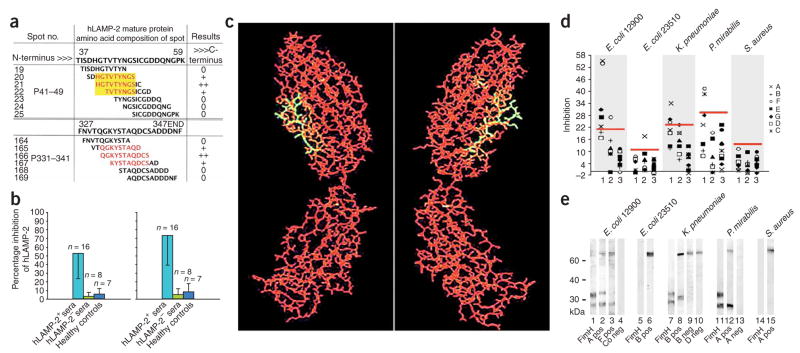
Figure 3.
Autoantibodies to hLAMP-2 cross-react with the bacterial adhesin FimH. (a) Two epitopes, P41–49 (HGTVTYNGS) and P331–341 (QGKYSTAQDCS) recognized by autoantibodies to hLAMP-2 in active FNGN are shown in red. The yellow box illustrates the homologous sequence shared by human LAMP-2 and FimH. Depicted are examples of overlapping peptides synthesized onto a SPOTs membrane aligned to the human LAMP-2 sequence. (b) Inhibition ELISA used to determine whether synthetic peptides corresponding to P41–49 (left) and P331–341 (right) prevented autoantibodies in active pauci-immune FNGN from binding to human LAMP-2. Both peptides significantly inhibited binding (P < 0.001). Values represent means ± s.d. (c) Three-dimensional projection of the structure of the FimH mannose binding pocket. Amino acids 72–80 of FimH, homologous to P41–49 (yellow), are exposed on the surface. (d) ELISA was used to determine whether bacterial lysates from FimH-expressing (E. coli 12900, K. pneumoniae., P. mirabilis) or non-expressing (E. coli 23510, S. aureus) organisms inhibit binding of antibodies to LAMP-2 from subjects (A–G) with active FNGN (lane 1), FNGN in remission (lane 2) or healthy controls (lane 3). Red bars, 99.5% confidence interval for inhibition of binding. (e) Western blot showing binding of antibodies from subjects with FNGN to lysates of bacteria that do (lanes 1–4, 7–10 and 11–13) or do not (lanes 5–6 and 14–15) express FimH. Two sera with specificity for hLAMP-2 (A pos, B pos and F pos) recognize the same bands as an antibody to FimH (fimH), whereas negative sera (A neg, B neg and D neg) and controls (Co neg) do not.
The specificity of the subjects’ antibodies for both human LAMP-2 epitopes was confirmed by competition ELISA. In these assays, a synthetic peptide corresponding to P41–49 specifically inhibited auto-antibody binding to human LAMP-2 by a mean of 53% (range 17–100%) and a peptide corresponding to P331–341 inhibited by a mean of 73% (range 29–100%). Neither peptide affected background binding in ELISAs of human LAMP-2–-negative sera from treated subjects (P41–49 median inhibition 2%, range 0–11%; P331–341 median inhibition 4%, range 0–20%) or healthy controls (P41–49 median inhibition 9%, range 0–13%; P331–341 median inhibition 9%, range 0–18%) (Fig. 3b). The degree of inhibition confirms that P41–49 and P331–341 are the two major epitopes recognized by autoantibodies to human LAMP-2 in FNGN.
LAMP-2 epitope P41–49 is homologous to bacterial FimH
Neither human LAMP-2 epitope had homologies to myeloperoxidase or proteinase-3 or to their complementary sequences (Supplementary Table 1 online). However, the P41–49 epitope has 100% homology with amino acids 72–80 of mature FimH (with eight of the nine amino acids identical), an adhesin located at the tip of type 1 fimbriae and crucial for attachment to host epithelia of Gram-negative pathogens such as Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis (Table 1). The homologous sequence lies on the surface of FimH in the mannose-binding pocket, an essential region for mannose binding and accessible to the host immune system (Fig. 3c). The P331–341 epitope was not homologous to FimH but had looser homologies to other bacterial and viral proteins (Table 1).
Table 1.
Homologies in hLAMP-2 peptides P41–49 and P331–341 to bacterial and viral proteins
| Protein | AA | AA | Species | Accession code or GenBank GI number |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAMP-2 P41–49 | 69 | H | G | T | V | T | Y | N | G | S | 77 | Homo sapiens | gi: 307110 | |
| FimH | 94 | G | T | V | K | Y | N | G | S | 101 | E. coli O18:K1:H7, O2:MT78, LF82 | gi: 10946257 | ||
| Minor fimbrial subunit | 94 | G | T | V | K | Y | S | G | S | 101 | E. coli K12, O157:H7 | NP 418740, AAC35864 | ||
| Fimbrial protein | 26 | G | T | V | T | F | N | G | K | 33 | Yersinia pestis | 16123128, 15980903 | ||
| Fimbrial adhesin | 96 | G | T | V | K | Y | N | G | T | 103 | Klebsiella pneumoniae | AAA25063 | ||
| FimH | 185 | Y | T | I | S | Y | S | G | K | 192 | Salmonella typhimurium | NP 455136 | ||
| Uroepithelial adherence protein | 25 | G | T | I | T | F | T | G | K | 32 | Proteus mirabilis | AAA68979 | ||
| Glucosyltransferase | 401 | G | T | V | T | F | N | G | Q | 407 | Streptococcus mutans | BAB83942 | ||
| ORF 1 | 170 | E | T | V | S | Y | N | G | S | 177 | Staphylococcus warneri | BAB83942 | ||
| Putative ABC transporter | 63 | G | T | I | T | Y | N | G | 69 | Streptomyces coelicolor | gi: 9967662 | |||
| ORF | 171 | T | V | S | Y | N | G | S | 177 | Staphylococcus warnerii | gi: 7804481 | |||
| Putative outer membrane protein | 593 | T | V | T | Y | N | G | S | 599 | E. coli CFT073 | gi: 26248725 | |||
| Outer membrane protein | 261 | V | T | Y | N | G | S | 266 | Bacteroides thetaiotaomicron | gi: 1100065 | ||||
| Myxobacterial hemagglutinin | 183 | G | T | M | T | Y | N | G | 189 | Myxococcus xanthus | gi: 126790 | |||
| Envelope glycoprotein | 139 | G | T | V | T | Y | N | 144 | HIV type 1 | gi: 17046905 | ||||
| Virion protein | 6 | H | S | T | V | S | Y | N | G | 13 | Human herpesvirus 3 | gi: 9625913 | ||
| Glycoprotein I | 170 | G | T | L | A | Y | N | G | S | Human herpesvirus 1 | gi: 9629448 | |||
| LAMP-2 P331–341 | 357 | Q | G | K | Y | S | T | A | Q | D | C | 366 | Homo sapiens | gi: 307110 |
| ORF | 71 | Y | S | T | V | Q | D | C | 77 | E. coli O157:H7 | gi: 15804774 | |||
| DMSO reductase | 193 | G | S | Y | S | T | A | Q | 199 | E. coli | gi: 1742611 | |||
| E1 protein | 163 | Y | N | T | A | Q | D | C | 169 | Hepatitis C virus | gi: 1469841 | |||
| Polyprotein | 143 | T | A | Q | D | C | 147 | Hepatitis C virus | gi: 1173700 | |||||
| Envelope protein 1 | 88 | T | A | Q | D | C | 92 | Hepatitis C virus | gi: 1737397 | |||||
| vif protein | 91 | Q | G | R | Y | S | T | 96 | HIV type 1 | gi: 17981621 | ||||
| E7 protein | 2 | H | G | K | Y | S | T | 7 | Human papillomavirus type 74 | gi: 1491799 |
Amino acid numbers correspond to those of preproteins published at Entrez, The National Center for Biotechnology Information (NCBI). AA, number of amino acids. ORF, open reading frame.
We next investigated whether autoantibodies from individuals with pauci-immune FNGN cross-reacted with FimH. Lysates from type 1 fimbriated bacteria inhibited autoantibody binding to human LAMP-2, whereas lysates from non–FimH-expressing bacteria (detailed in Supplementary Table 2 online) had no effect (Fig. 3d). In western blots, IgG from human LAMP-2–positive sera that recognized the P41–49 epitope consistently bound to the 28 kDa FimH and 32 kDa pre-FimH proteins in lysates of E. coli 12900, K. pneumoniae and P. mirabilis but did not react with proteins from FimH-negative bacteria (Fig. 3e). Finally, a purified recombinant FimH fusion protein specifically inhibited autoantibodies from subjects with FNGN, but not control sera, from binding human LAMP-2 in 14 of 15 (93.3%) subjects tested (median inhibition 83.9%, range 75–100%). Similarly, human LAMP-2 specifically inhibited binding of sera from FNGN-affected individuals to FimH (median inhibition 95%; range 59–100%). This competition was dependent on the homologous sequence, as peptide P41–49 but not peptide P331–341 was inhibitory (Supplementary Fig. 2a online). Neither human LAMP-2 nor FimH inhibited antibodies to myeloperoxidase or proteinase-3 from binding to their respective antigens in standard ELISAs (Supplementary Fig. 2b). This proves that autoantibodies to human LAMP-2 from individuals with FNGN cross-react with FimH.
FimH immunization induces antibodies to LAMP2 and FNGN
Rat and human LAMP-2 are identical at six of the nine P41–49 epitope residues, whereas this sequence in mice is very different29. Consequently, we immunized ten WKY rats with recombinant FimH fusion protein emulsified in Titermax, an adjuvant free of bacterial proteins. All of the rats developed antibodies to FimH that cross-reacted with human LAMP-2 (Fig. 4 and Supplementary Fig. 3a, b online). Two rats immunized with synthetic P41–49 human LAMP-2 peptide also developed cross-reactive antibodies, but assays were negative after immunization with Titermax alone (Fig. 4a,c). None of the rats developed antibodies to myeloperoxidase or proteinase-3 (Fig. 4a).
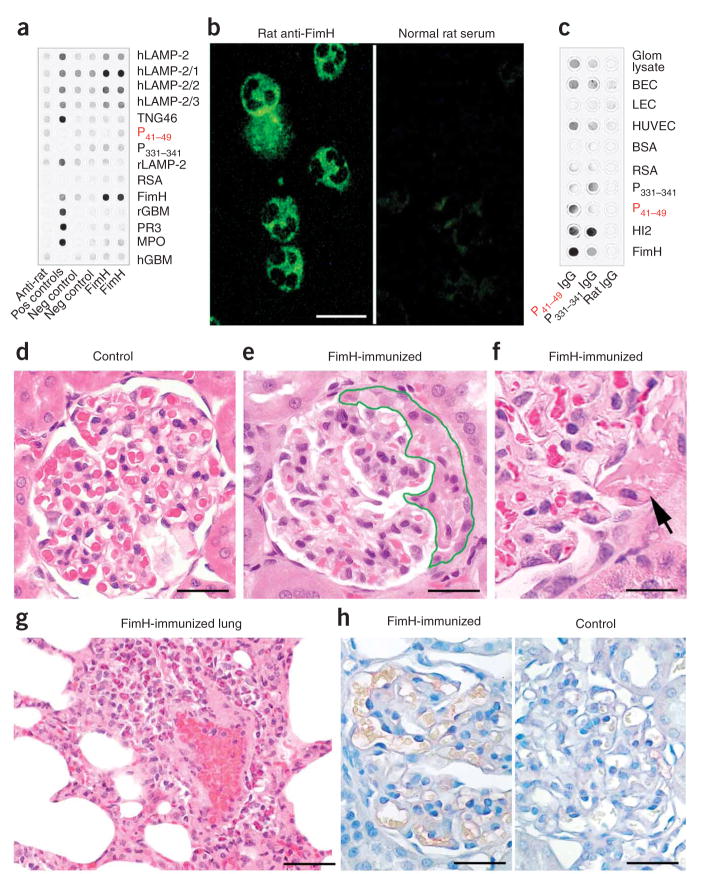
Figure 4.
Immunization with FimH induces antibodies to LAMP-2 and FNGN in rats. (a) Sera from FimH-immunized rats and controls were probed for FimH, full-length and N-terminal hLAMP-2 and 100–amino acid (hLAMP-2/1) fusion proteins. The specificity of rat IgG for human or rat glomerular basement membrane (rGBM and hGBM), proteinase-3 (PR3) or myeloperoxidase (MPO), trans-Golgi network protein 46 (TGN46) or rat serum albumin (RSA) was tested with purified proteins. Specific antibodies to these proteins, where available, were used as positive controls, and anti–rat IgG alone as negative control. (b) Sera from FimH-immunized WKY rats bound neutrophil granules, resulting in a cytoplasmic staining pattern similar to that observed with sera from subjects with FNGN (left). Sera from control rats remained negative. Scale bar, 25 μm. (c, d) IgG specific for P41–49 or P331–341, purified from rat serum after immunization with FimH was probed on purified FimH and hLAMP-2 fusion proteins, peptides P41–49 and P331–341, and proteins from isolated glomeruli, microvascular blood endothelial cells (BECs), HUVECs and lymphatic endothelial cells (LECs) (c). Purified normal rat IgG was used as control. Glomerular histology from un-immunized control animals appears normal (d). WKY rats immunized with FimH show glomerular crescent formation (e), segmental necrosis of capillaries (f) and marked neutrophilic capillaritis of the lung (g). Direct immunoperoxidase staining shows no glomerular IgG deposition in glomeruli from FimH immunized nephritic rats (h; left) nor from adjuvant treated controls (h; right). Scale bars in d–h, 50 μm.
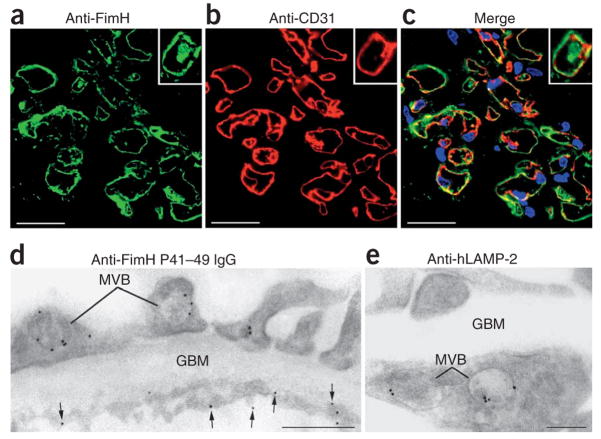
Sera from FimH-immunized rats were positive in standard ANCA assays using human (Fig. 4b) and rat (data not shown) neutrophils, and they cross-reacted with rat LAMP-2 (Supplementary Fig. 3c). IgG from rats immunized with FimH and affinity-purified on peptide P41–49 bound specifically to lysates of human umbilical vein endothelial cells (HUVECs) and human dermal blood micro-vascular endothelial cells (Fig. 4c). Immunoelectron microscopy (Fig. 5) showed that these antibodies targeted an antigen located on the endothelial plasma membrane, as well as within multivesicular bodies (Fig. 5d)—a binding pattern identical to that of authentic antibodies to human LAMP-2 (Fig. 5e). Similarly, rabbit IgG specific for FimH bound to glomerular endothelium and lysosomes in normal human kidney (Fig. 5a–c). Sera from rats immunized with Titermax alone were negative (data not shown).
Figure 5.
Antibodies induced by FimH immunization bind human glomerular endothelium. (a, b) Double labeling of frozen sections of normal human glomeruli with purified rabbit IgG specific for the FimH mannose binding pocket (green, a) and with an antibody to CD31 (red, b). (c) Overlay of a and b (yellow; scale bars, 25 μm). (d, e) IgG with specificity for peptide P41–49, purified from rat sera after immunization with FimH, was used for indirect immunolabeling of ultrathin frozen sections of human glomeruli. The staining pattern on endothelial cell membrane (arrows) as well as within multivesicular bodies (MVB) of endothelial and epithelial (E) is compared to that of a monoclonal antibody to hLAMP-2 (e). GBM, glomerular basement membrane. Scale bar in d, 500 nm; scale bar in e, 200 nm.
Nine of the FimH-immunized rats also developed antibodies to rat LAMP-2 and pauci-immune FNGN similar to the human disease (Fig. 4e,f), with 15–31% (mean 24%) of rat glomeruli affected by crescents without detectable immunoglobulin deposits, despite circulating antibodies to rat LAMP-2 (Fig. 4h). Two rats also developed hemorrhagic pulmonary vasculitis (Fig. 4g and Supplementary Fig. 3d). Rats immunized with peptide P41–49 alone developed milder disease, whereas controls injected with only Titermax remained healthy (Fig. 4d). Thus, immunizing rats with FimH breaks immunological tolerance to LAMP-2 and provokes synthesis of autoanti-bodies to it through molecular mimicry. These antibody responses are accompanied by pauci-immune FNGN and pulmonary vasculitis.
Infection with fimbriated pathogens is common before FNGN
Finally, data available from samples sent by primary care physicians showed that nine of the most recent thirteen (69%) subjects in our cohort had microbiologically confirmed diagnoses of infection with FimH-expressing bacteria during the 12 weeks before presentation with pauci-immune FNGN (eight E. coli and one K. pneumoniae infection), indicating that they had been exposed to the relevant pathogens. The autoantibodies in these individuals bound the human LAMP-2 region containing the cross-reactive P41–49 epitope. By contrast, subjects recovering from systemic infections with the relevant bacteria without evidence of renal disease had high titers of antibodies to FimH that did not recognize the P41–49 epitope or cross-react with human LAMP-2 (data not shown).
DISCUSSION
The results presented here lead us to propose a previously unrecognized molecular explanation for the origin and development of injury in pauci-immune FNGN. We show that (i) autoantibodies to human LAMP-2 are present in over 90% of individuals with active pauci-immune FNGN; (ii) antibodies to human LAMP-2 injure human microvascular endothelium in vitro and induce FNGN when administered to rats; (iii) autoantibodies from individuals with pauci-immune FNGN commonly bind an epitope shared by human LAMP-2 and the bacterial adhesin FimH and cross-react with it; (iv) immunization with FimH induces pauci-immune FNGN associated with antibodies that bind human and rat LAMP-2; and (v) individuals with pauci-immune FNGN commonly have infections with FimH-expressing bacteria shortly before presentation of FNGN. Thus, FimH-triggered autoimmunity to human LAMP-2 could be responsible for the initiation and development of injury in pauci-immune FNGN, either operating alone or synergistically with antibodies to myeloperoxidase and proteinase-3.
Our hypothesis depends on the high prevalence of antibodies to human LAMP-2 in individuals with active pauci-immune FNGN. Consequently, we validated the results of our standard ELISA, not only by western blotting but also in other assays using mammal-expressed human LAMP-2. Thus, we can be certain that over 90% of our subject cohort with active pauci-immune FNGN had circulating autoantibodies to human LAMP-2. This exceeds the prevalence of antibodies to myeloperoxidase and proteinase-3, which, as in other series, were each present in around half of the subjects. Thus, autoantibodies to human LAMP-2 are the first to be found in all types of pauci-immune FNGN. Furthermore, they bind fully glycosylated human LAMP-2 on the cell surface, suggesting that they could cause injury.
The frequent co-existence of autoantibodies to LAMP-2 with those to myeloperoxidase and proteinase-3 precluded the use of patients’ sera for testing whether the autoantibodies to LAMP-2 cause injury. However, we showed that pathologically plausible concentrations of monoclonal and polyclonal antibodies to human LAMP-2 cause injury both in vitro and in vivo without the need for additional stimuli—such as LPS or cytokines—that are commonly required in such experiments7,30,31. Uniquely among autoantibodies found in pauci-immune FNGN, the monoclonal human LAMP-2–specific antibody H4B4 injured human blood microvascular endothelium, the primary target of damage in pauci-immune FNGN. Although the molecular mechanisms for this have yet to be defined, activation of endothelial caspase-3 is consistent with the role of LAMP-2 in apoptotic and autophagocytic cell death32,33. Antibodies to human LAMP-2 cause pauci-immune FNGN with up to 25% crescents when injected into WKY rats. Taken together, our data provide strong, though indirect, evidence that autoantibodies to human LAMP-2 contribute to glomerular injury in individuals with pauci-immune FNGN.
The data raise the intriguing question as to why autoantibodies to human LAMP-2 so commonly occur together with antibodies to either myeloperoxidase or proteinase-3. An essential synergistic effect on injury provides an obvious explanation, but an alternative (and not mutually exclusive) possibility is that the autoantibodies to human LAMP-2 dysregulate the role of LAMP-2a in presentation of cytoplasmic antigens, including lysosomal proteins15,34,35. This, in turn, could facilitate the generation of autoantibodies to myeloperoxidase and proteinase-3.
Characterization of the epitopes recognized by autoantibodies from subjects with pauci-immune FNGN provided our most unexpected result. It uncovered a hitherto unsuspected homology between one of these epitopes (P41–49) and the bacterial fimbrial adhesin FimH. The homology has profound functional consequences, first because auto-antibodies that recognize P41–49 cross-react with FimH, as proved by our reciprocal inhibition experiments, and second because WKY rats immunized with FimH develop antibodies to the shared epitope. The immunized rats also developed autoantibodies to rat LAMP-2 and pauci-immune FNGN with pulmonary small vessel vasculitis similar to the human disease. This clearly demonstrates that FimH can induce FNGN by operating as a molecular mimic for LAMP-2, at least when administered together with adjuvant. These results identify P41–49 as a pathogenic epitope and support our proposal that autoimmunity to LAMP-2 causes FNGN, even in the absence of antibodies to myeloperoxidase or proteinase-3.
Molecular mimicry has frequently been proposed as a mechanism for autoimmune disease, but convincing evidence has been difficult to obtain, as has been previously reviewed21, except possibly in neurological diseases in the presence of cross-reacting antibodies36,37. Our demonstration that human LAMP-2 cross-reacts with FimH and that immunization with FimH induces antibodies to LAMP-2 and FNGN shows the molecular mimicry between the two proteins. This conclusion is supported by two additional pieces of data: it has previously been shown that FNGN is induced in rats immunized with attenuated E. coli but not with Staphylococcus aureus38, and we have found that nine of thirteen subjects had microbiologically proven infections with FimH-bearing organisms shortly before the clinical appearance of FNGN. Although this provides some of the most complete evidence yet for molecular mimicry as mechanism of human autoimmune disease, it doesn’t identify the exceptional circumstances needed for FimH to induce the cross-reactive auto-antibodies. FimH is required for bacterial colonization of bladder epithelium39,40 and so has been proposed as an attractive target for immunization programs for urinary tract infections in humans41,42. Our data clearly question the safety of this approach.
In conclusion, we propose that infection with fimbriated bacteria induces autoantibodies to human LAMP-2 through molecular mimicry and that these antibodies bind microvascular endothelium and cause injury (Supplementary Fig. 4). This presents a new model for the pathogenesis of pauci-immune FNGN and related ANCA-associated diseases and could provide the foundation of a new therapeutic strategy for this devastating disease.
METHODS
Study subjects and controls
We studied sera from 84 individuals with biopsy-proven active pauci-immune FNGN, diagnosed according to Chapel Hill definitions43. We assessed disease activity with a validated renal biopsy scoring system44 supplemented by clinical indices (Supplementary Table 3 online). Remission sera were available from 54 individuals who had no clinical evidence of disease activity for at least 2 months before and after the samples were taken. Control sera were from individuals with glomerulonephritis other than FNGN (n = 30); ANCA-positive individuals with other types of FNGN (n = 8; two each of Goodpasture’s, systemic lupus erythematosus, immune-complex glomerulonephritis and thrombotic thrombocytopenic purpura and hemolytic uremic syndrome); individuals with localized vasculitis without ANCA or renal involvement (n = 20); and healthy volunteers (n = 50). Subject samples were taken for routine clinical management, and use of human samples conformed to legal regulations in Austria and the United Kingdom and were approved by the Ethics Committees of the Medical University of Vienna and the Grampian Research Ethics Committee. Informed consent was sought for the use of the samples, where required.
Reagents
We used the following monoclonal (except where stated) antibodies: human LAMP-2 (clone H4B4, Developmental Studies Hybridoma Bank), proteinase-3 (clone 1F11, HyTest), CD4 (clone RPA-T4, BD-Pharmingen), actin (Chemicon) and E-selectin (CD62E, HyCult Biotechnology) and rabbit antibody to cleaved caspase-3 (Cell Signaling Technology).
Generation of recombinant human LAMP-2
We amplified the extracellular domain of human LAMP-2 from bases −83 to 1026 of the open reading frame by PCR and cloned it into pRSETA or pGEX6P1 (His-tagged (Invitrogen) or GST fusion (Amersham, respectively) for bacterial protein expression and into pCDNAI (Invitrogen) for mammalian expression. We confirmed the sequences and cloning sites of all constructs as well as the size and immunoreactivity of recombinant human LAMP-2 by methods described previously10,45,46 (Supplementary Methods online).
Enzyme-linked immunosorbent assay for human LAMP-2 and canonical autoantibodies to neutrophil cytoplasmic antigens
We assayed serum samples at a dilution of 1:100 on ELISA plates coated with purified human LAMP-2 extracellular domain. We calculated the 99.5% confidence limit for the upper limit of normal as three standard deviations above the mean absorbance for healthy individuals. We confirmed ELISA results by western blotting and indirect immunofluorescence on ldlD47 cells expressing human LAMP-2 on their surface (Supplementary Methods). We assayed ANCA staining patterns by immunofluorescence in accordance with published guidelines10,48. We tested sera from an active and inactive stage of disease with commercially available ELISAs for myeloperoxidase and proteinase-3 (Varelisa, Phadia).
Human LAMP-2 epitope analysis
We characterized human LAMP-2 epitopes recognized by subjects’ IgG by SPOTscan analysis (Sigma-Genosys)28 (Supplementary Methods), validated positive results by sequential alanine substitution (SPOTsalogue, Sigma-Genosys) and confirmed them by specific inhibition with synthetic peptides (gift from A. Jungbauer).
Recognition of bacterial proteins by autoantibodies
We probed lysates of E. coli, K. pneumoniae, P. mirabilis, Pseudomonas aeruginosa and S. aureus (Supplementary Table 2) by western blotting with sera from subjects with pauci-immune FNGN and control sera. We analyzed FimH binding and cross-reactivity of human LAMP-2 autoantibodies by reciprocal cross-inhibition ELISA, using equimolar concentrations of soluble human LAMP-2, FimH fusion protein49 or synthetic human LAMP-2 peptides P41–49 or P331–341 (Supplementary Methods).
Interaction of antibodies to human LAMP-2 with human granulocytes and microvascular endothelium
We incubated human neutrophils10,26 for 30 min with 10 μg ml−1 or 20 μg ml−1 antibodies to human LAMP-2, proteinase-3 or CD4 or with TNF-α (2 ng ml−1), assessed shape change25 and F-actin condensation26 and measured myeloperoxidase concentrations in supernatants (Immundiagnostik). We incubated purified human dermal microvascular blood endothelial cells, grown to 60% confluence27, with the monoclonal antibodies described above for 16 h and assessed expression of E-selectin and activated caspase-3 by immunofluorescence (Supplementary Methods). All antibodies contained less than 2 pg ml−1 LPS (Limulus Amebocyte Lysate, Cambrex BioScience).
Immunization with antibodies to LAMP-2
We injected WKY rats intravenously with 10 mg of IgG to human and rat LAMP-2 extracellular domain purified using endotoxin-free reagents. Control rats received normal rabbit IgG or saline. We culled the rats at specified time points up to 120 h (Supplementary Methods) and assessed glomerular injury by hematuria (Combur-7 test, Roche Diagnostics), albuminuria (QuantiChrom BCG Albumin Assay Kit, BioAssay Systems), histomorphology and immunohistochemistry.
Immunization with FimH fusion protein or the cross-reactive epitope
We immunized WKY rats with 150 μg purified FimH fusion protein emulsified in Titermax Gold (Sigma-Aldrich) or with peptide P41–49 conjugated to rat serum albumin. Controls received PBS in Titermax alone. We culled the rats on day 39 and assessed glomerular injury as described above (Supplementary Methods).
Analysis of rat sera
We assayed ANCAs by immunofluorescence on human and rat granulocytes and antibodies to human LAMP-2, FimH, human myeloperoxidase and proteinase-3 by ELISA. We used dot-blot analysis to test whole sera for antibodies to human LAMP-2, rat LAMP-2, FimH, human myeloperoxidase and proteinase-3, serum albumin and glomerular basement membranes, and we probed rat IgG, affinity-purified on peptide P41–49 and P331–341 (ref. 50) for reactivity with lysates of blood microvascular endothelial cells or lymphatic endothelial cells (Supplementary Methods). We probed rat and rabbit IgG specific for FimH and LAMP-2 on 4-μm-thick unfixed frozen sections or ultrathin frozen sections of PLP-fixed normal human kidneys10.
Statistical analyses
We used two-way analysis of variance followed by a multiple range test (Tukey analysis) to estimate the significance of results from ELISA. We used the Wilcoxon rank sum test after an initial Kruskal Wallis test for the in vivo and in vitro experiments.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of Fokko van der Woude. We thank A. Jungbauer (University of Agriculture and Forestry, Vienna) for synthetic peptides. We thank Kidney Research UK, who generously funded R.K.’s Senior Research Fellowship (KRUK SF3/2000-2005) at the University of Aberdeen and Scottish Hospitals Endowment Research Trust (RG15/02) for supporting parts of this project. A.R. is funded by an EU Marie Curie Excellence Chair (MEXC-CT-2006-042742). Parts of this work were funded by the Austrian Federal Ministry of Science and Research. M.F.’s research is supported by grant RO1CA48737. We are indebted to H. Schachner for technical assistance and to A. Jäger for his help in preparing the figures. We also would like to thank the many physicians who provided sera and subject details.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
R.K.: design, execution, supervision and analysis of experiments, and manuscript writing. M.E.: development of human LAMP-2 ELISA and usage of SPOT assays. R.B.: cloning and generation of fusion proteins, western blots, human LAMP-2 and inhibition ELISA. R.Z.: collection and analysis of subject data. D.C.: in vivo experiments. C.A.L.: human LAMP-2 and inhibition ELISA of subject sera. A.D.: glycoepitope analysis. I.R.: experimental tissue culture work. R.J.: western blot analysis of antibody binding to bacterial proteins. O.A.: in vitro experiments with polymorphonuclear and endothelial cells. S.S.: myeloperoxidase and proteinase-3 ELISA. G.S.-P.: collection and analysis of subject data. M.F.: provision of human LAMP-2 complementary DNA constructs, antibodies and advice. P.K.: provision of FimH cDNA construct, antibodies and advice. A.J.R.: design and analysis of experiments and manuscript writing. D.K.: design and analysis of experiments and manuscript writing.
Published online at http://www.nature.com/naturemedicine/
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 2.Morgan MD, Harper L, Williams J, Savage C. Anti-neutrophil cytoplasm associated glomerulonephritis. J Am Soc Nephrol. 2006;17:1224–1234. doi: 10.1681/ASN.2005080882. [DOI] [PubMed] [Google Scholar]
- 3.Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotizing glomerulo-nephritis with antineutrophil antibody: possible arbovirus aetiology? . Br Med J (Clin Res Ed) 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Woude FJ, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1:425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 5.Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235–1242. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- 6.Xiao H, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huugen D, et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-α. Am J Pathol. 2005;167:47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfister H, et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 9.van der Geld YM, et al. Rats and mice immunised with chimeric human/mouse proteinase 3 produce autoantibodies to mouse PR3 and rat granulocytes. Ann Rheum Dis. 2007;66:1679–1682. doi: 10.1136/ard.2006.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kain R, et al. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies (ANCA) in necrotizing and crescentic glomerulonephritis. J Exp Med. 1995;181:585–597. doi: 10.1084/jem.181.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsson SR, Roth J, Piller F, Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-LAMP-1 and h-LAMP-2. Major sialoglyco-proteins carrying polylactosaminoglycan. J Biol Chem. 1988;263:18911–18919. [PubMed] [Google Scholar]
- 12.Eskelinen EL, et al. Unifying nomenclature for the isoforms of the lysosomal membrane protein LAMP-2. Traffic. 2005;6:1058–1061. doi: 10.1111/j.1600-0854.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 13.Sawada R, Lowe JB, Fukuda M. E-selectin–dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem. 1993;268:12675–12681. [PubMed] [Google Scholar]
- 14.Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D, et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Gough NR, Fambrough DM. Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J Cell Biol. 1997;137:1161–1169. doi: 10.1083/jcb.137.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wegener F. Über generalisierte septische Gefäßerkrankungen. Verh Dtsch Pathol Ges. 1936;29:202–209. [Google Scholar]
- 18.Pinching AJ, et al. Relapses in Wegener’s granulomatosis: the role of infection. BMJ. 1980;281:836–838. doi: 10.1136/bmj.281.6244.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 20.Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–1104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fourneau JM, Bach JM, van Endert PM, Bach JF. The elusive case for a role of mimicry in autoimmune diseases. Mol Immunol. 2004;40:1095–1102. doi: 10.1016/j.molimm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Pendergraft WF, III, et al. Autoimmunity is triggered by cPR-3105–201, a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–79. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- 23.Franssen CF, et al. In vitro neutrophil activation by antibodies to proteinase 3 and myeloperoxidase from patients with crescentic glomerulonephritis. J Am Soc Nephrol. 1999;10:1506–1515. doi: 10.1681/ASN.V1071506. [DOI] [PubMed] [Google Scholar]
- 24.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 26.Tse WY, Nash GB, Hewins P, Savage CO, Adu D. ANCA-induced neutrophil F-actin polymerization: implications for microvascular inflammation. Kidney Int. 2005;67:130–139. doi: 10.1111/j.1523-1755.2005.00063.x. [DOI] [PubMed] [Google Scholar]
- 27.Kriehuber E, et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerjaschki D, Ullrich R, Exner M, Orlando RA, Farquhar MG. Induction of passive Heymann nephritis with antibodies specific for a synthetic peptide derived from the receptor-associated protein. J Exp Med. 1996;183:2007–2015. doi: 10.1084/jem.183.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granger BL, et al. Characterization and cloning of lgp110, a lysosomal membrane glycoprotein from mouse and rat cells. J Biol Chem. 1990;265:12036–12043. [PubMed] [Google Scholar]
- 30.Little MA, et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106:2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- 31.Ruth AJ, et al. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1940–1949. doi: 10.1681/ASN.2006020108. [DOI] [PubMed] [Google Scholar]
- 32.González-Polo RA, et al. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- 33.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dengjel J, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from auto-phagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuki N, et al. Carbohydrate mimicry between human ganglioside GM1 and Campylo-bacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci USA. 2004;101:11404–11409. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin MC, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8:509–513. doi: 10.1038/nm0502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savige J, et al. Antineutrophil cytoplasmic antibody (ANCA)-associated systemic vasculitis after immunisation with bacterial proteins. Clin Exp Rheumatol. 2002;20:783–789. [PubMed] [Google Scholar]
- 39.Connell I, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus–mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langermann S, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin–based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 42.Langermann S, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 43.Jennette JC, et al. Nomenclature of systemic vasculitides Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 44.Neumann I, et al. Histological and clinical predictors of early and late renal outcome in ANCA-associated vasculitis. Nephrol Dial Transplant. 2005;20:96–104. doi: 10.1093/ndt/gfh563. [DOI] [PubMed] [Google Scholar]
- 45.Skrincosky D, et al. Altered Golgi localisation of Core 2 β-1,6-N-Acetylglucosaminyl-transferase leads to decreased synthesis of branched O-glycans. J Biol Chem. 1997;272:22695–22702. doi: 10.1074/jbc.272.36.22695. [DOI] [PubMed] [Google Scholar]
- 46.Kain R, Angata K, Kerjaschki D, Fukuda M. Molecular cloning and expression of a novel human trans-Golgi network glycoprotein, TGN51, that contains multiple tyrosine-containing motifs. J Biol Chem. 1998;273:981–988. doi: 10.1074/jbc.273.2.981. [DOI] [PubMed] [Google Scholar]
- 47.Kozarsky K, Kingsley D, Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci USA. 1988;85:4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savige J, et al. International group for consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA) Am J Clin Pathol. 2003;120:312–318. doi: 10.1309/WAEP-ADW0-K4LP-UHFN. [DOI] [PubMed] [Google Scholar]
- 49.Schembri MA, Hasman H, Klemm P. Expression and purification of the mannose recognition domain of the FimH adhesin. FEMS Microbiol Lett. 2000;188:147–151. doi: 10.1111/j.1574-6968.2000.tb09186.x. [DOI] [PubMed] [Google Scholar]
- 50.Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D. Endothelial cell membranes contain podocalyxin—the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986;102:484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.