Abstract
Background
Previously, the authors found that risk of spontaneous abortion was increased in the pregnancies of women with epilepsy compared with their same-sex siblings, which could have implications for risk of epilepsy in their offspring. An association between a history of spontaneous abortion in the mother and risk of epilepsy in her live-born offspring may arise through selective loss of fetuses with a genetic susceptibility to epilepsy or through intrauterine environmental factors that may predispose the mother to a spontaneous abortion and to epilepsy in her live-born children.
Methods
The authors examined the relation of a history of spontaneous abortion to the risk of idiopathic or cryptogenic epilepsy in 791 live-born offspring of 385 women with cryptogenic localization-related epilepsy (probands) ascertained from voluntary organizations. A semistructured telephone interview with probands and additional family informants, supplemented by medical record review, was used to obtain information on seizures and other risk factors in probands and relatives.
Results
Live-born offspring of women with a history of spontaneous abortion were four or five times as likely to develop epilepsy as were children of women without (12.8% versus 4.7%; rate ratio = 4.6, 95% CI: 2.3–9.0). Cumulative incidence of epilepsy was 21.9% in offspring of women with a history of spontaneous abortion and a family history of epilepsy, compared with 4.7% in offspring of women with neither risk factor.
Conclusions
These results suggest that a history of spontaneous abortion is associated with increased risk of epilepsy in live-born offspring and may be a marker for genetic susceptibility for epilepsy in the mother.
The risk of reproductive loss in patients with epilepsy is not well understood, despite concerns regarding the possible teratogenic effects of gestational seizures and antiepileptic drugs (AED). Several studies have found that increases in spontaneous abortion were associated with AED use in the first trimester, a result consistent with the possible teratogenic effects of AED.1–3 However, in population-based data from Rochester, Minnesota, neither epilepsy per se nor AED use during pregnancy was associated with an increased risk for spontaneous abortion.4 The cumulative risk of spontaneous abortion in AED-exposed pregnancies was 18% compared with 17.4% in the wives of men with epilepsy. Population-based estimates of the rate of spontaneous abortion in clinically recognized pregnancies have ranged from 5.5% to 15.3%; hence, the rate in affected women is not excessive.5–10 In contrast, we found that the risk of spontaneous abortion was significantly increased in the pregnancies of women with epilepsy and the wives of men with epilepsy, compared with their same-sex siblings.11 In that study of risk factors for spontaneous abortion in patients with epilepsy, the frequency of spontaneous abortion was highest in the pregnancies of women with a family history of epilepsy (29.7% in women with epilepsy versus 16.9% in unaffected siblings).11
This increased risk of spontaneous abortion in women with epilepsy could have implications for the risk of epilepsy in live-born offspring. An association between a history of spontaneous abortion in the mother and risk of epilepsy in her live-born offspring may arise in several ways. First, the spontaneous abortion may result from selective loss of fetuses with a genetic susceptibility to epilepsy. This would imply a greater risk of spontaneous abortion among women with a genetic susceptibility to epilepsy than women without and a lower risk of epilepsy among live-born offspring of affected women with a history of spontaneous abortion than among live-born offspring of those without. Second, a history of spontaneous abortion may be a marker for a genetic susceptibility to epilepsy in the mother, without selective loss of genetically susceptible fetuses. In this case, we would expect a greater risk of spontaneous abortion among women with a genetic susceptibility to epilepsy than among those without and a greater risk of epilepsy among offspring of women with a history of spontaneous abortion than among offspring of those without. Finally, the increased risk of spontaneous abortion may be unrelated to the genotype of the mother or fetus and may be caused by intrauterine environmental exposures, such as gestational seizures or exposure to AED, that increase the risk for a spontaneous abortion and epilepsy in live births. In this case, we would expect an increased risk of epilepsy in children born to mothers with a history of spontaneous abortion, but only in children born after, not before, the mother’s onset of epilepsy.
In the current study, we examined the relation of a history of spontaneous abortion to the risk of idiopathic or cryptogenic epilepsy in live-born offspring of women with cryptogenic localization-related epilepsy. First, we investigated whether an association between a history of spontaneous abortion and risk of epilepsy in live-born offspring was the result of confounding with other risk factors for increased risk of epilepsy in offspring. Confounding could occur if the proportion with other risk factors differed between mothers with and without a history of spontaneous abortion. Potential confounding factors included the mother’s level of education, mother’s ethnicity, mother’s age at onset of epilepsy, maternal history of gestational seizures, family history of epilepsy, and total number of children. Second, we examined whether the effect of a history of spontaneous abortion on risk of epilepsy in live-born offspring varied by risk factors for spontaneous abortion or the clinical characteristics of the mother’s epilepsy, including age at onset of epilepsy, family history, history of gestational seizures in the pregnancy and maternal education and ethnicity. Third, we compared children born before and after the onset of the mother’s epilepsy, in terms of the association between epilepsy risk and maternal history of spontaneous abortion.
Methods
Subjects
The data for this study were collected as part of the Epilepsy Family Study of Columbia University.12 The design of the study, subjects, and methods have been described in detail elsewhere.12 Briefly, 1957 adults with epilepsy (probands) were ascertained from 10 voluntary organizations through a telephone survey conducted between 1985 and 1988. Semistructured telephone interviews were used to obtain detailed information on seizure histories of the probands and their parents, full and half siblings, children, and spouses. To improve the sensitivity of the family history data, whenever possible (67% of families), an additional family informant, usually the mother of the proband, was also interviewed regarding the same relatives reported on by the proband. To confirm and augment the clinical detail on the family histories, 51% of living adult relatives who were reported to have had seizures when they were at least 5 years old were also interviewed. Medical records were requested for all probands and were obtained for 60% of probands.
Clinical diagnosis and classification
Probands were interviewed regarding seizure semiology and potential causal factors with respect to themselves and to any relative they reported as having had seizures. Seizure semiology was obtained through verbatim descriptions of seizures and structured questions regarding signs and symptoms (e.g., specific aura, unilateral signs, and alteration in consciousness). Etiology was derived from questions regarding a history of specific risk factors previously shown for epilepsy, the age at which each occurred, and their timing in relation to seizure onset. The interviews with other family informants included the same questions regarding seizure type and etiology relating to the proband and to the informant and any other relatives reported by the informant to have had seizures. Diagnoses of seizure disorders were based on a consensus review of all information collected on each proband or relative (proband interview, informant interview, direct interview, and medical record). Information from EEG, MRI, or CT scans was seldom available; hence, in most cases, the diagnosis was based solely on historical information. For each subject (proband or relative), the consensus review was carried out blindly with respect to the information collected on other relatives. This independent diagnosis and classification of epilepsy in each subject removed the potential for bias.
Epilepsy was defined as a lifetime history of at least two unprovoked seizures.13 The proband’s report of epilepsy in parents and siblings had excellent validity (87% sensitivity and 99% specificity), with the mother’s report used as the gold standard.14 In probands and relatives with epilepsy, seizures were classified according to the 1981 criteria of the International League Against Epilepsy.15 The resulting seizure classifications were reliable (reproducible) and valid compared with the diagnoses of expert physicians.16,17 Probands and relatives with generalized-onset seizures were classified as having generalized epilepsies, and those with partial-onset seizures, including those with secondary generalization, were classified as having localization-related epilepsies.18 Few probands had idiopathic epilepsy syndromes; hence, this is essentially a study of the families of probands with cryptogenic epilepsy. The diagnosis of seizure disorders in offspring was based on the same procedures used for the probands. Offspring were classified as affected if they had epilepsy, and unaffected if they had isolated unprovoked seizures, febrile convulsions, or other acute symptomatic seizures. In this study, we restricted the analysis to offspring of women with cryptogenic localization-related epilepsy because previous analyses of the data set had shown that patterns of transmission differed between families of probands with generalized versus localization-related epilepsy, and localization-related epilepsy accounted for the most.19 In addition, we classified only offspring with idiopathic or cryptogenic epilepsy as affected. We investigated how the risk of idiopathic or cryptogenic epilepsy in 791 live-born offspring of 385 mothers with cryptogenic localization-related epilepsy varied by history of spontaneous abortion in the mother.
Pregnancy outcomes
To ascertain a history of spontaneous abortion, women were asked their age at each pregnancy and the number, paternity, and outcome of each pregnancy. Spontaneous abortion was defined as fetal death before 20 weeks of gestational age.
Confounders
Factors that could influence the risk of spontaneous abortion and risk of epilepsy in offspring were included in the analyses as potential confounders, including the mother’s level of education, mother’s ethnicity, maternal history of gestational seizures, mother’s age at onset of epilepsy, family history of epilepsy, and total number of children. We used a conservative approach and included as potential confounders several variables that were only weakly associated with spontaneous abortion in this data set (e.g., ethnicity, mother’s age at onset of epilepsy, maternal history of gestational seizures, and total number of children), although some were related to risk of epilepsy in offspring. The mother’s level of education was classified as less than high school or high school or more. Ethnicity was classified as nonwhite or white. Maternal history of gestational seizures during the pregnancy of each offspring was classified as yes or no, because in almost all cases, the gestational seizures occurred throughout the pregnancy. The age at onset of epilepsy was assigned to one of four groups: 0 to 9 years, 10 to 19 years, 20 to 35 years, and after 35 years. Mothers with a parent or at least one sibling with idiopathic or cryptogenic epilepsy were classified as familial cases (n = 29). We did not include the occurrence of affected offspring in the classification of familial cases, because the risk in offspring was the outcome for these analyses.
Statistical analyses
The risk of epilepsy was examined only for offspring of affected women, because men were less likely to report accurately on the outcome of the pregnancies that they had fathered. We restricted our analyses to women who had at least one live birth. In preliminary analyses, we used χ2 tests to compare the frequency of epilepsy in the offspring of mothers with and without a history of spontaneous abortion.20 We used survival analysis to assess the risk of idiopathic or cryptogenic epilepsy in the offspring of women with cryptogenic localization-related epilepsy. We estimated the cumulative incidence of epilepsy using a reconstructed cohort design in which each offspring was taken to be at risk of epilepsy from birth until current age or age at death (if unaffected) or age at onset of epilepsy.21 We used Kaplan–Meier life table methods and Cox proportional hazards analysis22 to estimate age-specific cumulative incidence to age 25 and rate ratios (RR) for epilepsy in live-born offspring according to the mother’s history of spontaneous abortion, maternal education, maternal ethnicity, history of gestational seizures, family history of epilepsy, age at onset of epilepsy, and total number of children. We examined the potential interaction between these risk factors and history of spontaneous abortion by comparing risk of epilepsy in offspring of women with and without each risk factor. Finally, we examined the effect of a history of spontaneous abortion on risk of epilepsy in offspring born before and after their mother’s onset of epilepsy, to evaluate the influence of environmental factors, such as gestational seizures, which would be expected to exert their effects after, but not before, the mother’s onset of epilepsy.
Mothers who have a child with epilepsy may be more likely to recall or report early-gestation spontaneous abortions than are mothers of healthy children. We examined the impact of such potential recall bias on the association between spontaneous abortion and risk of epilepsy in offspring in two ways. First, we compared women with an affected child versus those without an affected child in terms of the proportion who reported a spontaneous abortion within three age groups at the time of interview: 35 years or younger, 36 to 45 years, and older than 45 years. In each of these age groups, we used Cox proportional hazards modeling to compute the RR for a spontaneous abortion in women with versus without an affected child. We would expect reporting by the younger women to be the most accurate because they are closest to their reproductive years. Thus, the effect of recall bias would be reflected in a higher RR in women who had remote spontaneous abortions (older women) than in women who had recent spontaneous abortions (younger women). Second, we examined the effect of time since the mother’s spontaneous abortion on the risk of epilepsy in her offspring, in women aged 36 to 45 years, whose reproductive years could span recent and remote spontaneous losses. The median interval between first spontaneous abortion and time at the interview was 16 years. We selected eight years (25th percentile) as the cutoff point for these comparisons and classified each woman’s first spontaneous abortion as occurring less than 8 years, 8 to 16 years, or more than 16 years before the interview. We then used Cox proportional hazards modeling to estimate the RR for each of these periods of spontaneous abortion in women with versus women without an affected child. Again, we would expect that reporting of recent spontaneous abortion would be most accurate and least subject to recall bias. Thus, if the association between spontaneous abortion and risk of epilepsy in live-born offspring were the result of recall bias, we would expect a higher RR for remote than for recent spontaneous abortion among women with an affected child versus women without an affected child. All analyses were conducted using the Kaplan–Meier and Cox regression procedures in SPSS for Windows, version 9.0.23
Results
Proband characteristics
The analysis included 791 live births of 385 mothers with cryptogenic localization-related epilepsy for whom data on all variables were complete. Most (84%) mothers were white and had relatively high levels of education; 52.5% had at least 1 year of college. Twenty-nine (7.5%) probands had a positive family history of epilepsy, and 115 (29.9%) probands had a history of spontaneous abortion (table 1). Among the 791 live-born offspring, there was a history of gestational seizures in 251 (31.7%) pregnancies.
Table 1.
Proband demographic characteristics
| Characteristic | No. of female probands (%) |
|---|---|
| Idiopathic or cryptogenic epilepsy | 385 |
| Age, y (mean ± SD) | 40.6 ± 11.2 |
| Education | |
| No college | 183 (47.5) |
| At least 1 year of college | 202 (52.5) |
| Ethnicity | |
| White | 323 (84.3) |
| Nonwhite | 60 (15.7) |
| Age at onset of epilepsy | |
| 0–9 y | 71 (18.4) |
| 10–19 y | 152 (39.5) |
| 20–35 y | 112 (29.1) |
| >35 y | 50 (13.0) |
| Family history of epilepsy in a parent or sibling | |
| Yes | 29 (7.5) |
| No | 356 (92.5) |
| History of spontaneous abortion | |
| Yes | 115 (29.9) |
| No | 270 (70.1) |
Risk of epilepsy in offspring
The overall prevalence of a history of epilepsy in offspring was 5.7%. The cumulative incidence of epilepsy to age 25 among offspring of women with a history of spontaneous abortion was 12.8%, compared with 4.7% in offspring of women without a history of spontaneous abortion (table 2, figure). Offspring of women with a history of spontaneous abortion were three times as likely to develop epilepsy as offspring of female probands without a history of spontaneous abortion in univariate analysis (RR = 3.2, 95% CI: 1.8 –5.7) (see table 2). The risk of epilepsy in live-born offspring was also associated with early age at onset in the proband, a history of gestational seizures in the pregnancy, and mother’s total number of children, but not with family history of epilepsy in parent or sibling, mother’s level of education, or mother’s ethnicity (see table 2). The high relative risk of epilepsy to offspring of women with an age at onset of epilepsy of 35 years or younger was primarily attributable to the extremely low risk of epilepsy (0.8%) in offspring of women with an older age at onset (older than 35 years). Comparison of univariate with multivariate RR, in which all variables were included in the analysis, showed that the association of a history of spontaneous abortion and risk of epilepsy in offspring was not changed when we adjusted for potential confounding factors that may influence risk of epilepsy in offspring (unadjusted and adjusted RR for epilepsy in offspring = 3.2 versus 4.6) (see table 2).
Table 2.
Rate ratio for epilepsy in offspring of women with localization-related epilepsy
| Proband characteristics | No. of offspring* (No. affected) | Cumulative incidence to age 25 (%) | Univariate rate ratio (95% CI) | Multivariate rate ratio (95% CI) |
|---|---|---|---|---|
| History of spontaneous abortion† | ||||
| Yes | 246 (27) | 12.8 | 3.2 (1.8–5.7)‡ | 4.6 (2.3–9.0)‡ |
| No | 545 (19) | 4.7 | 1.0 (reference) | 1.0 (reference) |
| Family history of epilepsy | ||||
| Yes | 54 (5) | 13.1 | 1.8 (0.7–4.5) | 1.5 (0.6–3.8) |
| No | 737 (41) | 6.9 | 1.0 (reference) | 1.0 (reference) |
| Age at onset of epilepsy | ||||
| 0–9 y | 133 (12) | 12.6 | 15.8 (2.1–121.6)‡ | 12.1 (1.5–97.5)‡ |
| 10–19 y | 291 (16) | 7.2 | 8.8 (1.7–66.4)‡ | 9.3 (1.2–73.6)‡ |
| 20–35 y | 239 (17) | 9.6 | 11.3 (1.5–85.1)‡ | 7.7 (1.0–60.5) |
| >35 y | 124 (1) | 0.8 | 1.0 (reference) | 1.0 (reference) |
| Education | ||||
| <High school | 370 (24) | 7.9 | 1.4 (0.8–2.4) | 1.8 (0.9–3.6) |
| ≥High school | 421 (22) | 9.6 | 1.0 (reference) | 1.0 (reference) |
| Ethnicity | ||||
| Nonwhite | 136 (13) | 11.2 | 1.8 (0.9–3.4) | 1.3 (0.5–3.2) |
| White | 648 (33) | 6.5 | 1.0 (reference) | 1.0 (reference) |
| Gestational seizures | ||||
| Yes | 251 (19) | 9.8 | 2.1 (1.1–3.6)‡ | 1.3 (0.7–2.5) |
| No | 492 (21) | 5.5 | 1.0 (reference) | 1.0 (reference) |
| Total no. of children | 791 (46) | 7.3 | 1.4 (1.3–1.5)‡ | 1.4 (1.1–1.7)‡ |
Offspring with unknown history excluded.
History of spontaneous abortion considered positive if at least one pregnancy was terminated in spontaneous abortion.
p < 0.05.
Figure.
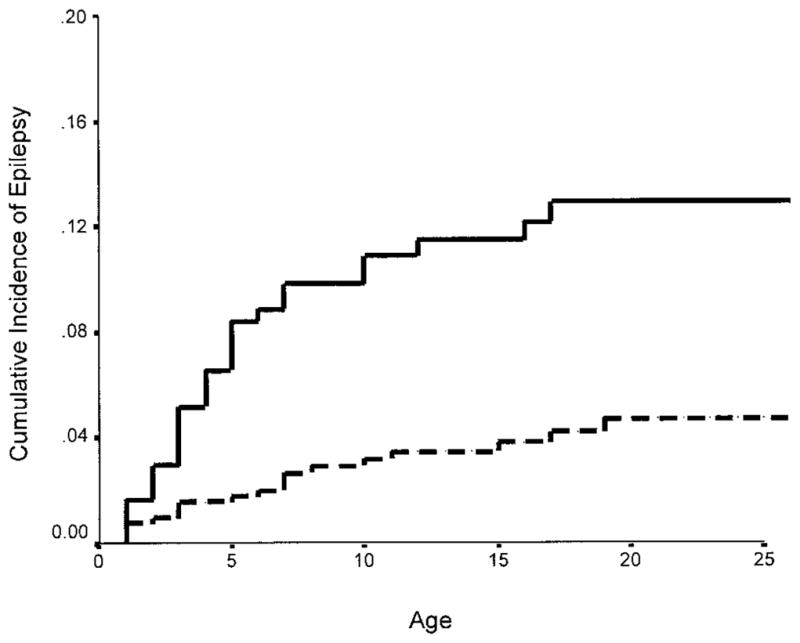
Cumulative incidence of idiopathic or cryptogenic epilepsy to age 25 in offspring of women with cryptogenic epilepsy with and without a history of spontaneous abortion. Solid line = offspring of women with a history of spontaneous abortion. Dashed line = offspring of women without a history of spontaneous abortion.
To examine the interaction between a history of spontaneous abortion and other risk factors for epilepsy in offspring, we compared RR for epilepsy in offspring of probands with and without a history of spontaneous abortion within strata defined by the clinical characteristics of the mother’s epilepsy and other risk factors (table 3). Offspring of women with a history of spontaneous abortion were more likely to develop epilepsy than offspring of those without a history of spontaneous abortion in almost all risk categories (see table 3). The influence of a history of spontaneous abortion was greater in offspring of female probands with than without a family history of epilepsy (RR = 7.5 versus 2.9), greater in offspring with than without a history of gestational seizures (RR = 7.6 versus 3.1), greater in offspring of women with low than with high levels of education (RR = 5.4 versus 1.8), and greater in offspring of white than nonwhite women (RR = 5.6 versus 0.9). However, the influence of a history of spontaneous abortion on the risk of epilepsy in offspring did not vary systematically by mother’s age at onset of epilepsy (see table 3).
Table 3.
Rate ratio for epilepsy in offspring of women with and without a history of spontaneous abortion
| History of spontaneous abortion* |
No history of spontaneous abortion |
||||
|---|---|---|---|---|---|
| Proband characteristics | No. of offspring (No. affected) | Cumulative incidence to age 25 | No. of offspring (No. affected) | Cumulative incidence to age 25 | Rate ratio (95% CI) |
| Family history of epilepsy† | |||||
| Yes | 21 (4) | 21.9 | 33 (1) | 7.6 | 7.5 (0.8–67.5) |
| No | 225 (23) | 12.1 | 512 (18) | 4.6 | 2.9 (1.6–5.3)‡ |
| Age at onset of epilepsy | |||||
| 0–9 y | 44 (16) | 16.7 | 89 (6) | 11.1 | 2.9 (1.6–5.3)‡ |
| 10–19 y | 93 (12) | 16.3 | 198 (4) | 2.7 | 6.4 (2.1–20.0)‡ |
| 20–35 y | 65 (8) | 14.7 | 274 (9) | 7.7 | 2.3 (0.9–5.9) |
| >35 y | 43 (1) | 2.4 | 81 (0) | — | — |
| Education | |||||
| <High school | 136 (18) | 15.1 | 234 (6) | 3.8 | 5.4 (2.2–13.7)‡ |
| ≥High school | 110 (9) | 10.0 | 311 (13) | 5.3 | 1.8 (0.8–4.3) |
| Ethnicity | |||||
| Nonwhite | 32 (3) | 12.4 | 104 (10) | 10.8 | 0.9 (0.2–3.3) |
| White | 210 (24) | 13.4 | 438 (9) | 3.2 | 5.6 (2.6–12.2)‡ |
| Gestational seizures | |||||
| Yes | 82 (15) | 21.7 | 169 (4) | 3.5 | 7.6 (2.5–23.0)‡ |
| No | 149 (12) | 9.7 | 343 (9) | 3.6 | 3.1 (1.3–7.4)‡ |
History of spontaneous abortion considered positive if at least one pregnancy was terminated in spontaneous abortion.
Family history of epilepsy considered positive if at least one parent or sibling was affected.
p < 0.05.
To assess the impact of reporting or recall bias on the association between spontaneous abortion and risk of epilepsy in offspring, we compared the RR for spontaneous abortion in women with and without an affected child in two ways: 1) within strata defined by maternal age at the interview or 2) by time since the first spontaneous abortion in women aged 36 to 45 years at the time of the interview. When we stratified by maternal age at the interview, the RR for spontaneous abortion in women with versus women without an affected child was similar among all maternal age groups (RR for women 35 years and younger at the interview = 5.1, 95% CI: 1.8 –14.7; RR for women 36 to 45 years of age at the interview = 2.0, 95% CI: 0.9 –4.5; RR for women older than 45 years at the interview = 3.0, 95% CI: 1.3–6.6). Similarly, among women 36 to 45 years of age at the interview, the RR for spontaneous abortion in women with versus women without an affected child did not vary by time since the first spontaneous abortion (RR for first spontaneous abortion less than 8 years before interview = 2.9, 95% CI: 0.6 –13.4; 8 –16 years before interview = 2.1, 95% CI: 0.5–9.3; more than 16 years before interview = 2.2, 95% CI: 0.6 –7.8).
Finally, we examined the effect of a history of spontaneous abortion on the risk of epilepsy in offspring born before and after onset of epilepsy. We restricted this analysis to the offspring of probands with an age at onset of epilepsy between 20 and 35 years (table 4). We used 20 years as the youngest age because almost all births among those with earlier ages at onset occurred after the onset of epilepsy. We selected the upper limit of 35 years because almost all births to probands with an age at onset older than 35 years occurred before the onset and there was an extremely low risk of epilepsy in these offspring. The association between a history of spontaneous abortion and risk of epilepsy was stronger in offspring born after the mother’s onset (RR = 4.4, 95% CI: 0.9 –17.1) than in offspring born before the mother’s onset of epilepsy (RR = 2.2, 95% CI: 0.6 –8.1) (see table 4). Among offspring born after the mother’s onset of epilepsy, the cumulative incidence of epilepsy to age 25 was 19.1% in offspring of women with a history of spontaneous abortion compared with 5.7% in offspring of women without a history of spontaneous abortion. Among offspring born before the mother’s onset of epilepsy, the cumulative incidence of epilepsy to age 25 was 12.8% in offspring of women with a history of spontaneous abortion compared with 6.9% in offspring of women without a history of spontaneous abortion (see table 4).
Table 4.
Rate ratio for epilepsy in offspring born before and after onset of mother’s epilepsy
| Proband characteristics | No. of offspring* (No. affected) | Cumulative incidence to age 25 | Rate ratio† (95% CI) |
|---|---|---|---|
| Before onset of epilepsy | |||
| History of spontaneous abortion‡ | 39 (4) | 12.8 | 2.2 (0.6–8.1) |
| No history of spontaneous abortion | 111 (5) | 6.9 | 1.0 (reference) |
| After onset of epilepsy | |||
| History of spontaneous abortion | 31 (5) | 19.1 | 4.4 (0.9–17.1) |
| No history of spontaneous abortion | 94 (4) | 5.7 | 1.0 (reference) |
Rate ratio for epilepsy in offspring of women with and without a history of spontaneous abortion. Risk of epilepsy in offspring of women with age at onset 20 –35 years, before and after onset of epilepsy.
Offspring with unknown history of epilepsy excluded.
Rate ratio calculated using Cox proportional hazards modeling.
History of spontaneous abortion considered positive if at least one pregnancy was terminated in spontaneous abortion.
Discussion
Offspring of women who had at least one spontaneous abortion were four or five times as likely to develop idiopathic or cryptogenic epilepsy as were offspring of women who had not had a spontaneous abortion. In multivariate analyses, early age at onset of the mother’s epilepsy and total number of children were also associated with a significant increase in the risk of epilepsy in offspring (see table 2). We used multiple offspring from each family, which might alter the risk estimates because of families with several affected children. To examine the effect of including multiple offspring from each family, we repeated the analysis using only the first-born child from each family. Offspring of women who had one or more spontaneous abortions were 10 times as likely to develop idiopathic or cryptogenic epilepsy as were offspring of women who had not had a spontaneous abortion (adjusted RR = 9.8, 95% CI: 2.6 –37.8; cumulative incidence in offspring of women with a history of spontaneous abortion = 12.1% versus 2.15% in offspring of women without a history of spontaneous abortion), indicating that the association was not the result of the presence of families with several affected children. The risk of epilepsy was increased moderately in offspring of mothers with other risk factors, including family history of epilepsy, gestational seizures, maternal education, and ethnicity. However, there was essentially no change in the strength of the association between a history of spontaneous abortion and increased risk of epilepsy in offspring when we compared univariate and multivariate RR, indicating no effect of confounding with other risk factors for epilepsy, although we were not able to consider factors such as placental abruption, intrauterine growth retardation, or a history of prematurity in the offspring. We conclude that a history of spontaneous abortion is an independent risk factor for epilepsy in offspring of women with cryptogenic localization-related epilepsy.
We conducted stratified analyses to examine possible interactions between the levels of risk factors and the effects of spontaneous abortion on risk of epilepsy in offspring. Although the cumulative incidence and RR associated with a maternal history of spontaneous abortion were higher in offspring of women with high-risk characteristics, such as a family history of epilepsy, the occurrence of gestational seizures, earlier age at onset of epilepsy, and low education, the degree of interaction was modest and the effect of spontaneous abortion was seen in almost all strata.
To examine the impact of reporting or recall bias on the association between spontaneous abortion and risk of epilepsy in offspring, we determined whether there was a relative excess of reported spontaneous abortions by mothers with versus without an affected child, depending on how old the mothers were at the interview or on how long ago the first spontaneous abortion had occurred. Mothers who have a child with epilepsy might be more likely to recall or to report early-gestation spontaneous abortions than are mothers of healthy children. However, RR of spontaneous abortion among women with versus without an affected child did not differ between women with a recent and remote history of spontaneous abortion in either analysis, suggesting that the relationship between a maternal history of spontaneous and increased risk of epilepsy in offspring is not substantially influenced by reporting bias. A limitation of the study is that we used maternal history as the gold standard for reporting of epilepsy in offspring because we were able to review medical records for only 60% of the probands and none of the offspring. We think that accuracy of recall is more important for reporting of spontaneous abortion than for reporting of epilepsy in offspring; mothers are likely to report the occurrence of epilepsy in their children and not likely to differ according to whether the mother had a spontaneous abortion.
Spontaneous abortion is a relatively common occurrence, with rates ranging from 5% to 15% in population-based studies of clinically recognized pregnancies.5–10 Little is known regarding factors associated with spontaneous abortion. Maternal age, smoking, and a family history of recurrent miscarriage have been found to increase risk,24,25 but we are not aware of any studies that have examined the association between epilepsy and spontaneous abortion in the general population. In a previous study, we found that rates of spontaneous abortion in women with epilepsy were high compared with rates in the general population.11 The rates of spontaneous abortion observed in this study are likely to reflect the effects of known risk factors and factors specific to women with epilepsy.
The generalizability of our findings is limited by the nature of our case sample. Probands in our series (adults with epilepsy who contacted voluntary organizations for epilepsy) are not representative of the general population of persons with epilepsy in terms of seizure type, age at onset, and education. Our proband sample is likely to be better educated, to be more severely affected, and to have poorer seizure control than the total population with epilepsy. Women who had affected children and pregnancy loss and epilepsy may be more likely to join a voluntary organization, and we may have overestimated the prevalence of spontaneous abortion in women with epilepsy, but this would not change the estimate of relative risk of epilepsy in offspring of women with versus without a history of spontaneous abortion. Our findings indicate that there may be a subgroup of women with a high risk for epilepsy in their offspring who can be identified by their reproductive history.
The higher risk of epilepsy in offspring of women with a history of spontaneous abortion could result from intrauterine, neonatal, or early childhood environments or genetic susceptibility factors provided by the mother. The two intrauterine exposures most likely to be associated with an increased frequency of spontaneous abortion and increased risk of epilepsy in live-born offspring are AED and maternal seizures. In a previous study, we showed that intrauterine exposure to AED was not associated with a risk of epilepsy in offspring, whereas seizures during pregnancy were associated with increased risk.26 In this study, we also found that seizures during pregnancy were associated with an increased risk of epilepsy in offspring. However, there was an increased risk of epilepsy in offspring of women with a history of spontaneous abortion among those with and without gestational seizures (RR = 7.6 and 3.1) (see table 3), suggesting that gestational seizures contribute to the risk of epilepsy in offspring, but cannot entirely account for the association of a history of spontaneous abortion and epilepsy in offspring. An influence of neonatal and early childhood environments is suggested also by the increased risk of epilepsy in offspring of women with a low versus high level of education (RR = 1.8, 95% CI: 0.9 –3.6), which may be a surrogate for socioeconomic status and for exposure to environmental risk factors that are not yet identified.
One possibility is that the occurrence of a spontaneous abortion is a marker for the effect of an intra-uterine exposure to offspring in women with epilepsy that increases the risk for spontaneous abortion and epilepsy. Although the relative risk of epilepsy was greater in children born after than before the mother’s onset of epilepsy (RR = 4.4 versus 2.2), the small number of affected offspring makes it difficult to determine whether risk of epilepsy is increased in offspring born before the onset.
Another possibility is that a history of spontaneous abortion is a marker for a genetic susceptibility to epilepsy in the mother, leading to increased risk in live-born offspring. Several genetic models may account for the pattern of results we observe. Selective abortion of genetically susceptible fetuses is ruled out by our finding that risk of epilepsy is higher, rather than lower, among live-born offspring of affected women with than without a history of spontaneous abortion. If a maternal epilepsy susceptibility genotype increased risk for spontaneous abortion in addition to epilepsy, and the increased risk for spontaneous abortion were not selective with respect to the offspring’s genotype, we would expect 1) a greater risk of spontaneous abortion among women with a genetic susceptibility to epilepsy than among those without and 2) a greater risk of epilepsy in offspring of women with a history of spontaneous abortion and a family history of epilepsy than with a family history alone. Among women with a family history of epilepsy, 12 (41.4%) of 29 had a history of spontaneous abortion, whereas among women without a family history of epilepsy 103 (29%) of 356 had a history of spontaneous abortion. The cumulative incidence of epilepsy to age 25 was 21.9% among offspring of women with a family history of epilepsy and a history of spontaneous abortion, compared with a cumulative incidence of 12.1% among offspring of women with a history of spontaneous abortion, but without a family history of epilepsy, 7.6% among offspring of women with a family history of epilepsy alone, and 4.6% among offspring of women with neither a family history of epilepsy nor a history of spontaneous abortion (see table 3). These results are consistent with the hypothesis that a history of spontaneous abortion is a marker for genetic susceptibility for epilepsy in the mother, with increased risk to the offspring, suggesting that molecular studies may be warranted in women with epilepsy with a history of spontaneous abortion and affected offspring.
Acknowledgments
Supported by NIH grant R01 NS20656 and by funds from the New York State Office of Mental Retardation and Developmental Disabilities. Received October 2, 2000. Accepted in final form July 19, 2001.
References
- 1.Nakane Y, Okuma T, Takahashi R, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia. 1980;21:663–680. doi: 10.1111/j.1528-1157.1980.tb04320.x. [DOI] [PubMed] [Google Scholar]
- 2.Andermann E, Dansky L, Kinch RA. Complications of pregnancy, labour and delivery in epileptic women. In: Janz D, Dams M, Richens A, Bossi L, Helge H, Schmidt D, editors. Epilepsy, pregnancy and the child. New York: Raven Press; 1982. pp. 61–74. [Google Scholar]
- 3.Ogawa Y, Fukushi A, Nomura Y, et al. Complications of pregnancy, labor and delivery in women with epilepsy. In: Sato T, Shinagawa S, editors. Antiepileptic drugs and pregnancy. Amsterdam: Excerpta Medica; 1984. pp. 87–97. [Google Scholar]
- 4.Annegers JF, Baumgartner KB, Hauser WA, Kurland LT. Epilepsy, antiepileptic drugs, and the risk of spontaneous abortion. Epilepsia. 1988;29:451–458. doi: 10.1111/j.1528-1157.1988.tb03745.x. [DOI] [PubMed] [Google Scholar]
- 5.Yerushalamy J, Bierman JM, Kemp DH, Connor A, French FE. Longitudinal studies of pregnancy on the island of Kauai, Territory of Hawaii. Am J Obstet Gynecol. 1956;71:80–85. doi: 10.1016/0002-9378(56)90682-2. [DOI] [PubMed] [Google Scholar]
- 6.Warburton D, Fraser FC. Spontaneous abortion risks in man: data from reproductive histories collected in a medical genetics unit. Am J Hum Genet. 1965;16:1–24. [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro S, Levine HS, Abramowicz M. Factors associated with early and late fetal loss. Adv Planned Parent. 1971;6:45–63. [Google Scholar]
- 8.Naylor AF. Sequential aspects of spontaneous abortion: maternal age, parity, and pregnancy compensation artifact. Soc Biol. 1974;21:195–204. doi: 10.1080/19485565.1974.9988106. [DOI] [PubMed] [Google Scholar]
- 9.Leridon H. Facts and artifacts in the study of intra-uterine mortality: a reconsideration from pregnancy histories. Pop Studies. 1976;30:319 –336. doi: 10.1080/00324728.1976.10412738. [DOI] [PubMed] [Google Scholar]
- 10.Miller JF, Williamson E, Glue J, Gordon YB, Grudsinskas JG, Sykes A. Fetal loss of implantation. A prospective study. Lancet. 1980;2:554 –556. doi: 10.1016/s0140-6736(80)91991-1. [DOI] [PubMed] [Google Scholar]
- 11.Schupf N, Ottman R. Reproduction among individuals with idiopathic/cryptogenic epilepsy: risk factors for spontaneous abortion. Epilepsia. 1997;38:824 –829. doi: 10.1111/j.1528-1157.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 12.Ottman R, Susser M. Data collection strategies in genetic epidemiology: the Epilepsy Family Study of Columbia University. J Clin Epidemiol. 1992;45:721–727. doi: 10.1016/0895-4356(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 13.ILAE Commission Report. The epidemiology of the epilepsies: future directions. Epilepsia. 1997;38:614 –618. [PubMed] [Google Scholar]
- 14.Ottman R, Hauser WA, Susser M. Validity of family history data on seizure disorders. Epilepsia. 1993;34:469 –475. doi: 10.1111/j.1528-1157.1993.tb02587.x. [DOI] [PubMed] [Google Scholar]
- 15.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489 –501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 16.Ottman T, Lee JH, Hauser WA, et al. Reliability of seizure classification using a semi-structured interview. Neurology. 1993;43:2526 –2530. doi: 10.1212/wnl.43.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottman R, Hauser WA, Stallone L. Semi-structured interview for seizure classification: agreement with physician’s diagnosis. Epilepsia. 1990;31:110 –115. doi: 10.1111/j.1528-1157.1990.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;22:489 –501. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 19.Ottman R, Lee JH, Risch N, Hauser WA, Susser M. Clinical indicators of genetic susceptibility to epilepsy. Epilepsia. 1996;37:353–361. doi: 10.1111/j.1528-1157.1996.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ. Modern epidemiology. Boston: Little, Brown and Company; 1982. [Google Scholar]
- 21.Susser E, Susser MW. Familial aggregation studies: a note on their epidemiologic properties. Am J Epidemiol. 1989;79:557–563. doi: 10.1093/oxfordjournals.aje.a115119. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life tables. J Royal Statis Soc. 1972:187–220. series B. [Google Scholar]
- 23.SPSS for Windows, version 9.0. Chicago: SPSS; 1998. [Google Scholar]
- 24.Kline J, Stein Z, Susser M. Conception to birth. New York: Oxford University Press; 1989. [Google Scholar]
- 25.Parazzini F, Bocciolone L, Fedele L, Negri E, La Vecchia C, B Acaia. Risk factors for spontaneous abortion. Int J Epidemiol. 1991;20:157–161. doi: 10.1093/ije/20.1.157. [DOI] [PubMed] [Google Scholar]
- 26.Ottman R, Annegers JF, Hauser WA, Kurland LT. Higher risk of seizures in offspring of mothers than of fathers with epilepsy. Am J Hum Genet. 1988;43:257–264. [PMC free article] [PubMed] [Google Scholar]


