Abstract
A kinase anchoring proteins (AKAPs) compose a growing list of diverse but functionally related proteins defined by their ability to bind to the regulatory subunit of protein kinase A. AKAPs perform an integral role in the spatiotemporal modulation of a multitude of cellular signaling pathways. This review highlights the extensive role of AKAPs in cardiac excitation/contraction coupling and cardiac physiology. The literature shows that particular AKAPs are involved in cardiac Ca2+ influx, release, re-uptake, and myocyte repolarization. Studies have also suggested roles for AKAPs in cardiac remodeling. Transgenic studies show functional effects of AKAPs, not only in the cardiovascular system, but in other organ systems as well.
AKAPs: multiprotein signal integration complexes
PKA signaling plays a prominent role in the modulation of cardiac function. Extensive research indicates that AKAPs, which bind and sequester PKA to specific subcellular locations, also nucleate multicomponent protein signaling complexes. Thus, AKAPs are central mediators of crosstalk and integration of cAMP/PKA signaling with other signaling pathways. Most known AKAPs bind to the type II regulatory subunit of PKA (RII); however, several `dual-AKAPs' also bind type I regulatory subunit (RI). A survey of some of the AKAPs found in the heart (Table 1) reveals that multiple signaling proteins including kinases, protein phosphatases, and phosphodiesterases, can be found in complex with different AKAPs. The targeting of phosphodiesterases together with PKA is believed to enable finite control over local cAMP levels, and thus, the extent and duration of PKA activation (30, 31, 79, 87), see also (4, 88). A comprehensive list of AKAPs is found in a recent review (51). Based upon distinct complements of signaling molecules bound to each AKAP, the local spatial and temporal activation of PKA bound to each AKAP is likely to be unique. The ability of AKAPs to sequester discrete sets of signaling molecules to particular regions of the cell therefore allows for specificity and diversity of local cellular signaling dynamics (12).
Table 1.
Partial list of cardiac AKAPs and their heterogeneous binding partners
Examples of signal integration and crosstalk are seen with mAKAP and AKAPLbc. mAKAP is expressed in cardiac and brain tissue and is known to form a multienzyme complex that includes PKA, PDE4D3, Epac, Erk5 and PDK1 (57, 74, 77, 108). These components interact to form a local signal cascade that can positively or negatively modulate cAMP metabolism (30). Another example of signal integration is seen with AKAP-Lbc, which associates with Rho, PKC, and PKD, in addition to PKA. AKAP-Lbc demonstrates a Rho-specific guanine nucleotide exchange factor activity (GEF) at the C-terminus (27, 29, 60). PKA-dependent phosphorylation of Ser1565 of AKAP-Lbc facilitates binding of an accessory protein (14-3-3) which, in turn, results in inhibition of GEF activity (26). Moreover, AKAP-Lbc positions PKC to phosphorylate and activate PKD (17). Thus, AKAPs not only localize signaling peptides to particular locations in the cell, but also provide a mechanism for a diverse array of signal integration.
AKAPs bind PKA Regulatory subunit dimers
Canonical AKAPs bind the regulatory (R) subunits of the PKA holoenzyme via an amphipathic α-helix, typically 14 to 18 amino acids in length (20). A wide range of sequence variation is observed between the RII binding domains of individual proteins; therefore, AKAPs are typically defined as a functionally homologous family of proteins. A loose consensus motif (X[L,I,V]XX[L,I,V] [L,I,V]XX[L,I,V][L,I,V]XX[A,S][L,I,V]) has been proposed, (103) but the critical feature of the A-kinase anchoring domain is a concentration of hydrophobic amino acids on one face of the helix that forms a `hydrophobic ridge'. Introduction of a proline residue, with its rigid structure, introduces a `kink' that disrupts the amphipathic α-helix and eliminates binding, which highlights the importance of secondary structure in RII:AKAP interaction (19, 20). Binding to a general hydrophobic ridge rather than a sequence-specific motif permits high affinity binding between diverse AKAPs and the RII subunit dimer (82). Despite the proposed consensus motif, `unconventional' RII binding proteins such as pericentrin are not predicted to contain the hallmark helix; however, they are believed to anchor RII in a manner still dependent on hydrophobic residues in the putative RII binding site (28).
Crystallographic and NMR data have shown that R subunit dimers form an x-type, four helix bundle containing a `hydrophobic groove' that is the binding site for AKAPs (58). Thus the `hydrophobic ridge' in the PKA anchoring domain of AKAPs fits the `hydrophobic groove' formed by R subunit dimers. While a portion of the groove formed by RIα dimers contains a cavity that can accept bulky side chains, the corresponding sequence on RIIα dimers generate a relatively flatter hydrophobic surface. The difference in the hydrophobic grooves has been proposed as a mechanism that allows AKAP peptides to differentially interact with RI and RII dimers (58).
Highly localized dynamics of PKA are determined by the unique complement of signaling molecules associated with each AKAP. The affinity of individual AKAPs for RII differs, likely due in part to sequence variations within the PKA binding domain that correlate with differences in 3D structure of the R binding site (2, 111). For example, the 24 amino acid peptide called Ht31, which constitutes the RII binding domain of AKAPLbc, is used as an experimental tool to disrupt RII:AKAP interaction. Ht31 binds RIIα with much higher affinity (Kd=2-10nM), than RIα (Kd=1030-1277nM) (19, 46, 111). Differences in binding affinity between different R isoforms and other AKAPs have also been determined. Protein interaction can be measured via surface plasmon resonance analysis, and work by our lab and others has demonstrated that AKAP proteins can have varying affinities for specific R isoforms. S-AKAP84/D-AKAP-1, a “dual AKAP”, binds both RI and RII with high (nM) affinity; in contrast, AKAP79 preferentially binds RII isoforms α and β (Kd=1.5nM and 4.5nM, respectively), and binds RI with a Kd exceeding 1μM (46). AKAP95, mAKAP, and AKAP15/18 also preferentially bind RII isoforms, but variations such as a three-fold difference in affinity for AKAP95 and RIIα versus RIIβ suggest that isoform affinity is highly relevant in PKA anchoring (46, 111). These binding preferences between various AKAPs and R subunits may represent an important component of the mechanism by which isoforms of PKA are organized into highly localized, discrete, signaling microdomains.
Phosphorylation of the R subunit
To date, limited information is available on the functional significance of different affinities of different AKAPs for the PKA holoenzyme, as discussed above. PKA redistribution upon RII phosphorylation may be one mechanism by which dynamic changes in micro-PKA distribution occurs. For example, this could take place upon down-regulation of the β-adrenergic signaling pathway in cardiac disease.
As cAMP levels increase, cAMP binds to cooperative sites on each R subunit, inducing a conformational change that releases the catalytic (C) subunits. The C subunit phosphorylates RII at Ser96, a residue within the PKA `inhibitory domain,' which then decreases the affinity of RII for C (42, 85). In contrast, phosphorylated RII binds the A-kinase anchoring domain of AKAPs with higher affinity than unphosphorylated RII, as indicated by surface plasmon resonance studies (111) and more recently, studies where RIIS96D and RIIS96A, mimicking phosphorylated and unphosphorylated RII, respectively, were expressed in isolated myocytes (71). Expression of RIIS96D also resulted in increased phosphorylation of PKA substrates, and altered Ca2+ signaling (71). Increased PKA substrate phosphorylation suggests that a local conformational change that occurs upon Ser96 phosphorylation is communicated to the AKAP binding domain at the N-terminal of the molecule.
Phosphorylation of RII at alternative sites can result in other downstream effects. For example, in quiescent cells, Yotiao localizes RIIα to the centrosome; however, as cells enter mitosis, RIIα is phosphorylated at Thr54 by cyclin B-p34cdc2 kinase (CDK1). Whereas Ser96 is located in the inhibitory domain of RII, Thr54 is adjacent to the dimerization/docking domain of RII. Phosphorylation at this latter site results in decreased affinity of RII for Yotiao and redistribution of PKA to the cytoplasm and chromatin (16). Interestingly, CDK1-dependent phosphorylation of RIIα at Thr54 also increases the binding affinity of RIIα for AKAP95. This has the effect of recruiting RIIα for proper chromatin remodeling during mitosis (65). Thus phosphorylation of at least two sites on the RII subunit represents additional, distinct mechanisms for regulating localization of PKA in different microdomains within the cell, ultimately affecting local kinase function.
AKAPs and cardiac physiology
PKA enzymatic activity is extensively involved in normal cardiac myocyte function. It is widely known that perturbations of PKA activity, including decreased PKA phosphorylation of phospholamban, myosin binding protein C, and troponin I, accompany remodeling of the heart, and heart failure (10, 45, 96, 104, 105, 112). The ryanodine receptor (RyR) Ca2+ release channel located in the sarcoplasmic reticulum and the α and β subunits of L-type Ca2+ channels are also phosphorylated by PKA. The functional effects resulting from PKA phosphorylation of RyR and the relative roles of different RyR phosphorylation sites are controversial. Recent conclusions from several investigators indicate that β-adrenergic stimulation has only a small effect on RyR phosphorylation, leading primarily to increased kinetics of Ca2+ release and affecting Ca2+ cycling (6, 40, 70). Others report that PKA phosphorylation of RyR does not change significantly in heart failure (6, 52). In contrast, Marks and colleagues have identified a more significant role of PKA-dependent RyR phosphorylation. They report that in heart failure RyR hyperphosphorylation takes place, with a resultant increase in RyR channel leak (32, 73, 86). Further discussion of the role of mAKAP in the regulation of RyR channel phosphorylation is described at a later point in this review.
The functional importance of PKA targeting by AKAPs is supported by experiments in which the targeting of PKA is disrupted via expression of a competing peptide (Ht31) which binds RII dimers in cells or tissues. Under these conditions, a spectrum of changes as a result of disruption of PKA anchoring to AKAPs has been observed, including impaired forskolin-stimulated Cl- current activity (61), decreased PKA- potentiation of L-type current (37), decreased PKA phosphorylation of troponin I, phospholamban and RyR, and altered contraction, upon β-adrenergic-stimulation of cardiomyocytes (33) or hearts in vivo (75). Thus, a substantial body of evidence highlights the involvement of multiple AKAPs in the heart.
Cardiac Hypertrophy and AKAPs
Cardiac hypertrophy may be viewed as a graded adaptive response of the heart to systemic demands. Left unchecked, however, cardiac growth can lead to maladaptive remodeling of the heart that leads to poor performance and, eventually, failure. Recent studies have suggested that two AKAPs in particular (AKAP-Lbc, mAKAP) are involved in the hypertrophic response of the heart.
AKAP-Lbc
Chronic infusion of phenylephrine (PE) into mice increased cardiac weight index (ventricular weight/body weight) and levels of AKAP-Lbc mRNA from ventricular myocytes in vivo. Interestingly, downregulation of this AKAP, via RNA interference, inhibited the PE-stimulated activation of RhoA and cellular hypertrophy in rat neonatal cardiomyocytes (NCM). These investigators concluded that AKAP-Lbc is involved in the hypertrophic pathway activated by α1-adrenergic receptors in rat NCM (3). The downstream effector/s of activated RhoA in the hypertrophic response remain to be elucidated; however, putative pathways involved in participation of AKAP-Lbc in the hypertrophic process have been proposed (25). A recent report by Scott and colleagues (18) postulated that by means of binding of PKD1, AKAP-Lbc plays a significant role in the agonist-stimulated hypertrophic response. These investigators showed that gene silencing of AKAP-Lbc blunted the hypertrophic response to phenylephrine in NCM. Agonist-stimulated hypertrophy could be reinstated by introduction of AKAP-Lbc constructs that retained their ability to bind PKD. Furthermore, exogenous expression of AKAP-Lbc in NCM increased agonist-driven nuclear PKD activity and export of histone deacetylase 5 (HDAC5). The investigators postulated that AKAP-Lbc participates in the hypertrophic response by enhancing the efficiency of activation of the `fetal program' involving upstream activity of PKD/HDAC5/MEF2 (18).
mAKAP
Two separate studies have shown that adrenergic- and cytokine-induced hypertrophy in cultured NCM can be decreased by downregulation of mAKAP (30, 83). The study by Kapiloff and colleagues further showed that adrenergic stimulation facilitated activation and nuclear localization of NFATc1. The investigators proposed that mAKAP participates in hypertrophic gene expression via a mechanism involving activation of the pro-hypertrophic transcription factor NFATc (83).
Cardiac EC Coupling and AKAPs
As indicated above (33, 75), it is increasingly evident that AKAPs are intimately involved in modulation of cardiac EC coupling at many levels. Studies have revealed extensive participation of AKAPs in the generation of the cardiac action potential and Ca2+ transients. Multiple AKAPs associate with specific components of the cardiac EC coupling machinery and, along with other signaling proteins, facilitate modulation of the cardiac cycle.
AKAP15/18 and Ca2+ influx
AKAP15 (or AKAP18), named for slight differences in molecular weight following its discovery by two independent laboratories, has multiple isoforms with molecular weights ranging from 15-50 kDa (35, 43, 44, 76, 101). The αδ, and γ isoforms have all been reported to be expressed in the heart (68, 101). AKAP 15/18 colocalizes with, and co-immunoprecipitates L-type Ca2+ channels (14, 43, 44). Lipid modification is involved in the localization of AKAP15/18 to the plasma membrane (35). AKAP15/18 interacts with the C-terminal domain of the α1 subunit of skeletal L-type Ca2+ channel (48).
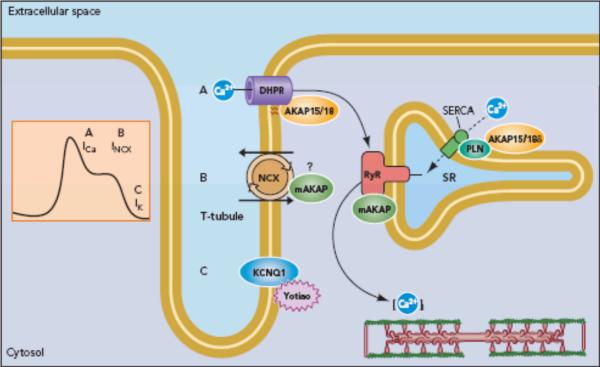
A number of studies have demonstrated that L-type Ca2+ currents are modulated by AKAP15/18-dependent mechanisms (Fig 1). PKA-mediated phosphorylation of the Ca2+ channel augments channel activity (24, 55, 110). PKA-dependent phosphorylation of both the α1C and β2a subunits has been reported, however the question of whether one or more of these sites is required for the increased Ca2+ flux through the channel in intact cardiac myocytes is still being debated, in part because of long standing difficulties encountered in reconstituting the AKAP-dependent signaling complexes in heterologous expression systems. For example, while Hosey and colleagues (37) identified Ser1928 of the α1C subunit as critical for PKA-mediated modulation of the channel, O'Rourke and colleagues reported that mutation of Ser1928 to an alanine did not significantly attenuate the beta-adrenergic response (36). However, when AKAP15/18 is expressed in HEK293 cells, it targets PKA to the channel and enhances L-type Ca2+ channel activity in response to activation of PKA. This effect is lost upon expression of the inactive AKAP15/18 mutant (35). Thus, both measurements of Ca2+ currents and colocalization/coimmunoprecipitation studies indicate a role for AKAP15/18 in the modulation of Ca2+ influx (Fig 1), and by extension, cytosolic Ca2+ concentration.
Figure 1. AKAPs modulate cardiac excitation/contraction coupling.
Different AKAPs are thought to be involved in the modulation of the cardiac action potential and Ca2+ transient. The processes of calcium entry via L-type calcium channels, calcium release from the sarcoplasmic reticulum via ryanodine receptors, calcium reuptake and cardiac repolarization employ AKAPs.
Interestingly, a report by Hosey and colleagues also suggested that AKAP79 can modulate activity of L-type Ca2+ channels (37). These investigators reported that when α1C subunit of L-type channels is expressed in HEK293 cells stably expressing wild type AKAP79, L-type Ca2+ channels are phosphorylated upon activation of PKA. Ser1928 of α1C was identified as the PKA phosphorylation site. PKA-dependent phosphorylation of Ser1928 did not increase in cells expressing an AKAP79 construct in which a proline residue disrupted the structure of the RII binding domain. The results suggested that AKAP79 can potentially substitute for AKAP15/18 in the facilitation of the phosphorylation of the α1C subunit of L-type Ca2+ channels. The question of whether AKAP79 plays a physiological role in the modification of this channel in the heart remains to be determined. This particular AKAP has also been reported to regulate L-type Ca2+ channel trafficking independently of PKA, though to date, this has only been observed in the brain (1).
mAKAP and Ca2+ release
mAKAP localizes to the junctional membrane of the sarcoplasmic reticulum and to the perinuclear region (56, 57, 73, 74, 108). In the heart, mAKAP forms a complex with type 2 ryanodine receptor (RyR2) and PKA (56, 108). Marks and colleagues reported that FKBP12.6, PP2A and PP1 form a complex with RyR2 and that PKA-dependent phosphorylation of RyR2 decreased the amount of FKBP12.6 that coimmunoprecipitated with RyR2 (73). Dissociation of FKBP12.6 was predicted to increase the open probability of the Ca2+ release channel (13, 53). Recently, we demonstrated that overexpression of a phosphomimic of RII, (with the phosphorylatable serine 96 substituted by an aspartate) increased the binding of RII for AKAPs (111) and resulted in increased PKA phosphorylation of RyR2 (Ser 2809) in NCMs (71). Taken together, these studies suggest that localization of PKA to RyR2, represents an important mechanism for modulating the activity of RyR2 and, ultimately, the cardiac Ca2+ transient (Fig 1). As indicated previously in this review, the functional significance of mAKAP-dependent, PKA phosphorylation of RyR2 continues to be actively investigated. mAKAP and Na+ Ca2+ exchange: mAKAP may participate in the modulation of Na+-Ca2+ exchange. The cardiac Na+-Ca2+ exchanger (NCX1), located on the plasma membrane and enriched in transverse tubules, plays a primary role in Ca2+ extrusion in the heart, as well as in other tissues (for review (7, 9)). The intracellular loop of NCX1 can be phosphorylated by PKA (50), but the functional outcome of PKA phosphorylation of NCX1 remains to be clarified (113). Whereas some investigators report effects of PKA-dependent phosphorylation of NCX1 (62, 89), others have not detected PKA-mediated modulation of NCX (39). Interestingly immunoprecipitation of NCX1 from rat ventricular cardiac lysates revealed that mAKAP, but not other AKAPs was present in the immunoprecipitated complex (94). In this study, Ruknudin and colleagues showed that the RI subunit of PKA was also found in complex with mAKAP. These findings differ from results of surface plasmon resonance studies which reported no appreciable binding of RI to mAKAP (111). The reason for this inconsistency is unknown, but differences may be due to the methods and conditions used for the two studies. Ruknudin and colleagues reported that the complex also contained protein phosphatase 1 and 2A (PP1 and PP2A), PKC and localized to the Z-line in rat cardiomyocytes. To date, no additional studies have described an mAKAP:NCX complex. As indicated earlier, the majority of studies demonstrate that mAKAP targets RII to the RyR at the junctional sarcoplasmic reticulum (SR) and nuclear membrane. Further investigation is needed to determine a potential role for mAKAP at the plasma membrane.
AKAP15/18δ and SR Ca2+ re-uptake
A recent study by Klussmann and colleagues described a role for AKAP15/18δ in modulation of Ca2+ re-uptake into the SR via SERCA in cardiomyocytes. Immunogold staining in neonatal heart tissue showed that SERCA2, phospholamban (PLN), and AKAP15/18δ colocalize, and that these three proteins were found to coimmunoprecipitate as a protein complex. Furthermore, disruption of the PLNAKAP15/18δ interaction, via expression of a short peptide derived from PLN, disrupted the striated distribution of AKAP15/18δ, decreased isoproterenol-induced phosphorylation of PLN (Ser 16), and reduced Ca2+ re-uptake. Knockdown of AKAP15/18δ abolished the effect of norepinephrine on Ca2+ re-uptake. The investigators concluded that AKAP15/18δ plays a significant role in PKA-mediated phosphorylation of PLN and Ca2+ re-uptake into cardiac SR (68) (Fig 1).
Yotiao and cardiac repolarization
Yotiao was initially identified in the brain, where it is involved in the regulation of NMDA receptors (66, 106). In cardiac tissue, Yotiao interacts with the α subunit of the IKs channel (KCNQ1) via two cooperative domains on Yotiao itself: a seventeen amino acid binding site at the amino terminus, and a leucine zipper motif in the carboxy-terminus, which is likely to interact with a complimentary leucine zipper domain on KCNQ1 (22, 72). The channel responsible for the IKs current is composed of two subunits, a regulatory subunit (KCNE1) and the α subunit (KCNQ1) (92). β-adrenergic receptor-mediated modulation of IKs is known to involve Yotiao (72). Yotiao anchors PKA near the channel, which in turn affects the function of IKs via phosphorylation of the α subunit at Ser-27 (Fig 1). In CHO cells heterologously expressing IKs, stimulation with cAMP resulted in phosphorylation of the α subunit and augmentation of the IKs current (63, 64). Interestingly, PKA phosphorylates Yotiao itself (Ser-43). Mutation of Ser-43 in Yotiao to an Ala decreased the response of IKs to cAMP; therefore, Yotiao may also modulate IKs via allosteric mechanisms (21).
Long-QT syndrome (LQTS) is a cardiac disorder characterized by a prolonged repolarization of the cardiac action potential. This temporal lengthening of the action potential can promote arrhythmia and increase risk of sudden cardiac death (93). Some forms of congenital LQTS have been linked to mutations in either subunit of the slowly activating IKs channel (reviewed in (84) and (47)). Some mutations have been shown to affect channel current and endosomal recycling of IKs, while others disrupt the ability of IKs to associate with the regulatory signaling complex nucleated by Yotiao (22, 95).
Whereas a number of point mutations in the α subunit of IKs have been associated with development of LQTS, at least two (G598D and S1570L) directly disrupt the ability of the Yotiao signaling complex to associate with the α subunit of IKs. Computational analysis has demonstrated that the G598D mutation in KCNQ1 disrupts the targeting of the Yotiao-PKA-PP1 complex to the channel subunit. A study conducted in Finland identified this mutation in more than fifty percent of LQTS patients (34, 72). The mutant phenotype was observed after β-adrenergic stimulation, which promoted a prolonged QT wave and T wave abnormalities (72). When the S1507L was introduced into KCNQ1, the amount of Yotiao that co-immunoprecipitated with KCNQ1 was significantly decreased (22). Furthermore, PKA-dependent phosphorylation of the α subunit in response to elevated cAMP was decreased in cells expressing the S1570L mutant subunit when compared to wild type (21, 22). Computational analysis indicated that heterozygous and homozygous S1570L mutant mice have prolonged duration of the cardiac action potential, suggesting a link between development of LQTS and disruption of the Yotiao scaffolding protein complex (22).
AKAP mutations and knockouts
Members of the diverse AKAP family participate in a wide array of signaling processes. Perturbations of the signaling complexes nucleated by different AKAPs can potentially disrupt cellular homeostasis. Considering the multiple roles of AKAPs and the vital nature of cAMP-dependent signaling, deficiencies in these proteins are likely to be associated with human disease; however, interestingly, to date, few AKAPs have been definitively linked to human disease. There is also a lack of published studies that describe ramifications of a loss of function of particular AKAP family members.
Single nucleotide polymorphism in D-AKAP2
D-AKAP2 (AKAP10) is a dual function AKAP associated with mitochondria, membrane and cytosolic cell fractions. Analysis of approximately 6500 single nucleotide polymorphism (SNPs) in a population of `healthy' European-Americans revealed a polymorphism at amino acid 646 of D-AKAP2, in which an Ile residue was replaced with a Val. This SNP is located within the PKA binding domain of the AKAP. Biochemical analysis of each variant demonstrated that the Ile-646 D-AKAP2 bound RIα with three-fold lower affinity than the Val-646 variant. Interestingly, binding of both variants to RII remained unaffected. The Val-646 variant was associated with a shorter cardiac P-R interval than that of Ile-646 (54). The investigators suggested that the cardiac phenotype resulted from altered localization of RIα from the sarcolemma. In a separate study, Conklin and colleagues (100) examined the Val-646 polymorphism in a cohort of 122 patients. Patients with the Val-646 SNP had an elevated heart rate, but low heart rate variability, which is considered a risk factor for sudden cardiac death (11, 38, 59, 100). Interestingly, the study by Conklin and colleagues, conducted on patients who already had coronary heart disease, found no correlation between the SNP occurrence and other factors, such as age, that were highlighted in the study by Braun and colleagues (54). Clearly, additional studies are needed to elucidate the role of this AKAP in cardiac function and disease.
Gene trapping, a technique used to insert a deleterious fragment into the mouse genome (for review (97)), was employed by Conklin and colleagues to disrupt a C-terminal fifty one amino acid section of D-AKAP2 which contains the A-kinase anchoring domain. The resulting phenotype displayed severe functional abnormalities in the cardiovascular and nervous systems, including changes in heart rate, baroreceptor function, and abnormal conduction at the sinoatrial and atrioventricular nodes. These mice exhibited arrhythmias characterized by extended P-P and P-R intervals. These factors suggest a potential risk of sudden cardiac death in organisms lacking a complete, functional D-AKAP2 (100).
AKAP Knockout studies
To better define the role of individual AKAPs in the whole organism, efforts have been made to generate knockout mice. Table 2 summarizes the phenotypes observed in a selection of AKAP knockout studies. Selective knockout of particular AKAPs have functional outcomes, not only in the cardiovascular system, but in multiple organ systems. Transgenic studies have also shown that examination of the effects of AKAP knockout in mice can be a complex undertaking. For example, loss of AKAP95, a nuclear protein that recruits RII to chromatin during mitosis, resulted in no observable phenotype; however, crossing AKAP95-null mice with mice lacking the chaperone protein, fidgetin, resulted in poor survival in neo- and post-natal periods, cleft palate, and respiratory distress (23, 109).
Table 2.
AKAP knockouts/mutations and associated phenotypes
| Phenotype | References | |
|---|---|---|
| AKAP10 (D-AKAP2) | ↑ cardiac cholinergic response cardiac arrhythmia human; 646Vassoc ↑ basal heart rate | (100) |
| D-AKAP1 | oocyte meiosis defects, ↓ female fertility | (81) |
| AKAP4 | sperm morphology, motility and viability defects | (78) |
| AKAP5(AKAP150) | LTP defects, motor coordination spatial memory | (67, 102) |
In a study designed to examine the role of mAKAPα in the brain, knockout of mAKAPα, which is 244 amino acids longer than the β isoform, resulted in significant postnatal lethality, low body weight, and craniofacial defects. Interestingly, loss of mAKAPα, the preferred isoform expressed in brain, induced expression of the normally heart-specific β isoform in the brains of mAKAPα knockout mice (77). Thus, the effects of total knockout of mAKAP or of loss of its β isoform, a significant A-kinase anchoring protein in the heart, remain unresolved.
Reports from other investigators show more straightforward effects of other AKAP knockouts. Homozygous strains of mice deficient in D-AKAP1 exhibited disrupted oocyte meiosis, decreased female fertility and litter size (81). Loss of AKAP4 (AKAP82), a sperm-specific AKAP, resulted in altered sperm morphology, motility and viability (78). While the phenotypic knockout of AKAP5 (AKAP75/79) was not analyzed, knock-in of a mutant protein on this mouse background that lacked the PKA-anchoring domain affected neural function in the hippocampus, suggesting a role for this AKAP in long-term memory (67). In contrast, loss of the P1 isoform of AKAP250 (gravin) from the nervous system resulted in no observable phenotype (15).
The AKAP family has rapidly expanded in number and diversity over the last two decades, yet limited progress has been made in defining the role of these proteins using mouse knockout models. Even when null mice are successfully generated, differences between mice and humans can render interpretation of these phenotypes difficult. SNP analysis of normal human populations versus those at risk for conditions such as cardiac disease is a promising tool through which the functional significance of AKAP-mediated signaling may be examined. To date, D-AKAP2 is the only AKAP, of which we are aware, with a phenotype-associated polymorphism within the PKA binding domain. It is possible that the vital nature of PKA anchoring to growth and development precludes introduction of deleterious SNPs in populations that have been analyzed to date. For example, loss of AKAP function could compromise survival of carriers of critical mutations. Given the number of AKAPs that sequester PKA to similar areas within the cell, it is reasonable to assume that redundancy is built into the PKA-anchoring system. The isoform switch observed following knockout of mAKAPα is an example of an adaptation that arises to accommodate disrupted PKA targeting. Future studies focusing on the role of AKAP SNPs and isoforms in the context of disease should elucidate a link between disrupted AKAP function and human pathology.
Future Directions
AKAPs in the heart
Studies to date show that AKAPs play an integral role in cardiac function. Two cardiac AKAPs are currently implicated in agonist-induced cardiac myocyte hypertrophy. It would be very interesting to explore whether the same AKAPs are involved in other paradigms of hypertrophy (e.g. aortic banding, etc). Certain AKAPs are also of interest for development of therapeutic strategies. The delta isoform of AKAP15/18 has been proposed as a therapeutic target for modulating Ca2+ reuptake, and thus cardiac relaxation, in patients with heart failure (68). Such strategies may be combined with existing therapies to enhance cardiac function and prevent progression into heart failure (49, 68, 69).
AKAPs in general
The earlier studies utilizing oligopeptide constructs (Ht31, AKAP-is, etc) to effect broad and global disruption of the distribution of PKA have identified a multitude of cellular processes involving PKA activity. Since this disruption is global for all AKAPs and thus nonselective, the studies have not manipulated specific PKA:AKAP interactions and interpretation of results have always included this caveat. The studies that followed, employing RNA interference-mediated downregulation of specific AKAPs in cells, as well as generation of AKAP knockout mice, have explored and revealed functional roles of particular AKAPs at the cellular and organismal level. AKAPs can have diverse roles, well beyond anchoring of PKA. Moreover, AKAPs may behave in a modular fashion, wherein peptide domains execute particular functions, or as signal integration complexes. Wholesale knockdown or downregulation of AKAPs may not differentiate between modular and integrative functions of AKAPs. Which direction should future inquiries into AKAP biology take? We would suggest that whereas studies utilizing RNAi and gene knockout should continue to provide valuable insights, the future of AKAP research should be one of “increased specificity”. Effective future approaches could include genetic manipulations of functional domains on individual AKAPs. Alternatively, future studies should include design and development of peptides or small molecules that bind with high affinity and specificity to certain domains on individual AKAPs. These tactics could then be employed to selectively examine various functional domains on the AKAP. Examples of the abovementioned strategies have been presented for AKAP-Lbc, AKAP15/18δ and AKAP15/18α (18, 48, 68). In addition, studies can be designed to incorporate cell- and organ system-specific modifications of AKAP protein expression. These combined strategies will enable researchers to both tease out and piece together the functional roles of this fascinating and diverse group of proteins.
Acknowledgments
We thank Dr. Linda Lund for helpful suggestions on the manuscript. Dedicated to Ken Bond and Ate Tayen; thank you for a lifetime of memories, laughter, and caring.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AG16613 and R01 HL79134 (to M. B.), F31 AG032162 (to M. O.) and an AHA Postdoctoral Fellowship (to J. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Altier C, Dubel SJ, Barrere C, Jarvis SE, Stotz SC, Spaetgens RL, Scott JD, Cornet V, De Waard M, Zamponi GW, Nargeot J, Bourinet E. Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. Journal of Biological Chemistry. 2002;277:33598–33603. doi: 10.1074/jbc.M202476200. [DOI] [PubMed] [Google Scholar]
- 2.Alto NM, Soderling SH, Hoshi N, Langeberg LK, Fayos R, Jennings PA, Scott JD. Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc Natl Acad Sci U S A. 2003;100:4445–4450. doi: 10.1073/pnas.0330734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10140–10145. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellin RM, Sernett SW, Becker B, Ip W, Huiatt TW, Robson RM. Molecular characteristics and interactions of the intermediate filament protein synemin. Interactions with alpha-actinin may anchor synemin-containing heterofilaments. Journal of Biological Chemistry. 1999;274:29493–29499. doi: 10.1074/jbc.274.41.29493. [DOI] [PubMed] [Google Scholar]
- 6.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circulation Research. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. see comment. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 8.Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM. Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochemical & Biophysical Research Communications. 2006;346:768–777. doi: 10.1016/j.bbrc.2006.05.192. [DOI] [PubMed] [Google Scholar]
- 9.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiological Reviews. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 10.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 11.Bonaduce D, Petretta M, Marciano F, Vicario ML, Apicella C, Rao MA, Nicolai E, Volpe M. Independent and incremental prognostic value of heart rate variability in patients with chronic heart failure. American Heart Journal. 1999;138:273–284. doi: 10.1016/s0002-8703(99)70112-2. see comment. [DOI] [PubMed] [Google Scholar]
- 12.Bornfeldt KE. A single second messenger: several possible cellular responses depending on distinct subcellular pools. Circulation Research. 2006;99:790–792. doi: 10.1161/01.RES.0000247760.34779.f5. comment. [DOI] [PubMed] [Google Scholar]
- 13.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 14.Burton KA, Johnson BD, Hausken ZE, Westenbroek RE, Idzerda RL, Scheuer T, Scott JD, Catterall WA, McKnight GS. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca2+ channel activity by cAMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11067–11072. doi: 10.1073/pnas.94.20.11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camus A, Mesbah K, Rallu M, Babinet C, Barra J. Gene trap insertion reveals two open reading frames in the mouse SSeCKS gene: the form predominantly detected in the nervous system is suppressed by the insertion while the other, specific of the testis, remains expressed. Mechanisms of Development. 2001;105:79–91. doi: 10.1016/s0925-4773(01)00384-7. [DOI] [PubMed] [Google Scholar]
- 16.Carlson CR, Witczak O, Vossebein L, Labbe JC, Skalhegg BS, Keryer G, Herberg FW, Collas P, Tasken K. CDK1-mediated phosphorylation of the RIIalpha regulatory subunit of PKA works as a molecular switch that promotes dissociation of RIIalpha from centrosomes at mitosis. Journal of Cell Science. 2001;114:3243–3254. doi: 10.1242/jcs.114.18.3243. [DOI] [PubMed] [Google Scholar]
- 17.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Molecular Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Molecular Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. Journal of Biological Chemistry. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 20.Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, Scott JD. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. Journal of Biological Chemistry. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 21.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem. 2005;280:31347–31352. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox GA, Mahaffey CL, Nystuen A, Letts VA, Frankel WN. The mouse fidgetin gene defines a new role for AAA family proteins in mammalian development. Nature Genetics. 2000;26:198–202. doi: 10.1038/79923. [DOI] [PubMed] [Google Scholar]
- 24.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3',5'-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 25.Diviani D. Modulation of cardiac function by A-kinase anchoring proteins. Current Opinion in Pharmacology. 2008;8:166–173. doi: 10.1016/j.coph.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO Journal. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diviani D, Baisamy L, Appert-Collin A. AKAP-Lbc: a molecular scaffold for the integration of cyclic AMP and Rho transduction pathways. European Journal of Cell Biology. 2006;85:603–610. doi: 10.1016/j.ejcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Diviani D, Langeberg LK, Doxsey SJ, Scott JD. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr Biol. 2000;10:417–420. doi: 10.1016/s0960-9822(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 29.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. Journal of Biological Chemistry. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 30.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO Journal. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi M, Yano M, Kobayashi S, Kohno M, Tokuhisa T, Okuda S, Suetsugu M, Hisamatsu Y, Ohkusa T, Kohno M, Matsuzaki M. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation. 2002;105:1374–1379. doi: 10.1161/hc1102.105270. [DOI] [PubMed] [Google Scholar]
- 33.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, Damron DS, Bond M. AKAP-mediated targeting of protein kinase a regulates contractility in cardiac myocytes. Circ Res. 2001;88:291–297. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 34.Fodstad H, Swan H, Laitinen P, Piippo K, Paavonen K, Viitasalo M, Toivonen L, Kontula K. Four potassium channel mutations account for 73% of the genetic spectrum underlying long-QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann Med. 2004;36(Suppl 1):53–63. doi: 10.1080/17431380410032689. [DOI] [PubMed] [Google Scholar]
- 35.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO Journal. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesan AN, Maack C, Johns DC, Sidor A, O'Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circulation Research. 2006;98:e11–18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 38.Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study) American Journal of Cardiology. 2005;95:1474–1477. doi: 10.1016/j.amjcard.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsburg KS, Bers DM. Isoproterenol does not enhance Ca-dependent Na/Ca exchange current in intact rabbit ventricular myocytes. Journal of Molecular & Cellular Cardiology. 2005;39:972–981. doi: 10.1016/j.yjmcc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. Journal of Physiology. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granger BL, Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980;22:727–738. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- 42.Granot J, Mildvan AS, Hiyama K, Kondo H, Kaiser ET. Magnetic resonance studies of the effect of the regulatory subunit on metal and substrate binding to the catalytic subunit of bovine heart protein kinase. J Biol Chem. 1980;255:4569–4573. [PubMed] [Google Scholar]
- 43.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, 3rd, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 44.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. Journal of Biological Chemistry. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 45.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 46.Herberg FW, Maleszka A, Eide T, Vossebein L, Tasken K. Analysis of A-kinase anchoring protein (AKAP) interaction with protein kinase A (PKA) regulatory subunits: PKA isoform specificity in AKAP binding. J Mol Biol. 2000;298:329–339. doi: 10.1006/jmbi.2000.3662. [DOI] [PubMed] [Google Scholar]
- 47.Herbert E, Trusz-Gluza M, Moric E, Smilowska-Dzielicka E, Mazurek U, Wilczok T. KCNQ1 gene mutations and the respective genotype-phenotype correlations in the long QT syndrome. Med Sci Monit. 2002;8:RA240–248. [PubMed] [Google Scholar]
- 48.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. Journal of Biological Chemistry. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 49.Hundsrucker C, Klussmann E. Direct AKAP-mediated protein-protein interactions as potential drug targets. Handbook of Experimental Pharmacology. 2008:483–503. doi: 10.1007/978-3-540-72843-6_20. [DOI] [PubMed] [Google Scholar]
- 50.Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. Journal of Biological Chemistry. 1996;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- 51.Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochemical Society Transactions. 2007;35:931–937. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- 52.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circulation Research. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. see comment. [DOI] [PubMed] [Google Scholar]
- 53.Kaftan E, Marks AR, Ehrlich BE. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circulation Research. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- 54.Kammerer S, Burns-Hamuro LL, Ma Y, Hamon SC, Canaves JM, Shi MM, Nelson MR, Sing CF, Cantor CR, Taylor SS, Braun A. Amino acid variant in the kinase binding domain of dual-specific A kinase-anchoring protein 2: a disease susceptibility polymorphism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4066–4071. doi: 10.1073/pnas.2628028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circulation Research. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 56.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. Journal of Cell Science. 2001;114:3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 57.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. Journal of Cell Science. 1999;112:2725–2736. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 58.Kinderman FS, Kim C, von Daake S, Ma Y, Pham BQ, Spraggon G, Xuong NH, Jennings PA, Taylor SS. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Molecular Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kleiger RE, Miller JP, Bigger JT, Jr., Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. American Journal of Cardiology. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 60.Klussmann E, Edemir B, Pepperle B, Tamma G, Henn V, Klauschenz E, Hundsrucker C, Maric K, Rosenthal W. Ht31: the first protein kinase A anchoring protein to integrate protein kinase A and Rho signaling. FEBS Letters. 2001;507:264–268. doi: 10.1016/s0014-5793(01)02995-7. [DOI] [PubMed] [Google Scholar]
- 61.Kockskamper J, Sendhoff K, Erlenkamp S, Bordusa F, Cerovsky V, Glitsch HG. Differences in the protein-kinase-A-dependent regulation of CFTR Cl-channels and Na+-K+ pumps in guinea-pig ventricular myocytes. Pflugers Archiv -European Journal of Physiology. 2001;441:807–815. doi: 10.1007/s004240000485. [DOI] [PubMed] [Google Scholar]
- 62.Kravtsov GM, Kam KW, Liu J, Wu S, Wong TM. Altered Ca(2+) handling by ryanodine receptor and Na(+)-Ca(2+) exchange in the heart from ovariectomized rats: role of protein kinase A. American Journal of Physiology - Cell Physiology. 2007;292:C1625–1635. doi: 10.1152/ajpcell.00368.2006. [DOI] [PubMed] [Google Scholar]
- 63.Kurokawa J, Chen L, Kass RS. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc Natl Acad Sci U S A. 2003;100:2122–2127. doi: 10.1073/pnas.0434935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc Natl Acad Sci U S A. 2004;101:16374–16378. doi: 10.1073/pnas.0405583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landsverk HB, Carlson CR, Steen RL, Vossebein L, Herberg FW, Tasken K, Collas P. Regulation of anchoring of the RIIalpha regulatory subunit of PKA to AKAP95 by threonine phosphorylation of RIIalpha: implications for chromosome dynamics at mitosis. Journal of Cell Science. 2001;114:3255–3264. doi: 10.1242/jcs.114.18.3255. [DOI] [PubMed] [Google Scholar]
- 66.Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. Journal of Neuroscience. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO Journal. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Tasken K, Klussmann E. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Reports. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lygren B, Tasken K. The potential use of AKAP18delta as a drug target in heart failure patients. Expert Opinion on Biological Therapy. 2008;8:1099–1108. doi: 10.1517/14712598.8.8.1099. [DOI] [PubMed] [Google Scholar]
- 70.MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circulation Research. 2008;102:e65–72. doi: 10.1161/CIRCRESAHA.108.174722. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manni S, Mauban JH, Ward CW, Bond M. Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. Journal of Biological Chemistry. 2008;283:24145–24154. doi: 10.1074/jbc.M802278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 73.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 74.McCartney S, Little BM, Langeberg LK, Scott JD. Cloning and characterization of A-kinase anchor protein 100 (AKAP100). A protein that targets A-kinase to the sarcoplasmic reticulum. Journal of Biological Chemistry. 1995;270:9327–9333. doi: 10.1074/jbc.270.16.9327. [DOI] [PubMed] [Google Scholar]
- 75.McConnell B, Popovic Z, Mal N, Lee K, Bautista J, Forudi F, Schwartzman R, Jin JP, Penn M. M. B. Disruption of protein kinase-a interaction with a-kinase anchoring proteins in the heart in vivo: Effects on cardiac contractility, PKA phosphorylation and troponin-I proteolysis. J Biol Chem. 2008 doi: 10.1074/jbc.M806321200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McSorley T, Stefan E, Henn V, Wiesner B, Baillie GS, Houslay MD, Rosenthal W, Klussmann E. Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells. European Journal of Cell Biology. 2006;85:673–678. doi: 10.1016/j.ejcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Michel JJ, Townley IK, Dodge-Kafka KL, Zhang F, Kapiloff MS, Scott JD. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Molecular Cell. 2005;20:661–672. doi: 10.1016/j.molcel.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 78.Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Developmental Biology. 2002;248:331–342. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- 79.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circulation Research. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 80.Nauert JB, Klauck TM, Langeberg LK, Scott JD. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Current Biology. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 81.Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS. Dynamic anchoring of PKA is essential during oocyte maturation. Current Biology. 2006;16:321–327. doi: 10.1016/j.cub.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newlon MG, Roy M, Morikis D, Carr DW, Westphal R, Scott JD, Jennings PA. A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. Embo J. 2001;20:1651–1662. doi: 10.1093/emboj/20.7.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. Journal of Cell Science. 2005;118:5637–5646. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 84.Peroz D, Rodriguez N, Choveau F, Baro I, Merot J, Loussouarn G. Kv7.1 (KCNQ1) properties and channelopathies. J Physiol. 2008;586:1785–1789. doi: 10.1113/jphysiol.2007.148254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rangel-Aldao R, Kupiec JW, Rosen OM. Resolution of the phosphorylated and dephosphorylated cAMP-binding proteins of bovine cardiac muscle by affinity labeling and two-dimensional electrophoresis. J Biol Chem. 1979;254:2499–2508. [PubMed] [Google Scholar]
- 86.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. Journal of Biological Chemistry. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 87.Rich TC, Tse TE, Rohan JG, Schaack J, Karpen JW. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. Journal of General Physiology. 2001;118:63–78. doi: 10.1085/jgp.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruehr ML, Russell MA, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol. 2004;37:653–665. doi: 10.1016/j.yjmcc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Ruknudin A, He S, Lederer WJ, Schulze DH. Functional differences between cardiac and renal isoforms of the rat Na+-Ca2+ exchanger NCX1 expressed in Xenopus oocytes. Journal of Physiology. 2000;529(Pt 3):599–610. doi: 10.1111/j.1469-7793.2000.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russell MA, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M. The intermediate filament protein, synemin, is an AKAP in the heart. Archives of Biochemistry & Biophysics. 2006;456:204–215. doi: 10.1016/j.abb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 91.Sacchetto R, Damiani E, Margreth A. Clues to calcineurin function in mammalian fast-twitch muscle. Journal of Muscle Research & Cell Motility. 2001;22:545–559. doi: 10.1023/a:1015010914328. [DOI] [PubMed] [Google Scholar]
- 92.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 93.Saucerman JJ, Healy SN, Belik ME, Puglisi JL, McCulloch AD. Proarrhythmic consequences of a KCNQ1 AKAP-binding domain mutation: computational models of whole cells and heterogeneous tissue. Circ Res. 2004;95:1216–1224. doi: 10.1161/01.RES.0000150055.06226.4e. [DOI] [PubMed] [Google Scholar]
- 94.Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM. Sodium/calcium exchanger (NCX1) macromolecular complex. Journal of Biological Chemistry. 2003;278:28849–28855. doi: 10.1074/jbc.M300754200. [DOI] [PubMed] [Google Scholar]
- 95.Seebohm G, Strutz-Seebohm N, Ureche ON, Henrion U, Baltaev R, Mack AF, Korniychuk G, Steinke K, Tapken D, Pfeufer A, Kääb S, Bucci C, Attali B, Merot J, Tavare JM, Hoppe UC, Sanguinetti MC, F. L Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels. Circ Res. 2008;103:1451–1457. doi: 10.1161/CIRCRESAHA.108.177360. [DOI] [PubMed] [Google Scholar]
- 96.Sipido KR, Eisner D. Something old, something new: changing views on the cellular mechanisms of heart failure. Cardiovasc Res. 2005;68:167–174. doi: 10.1016/j.cardiores.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 97.Stanford WL, Cohn JB, Cordes SP. Gene-trap mutagenesis: past, present and beyond. Nature Reviews Genetics. 2001;2:756–768. doi: 10.1038/35093548. [DOI] [PubMed] [Google Scholar]
- 98.Sun N, Critchley DR, Paulin D, Li Z, Robson RM. Human alpha-synemin interacts directly with vinculin and metavinculin. Biochemical Journal. 2008;409:657–667. doi: 10.1042/BJ20071188. see comment. [DOI] [PubMed] [Google Scholar]
- 99.Tao J, Wang HY, Malbon CC. Src docks to A-kinase anchoring protein gravin, regulating beta2-adrenergic receptor resensitization and recycling. Journal of Biological Chemistry. 2007;282:6597–6608. doi: 10.1074/jbc.M608927200. [DOI] [PubMed] [Google Scholar]
- 100.Tingley WG, Pawlikowska L, Zaroff JG, Kim T, Nguyen T, Young SG, Vranizan K, Kwok PY, Whooley MA, Conklin BR. Gene-trapped mouse embryonic stem cell-derived cardiac myocytes and human genetics implicate AKAP10 in heart rhythm regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8461–8466. doi: 10.1073/pnas.0610393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trotter KW, Fraser ID, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. Journal of Cell Biology. 1999;147:1481–1492. doi: 10.1083/jcb.147.7.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vijayaraghavan S, Liberty GA, Mohan J, Winfrey VP, Olson GE, Carr DW. Isolation and molecular characterization of AKAP110, a novel, sperm-specific protein kinase A-anchoring protein. Mol Endocrinol. 1999;13:705–717. doi: 10.1210/mend.13.5.0278. [DOI] [PubMed] [Google Scholar]
- 104.Waggoner JR, Kranias EG. Role of phospholamban in the pathogenesis of heart failure. Heart Fail Clin. 2005;1:207–218. doi: 10.1016/j.hfc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 105.Wehrens XH, Marks AR. Molecular determinants of altered contractility in heart failure. Ann Med. 2004;36(Suppl 1):70–80. doi: 10.1080/17431380410032481. [DOI] [PubMed] [Google Scholar]
- 106.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatasekinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 107.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO Journal. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang J, Drazba JA, Ferguson DG, Bond M. A-kinase anchoring protein 100 (AKAP100) is localized in multiple subcellular compartments in the adult rat heart. Journal of Cell Biology. 1998;142:511–522. doi: 10.1083/jcb.142.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y, Mahaffey CL, Berube N, Frankel WN. Interaction between fidgetin and protein kinase A-anchoring protein AKAP95 is critical for palatogenesis in the mouse. Journal of Biological Chemistry. 2006;281:22352–22359. doi: 10.1074/jbc.M603626200. [DOI] [PubMed] [Google Scholar]
- 110.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zakhary DR, Fink MA, Ruehr ML, Bond M. Selectivity and regulation of A-kinase anchoring proteins in the heart. The role of autophosphorylation of the type II regulatory subunit of cAMP-dependent protein kinase. J Biol Chem. 2000;275:41389–41395. doi: 10.1074/jbc.M004212200. [DOI] [PubMed] [Google Scholar]
- 112.Zakhary DR, Moravec CS, Bond M. Regulation of PKA binding to AKAPs in the heart: alterations in human heart failure. Circulation. 2000;101:1459–1464. doi: 10.1161/01.cir.101.12.1459. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y, Hancox J. Regulation of cardiac Na+-Ca2+ exchanger activity by protein kinase phosphorylation-Still a paradox? Cell Calcium. 2008 doi: 10.1016/j.ceca.2008.05.005. doi:10.1016/j.ceca.2008.1005.1005. [DOI] [PubMed] [Google Scholar]