Abstract
Oval cells which become apparent in the liver after chronic injury, serve as bi-potent progenitors for differentiated hepatocytes and cholangiocytes. We found that in the liver of adult transgenic mice in which expression of green fluorescent protein (GFP) is driven by regulatory elements of the nestin gene, the GFP signal marks a subpopulation of small epithelial cells which meet the criteria for oval cells, including morphology, localization, antigenic profile, and reactivity in response to injury. In the regenerating and developing liver we also found nestin-GFP-positive cells which express hepatocyte markers; such cells may correspond to transiently appearing differentiating progeny of oval cells. During development, GFP-expressing cells in the liver emerge relatively late, after the appearance of differentiated hepatocytes and cholangiocytes. Our results suggest that nestin-GFP cells in the liver correspond to a specialized cell type whose primary function may be to serve as a reserve for adult liver epithelial cell types.
Keywords: liver, stem cells, oval cells, nestin, neuro-specific enhancer, GFP, A6, EpCAM, Bgp1, alpha-fetoprotein
Introduction
The liver contains two major epithelial cells types, both derived from embryonic endoderm: hepatocytes, which form the liver parenchyma and perform all of the major liver functions (production of plasma proteins, detoxification, bile production, glycogen storage), and cholangiocytes, which form the bile ducts, specialized epithelial structures through which bile is transported from the liver to the duodenum. In addition, the liver contains several types of cells of mesenchymal origin, such as Kupfer cells, Ito cells, and endothelial cells. It is now generally believed that in adult liver both epithelial cell types, hepatocytes and cholangiocytes, can arise from common precursors, adult liver stem cells. However, the origin, heterogeneity, and properties of such putative stem cells are still unclear.
In mammals, regeneration of liver can be accomplished in two modes. In response to partial hepatectomy, differentiated hepatocytes rapidly divide and replace the lost cells without stem cell involvement. However, when the proliferative response of hepatocytes is suppressed or after exposure to carcinogens, undifferentiated progenitor cells can be activated and can contribute to the restoration of damaged hepatic tissue (for review see Sell, 2001; 2003). These progenitor cells have been originally observed in rat liver after treatment with carcinogens and were termed oval cells because of their shape and the absence of any specific morphological features (Farber, 1957). Oval cells are usually located in the periportal area of the hepatic lobule and are associated with terminal bile ductules (canals of Hering), corresponding to junctions between bile ducts and bile canaliculi. Oval cells act as bi-potent progenitors and can produce both hepatocytes and cholangiocytes. Oval cell proliferation and bi-directional differentiation in response to certain types of injury seems to be a universal phenomenon in mammals [note however, there are important differences between mouse and rat oval cells in response to carcinogens (see Engelhardt, 1997)]. Despite a long history of investigation, the origin of oval cells is still unclear. Although studies imply that they arise from precursor cells residing in the liver (Wang et al., 2003), it has also been proposed that under certain conditions oval cells or their precursors originate in the bone marrow and are relocated to and retained in liver tissue (see Sell, 2001; 2003; Oh et al., 2002).
One of the drawbacks in studying the role of oval cells as putative liver stem cells under normal conditions and upon regeneration is the lack of molecular markers which would allow them to be easily identified, isolated, and studied. We here report that a population of cells behaving as oval cells can be identified in the developing and adult liver and in the regenerating following exposure to carbon tetrachloride (CCl4). These cells can be identified by their expression of green fluorescent protein (GFP) driven by the neural stem cell-specific regulatory elements of the nestin gene. Our results present an approach for the study of liver stem cells under normal and pathological conditions. Furthermore, our results suggest a more general role for nestin as a marker of cells with stem-like properties in diverse tissues.
Results
Nestin-GFP expression in the adult quiescent liver
The intermediate filament nestin is expressed in neuroepithelial cells of the embryo and in the neurogenic areas of adult mammalian brain, among other sites (Lindahl et al., 1990). Regulatory elements of the nestin gene, including enhancers located in the introns of the gene, can drive the expression of reporter genes in a range of cell types (Zimmerman et al., 1994; Josephson et al., 1998; Yaworsky, Kappen, 1999; Yamaguchi et al., 2000; Kawaguchi et al., 2001; Sawamoto et al., 2001; Mignone et al., 2004). We have recently generated transgenic animal lines in which expression of green fluorescent protein (GFP) is driven by the promoter and the second intron of the nestin gene (Mignone et al., 2004). In these animals, GFP is expressed in the stem and progenitor cells of the adult nervous system (Mignone et al., 2004) as well as in putative stem cells in the hair follicle (Li et al., 2003).
We have examined the quiescent liver of nestin-GFP transgenic animals and found a small number of cells with bright GFP fluorescence. These cells were located exclusively in the periportal areas (Fig. 1A) and were found either within typical bile ducts or in structures resembling the canals of Hering (Fig. 1B). Not every bile duct and not every liver lobule in the quiescent liver contained GFP-expressing cells. Such cells were found both in terminal interlobular bile ducts and in the bile ducts of higher caliber (Fig. 1C). GFP-expressing cells in the bile ducts had a cuboidal shape typical of cholangiocytes, whereas GFP-expressing cells in the canals of Hering had a more elongated shape typical of the cells in those structures (Fig. 1B). The morphology and the distribution of these cells correspond to the “ductular” and “periductular” liver progenitors (see Sell, 2001; 2003).
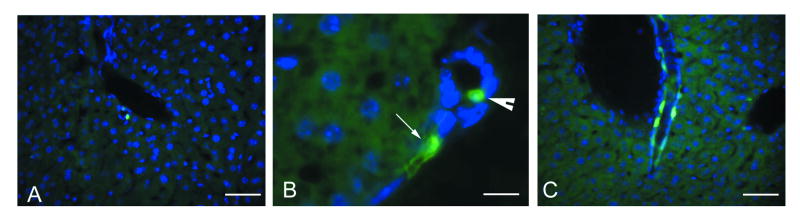
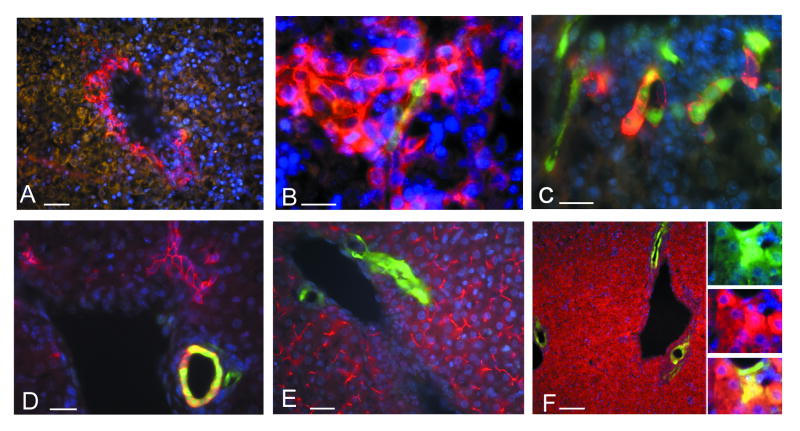
Figure 1.
GFP expression in the quiescent liver of adult nestin-GFP transgenic mice. Cell nuclei are stained with DAPI (blue).
A – General view of quiescent nestin-GFP mouse liver. Few GFP-positive cells are seen inside an intralobular bile duct.
B – GFP-positive cells inside a bile duct (arrowhead) and a canal of Hering (arrow).
C – Bile ducts of higher caliber contain a larger number of GFP-positive cells.
Scale bar is 20 μm in A and C, 50 μm in B.
Nestin-GFP-expressing cells in the regenerating liver
Since oval cells have been defined by their appearance in the liver in response to injury, we examined the changes in nestin-GFP cells during liver regeneration. We induced liver regeneration using a CCl4 poisoning regimen which is known to induce centrolobular necroses in every liver lobule, killing 40-45% of all hepatocytes within 18 hours. In this model, fast liver regeneration after a single CCl4 administration is due to the high proliferative activity of hepatocytes. Although the involvement of oval cells in this process of regeneration is considered to be quite marginal (see Sell, 2001;2003), a measurable proliferative activity of oval cells has been reported in this CCl4 mouse liver regeneration model (Engelhardt et al., 1984) (this stands in contrast to the partial hepatectomy model, where no contribution of oval cells has been reported). Moreover, several rounds of CCl4 administration induce the appearance of small aggregates of proliferating oval cells (Factor et al., 1982) (note that prolonged, every 3-4 days for several months, administration of CCl4 can induce hepatic tumors). Thus, we used both single and multiple (4 times within 10 days) CCl4 treatment regimens to induce liver regeneration and proliferation of oval cells.
We found that even a single administration of CCl4 resulted in an increase in the number of GFP-expressing cells in the periportal areas 72 hours after treatment, and the increase was evident for the next 2-3 days (Fig. 2 A). After four rounds of CCl4 treatment, groups of GFP-expressing cells were regularly seen either in growing bile ducts with a poorly defined lumen in the periportal areas (Fig.2 B, C) or as small aggregates without a distinct ductular structure (Fig.2 D).
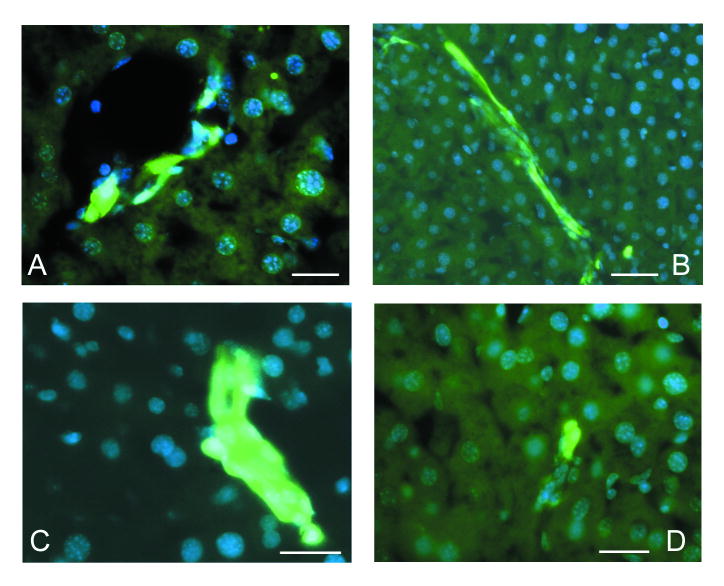
Figure 2.
GFP expression in the liver of adult nestin-GFP transgenic mice after CCl4 treatment. Cell nuclei are stained with DAPI (blue).
A – Aggregates of GFP-positive cells in the periportal area of the liver 3 days after a single administration of CCl4.
B – Growing primitive ductular structures after 4 rounds of CCl4 treatment contain large number of GFP-expressing cells.
C – A poorly organized ductular structure containing GFP-expressing cells after 4 rounds of CCl4
D – A small aggregate of GFP-expressing cells without an obvious ductular organization seen after 4 rounds of CCl4 treatment.
Scale bar is 20 μm in A, C and D; 40 μm in B.
Nestin-GFP-positive cells express epithelial and oval cell markers
To further characterize GFP-expressing cells in the liver of nestin-GFP transgenic animals, we examined them for expression of a set of markers characteristic for cholangiocytes and oval cells.
Epithelium-specific cell adhesion molecule EpCAM is expressed by almost all epithelial cells except for hepatocytes and differentiated keratinocytes. In adult mouse liver, EpCAM is expressed in all cholangiocytes (Tarmann et al., 1990; see for review Balzar et al., 1999). Most GFP-positive cells in the quiescent liver of nestin-GFP animals are EpCAM positive (Fig.3 A, B). In the regenerating liver after single or multiple treatments with CCl4 the majority of GFP-positive cells were also EpCAM-positive. Co-expression of GFP and EpCAM was observed both for the cells located in the ductular structures (Fig. 3 C) and for cells in small clusters in the periportal area that did not have a clear ductular structure (Fig.3 D). We also found a number of weakly GFP-positive cells that did not express EpCAM; their properties are described below (see Mesenchymal GFP-positive cells).
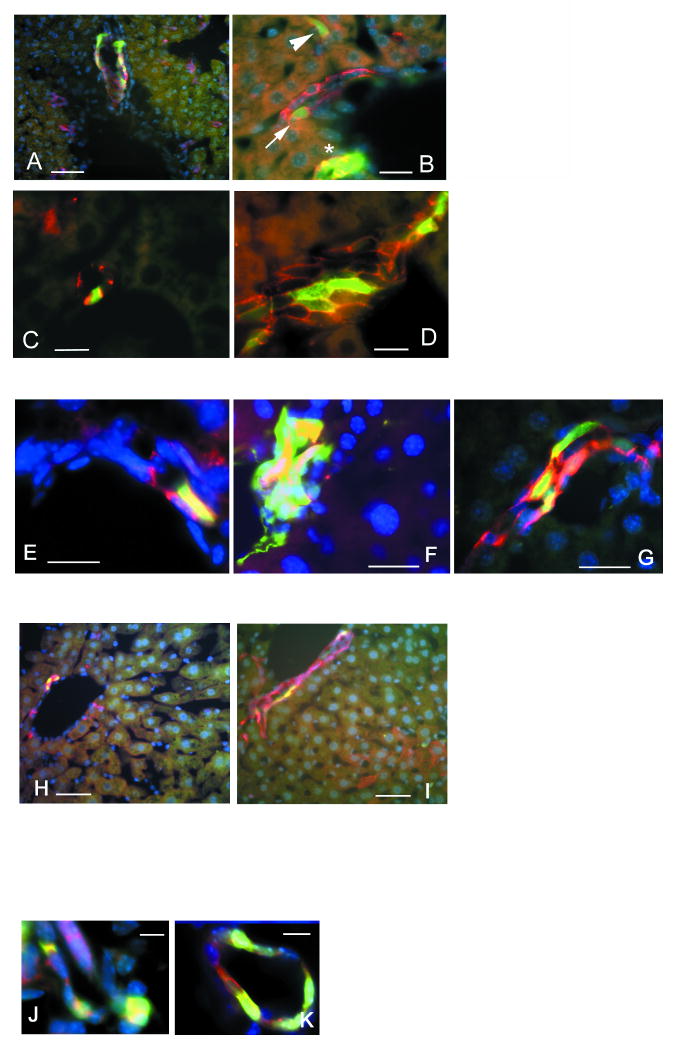
Figure 3.
Co-expression of the nestin-GFP transgene with cholangiocyte/oval cell markers in the liver of adult nestin-GFP transgenic mice. EpCAM, A6 and keratin 8 are in red, nestin-GFP in green, and DAPI-stained nuclei in blue.
A – EpCAM/GFP-positive cells inside an interlobular bile duct of the quiescent liver.
B – EpCAM/GFP-positive cells in bile ducts (asterisk and arrow) and in a canal of Hering (arrowhead).
C – EpCAM/GFP-positive cell in the bile duct in the liver regenerating after a single CCl4 treatment.
D – Poorly organized EpCAM-positive structure contains many GFP-positive cells after 4 rounds of CCl4 treatment.
E – An A6/GFP-positive oval cell located in the junction between bile duct and canal of Hering in the quiescent liver.
F – An aggregate of A6/GFP-positive cells without clear ductular structure after 4 rounds of CCl4 treatment.
G – Primitive A6-positive ductular structures formed after 4 rounds of CCl4 treatment contain GFP-expressing cells.
H – Co-localization of keratin 8 and nestin-GFP in the quiescent liver.
I – Keratin 8/GFP-positive cells in a primitive ductular structure after 4 rounds of CCl4 treatment.
J – c-kit/GFP-positive cells in a poorly organized ductular structure 6 days after a single CCl4 treatment.
K – Thy-1/GFP-positive cells in the bile duct 6 days after a single CCl4 treatment.
Scale bar is 50 μm in A, H, I; 20 μm in B, C, D, E, F, G; 15 μm in J, K.
The relationship between the nestin-GFP-expressing cells and oval cells was further examined using antibodies to A6, an epitope highly specific for cholangiocytes and oval cells (Engelhardt et al., 1990; 1993; Petersen et al., 2003). The distribution of A6-expressing cells in the quiescent and in regenerating liver was virtually identical to that of EpCAM. A6 antibodies stained GFP-expressing cells located in the bile ducts of different caliber as well as in the canals of Hering of the quiescent liver (Fig. 3 E, F). In the regenerating liver, after multiple administration of CCl4, the GFP-positive cells both in the newly formed primitive ductular structures (Fig. 3 G) and in small aggregates without defined ductular structure were also positive for the A6 epitope.
GFP-expressing cells both in the quiescent and regenerating liver were also positive for cytokeratin 8, an intermediate filament protein found in many epithelial cell types but expressed relatively poorly in hepatocytes (Fig. 3 H, I). Furthermore, we found that GFP-positive cells in the regenerating liver stain for Thy-1 and c-kit; their expression marks several types of adult stem and progenitor cells and has also been reported for oval cells in the liver (Petersen et al., 1999) (Fig. 3 J, K).
We also found that in the quiescent liver or after one round of treatment with CCl4, GFP-expressing cells were never found to stain positively for Bgp1 (Fig.4 A, B), a membrane glycoprotein which in the liver is expressed only by adult differentiated hepatocytes but not by cholangiocytes or by cells in the canals of Hering (Kuprina et al., 1990; Daniels et al., 1996). However, after multiple administrations of CCl4, we found rare GFP-positive cells which also express Bgp1 These cells have been found inside expanding primitive ductular structures (Fig.4 C). The dynamics of Bgp1 expression during liver regeneration and development has been thoroughly studied (Gleiberman et al., 1983; Kuprina et al., 1990; Daniels et al., 1996). Its expression in transgene-positive cells indicates the formation of a junction complex associated with bile canaliculi. Thus, nestin-GFP-expressing Bgp1-positive cells may represent a transitional cell type between oval cells and hepatocytes. Such transitory cells derived from oval cells have been found to express some other hepatocyte markers, for example alpha-fetoprotein (AFP) (Tchipysheva et al, 1977; Engelhardt et al., 1984), a serum glycoprotein produced by hepatocytes in the embryonic and regenerating liver as well as by hepatocellular tumors (see Abelev, 1971). We could not detect AFP in GFP-expressing cells in the regenerating liver after single or multiple rounds of administration of CCl4 (Fig.4 D) [note, however, that the C57Bl6 mouse strain we are using in this study shows a low level of AFP expression due to a set of modifier genes which have been shown to downregulate the levels of AFP expression in adult liver (Pachnis et al., 1984)].
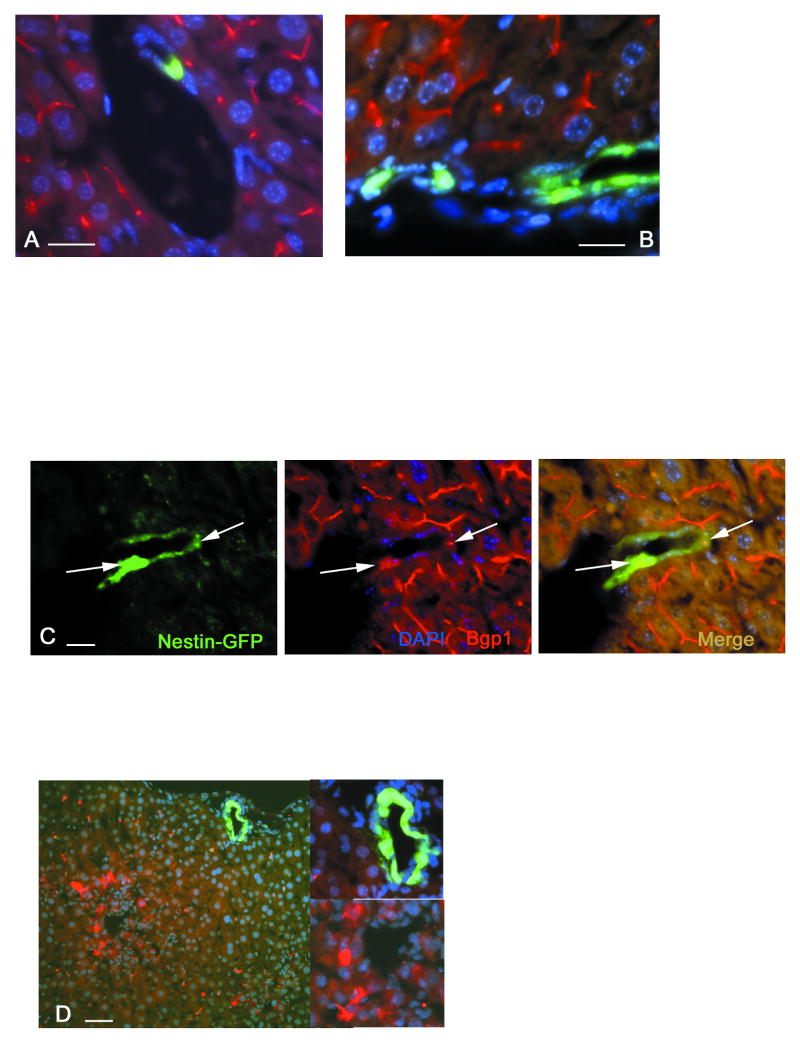
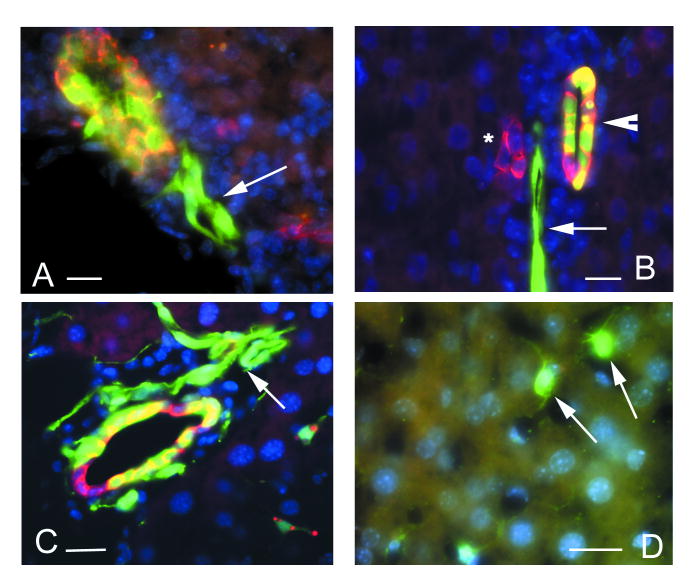
Figure 4.
Bgp1 and nestin-GFP expression marks cells with a transitory phenotype. Bgp1 and AFP signals are in red, nestin-GFP in green, and DAPI-stained nuclei in blue.
A – GFP-positive cells in quiescent liver are Bgp1 negative. Bgp1 is present on the surface of hepatocytes and marks bile canaliculi.
B – 3 days after a single CCl4 treatment, proliferating periportal nestin-GFP-positive cells do not express Bgp1.
C - Primitive ductular structures formed after 4 rounds of CCl4 treatment contain cells that co-express nestin-GFP and Bgp1, indicating a transitory phenotype between nestin-GFP-positive precursors and Bgp1-positivie hepatocytes (arrows).
D – Absence of co-expression between GFP and AFP. All of the AFP-producing cells have been found in the centrolobular areas, while nestin-GFP-positive cells are localized in the periportal areas. Inserts show a high magnification view of the periportal area with nestin-GFP-positive cells in a ductular structure (top) and the centrolobular area with AFP-positive hepatocytes (bottom).
Scale bar is 20 μm in A, B, C; 30 μm in D.
Together, based on the localization, morphology, antigenic profile, and response to CCl4 treatment, our results indicate that in the adult liver under both quiescent and regenerating conditions, nestin-GFP-expressing cells correspond to oval cells, the purported stem/progenitor cells of the liver.
Nestin-GFP expressing cells in developing liver
While participation of oval cells has been extensively studied in the events associated with liver repair and hepatocarcinogenesis, little is known about the role of these cells in normal liver development. Both the differentiated hepatocytes and bile duct epithelium originate from the liver bud, the endodermal liver primordium, and form the liver with active participation of mesenchymal cells from the hepato-cardial mesoderm (see Zaret, 2002). The exact relationship between hepatocytes and cholangiocytes is not sufficiently clear; recently, it has been proposed that primitive embryonic hepatoblasts give rise to both cell types (see Zaret, 2002). However, the position of oval cells within the developmental hepatic lineage has not been investigated. Since our results indicate that GFP-positive cells in the liver correspond to oval cells, we followed the distribution of GFP-expressing cells and expression of oval cell markers in these cells in the developing and early postnatal liver.
After embryonic day 15 (e15), groups of newly formed cholangiocytes are visible in the developing liver. These groups of cholangiocytes expand during the following days and at day e18.5 relatively large areas of cholangiocytes forming bile duct structures can be identified by staining with antibodies to EpCAM (Fig. 5 A) or keratin 8 (not shown). GFP expression is first detected in rare single cells inside these EpCAM-positive areas at day e18.5 (Fig.5 B). In the liver of newborn nestin-GFP mice, the number of GFP-positive cells is increased. Most of these cells were also positive for A6 (Fig. 5 C), EpCAM, and keratin 8 (data not shown), and were located within the groups of cholangiocytes. Later, near the end of the first week of postnatal development, the majority of GFP-positive cells were located inside developing bile duct structures (Fig. 5 D).
Figure 5.
Expression of nestin-GFP transgene in the developing mouse liver.
A - At day e18.5, EpCAM positive fields of cholangiocytes are clearly seen in the periportal areas.
B – Rarely, a few GFP-positive cells can be found in these fields (red – EpCAM).
C - Shortly after birth (postnatal day p1), the number of GFP-positive cells in these aggregates of cholangiocytes is increased (red – A6).
D - At day p6, the majority of GFP-positive cells reside in the newly formed EpCAM-positive ductular structures.
E - At day p6 (and later in the adult quiescent liver), GFP-positive cells do not express Bgp1.
F – At p6 the majority of hepatocytes produces AFP. A small number of periportal hepatocytes co-express AFP and GFP (insert).
Scale bar is 30 μm in A, B, C, D, E; 60 μm in F.
We also examined the possibility that nestin-GFP expression may mark transitional cell types with properties of both cholangiocytes and hepatocytes in the developing or postnatal liver. We did not detect any GFP-positive cells that co-expressed Bgp1, a marker of differentiated hepatocytes (Fig. 5 E). However, on postnatal day 6 we found a small number of cells in the periportal areas that co-expressed GFP and AFP (Fig.5 F); such cells may correspond to a transitory form between transgene-positive cells and hepatocytes.
Taken together, our data suggest that nestin-GFP-positive cells in mouse liver are a subset of cholangiocyte cell lineage with bi-directional differentiation potential, i.e. under appropriate stimuli these cells can differentiate into both hepatocytes and cholangiocytes.
Mesenchymal GFP-expressing cells in the developing and adult liver
In the early postnatal liver of transgenic mice we found some GFP-positive cells that did not express cholangiocyte- or oval cell-specific antigens (EpCAM, A6, cytokeratin 8). These cells were usually associated with developing blood vessels (Fig.6 A, B). The level of GFP expression in these cells was significantly lower that in epithelial GFP-positive cells and, in most cases, they could only be revealed with antibodies against GFP.
Figure 6.
Staining with anti-GFP antibody reveals transgene expression in mesenchymal cell types.
A – GFP-positive cells in the wall of a blood vessel (arrow) and in an EpCAM positive bile duct in the developing liver at postnatal day 1.
B – A6-positive bile duct without GFP positive cells (asterisk), a bile duct of the higher caliber containing GFP-positive cells (arrowhead), and an A6-negative vascular structure containing GFP-positive cells (arrow) at postnatal day 6.
C – GFP positive cells associated with blood vessels in the liver regenerating after four rounds of CCl4 treatment (arrow).
D – GFP positive cells resembling stellate cells in the parenchyma of the regenerating liver (arrows).
Scale bar is 30 μm in A, B; 20 μm in C, D
In adult liver following repeated CCl4 administration, we found two additional types of GFP-expressing cells which are clearly distinct from the GFP-expressing oval cells. These cells do not express either EpCAM, A6, or cytokeratin 8, and, as in the case of the developing liver, the level of GFP expression in these cells was low and had to be revealed using anti-GFP antibody. The first type was found in association with blood vessels (most probably, the branches of hepatic artery located in the periportal area in the close vicinity of bile ducts) (Fig.6 C). These cells may correspond to activated pericytes, undifferentiated cells of mesenchymal origin that are close to mesenchymal or stromal stem cells. The second cell type had a distinct stellate morphology. Such cells were dispersed throughout the liver parenchyma (Fig.6 D) and may correspond to activated Kupfer cells or to stellate cells, another member of reticulo-endothelial hepatic system.
Discussion
Here we describe a population of liver epithelial cells that can be identified by the expression of GFP driven by the regulatory elements of the nestin gene. Several features of these cells suggest that they represent liver stem or progenitor cells, or at least a subset of such cells: a) GFP-expressing cell morphologically s resemble oval cells, the purported stem/progenitor cells of the liver; b) they are detected in the periportal areas in close association with bile transporting structures, where oval cells reside; c) their number is low in quiescent liver but increases in regenerating liver, after exposure to CCl4, which is known to induce the appearance of oval cells; d) their antigenic profile corresponds to that of oval cells; e) their gradual appearance during development is compatible with their function as stem/progenitor cells of the adult, but not embryonic tissue; f) they include a small subpopulation of cells that also express hepatocyte markers and may correspond to a transitory cells between progenitor cells and their hepatocyte progeny. Together, these similarities support the notion that GFP-expressing cells in our nestin-GFP transgenic animals correspond to oval cells and serve as stem/progenitor cells in the adult liver.
Interestingly, the first nestin-GFP-positive cells appear relatively late during development, well after the appearance of hepatocytes and cholangiocytes. This suggests that the GFP-expressing cells are not primitive developmental liver cell precursors but a specialized cell type characteristic of a definite liver tissue. It is conceivable that a bona fide stem cell of the adult liver can arise and persist only after establishment of a specific niche that supports their stem cell state (Fuchs et al., 2004). For liver stem cells, such a specific niche is thought to be provided by the bile ducts and canals of Hering (Sell, 2001; 2003; Wang et al., 2003). Thus, it seems plausible that adult liver stem cells appear after cholangiocyte differentiation and bile duct formation.
Our results are compatible with the idea of a lineage hierarchy for liver cells, where rare nestin-expressing cells with oval cell characteristics originate as a specialized subset of the cholangiocyte lineage, are located in the bile ducts and the canals of Hering, and serve as facultative stem cells for adult liver (see Fig.7). These cells can further expand in number to give rise to a similar population of oval cells which then act as bi-potent progenitors for hepatocytes and cholangiocytes.
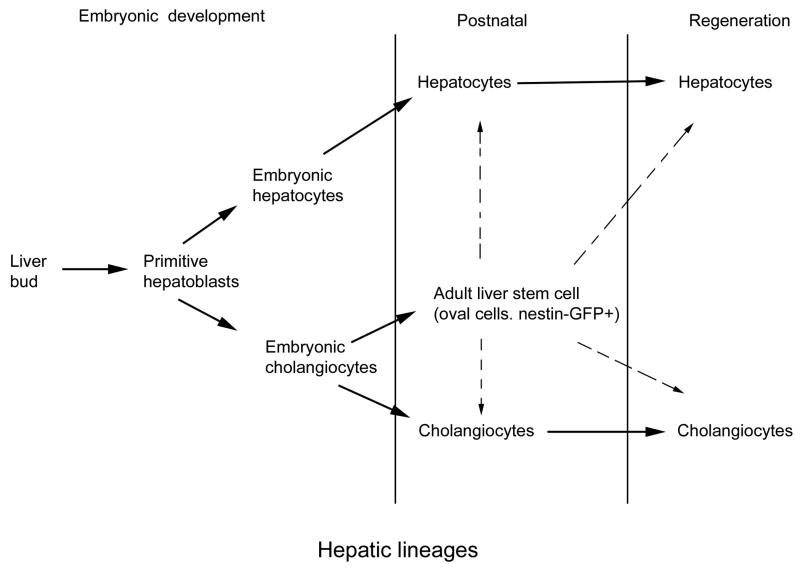
Figure 7.
Model for liver stem/progenitor cells. During development, endodermal cells from the liver bud differentiate into embryonic hepatoblasts. Later, these hepatoblasts give rise to two separate lineages, hepatocytes and cholangiocytes. Each of these two lineages is capable of self-renewal under physiological conditions. At the end of embryonic development and at the beginning of postnatal development, cholangiocyte cell lineage subdivides into two cell types that can be distinguished by the expression of the nestin-GFP transgene. We suggest that nestin-GFP expressing cells act as facultative stem cells in the adult liver.
A widely discussed possibility is that bone marrow stem-like cells can colonize the liver and give rise to intrahepatic liver stem cells (Oh et al., 2002; Sell, 2001; 2003). We have identified a small subpopulation of nestin-GFP-expressing cells of mesenchymal origin both in the developing and the adult liver, which may correspond to such bone marrow-derived cells. Note, however, that recent results strongly argue against the notion of hematopoietic stem cells as precursors to oval cells or their putative precursors (Wang et al., 2003).
GFP expression in the developing, quiescent, and regenerating liver of the nestin-GFP transgenics is driven by the regulatory elements located in the promoter and the second intron of the nestin gene. The same elements direct the expression of the reporter in neuroepithelial cells of the developing nervous system (Zimmerman et al., 1998; Yamagichi et al., 2000; Kawaguchi et al., 2001; Mignone et al., 2004), in stem/progenitor cells of the adult nervous system (Yamagichi et al., 2000; Kawaguchi et al., 2001 Mignone et al., 2004), in stem cells residing in the bulge region of the hair follicle (Li et al., 2003), in progenitors of steroidogenic Leydig cells (Davidoff et al., 2004), in progenitors of pancreatic β-cells (Seaberg et al., 2004), and in several other types of adult tissue-specific stem cells (Enikolopov, Gleiberman, unpublished observations). The crucial regulatory elements that drive reporter expression in these cells are, apparently, located within the second intron of the nestin gene (Josephson et al; 1998; Yaworsky and Kappen, 1999). This intron may contain several independent transcriptional elements that independently direct the expression in each of the stem-like cell types. It is also possible that the same elements (e.g., located within the 257bp that can confer neural stem cell-specific expression) are responsible for the activity of the reporter in all types of nestin-GFP-expressing stem/progenitor cells, which may indicate the presence of a common set of transcriptional regulators characteristic for the stem status of these cells.
Furthermore, these observations raise the possibility that nestin may not simply mark cells that possess stem potential but may directly contribute to this potential. Although the major role of intermediate filaments has been traditionally considered to be architectural (see Fuchs and Cleveland, 1998), recent evidence suggests that changes in intermediate filaments can have a profound effect on signal transduction and gene expression (see Magin et al., 2004 for review). The types of stem/progenitor cells which express nestin are dissimilar in shape and origin; however, it is conceivable that the presence of nestin confers similar stem-like properties on these cells.
The transgenic approach described here can be used not only for identification of liver-specific stem/progenitor cells but also for their isolation using standard sorting techniques. Oval cells have been isolated from rat and mouse liver based on their physical characteristics (Petersen et al., 1999; 2003; Wang et al., 2003). These approaches employ the paradigm of carcinogenesis in which oval cells are already activated and actively proliferate; furthermore, they may represent a class of advanced progenitors. Identification of the nestin-GFP transgene as a marker of liver stem/progenitor cells may allow direct isolation of non-activated stem cells from the developing or quiescent adult liver, analysis of their lineage and their repopulation ability, and elucidation of their relationship to the progenitor-like cells arising in response to a specific injury.
Material and Methods
Animals
Generation of transgenic mice has been described earlier (Mignone et al., 2004). Briefly, a recombinant construct that contained 5.8 kb 5′ sequence of the nestin gene followed by eGFP, polyadenylation signal from SV40 virus, and 1.8 kb second intron from the nestin gene was injected into pronuclei of the mouse fertilized oocytes. Positive offspring were selected with help of hand-held UV illuminator and confirmed by PCR. Transgene-positive males were crossed to C57Bl6 female. Subsequently, transgene positive mouse strain was kept on the C57Bl6 genetic background. Procedure for CCl4-induced liver regeneration using inhalation of CCl4 vapors has been described previously (Bakirov, 1968). For the multiple CCl4 treatment regimen, the procedure was performed at days 1, 4, 7, and 10. Mice were sacrificed each day during the 6 day period after single CCl4 treatment and on the 3rd day after multiple treatments. For staged pregnancies, transgene positive males were caged overnight with wild-type females and the vaginal plugs were checked next morning. The day of vaginal plug appearance was marked as E0.5. All mice were maintained identically following the recommendations from the UCSD Office of Animal Resources. The health of mice was monitored daily.
Immunohistochemistry
Adult livers were fixed by the perfusion with 4% formaldehyde in PBS and postfixed for 1 h. Embryos and livers from early postnatal animals were fixed by immersion in 4% formaldehyde in PBS during for 2-4 h. After fixation the specimens were washed in PBS, embedded in OCT compound, and serially sectioned with the Leica CM1850 cryotome. The thickness of the sections was 12 μm.
Sections mounted on Superfrost slides (Fisher) were incubated for 30 min with blocking solution (10% bovine serum, 0.4% triton X-100 in PBS) and incubated for 2 hr at room temperature with one of following antibodies: anti-EpCAM (rat monoclonal antibody G8.8 from Developmental Studies Hybridoma Bank, dilution 1:100); anti-cytokeratin 8 (rat monoclonal antibody Troma-1, Developmental Studies Hybridoma Bank, dilution 1:100); B10 (rat monoclonal antibody against Bgp1, dilution 1:20, Daniels et al., 1996); A6 (rat monoclonal antibody, dilution 1:20, Engelhardt et al., 1993); anti-GFP (chicken polyclonal antibody, Aves Inc., dilution 1:500); anti-alpha-fetoprotein (affinity purified rabbit polyclonal antibody, dilution 1:100, Gleiberman et al., 1989); anti-c-kit (rat monoclonal antibody, Santa Cruz Biotechnology, dilution 1:50); anti-thy1 (rabbit polyclonal antibody, Santa Cruz Biotechnology, dilution 1:50). As controls, primary antibodies were omitted, or normal rat or rabbit serum were substituted for the primary antibodies at comparable dilutions. After thorough washing in PBS and additional blocking, sections were incubated for 1.5 hr with the appropriate secondary antibodies: TRITC-conjugated anti-rat or anti-rabbit antibodies (from Jackson ImmunoResearch Labs, dilution 1:100) and Alexa Fluor 480-conjugated anti-chicken antibody (from Molecular Probes, dilution 1:500). Sections were counterstained with DAPI and analyzed under Axioplan-2 microscope (Zeiss) equipped with UV illuminator, an appropriate set of filters, and Hamamatsu ORCA-ER digital camera. Images were collected with AxioVision 3.1 software (Zeiss) and processed with Adobe Photoshop 5.5 software.
Acknowledgments
We thank Dr. Kristen Jepsen (UCSD, La Jolla CA), Dr. Natalia Engelhardt (Cancer Research Center, Moscow, Russia), Dr. Gregory Bannikov (OSU, Columbus OH), and Dr. Julian Banerji (MGH, Boston MA) for critical reading of the manuscript and insightful comments. We are grateful to Drs. Natalia Engelhardt, Nelly Kuprina and Tatyana Rudinskaya for the generous gift of monoclonal antibodies against liver markers.
Grant sponsor: National Institute of Health (Grant numbers: R01 DK018477 and R01 NS032764) Ira Hazan Fund, Seraph Foundation
Footnotes
M.G.R. is HHMI Investigator.
References
- Abelev GI. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Bakirov RD. The appearance of embryonic serum alpha-globulin in adult mice following carbon tetrachloride poisoning. Biull Eksp Biol Med. 1968;65:45–47. [PubMed] [Google Scholar]
- Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- Bennoun M, Rissel M, Engelhardt N, Guillouzo A, Briand P, Weber-Benarous A. Oval cell proliferation in early stages of hepatocarcinogenesis in simian virus 40 large T transgenic mice. Am J Pathol. 1993;143:1326–1336. [PMC free article] [PubMed] [Google Scholar]
- Daniels E, Letourneau S, Turbide C, Kuprina N, Rudinskaya T, Yazova AC, Holmes KV, Dveksler GS, Beauchemin N. Biliary glycoprotein 1 expression during embryogenesis: correlation with events of epithelial differentiation, mesenchymal-epithelial interactions, absorption, and myogenesis. Dev Dyn. 1996;206:272–290. doi: 10.1002/(SICI)1097-0177(199607)206:3<272::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt NV. Oval cells in rodent liver, rat. In: Jones TC, Popp JA, Mohr U, editors. Monographs on Pathology of Laboratory Animals, Digestive System. 2nd. Berlin-Heidelberg-New York: Springer-Verlag; 1997. pp. 162–166. [Google Scholar]
- Engelhardt NV, Baranov VN, Lazareva MN, Goussev AI. Ultrastructural localisation of alpha-fetoprotin (AFP) in regenerating mouse liver poisoned with CCl4. 1. Reexpression of AFP in differentiated hepatocytes. Histochemistry. 1984;80:401–407. doi: 10.1007/BF00495425. [DOI] [PubMed] [Google Scholar]
- Engelhardt NV, Factor VM, Medvinsky AL, Baranov VN, Lazareva MN, Poltoranina VS. Common antigen of oval and biliary epithelial cells (A6) is a differentiation marker of epithelial and erythroid cell lineages in early development of the mouse. Differentiation. 1993;55:19–26. doi: 10.1111/j.1432-0436.1993.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt NV, Factor VM, Yasova AK, Poltoranina VS, Baranov VN, Lasareva MN. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation. 1990;45:29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Faktor VM, Poltoranina VS, Uryvaeva IV. Changes in the nature of the hepatocyte proliferation in the liver and adenomatous nodes of mice in CC14-induced carcinogenesis. Bull Eksp Biol Med. 1982;93:79–82. [PubMed] [Google Scholar]
- Farber E. Similarities in the sequence of early histological changes induced in the livers by ethionine, 2-acetylamino-fluorene and 3-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Kuprina-Khramkova NI, Rudinskaya-Beloshapkina TD, Abelev GI. Alpha-fetoprotein synthesis in relation to structural peculiarities in postnatal and regenerating mouse liver. Int J Cancer. 1983;32:85–92. doi: 10.1002/ijc.2910320114. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Sharovskaya YuYu, Chailakhjan LM. “Contact inhibition” of alpha-fetoprotein synthesis and junctional communication in adult mouse hepatocyte culture. Exp Cell Res. 1989;184:228–234. doi: 10.1016/0014-4827(89)90380-7. [DOI] [PubMed] [Google Scholar]
- Josephson R, Muller T, Pickel J, Okabe S, Reynolds K, Turner PA, Zimmer A, McKay RD. POU transcription factors control expression of CNS stem cell-specific genes. Development. 1998;125:3087–3100. doi: 10.1242/dev.125.16.3087. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, Okabe M, Tano Y, Goldman SA, Okano H. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–273. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- Kuprina NI, Baranov VN, Yazova AK, Rudinskaya TD, Escribano M, Cordier J, Gleiberman AS, Goussev AI. The antigen of bile canaliculi of the mouse hepatocyte: identification and ultrastructural localization. Histochemistry. 1990;94:179–186. doi: 10.1007/BF02440185. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A. 2003;100:9958–9961. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin TM, Reichelt J, Hatzfeld M. Emerging functions: diseases and animal models reshape our view of the cytoskeleton. Exp Cell Res. 2004;15:91–102. doi: 10.1016/j.yexcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Oh SH, Hatch HM, Petersen BE. Hepatic oval “stem” cell in liver regeneration. Semin Cell Dev Biol. 2002;13:405–409. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Belayew A, Tilghman SM. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci U S A. 1984;81:5523–5527. doi: 10.1073/pnas.81.17.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kakishita K, Ogawa Y, Toyama Y, Yamamoto A, Yamaguchi M, Mori K, Goldman SA, Itakura T, Okano H. Generation of dopaminergic neurons in the adult brain from mesencephalic precursor cells labeled with a nestin-GFP transgene. J Neurosci. 2001;21:3895–3903. doi: 10.1523/JNEUROSCI.21-11-03895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- Sell S. The role of progenitor cells in repair of liver injury and in liver transplantation. Wound Repair Regen. 2001;9:467–482. doi: 10.1046/j.1524-475x.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Sell S. The hepatocyte: heterogeneity and plasticity of liver cells. Int J Biochem Cell Biol. 2003;35:267–271. doi: 10.1016/s1357-2725(02)00182-6. [DOI] [PubMed] [Google Scholar]
- Tarmann T, Dohr G, Schiechl H, Barth S, Hartmann M. Immunohistochemical detection of an epithelial membrane protein in rat embryos at different stages of development. Acta Anat (Basel) 1990;137:141–145. doi: 10.1159/000146874. [DOI] [PubMed] [Google Scholar]
- Tchipysheva TA, Guelstein VI, Bannikov GA. Alpha-fetoprotein-containing cells in the early stages of liver carcinogenesis induced by 3′-methyl-4-dimethyl-aminoazobenzene and 2-acetylaminofluorene. Int J Cancer. 1977;20:388–393. doi: 10.1002/ijc.2910200310. [DOI] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci U S A. 2003;100 1:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- Yaworsky PJ, Kappen C. Heterogeneity of neural progenitor cells revealed by enhancers in the nestin gene. Dev Biol. 1999;205:309–321. doi: 10.1006/dbio.1998.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]