Abstract
Background and Purpose
This study utilized middle cerebral artery occlusion (MCAO) with tissue plasminogen activator (tPA) to assess inhibition of the NOX2 isoform of NADPH oxidase on brain injury and functional recovery in aged rats.
Methods
Effects of NOX2 on the degree of brain injury and functional recovery following MCAO and tPA reperfusion was assessed in young adult and aged rats. Rats received apocynin (NOX2 inhibitor; 5 mg/kg) or saline 30 min prior to MCAO. At 24 h following MCAO, blood-brain barrier permeability (BBB), stroke infarct volume, edema formation, and oxidative damage were measured.
Results
Apocynin treatment in aged rats increased mortality rate and failed to improve functional outcome, total infarct volume, edema formation, and BBB permeability. Aged rats displayed increased BBB permeability to sucrose in the contralateral hemisphere following MCAO and diminished antioxidant capacity in the brain as compared to young adult rats.
Conclusions
We conclude that inhibition of NOX2 in the aged rat exacerbates stroke injury and diminishes functional outcome. These results suggest age is an important factor in stroke damage and more rigorous examination of apocynin as a therapeutic agent for treatment of stroke must be done.
Keywords: neurovascular unit, apocynin, MCAO, blood brain barrier, NADPH oxidase
1. Introduction
NADPH oxidases are a damaging source of reactive oxygen species during the aging process (Park L et al., 2007;Hamilton CA et al., 2001). Inhibition of the NOX2 isoform of NADPH oxidase reduces superoxide formation (Tang et al., 2007), prevents oxidative damage in the vasculature(Godbole et al., 2009;Park et al., 2008), and was recently touted as a promising therapeutic target for treatment of stroke(Kahles T et al., 2007). Studies using embolic models of stroke have identified many neuroprotective agents; however, none of these drugs have improved stroke outcome in humans (Gladstone DJ et al., 2002). Most preclinical studies have been conducted in young rodents. We contend aging impacts brain damage and functional recovery following stroke and that by not focusing on age-related processes, a vast area of potential therapeutic growth has been overlooked. This study utilized middle cerebral artery occlusion (MCAO) with tissue plasminogen activator (tPA) reperfusion to assess inhibition of NOX2 on brain injury and functional recovery in aged rats. Our results demonstrated that age is an important factor in stroke damage and suggest more rigorous examination of NOX2 inhibition as a therapeutic strategy for treatment of acute ischemic stroke is needed.
2. RESULTS
A total of 92 young adult and 120 aged rats were used in this study. At 24 h following MCAO, apocynin treatment led to increased mortality in aged rats from 14% (7 of 51) to 36% (25 of 69). Vehicle-treated aged rats suffered higher mortality than young adult rats (6%; 3 of 45). In young adult rats, treatment with apocynin (10%; 5 of 47) had no effect on mortality. No rats were excluded from the study for not meeting the criteria for ischemic depth and reperfusion. No deleterious signs of hemorrhage from tPA use were observed in the study. These findings were consistent with prior studies showing that administration of tPA at 5 mg/kg at 2 h after start of vessel occlusion did not worsen and, in fact, improved infarct volume compared to ischemic injury following suture occlusion and reperfusion (DiNapoli VA et al., 2006; DiNapoli VA et al., 2008; Tan Z et al., 2009).
2.1 Functional Score
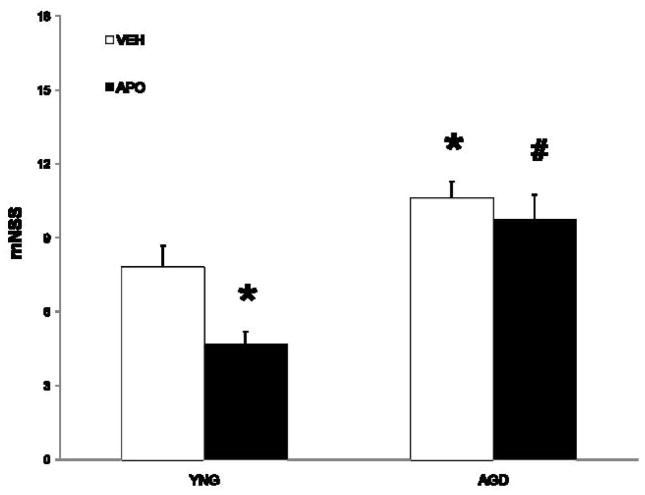
At 24 h following MCAO, neurological function was assessed using a modified mNSS (Figure 1). Aged rats (11±1) suffered worsened functional outcome compared to young adult rats (8±0.5). While apocynin improved functional outcome in young adult rats (5±1), no improvement from apocynin was noted in aged rats (10±0.5).
Figure 1.
Functional outcome was measured utilizing the modified Neurological Severity Score (mNSS). Values are expressed as mean ± S.E. Statistical significance was determined as * p < 0.05 as compared to vehicle treated MCAO young adult rats and # p < 0.05 compared to apocynin-treated young adult rats using two-way ANOVA with Tukey’s post hoc analysis.
2.2 Stroke Volume
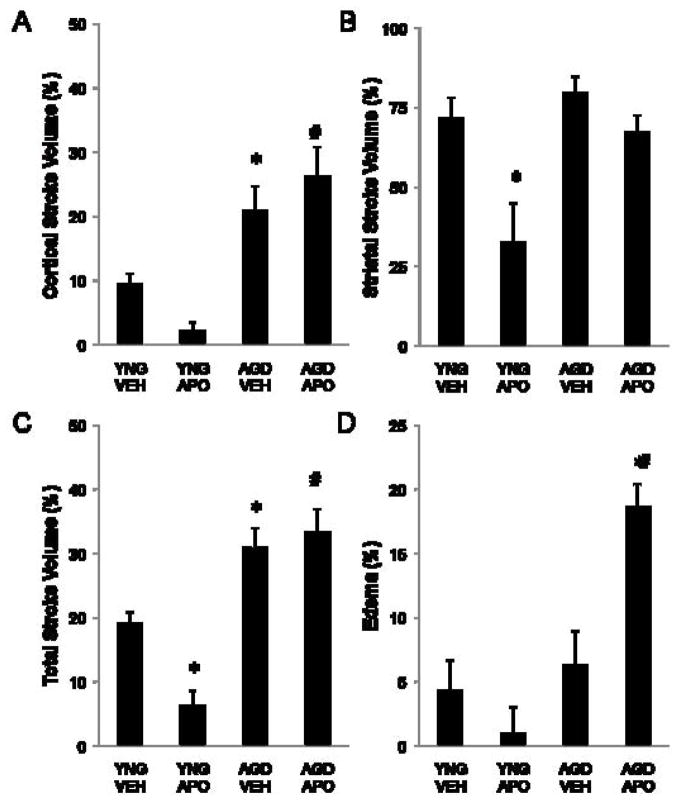
At 24 h following MCAO, cortical, striatal and total stroke infarct volumes were calculated from TTC stained brain sections (Figure 2A–C). Apocynin-treated aged rats displayed no improvement compared to vehicle-treated aged rats in cortical (27±4% and 21±4%, respectively), striatal (62±7% and 80±5%, respectively) or total (33±3% and 31±3%, respectively) stroke volumes. Aged rats exhibited significantly worsened cortical (21±4% and 10±2%, respectively) and total (31±1% and 19±2%, respectively) stroke volumes compared to young adult rats. Treatment with apocynin significantly decreased striatal (33±10% and 72±7%, respectively) and total (6±2% and 19±2%, respectively) stroke volume in young adult rats compared to vehicle-treated young adult rats.
Figure 2.
TTC stained brain sections were used to calculate (A) cortical, (B) striatal, and (C) total stroke volume and (D) edema formation at 24 h following MCAO in vehicle and apocynin-treated young adult and aged rats. Bars represent mean ± S.E. (n=6 rats per group). Statistical significance was set at *p<0.05 compared to vehicle-treated young adult rats, #p<0.05 as compared to apocynin-treated young adult rats using two-way ANOVA with Tukey’s post hoc analysis.
2.3 Edema Formation
At 24 h following MCAO, edema formation was measured (Figure 2D). Apocynin treated aged rats (20±2%) had exacerbated edema as compared to vehicle-treated aged rats (6±3%). No difference in edema formation between young adult, young adult apocynin treated, and aged rats was observed.
2.4 BBB Permeability
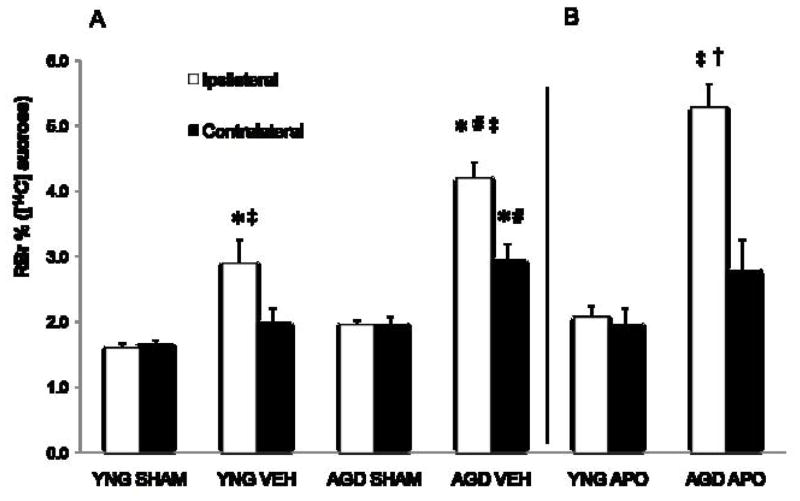
At 24 h following MCAO, BBB permeability was measured utilizing a 20 min in situ brain perfusion with [14C]sucrose (Figure 3). Aged rats treated with apocynin showed no difference in BBB permeability compared to vehicle-treated aged rats. The ipsilateral hemisphere of aged rats (4.2±0.2%) showed increased permeability of [14C]sucrose compared to both young adult MCAO rats (2.9±0.4%) and aged sham rats (2.0±0.1%). Following MCAO, aged rats also demonstrated increased permeability to [14C]sucrose in the contralateral hemisphere (2.9±0.2%) as compared to aged sham rats (2.0±0.1%). The ipsilateral hemisphere of young rats (2.9±0.4%) exhibited increased permeability of [14C]sucrose as compared to young adult sham rats (1.6±0.1%). When treated with apocynin 30 min prior to MCAO, young adult rats exhibited attenuated BBB permeability (2.0±0.2%).
Figure 3.
At 24 h following MCAO, (A) in situ brain perfusion using [14C]sucrose (342 Da) was used to determine the degree of BBB permeability and (B) the effect of apocynin treatment on changes in BBB permeability in young adult and aged rats. Brains were separated into infarcted and non-infarcted hemispheres. Bars represent mean ± S.E. (n=6 rats per group). Statistical significance was set at *p<0.05 compared to vehicle-treated age-matched sham, #p<0.05 compared to respective hemisphere of the vehicle-treated young adult MCAO rats, ‡p<0.05 compared to the respective non-infarct hemisphere, and †p<0.05 as compared to apocynin-treated young adult rats using three-way ANOVA with Tukey’s post hoc analysis.
2.5 Antioxidant enzyme activity
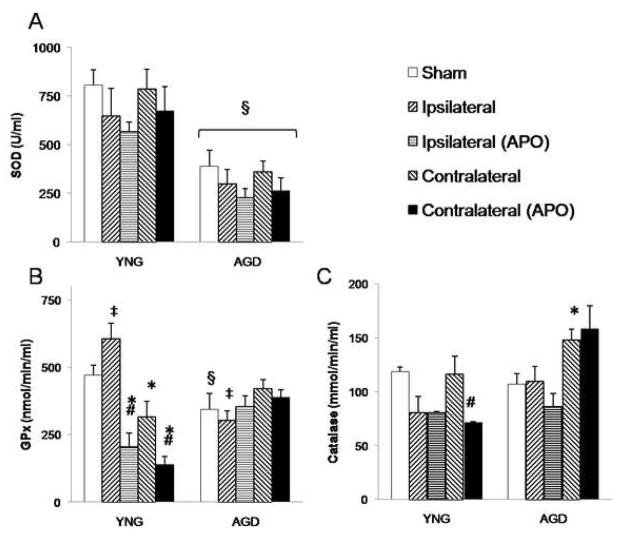
At 24 h following MCAO, aged rats treated with apocynin showed reduced SOD activity compared to respective young adult rats (Figure 4). Following MCAO, young adult rats had reduced GPx in the contralateral hemisphere (321±66 nmol/min/ml) compared to young adult sham rats (471±36 nmol/min/ml). Treatment with apocynin in young adult rats reduced GPx in the ipsilateral hemisphere (205±55 nmol/min/ml and 605±57 nmol/min/ml respectively), and GPx (138±35 nmol/min/ml and 321±68, respectively) and CAT (71±0.6 mmol/min/ml and 115±15 mmol/min/ml, respectively) in the contralateral hemisphere compared to vehicle-treated young adult rats. When compared to young adult sham rats, aged sham rats had decreased SOD (805±80 U/ml and 388±82 U/ml respectively) and GPx (471±36 nmol/min/ml and 348±66 nmol/min/ml respectively).
Figure 4.
(A) Superoxide dismutase (SOD), (B) glutathione peroxidase (GPx), and (C) catalase (CAT) activities were spectrophotometrically measured in infarcted and non-infarcted hemispheres at 24 h following MCAO in vehicle and apocynin-treated young adult and aged rats. Bars represent mean ± S.E. (n=6 rats per group). Statistical significance was set at *p<0.05 compared to age-matched sham, #p<0.05 compared to vehicle-treated young adult MCAO rats, ‡p<0.05 compared to respective non-infarct hemisphere, †p<0.05 compared to apocynin-treated young adult rats and §p<0.05 compared to respective young adult group using three-way ANOVA with Tukey’s post hoc analysis.
2.6 Oxidative Damage
At 24 h following MCAO, lipid peroxidation was assessed by measurement of its byproduct, MDA (Figure 5A). No significant difference was found between sham and MCAO rats. Apocynin treatment significantly decreased MDA concentration in aged rats as compared to aged sham rats.
Figure 5.
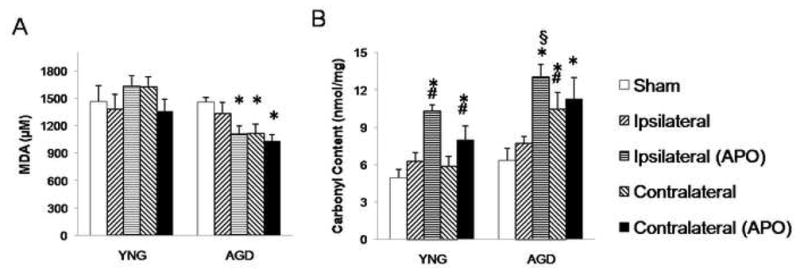
Downstream products of lipid peroxidation (A) malondialdehyde (MDA) and (B) protein carbonyl were spectrophotometrically measured to assess the effect of apocynin treatment on oxidative stress at 24 h following MCAO in young adult and aged rats. Brains were separated into infarcted and non-infarcted hemispheres. Bars represent mean ± S.E. (n=6 rats per group). Statistical significance was set at *p<0.05 compared to age-matched sham, #p<0.05 compared to vehicle-treated young adult MCAO, and §p<0.05 compared to respective young adult group using three-way ANOVA with Tukey’s post hoc analysis.
Protein oxidation was measured by assessment of protein carbonyl content (Figure 5B). In aged rats, apocynin treatment significantly increased protein carbonyl content in the ipsilateral (13±1 nmol/mg) and contralateral (11±2 nmol/mg) hemispheres as compared to aged sham rats (6±1 nmol/mg). Aged rats showed significantly higher protein carbonyl content in the contralateral hemisphere post MCAO (11±2 nmol/mg) than aged sham rats (6±1 nmol/mg). When treated with apocynin there was a significant increase in protein carbonyl content in young adult rats in both the ipsilateral (10±0.5 nmol/mg and 7±1 nmol/mg, respectively) and contralateral (8±1 nmol/mg and 6±0.7 nmol/mg, respectively) hemispheres as compared to young adult vehicle-treated rats. At 24 h following MCAO, young adult vehicle-treated rats showed no change in protein carbonyl content as compared to sham rats.
3. DISCUSSION
The primary finding of this study was that inhibition of the NOX2 isoform of NADPH oxidase prior to MCAO elicited distinctly different brain injuries between young adult and aged rats. We report, for the first time, that treatment with apocynin prior to MCAO in aged rats resulted in increased mortality at 24 h with no improvement in total or regional infarct volume, edema formation, or functional outcome. We showed that at 24 h following MCAO, both ipsilateral and contralateral hemispheres of aged rats had increased BBB permeability to sucrose (342 Da) and apocynin treatment did not improve BBB functional integrity. Often in stroke studies utilizing young animals, the contralateral hemisphere is either ignored or considered control. However, previous clinical studies have observed changes in the contralateral hemisphere following stroke (Carey et al., 2002; Blank et al., 2003). In addition to changes in BBB permeability in the contralateral hemisphere, significant changes in catalase activity, GPx activity, and protein carbonyl content after MCAO were found. This study, in correlation with others (Buga et al., 2008; Carey et al., 2002; Blank et al., 2003), highlights a potential role for the contralateral hemisphere as a compensatory mechanism following stroke. Utilizing aged rodents in stroke models provides a unique opportunity to gain a better understanding of changes in the contralateral hemisphere following stroke.
Our study confirmed the findings of previous studies that in young adult rats, NOX2 inhibition with apocynin prior to MCAO significantly improved total stroke volume and functional outcome. We demonstrated that much of the improvement in brain injury in young adult rats following MCAO was due to improved striatal infarct volume, lessened edema formation, and decreased BBB disruption. Understanding the effects of ischemic brain injury in reproductively senescent females is an understudied yet important area of research as females have an increased overall stroke burden (Lloyd-Jones D, et al. 2009). Recent studies indicate that gender plays a role in some mechanistic pathways following brain injury and recovery after stroke (De Silva et al., 2009; McCullough LD et al., 2005). Our current results argue against gender-related differences in NADPH oxidase activity, as results obtained from young adult female rats in our study were comparable to young adult male rats used in previous studies (Tang et al., 2007; Tang et al., 2008; Yenari MA et al., 2006; Kahles T et al., 2007); however, further investigation will need to be done to ascertain if these findings hold true in aged rats.
The decreased SOD and GPx activity observed in aged rats supports the “free radical theory of aging”, which contends that age-related diseases result from increased generation of reactive oxygen species (Harman D, 1994) and decreased antioxidant enzyme activity (Azhar S et al., 1995; Abete P et al., 1999). However, in concurrence with other studies (Meng et al., 2007; Goto et al., 1999), catalase activity and protein oxidation were unchanged due to the aging process. The brain, having a high metabolic rate and lack of metabolic reserves, possesses an increased concentration of mitochondria compared to other organs. Thus the brain is more resistant to lipid peroxidation (Meyer et al., 2004), perhaps due to break down of MDA by mitochondrial aldehyde dehydrogenase (Siu and Draper, 1982). Hypoxia and brain injury show elevated MDA, however, this increase is only seen at early time points, between 1 and 3 h (Lo W et al., 2007; Wang Q et al., 2006; Pantke et al., 1999). Due to the early formation and stability of protein carbonyl (Pantke et al., 1999), we propose that it is a better indicator than TBARS of oxidative damage in the brain at 24h after MCAO.
Inhibition of NOX2 with apocynin has recently gained momentum as a therapeutic option for treatment of acute ischemic stroke (Tang et al., 2007; Wang Q et al., 2006; Kahles T et al., 2007) as several studies demonstrate that administration of apocynin (5–50 mg/kg; i.p.) in young rodents prior to MCAO attenuated superoxide production, infarct volume (Tang et al., 2007), neuronal death, activated microglia (Wang Q et al., 2006) and edema formation (Kahles T et al., 2007). Interestingly though, route of administration may play a role in apocynin toxicity as a recent study reported that when apocynin (5 mg/kg) was administered intravenously prior to MCAO, rodents exhibited larger cerebral hemorrhages in both hemispheres and increased mortality (Tang et al., 2008). These opposing findings suggest that the activation, mechanism of action, and toxicity of apocynin are still poorly understood and further studies are necessary, especially before any therapeutic use of NOX2 inhibition is pursued in clinical trials.
In this study, young adult apocynin-treated rats exhibited decreased GPx activity and increased protein oxidation after MCAO and reperfusion, possibly as a consequence of unactivated apocynin, which acts as a pro-oxidant by decreasing the glutathione to glutathione disulfide ratio (Riganti et al., 2006). Unactivated apocynin promotes hydrogen peroxide production and increases protein carbonyl formation (Riganti et al., 2006; Ciolino and Levine, 1997). Unactivated apocynin must be activated to inhibit NOX2 (Stefanska and Pawliczak, 2008). Activation of apocynin by myeloperoxidase (MPO) forms an apocynin dimer, which prevents recruitment of the cytosolic proteins and thereby inhibits NADPH oxidase activation (Stefanska and Pawliczak, 2008). In pathologic inflammatory conditions MPO is elevated (Tsimikas, 2008). In particular, aged animals exhibit exacerbated inflammatory cell infiltration (DiNapoli VA et al., 2008) and worsened stroke volume which produces increased MPO expression (Romanos et al., 2007). The positive correlation between MPO expression and apocynin activation suggests enhanced NOX2 inhibition with age. Data gathered from young animals suggest that increased NOX2 inhibition results in a favorable outcome. In direct contrast, our data show that aged rats exhibit exacerbated edema formation and increased mortality. We conclude that inhibition of NOX2 with apocynin exacerbates stroke injury in the aged rat and would be a poor choice for clinical treatment of stroke.
Results from models utilizing aged animals are increasingly divergent from those in young adult animals. Evidence collected from aged animal models (DiNapoli VA et al., 2008; DiNapoli VA et al., 2006; Badan I et al., 2003; Popa-Wagner et al., 2007; Schroeder E et al., 2003) suggests that, in addition to exacerbated brain injury and persistent functional deficit after stroke, changes are occurring in the contralateral hemisphere. Disregarding the aging process in stroke research may be an important and yet overlooked mechanism resulting in failure to produce clinically applicable therapies.
4. EXPERIMENTAL PROCEDURE
4.1 MCAO and Apocynin Treatment
Female rats were used in this investigation due to the exaggerated sexual dimorphism of size and weight in young adult versus aged males. Female Sprague-Dawley rats [(3–4 months, 260–280 g, N=92) and (18–20 months, 275–350 g, N=120)] were received from Harlan (Indainapolis, IN) and housed under natural light/dark conditions with food and water available ad libitum. Procedures involving animals abided by the West Virginia University Animal Care and Use Committee. Before MCAO or sham surgery, rats from both age groups were randomly divided into apocynin (5 mg/kg; i.p.) and vehicle (0.9% saline; i.p.) treated groups. Treatment was administered 30 min prior to surgery. Rats were anesthetized with ketamine (90 mg/kg; i.p.; Webster Veterinary; Sterling, MA) and xylazine (5 mg/kg; Webster Veterinary) prior to surgery and then supplemented as needed. Rats underwent MCAO for 2 h followed by tPA (Genentech; San Francisco, CA)-induced reperfusion as previously described (DiNapoli VA et al., 2006). Briefly, a micro-catheter was inserted into the internal carotid artery (ICA) and advanced until its tip occluded the ipsilateral MCA. This mechanical occlusion was verified by laser Doppler (LD-CBF) monitoring of the cerebral blood flow in the MCA perfusion territory. A 25 mm fibrin-rich, autologous blood clot was then injected directly into the MCA. Sham surgery consisted of all steps above except for occlusion of the MCA. Ischemia was monitored continuously. At 2 h, tPA (5 mg/kg) was administered via the femoral vein, and restoration of blood flow through the MCA was verified by LD-CBF. Ischemia was defined as a perfusion drop across the MCA territory of >80% as determined by laser Doppler and successful reperfusion was denoted as a return to >80% of baseline perfusion rate by 30 min after tPA administration.
4.2 Functional Testing
At 24 h following MCAO, functional testing was performed using the modified Neurological Severity Scores (mNSS), which is a composite score of motor, sensory, balance and reflex measures ranging from 1–17, with higher scores indicating greater neurological injury (Chen J et al., 2001).
4.3 Determination of Infarct Volume and Edema Formation
At 24 h following MCAO, rats (n=6 rats per group) were anesthetized with ketamine and xylazine, sacrificed by decapitation, brains removed and sliced coronally at 2 mm intervals. Sections were incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 20 min at 37°C. Following TTC staining, infarct volumes were quantified according to method previously described (Yang ZW et al., 1998). For each brain section, ischemic area was outlined and infarct volume calculated. To avoid overestimation of infarct volume, the corrected infarction volume (CIV) was calculated: CIV=(LA-[RA-RI])xd, where LA was area of the left hemisphere (mm2), RA was area of the right hemisphere (mm2), RI was the infracted area (mm2), and d was slice thickness (2 mm). Edema index (%) was calculated according to previously described method (Maier et al., 1998). Briefly, edema index=(RV-LV)/LVx100%, where RV was volume of right hemisphere (mm3) and LV was volume of left hemisphere (mm3).
4.4 Blood-brain barrier (BBB) permeability
At 24 h following MCAO, In situ brain perfusion and capillary depletion were carried out as described previously (n=6 rats per group) (Preston JE et al., 1995; Triguero D et al., 1990). Briefly, rats were anesthetized with ketamine (90 mg/kg; i.p.) and xylazine (5 mg/kg; i.p.) and heparinized (10,000 U/kg; i.p.). The common carotid arteries were exposed, cannulated and perfused with modified Krebs-Henseleit Ringer’s (117 mmol/L NaCl; 4.7 mmol/L KCl; 0.8 mmol/ L MgSO4; 24.8 mmol/L NaHCO3; 1.2 mmol/L KH2PO4; 2.5 mmol/L CaCl2; 10mmol/L D-glucose; dextran (70,000 Da) 29 g/L; bovine serum albumin 10 g/L), aerated with 95% O2/5% CO2 and warmed to 37°C. With start of the perfusion jugular vein was sectioned to allow for drainage. Once both arteries were cannulated, [14C]-sucrose was infused (flow rate: 0.5 ml/min) into the inflowing Ringer’s solution (total flow rate: 3.6 ml/min/hemisphere). After 20 min, the brain was flushed for 20 s with unlabeled Ringer’s solution and the rat sacrificed by decapitation. The brain was removed, dissected into ipsilateral and contralateral hemispheres and the choroid plexis and meninges excised. Brain tissue samples (~150 mg wet weight) and 100 μl of perfusate samples were prepared for radioactive counting by addition of 1 ml of tissue solubilizer (TS-2; Research Products Inc.; Mount Prospect, IL). After 72 h in TS-2, 250 μl glacial acetic acid and 4 ml scintillation cocktail (Bio-Safe NA; Research Products Inc.) were added and samples analyzed by liquid scintillation counting (Beckman LS5801; Beckman Coulter; Fullerton, CA). Amount of [14C] radioactivity in the brain (Ctissue; dpm/g) was expressed as a percentage of that in artificial perfusate (Cperfusate; dpm/ml) and termed Rtissue% (μl/g) as follows: Rtissue% = (Ctissue / Cperfusate) × 100%.
Capillary depletion was carried out after in situ perfusion, the brain was removed dissected into ipsilateral and contralateral hemispheres and choroid plexis and meninges excised. Hemispheres were homogenized in 1.5 ml capillary depletion buffer (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid (HEPES); 141 mM NaCl; 4 mM KCl; 2.8 mM CaCl2; 1 mM MgSO4; 1 mM NaH2PO4; 10 mM D-glucose; pH 7.4) maintained at 4°C. An equal volume of ice-cold 26% dextran (60,000 Da) solution was added to homogenate. Aliquots of homogenate were taken and centrifuged at 5,400 × g for 15 min. Capillary depleted supernatant was separated from the vascular pellet. Homogenate, supernatant, and resuspended vascular pellet were assayed for radioactivity using a liquid scintillation counter.
4.5 Markers of oxidative stress
At 24 h following MCAO, rats (n=6 rats per group) were anesthetized with ketamine and xylazine and transcardially perfused with 0.9% saline with heparin (2 U/ml). Brains were removed, and the ipsilateral and contralateral hemispheres separated. Brain sections were weighed and stored at −80°C. Measurement of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activities as well as, thiobarbituric acid reactive substances (TBARS) in the form of malondealdehyde (MDA) and protein carbonyl were carried out according to manufacturer’s protocols(Cayman Chemicals; Ann Arbor, MI).
4.6 Statistics
Data are presented as mean ± S.E. BBB permeability and oxidative stress assays were compared by three-way (ANOVA) with Tukey’s post hoc analysis. Functional score, stroke infarct volume and edema formation were compared by two-way ANOVA with Tukey’s post hoc analysis. Statistical significance was set at p<0.05.
Abbreviations
- MCAO
middle cerebral artery occlusion
- tPA
tissue plasminogen activator
- TTC
2,3,5-triphenyl tetrazolium chloride
- BBB
blood-brain barrier
- SOD
superoxide dismutase
- GPx
glutathione peroxidase
- CAT
catalase
- MDA
malondealdehyde
- TBARS
thiobarbituric acid reactive substances
- MPO
myeloperoxidase
- ICA
internal carotid artery
- MCA
middle cerebral artery
- LD-CBF
laser Doppler monitoring of cerebral blood flow
- mNSS
modified neurological severity score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abete P, Napoli C, Santoro G, Ferrara N, Tritto I, Chiariello M, Rengo F, Ambrosio G. Age-related decrease in cardiac tolerance to oxidative stress. J Mol Cell Cardiol. 1999;31:227–236. doi: 10.1006/jmcc.1998.0862. [DOI] [PubMed] [Google Scholar]
- Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. J Clin Invest. 1995;96:1414–1424. doi: 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functonal recovery. Journal of Cerebral Blood Flow and Metabolism. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJ. Speech production after stroke: the role of the right pars opercularis. Ann Neurol. 2003;54:310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Buga AM, Sascau M, Pisoschi C, Herndon JG, Kessler C, Popa-Wagner A. The genomic response of the ipsilateral- and contralateral cortex to stroke in aged rats. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Levine RL. Modification of proteins in endothelial cell death during oxidative stress. Free Radic Biol Med. 1997;22:1277–1282. doi: 10.1016/s0891-5849(96)00495-9. [DOI] [PubMed] [Google Scholar]
- De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller AA. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke. 2009;40:1091–1097. doi: 10.1161/STROKEAHA.108.531707. [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser KA, Li X, Rosen CL. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiology of Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNapoli VA, Rosen CL, Nagamine T, Crocco T. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods. 2006;154:233–238. doi: 10.1016/j.jneumeth.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure. Lessons from neuroprotective stroke trails and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Godbole AS, Lu X, Guo X, Kassab GS. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol. 2009;296:H152–H158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Nakamura A, Radak Z, Nakamoto H, Takahashi R, Yasuda K, Sakurai Y, Ishii N. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech Ageing Dev. 1999;107:245–253. doi: 10.1016/s0047-6374(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Harman D. Free-radical theory of aging. Increasing the functional life span. Annals of the New York Academy of Sciences. 1994;717:1–15. doi: 10.1111/j.1749-6632.1994.tb12069.x. [DOI] [PubMed] [Google Scholar]
- Kahles T, Leudike P, Endres M, Galla H, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:000. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neuroscience Letters. 2007;414:228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. Journal of Cerebral Blood Flow and Metabolism. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech Ageing Dev. 2007;128:286–292. doi: 10.1016/j.mad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Meyer MJ, Mosely DE, Amarnath V, Picklo MJ., Sr Metabolism of 4-hydroxy-trans-2-nonenal by central nervous system mitochondria is dependent on age and NAD+ availability. Chem Res Toxicol. 2004;17:1272–1279. doi: 10.1021/tx049843k. [DOI] [PubMed] [Google Scholar]
- Pantke U, Volk T, Schmutzler M, Kox WJ, Sitte N, Grune T. Oxidized proteins as a marker of oxidative stress during coronary heart surgery. Free Radic Biol Med. 1999;27:1080–1086. doi: 10.1016/s0891-5849(99)00144-6. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. Journal of Cerebral Blood Flow and Metabolism. 2007:1–11. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Park YM, Lim BH, Touyz RM, Park JB. Expression of NAD(P)H Oxidase Subunits and Their Contribution to Cardiovascular Damage in Aldosterone/Salt-Induced Hypertensive Rat. J Korean Med Sci. 2008;23:1039–1045. doi: 10.3346/jkms.2008.23.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC. The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res. 2007;4:216–227. doi: 10.2174/156720207781387213. [DOI] [PubMed] [Google Scholar]
- Preston JE, al-Sarraf H, Segal MB. Permeability of the developing blood-brain barrier to 14C-mannitol using the rat in situ brain perfusion technique. Brain Res Dev Brain Res. 1995;87:69–76. doi: 10.1016/0165-3806(95)00060-q. [DOI] [PubMed] [Google Scholar]
- Riganti C, Costamagna C, Bosia A, Ghigo D. The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress. Toxicol Appl Pharmacol. 2006;212:179–187. doi: 10.1016/j.taap.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007;27:14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- Rosen CL, DiNapoli VA, Nagamine T, Crocco T. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg. 2005;103:687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- Schroeder E, Vogelgesang S, Popa-Wagner A, Kessler C. Neurofilament expression in the rat brain after cerebral infarction: effect of age. Neurobiology of Aging. 2003;24:135–145. doi: 10.1016/s0197-4580(02)00063-5. [DOI] [PubMed] [Google Scholar]
- Siu GM, Draper HH. Metabolism of malonaldehyde in vivo and in vitro. Lipids. 1982;17:349–355. doi: 10.1007/BF02535193. [DOI] [PubMed] [Google Scholar]
- Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Li X, Kelly KA, Rosen CL, Huber JD. Plasminogen activator inhibitor type 1 derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res. 2009;1281:84–90. doi: 10.1016/j.brainres.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Tsimikas S. In vivo markers of oxidative stress and therapeutic interventions. Am J Cardiol. 2008;101:34D–42D. doi: 10.1016/j.amjcard.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Research. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Zhang A, Altura BT, Altura BM. Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery: a potential new cerebral dilator mechanism. Brain Research Bulletin. 1998;47:257–263. doi: 10.1016/s0361-9230(98)00120-8. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]