Abstract
Objective
Surgical menopause has been associated with an increased risk of coronary heart disease events. In this study, we aimed to determine the associations between coronary artery calcium (CAC) and hysterectomy, oophorectomy, and hormone therapy use with a focus on the duration of menopause for which there was no hormone therapy use.
Design
In a substudy of the Women’s Health Initiative placebo-controlled trial of conjugated equine estrogens (0.625 mg/d), we measured CAC by computed tomography 1.3 years after the trial was stopped. Participants included 1,064 women with previous hysterectomy, aged 50 to 59 years at baseline. The mean trial period was 7.4 years. Imaging was performed at a mean of 1.3 years after the trial was stopped.
Results
Mean age was 55.1 years at randomization and 64.8 years at CAC measurement. In the overall cohort, there were no significant associations between bilateral oophorectomy, years since hysterectomy, years since hysterectomy without taking hormone therapy (HT), years since bilateral oophorectomy, and years of HT use before Women’s Health Initiative enrollment and the presence of CAC. However, there was a significant interaction between bilateral oophorectomy and prerandomization HT use for the presence of any CAC (P = 0.05). When multivariable analyses were restricted to women who reported no previous HT use, those with bilateral oophorectomy had an odds ratio of 2.0 (95% CI: 1.2–3.4) for any CAC compared with women with no history of oophorectomy, whereas among women with unilateral or partial oophorectomy, the odds of any CAC was 1.7 (95% CI: 1.0–2.8). Among women with bilateral oophorectomy, HT use within 5 years of oophorectomy was associated with a lower prevalence of CAC.
Conclusions
Among women with previous hysterectomy, subclinical coronary artery disease was more prevalent among those with oophorectomy and no prerandomization HT use, independent of traditional cardiovascular disease risk factors. The results suggest that factors related to oophorectomy and the absence of estrogen treatment in oophorectomized women may be related to coronary heart disease.
Keywords: Calcium, Coronary, Oophorectomy, Hormone therapy, Women, Coronary artery disease, Atherosclerosis
Results from the Women’s Health Initiative (WHI) observational study indicate that a history of hysterectomy is associated with a more adverse cardiovascular disease (CVD) risk factor profile than no history of hysterectomy.1 Furthermore, in a subsample of women in the WHI who had blood samples collected at baseline, hysterectomy with bilateral oophorectomy was an independent predictor of Framingham risk score.2 In the WHI observational study, those with a history of hysterectomy and any oophorectomy had significantly higher annualized rates for total CVD events over a mean follow-up period of 5.1 years compared with those with no history of hysterectomy. However, after adjustment for demographic variables, body mass index, diet, exercise, and CVD risk factors, those with a history of hysterectomy with any oophorectomy were not at significantly increased risk of incident CVD events.1 In the Nurses’ Health Study, women who had undergone bilateral oophorectomy and had never taken estrogens after menopause had an increased risk of coronary events, but there was no increased risk among women who used estrogens after oophorectomy.3
Calcified plaque is actively deposited in arteries and other vascular areas as part of the chronic inflammatory process of atherosclerosis.4,5 The calcified component of coronary atheroma can be measured using computed tomography (CT) in multiple vascular beds, including the coronary arteries.6–11 Previous studies have demonstrated that the amount of calcified plaque in the coronary arteries is highly correlated with the total plaque burden, both calcified and noncalcified, when measured histologically12 while also being significantly associated with incident CVD events independent of the traditional CVD risk factors.13–20 Accordingly, in a substudy nested within the WHI clinical trial of conjugated equine estrogens (CEE), we aimed to determine the associations between coronary artery calcium (CAC) and hysterectomy, oophorectomy, and hormone therapy (HT) use with a focus on the duration of menopause before enrollment in the WHI for which there was no hormone therapy use. We hypothesized there would be a significant association between a history of oophorectomy and CAC.
METHODS
Participants
Participants in the current study were women enrolled in the WHI clinical trial of CEE who underwent a one-time CT scan of the chest to determine CAC. Detailed descriptions of the WHI study design and baseline characteristics of participants in the WHI-CEE trial were previously published.21–23 In brief, participants were postmenopausal women aged 50 to 79 years at randomization and had previous hysterectomy. Study participants were randomized to receive CEE, 0.625 mg/day (Premarin, Wyeth Pharmaceuticals, St. Davids, PA) or a matching placebo. Participants had annual clinic visits and interim telephone contacts at 6-month intervals between visits. Methods for data collection, management, and quality assurance were published previously.24
The WHI-CEE trial was originally planned to have an average follow-up duration of 8 years. However, the trial was stopped approximately 1 year early due to an increased risk of stroke in the absence of apparent benefit for coronary heart disease (CHD), resulting in an average mean trial period of 6.8 years for the entire cohort and 7.4 years for women between the ages of 50 and 59 at enrollment into the WHI. The initial report of the CEE trial findings were published in April 2004.21 Analyses stratified on age showed a nonsignificantly reduced risk of CHD among the women aged 50 to 59 years who were randomized to CEE. A subsequent publication focused on CHD found a significant reduction in a composite endpoint of CHD plus coronary revascularization for women aged 50 to 59 randomized to CEE, but no effect in women aged 60 and older.25 Therefore, an explanatory ancillary study using CAC was proposed to provide mechanistic information that might elucidate this finding. The WHI Coronary Artery Calcium Study (WHI-CACS) obtained coronary calcium measurements using cardiac CT for WHI-CEE participants who were 50 to 59 years old at the time of randomization into the CEE trial. Results concerning the association of CAC and randomization status in the CEE trial were published previously and showed that women who received CEE had significantly reduced odds for the presence and extent of CAC compared with women who received a placebo.26
Survey data collection
At the baseline WHI clinic visit, CEE trial participants provided data on a wide range of factors, including dietary habits, medical history, physical activity, medications and supplements, and socioeconomic status. Hypertension was defined as systolic blood pressure of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or higher, or use of a blood pressure–lowering medication. The presence of high cholesterol or diabetes was identified by use of a medication for that condition. Ethnicity was determined by self-report with the following categories: non-Hispanic white, African American/black (non-Hispanic), Hispanic, Asian/Pacific Islander, American Indian/Alaska Native, or unknown (women who indicated “other” ethnicity or did not answer the question). Education and income were ascertained by self-report from a range of categories. Use of postmenopausal HT before WHI-CEE trial enrollment was ascertained via an interview on current and past HT use.
Hysterectomy status was determined by asking “Did you ever have a hysterectomy? (This is an operation to take out your uterus or womb.)” Oophorectomy status was determined by asking “Did you ever have an operation to have one or both of your ovaries taken out?” with response categories of “no,” “yes, one was taken out,” “yes, both were taken out,” “yes, part of an ovary was taken out,” “yes, unknown number taken out,” and “don’t know.” Women indicating either of the latter two categories had their bilateral oophorectomy status set as “missing.” If the woman answered that she had undergone an oophorectomy, she was then asked at what age this operation was performed. Age at menarche, age at hysterectomy, parity, and age at first birth were also ascertained via self-administered questionnaires.
Coronary artery calcified plaque measurements
After approval of the study by local institutional review boards, women in the CEE trial who were aged 50 to 59 at time of randomization to CEE or placebo at 28 WHI clinical centers (N = 1,742) were sent invitational mailings to participate in WHI-CACS. Exclusion criteria were a last measured or reported weight of 300 lb or more (due to technical and equipment-related restrictions), participant request for no further contact or clinic visits, or participant lost to follow-up or deceased since randomization (30.4% of participants were excluded for one or more of these reasons). A total of 1,079 women (61.6% of those eligible at the 28 clinical centers) provided informed consent and received cardiac CT examinations between May and September 2005.
Approximately 1.3 years after the CEE trial ended, each woman underwent a single noninvasive imaging study of the coronary arteries by electron beam or multidetector-row CT at one of 28 participating centers. A standardized protocol was developed based on previous multicenter experience with cardiac CT.27 Phantom and test images were obtained from each CT system to verify technical parameters and CT system performance. Analyses of the measurements were performed by the central reading center at Wake Forest University blinded to participants’ treatment assignment.27 The Agatston score was calculated on a computer workstation (TeraRecon Inc., San Mateo, CA) by experienced image analysts using established parameters (lesion size of >1 mm2, adjustment for slice thickness, and threshold of 130 Hounsfield units).28
Women with a history of coronary revascularization before randomization were excluded from the analysis (n = 12). The reading protocol specified exclusion of coronary stents, pacemakers, metallic clips, and other surgical remnants from the analysis process. Three women with incomplete scans were excluded. The final data set included 1,064 participants without previous revascularization and with nonmissing CAC scores.
Statistical analyses
Data collected from the baseline visit of the WHI-CEE trial were combined with CAC scores from the WHI-CACS project to form the data set for this study. The primary outcome variable was the presence of any coronary calcium. A CAC score of 10 or more was treated as a secondary outcome to evaluate potential false-positive results of using a CAC score of more than 0 as the only outcome measure. The primary predictor variable was a history of oophorectomy categorized as bilateral, unilateral, or partial. Baseline characteristics, including cardiovascular risk factors, were compared between participants with a CAC score of 0 versus more than 0. Differences between the groups were assessed using χ2 tests for categorical variables and t tests for continuous variables. Logistic regression models were used to evaluate the association of selected reproductive variables and the CAC score. The reproductive variables included oophorectomy status, years since bilateral oophorectomy, years since hysterectomy, years since hysterectomy without estrogen therapy, and years of estrogen therapy use before baseline. For the modeling, the CAC score was dichotomized two ways, either 0 versus more than 0 or less than 10 versus 10 or more. All the models were adjusted for baseline characteristics and subsequently for the CEE randomization assignment. The interaction between oophorectomy status (yes vs no) and use of HT before enrollment in the WHI (yes vs no) for CAC was prespecified and tested using multivariable logistic regression. Participants with missing data for any of the adjustment variables were excluded from analyses using those variables. A P value less than 0.05 was considered statistically significant. No adjustments were made for multiple comparisons, and exact P values are given. All reported P values are two sided. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics
Among women who participated in the WHI-CEE trial, those who enrolled in the WHI-CACS substudy were similar to those who did not enroll with respect to age, body mass index, oophorectomy status, family history of myocardial infarction, and medication use for hypertension and high cholesterol. However, those enrolled in WHI-CACS were more likely to be using HT and less likely to be current smokers and taking diabetes medications. WHI-CACS women had an average of 7.4 years of participation in the CEE trial and an average interval of 1.3 years from trial end to scanning.
There were 1,064 women available for this study. The mean (SD) age at WHI randomization was 55.1 (2.8) years and 64.8 (2.9) years at CAC measurement. Unilateral, bilateral, or partial oophorectomy was reported by 554 women (52.1%), with 378 having both ovaries removed. The mean duration since bilateral oophorectomy was 14 years. Pre-enrollment use of HT was reported by 49% of the cohort. By oophorectomy status, 72%, 44%, and 42% of women with a history of bilateral, unilateral/partial, or no oophorectomy, respectively, used HT before WHI randomization. The median CAC scores among those who reported any previous HT use were similar to those of women who reported no use (65 vs 76, P = 0.5).
Overall, the prevalence of a CAC score of more than 0 was 47%, whereas the prevalence of a CAC score of 10 or more was 39%. Those with any CAC had significantly older age at enrollment in the WHI (55.6 vs 54.8 y, P < 0.01). With adjustment for age at enrollment, those with any CAC had significantly greater body mass index (31.5 vs 29.8 kg/m2, P < 0.01), waist circumference (94.9 vs 88.8 cm, P < 0.01), waist-to-hip ratio (0.84 vs 0.80, P < 0.01), and pack-years of smoking (13.6 vs 7.1, P < 0.01) but lower reported physical activity (9.0 vs 11.0 METs per week, P = 0.06) (Table 1). The CAC score greater than 0 group also had greater prevalence of current smoking (17.1 vs 7.5%, P < 0.01), hypertension (48.3 vs 33.9%, P < 0.01), cholesterol medication use (14.8 vs 7.3%, P < 0.01), and medical treatment for diabetes (7.0 vs 3.2%, P < 0.01) but had a lower prevalence of achieving a college degree (24.9 vs 34.3%, P < 0.01). There were no significant differences between women with and without CAC for years since hysterectomy, years since bilateral oophorectomy, reported pre-enrollment use of HT, years since hysterectomy with no previous HT use, or duration of HT use.
TABLE 1.
Cohort characteristics by the presence and absence of coronary calcium
| Variable | CAC score > 0 (n = 499) | CAC score = 0 (n = 565) | Pa |
|---|---|---|---|
| Age at WHI enrollment, yb | 55.6 (2.8) | 54.8 (2.9) | <0.01 |
| Age at CAC scan, yb | 65.3 (2.8) | 64.4 (2.9) | <0.01 |
| Ethnicityc | 0.24 | ||
| White | 377 (75.6) | 422 (74.7) | |
| Black | 73 (14.6) | 104 (18.4) | |
| Hispanic | 33 (6.6) | 32 (5.7) | |
| American Indian | 8 (1.6) | 1 (0.2) | |
| Asian/Pacific Islander | 2 (0.4) | 1 (0.2) | |
| Unknown | 6 (1.2) | 5 (1.0) | |
| Body mass index (kg/m2)b | 31.5 (6.0) | 29.8 (6.1) | <0.01 |
| Waist circumference (cm)b | 94.9 (14.0) | 88.8 (13.9) | <0.01 |
| Waist-to-hip ratiob | 0.84 (0.1) | 0.80 (0.01) | <0.01 |
| Cigarette smoking, pack-yearsb | 13.6 (19.9) | 7.1 (14.4) | <0.01 |
| Physical activity, METsb | 9.0 (12.2) | 11.0 (13.8) | 0.06 |
| Educationc | <0.01 | ||
| 0–8 y | 6 (1.2) | 10 (1.8) | |
| Some high school | 22 (4.5) | 12 (2.1) | |
| High school diploma/GED | 124 (25.1) | 99 (17.7) | |
| School after high school | 219 (44.3) | 247 (44.1) | |
| College degree or higher | 123 (24.9) | 192 (34.3) | |
| Smoking status c | <0.01 | ||
| Never smoked | 212 (42.6) | 303 (54.0) | |
| Past smoker | 200 (40.2) | 216 (38.5) | |
| Current smoker | 85 (17.1) | 42 (7.5) | |
| Hypertensionc | 218 (48.3) | 176 (33.9) | <0.01 |
| High cholesterolc | 62 (14.8) | 36 (7.3) | <0.01 |
| Diabetes mellitusc | 35 (7.0) | 18 (3.2) | 0.01 |
| Oophorectomyc | 0.15 | ||
| None | 195 (39.3) | 264 (47.1) | |
| Part of an ovary removed | 10 (2.0) | 8 (1.4) | |
| One ovary removed | 85 (17.1) | 73 (13.0) | |
| Both ovaries removed | 184 (37.1) | 194 (34.6) | |
| Do not know | 13 (2.6) | 11 (2.0) | |
| Years since bilateral oophorectomyb | 14.1 (7.3) | 12.8 (7.0) | 0.26 |
| Years since hysterectomyb | 16.2 (6.9) | 15.5 (6.8) | 0.57 |
| Reported previous HT usec | 0.88 | ||
| Never used | 236 (47.3) | 267 (47.3) | |
| Past user | 161 (32.3) | 173 (30.6) | |
| Current user | 102 (20.4) | 125 (22.1) | |
| Duration of previous HT use, yb | 3.0 (5.0) | 3.2 (5.2) | 0.40 |
| Years of no HT after hysterectomyb | 13.3 (7.9) | 12.5 (7.9) | 0.39 |
CAC, coronary artery calcium; WHI, Women’s Health Initiative; HT, hormone therapy.
Adjusted for age at enrollment in the WHI.
Mean (SD).
Frequency (%).
The characteristics of the study cohort when they joined the WHI stratified by oophorectomy status and further stratified by reported HT use before WHI enrollment are shown in Table 2. The sample size for this table was 1,013 because 51 women were not able to be categorized by oophorectomy status (ie, missing or “don’t know” response on the questionnaire). In summary, there were differences in the mean values for some of the study variables by previous HT use and within an oophorectomy category. However, in general, the differences were not consistent across the oophorectomy groups.
TABLE 2.
Baseline characteristics of the Women’s Health Initiative Coronary Artery Calcium Study stratified by oophorectomy status
| Bilateral oophorectomy |
Unilateral or partial oophorectomy |
No oophorectomy |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous HT use: ever |
Previous HT use: ever |
Previous HT use: ever |
|||||||||||||||
| Yes |
No |
Yes |
No |
Yes |
No |
||||||||||||
| Continuous variables | Units | No. | Mean | No. | Mean | Pa | No. | Mean | No. | Mean | Pa | No. | Mean | No. | Mean | Pa | Pb |
| Age at WHI enrollment | y | 272 | 55.4 | 106 | 55.7 | 0.56 | 77 | 55.3 | 99 | 54.4 | 0.09 | 192 | 55.48 | 267 | 54.6 | <0.01 | 0.04 |
| Body mass index | kg/m2 | 271 | 30.5 | 105 | 31.8 | 0.05 | 76 | 31.1 | 99 | 31.7 | 0.58 | 190 | 29.90 | 267 | 30.1 | 0.79 | 0.03 |
| Cigarette smoking | Pack-years | 262 | 9.2 | 103 | 13.1 | 0.06 | 76 | 9.8 | 96 | 9.55 | 0.89 | 186 | 9.00 | 262 | 10.9 | 0.26 | 0.94 |
| Physical activity | MET-h/wk | 245 | 10.0 | 97 | 8.7 | 0.40 | 67 | 12.3 | 79 | 7.2 | 0.07 | 166 | 11.02 | 228 | 10.3 | 0.55 | 0.14 |
| Time since hysterectomy | y | 270 | 13.5 | 106 | 15.9 | <0.01 | 77 | 18.2 | 99 | 15.7 | 0.01 | 191 | 16.66 | 267 | 16.6 | 0.95 | <0.01 |
| Time since oophorectomy | y | 269 | 12.9 | 103 | 14.9 | 0.02 | 77 | 18.6 | 98 | 15.8 | <0.01 | – | – | – | – | – | <0.01 |
| Categorical variables | Group | No. | % | No. | % | Pa | No. | % | No. | % | Pa | No. | % | No. | % | Pa | Pb |
| Randomization (CEE) | CEE | 135 | 49.6 | 46 | 43.4 | 0.28 | 40 | 52.0 | 49 | 49.5 | 0.75 | 104 | 54.2 | 136 | 50.9 | 0.49 | 0.23 |
| Hypertension | Yes | 97 | 38.0 | 42 | 42.4 | 0.45 | 37 | 53.6 | 42 | 48.3 | 0.51 | 62 | 36.1 | 91 | 37.8 | 0.72 | 0.03 |
| High cholesterol | Yes | 16 | 6.7 | 11 | 12.1 | 0.11 | 11 | 16.4 | 9 | 11.1 | 0.35 | 22 | 13.3 | 24 | 10.5 | 0.39 | 0.32 |
| Diabetes | Yes | 9 | 3.3 | 8 | 7.55 | 0.08 | 5 | 6.5 | 9 | 9.09 | 0.53 | 4 | 2.1 | 14 | 5.24 | 0.16 | 0.11 |
| Hot flashes or night sweats | Yes | 174 | 63.9 | 39 | 37.1 | <0.01 | 55 | 71.4 | 56 | 57.1 | 0.05 | 138 | 73.4 | 141 | 53.6 | <0.01 | 0.09 |
HT, hormone therapy; WHI, Women’s Health Initiative; CEE, conjugated equine estrogens.
P value for comparison of HT use to no HT use within each oophorectomy status.
P value for comparison between oophorectomy groups.
Odds of any CAC for different reproductive variables
After adjustment for the traditional CVD risk factors and CEE randomization status in separate multivariable logistic regression models, there was a significant association between a history of single or partial oophorectomy and the presence of CAC (odds ratio = 1.50, 95% CI: 1.02–2.21) (Table 3). However, there were no significant associations between the presence of any CAC and bilateral oophorectomy, years since hysterectomy, years since hysterectomy without taking HT, years since bilateral oophorectomy, and years of HT use before WHI enrollment. Replacing body mass index with waist circumference and further adjustment for education level did not change these results. Moreover, these associations were not materially different when a CAC score of 10 or more versus less than 10 was used as the outcome.
TABLE 3.
Multivariable odds of a coronary artery calcium score >0 for different reproductive variables among the entire cohorta
| Characteristic | No. | OR | 95% CI |
|---|---|---|---|
| Unilateral or partial oophorectomy (compared with no oophorectomy) | 961 | 1.50 | 1.02–2.21 |
| Bilateral oophorectomy (compared with no oophorectomy) | 1.27 | 0.94–1.71 | |
| 1 y since hysterectomy | 1,006 | 0.99 | 0.97–1.01 |
| 1 y since bilateral oophorectomy | 353 | 1.01 | 0.98–1.04 |
| 1 y since hysterectomy without HT use | 1,006 | 0.99 | 0.98–1.01 |
| 1 y used HT before WHI enrollment | 1,006 | 1.00 | 0.97–1.03 |
| <5 y used HT before WHI enrollment (compared with no previous HT use) | 1,006 | 1.01 | 0.74–1.39 |
| 5–10 y used HT before WHI enrollment (compared with no previous HT use) | 1.51 | 0.98–2.33 | |
| >10 y used HT before WHI enrollment (compared with no previous HT use) | 0.91 | 0.59–1.40 |
Values adjusted for age at time of computed tomography scan, ethnicity, smoking, diabetes mellitus, hypertension, high cholesterol, body mass index, education, and conjugated equine estrogens trial randomization status. OR, odds ratio; HT, hormone therapy; WHI, Women’s Health Initiative.
Entire cohort except those with angioplasty or coronary artery bypass graft.
Differential association between oophorectomy and CAC
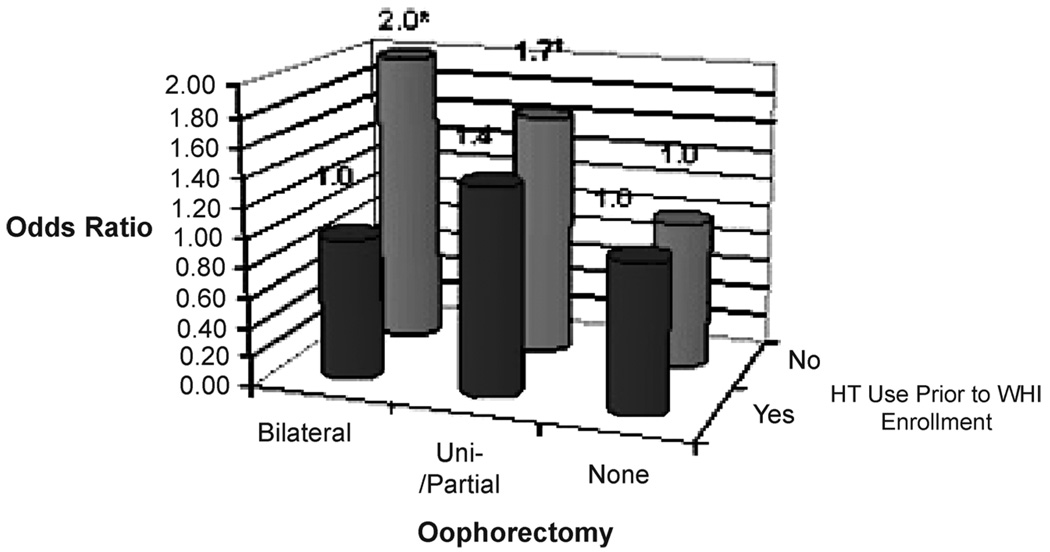
Among women who reported no HT use before WHI enrollment (n = 449), the median CAC scores were 12.0, 1.3, and 0.0 for those who had undergone a bilateral, unilateral/partial, and no oophorectomy, respectively. Among women who reported using any HT before enrollment, the median CAC scores were 0, 4.2, and 0 for the same groups, respectively. The interaction between any oophorectomy and reported use of HT before WHI enrollment for the presence of any CAC was of borderline significance (P = 0.06). However, we observed a significant interaction (P = 0.05) between bilateral oophorectomy status and reported previous use of HT for the presence of CAC. Specifically, those who reported no HT use and who had undergone an oophorectomy had higher odds for a CAC score of more than 0. Accordingly, when the analyses were restricted to women who reported not using any HT before enrollment and with adjustment for CHD risk factors, education, and CEE randomization status, women who had undergone bilateral oophorectomy had an odds ratio of 2.0 (95% CI: 1.2–3.4) for any CAC compared with women with no history of oophorectomy (Fig. 1). The odds of having any CAC for those who had a unilateral or partial oophorectomy were 1.7 (95% CI: 1.0–2.8). The magnitude and significance of these associations were not changed by adjustment for exercise, marital status, presence of vasomotor symptoms, history of depression, years since hysterectomy, or randomization status in the dietary modification trial of the WHI. Furthermore, the results were essentially unchanged when a CAC score of 10 or more versus less than 10 was used as the outcome.
FIG. 1.
Multivariable odds of a coronary calcium score >0 for oophorectomy stratified by prior hormone therapy group (except those with percutaneous transluminal coronary angioplasty or coronary artery bypass grafting). *P < 0.01; †P = 0.06; n = 961. Adjusted for age at time of computed tomography scan, ethnicity, smoking, diabetes, hypertension, high cholesterol, body mass index, conjugated equine estrogens, randomization status, and education. WHI, Women’s Health Initiative; HT, hormone therapy.
In comparison, when the analyses were restricted to women who reported using any HT before WHI enrollment (n = 513) and with the same adjustment for the risk factors listed above, women who had undergone bilateral oophorectomy had an odds ratio of 1.0 (95% CI: 0.6–1.4) for any CAC, whereas the odds ratio for any CAC score for those who had a unilateral or partial oophorectomy was 1.4 (95% CI: 0.8–2.5) (Fig. 1). As before, the results were not significantly different when a CAC score of 10 or more versus less than 10 was used as the outcome or additional covariates were included in the model.
We also examined the association between age at bilateral oophorectomy and CAC stratified by HT use before WHI enrollment. Among those who did not use HT before enrollment in the WHI and compared with those with no history of oophorectomy, those who had a bilateral oophorectomy between the ages of 35 and 44 years had an odds ratio of 2.1 for a CAC score of more than 0 (95% CI: 0.99–4.5). Similarly, those who had a bilateral oophorectomy between the ages of 45 and 54 years had an odds ratios of 2.4 (95% CI: 0.96–6.0). Conversely, among those who used HT before WHI enrollment, there were no significant associations between age at bilateral oophorectomy and CAC score.
To further explore the relationship between oophorectomy and HT use before enrollment in the WHI and the presence of CAC, we examined the timing of HT use by the oophorectomy group. With adjustment for age among women who had undergone a bilateral oophorectomy, women with a CAC score of more than 0 had a significantly lower prevalence of starting HT within 5 years of menopause than women with a CAC score of 0 (CAC score >0: 57.6%, CAC score of 0:67.3%, respectively) (P = 0.04). Conversely, there were no significant differences in the distribution of HT use by time since menopause among women with no history of oophorectomy or a history of unilateral or partial oophorectomy.
DISCUSSION
In this study of a subgroup of postmenopausal women from the WHI-CEE clinical trial who were 50 to 59 years of age at WHI baseline and studied for CAC, there were no significant associations between several reproductive variables and the presence of CAC. However, among women who had not used HT before enrollment in the WHI, those who had a history of bilateral oophorectomy had more than a twofold higher odds of the presence of any CAC as well as a CAC score of 10 or more, independent of traditional CVD risk factors, sociodemographic variables, and randomization status in the CEE trial. Women who reported a partial or unilateral oophorectomy and also reported not using any HT before enrollment in the WHI had higher odds of any CAC or a CAC score of 10 or more. Additionally, among women with bilateral oophorectomy but not those with unilateral/partial oophorectomy, HT use within 5 years of oophorectomy was associated with a lower prevalence of CAC. Conversely, there were no significant associations between any type of oophorectomy among women who reported using HT after this surgical procedure. Overall, these results suggest that a complex relationship exists between oophorectomy and HT for the presence and extent of coronary artery plaque and that the characteristics of women with oophorectomy, HT use, and the timing of HT use after menopause (ie, oophorectomy) may be clinically relevant.
Several previous studies have identified bilateral oophorectomy as a risk factor for coronary artery disease. For example, four studies reported more severe coronary atherosclerosis and/or clinical evidence of CHD among women who had a history of bilateral oophorectomy.29–32 This increased risk has been reported to depend on the time since oophorectomy.33 Studies on the potential association between reproductive factors such as hysterectomy and oophorectomy and subclinical atherosclerosis (eg, CAC) are more limited. In a study of African American and white women, hysterectomy was not associated with CAC,34 whereas a study of carotid intimal-medial thickness did not find a significant difference between those who had and did not have a bilateral oophorectomy.35 Notably, these studies did not consider the potential interaction between oophorectomy status and HT.
A potential explanation for the increased risk of coronary artery disease in women with oophorectomy is the resulting estrogen deficiency. Compared with women who have not undergone oophorectomy, even women who undergo a unilateral oophorectomy have significantly lower estrogen levels and higher follicle-stimulating hormone levels while also having an earlier onset of menopause.36–39 Of note, women who undergo bilateral oophorectomy are more likely to be prescribed HT after surgery than women who undergo unilateral oophorectomy.40 Therefore, women who undergo a unilateral oophorectomy would be more likely to have an untreated relative estrogen deficiency and therefore a higher odds of coronary artery disease. This is consistent with our finding of a significantly elevated odds of CAC among women with a history of unilateral, but not bilateral, oophorectomy in the analysis of the entire cohort.
Observational studies have shown that HT is associated with lower CHD, whereas clinical trials have not demonstrated a protective effect21,41; although recent findings in the parent study for the present analyses suggest a protective effect for women between the ages of 50 and 59 years.25 To further investigate this paradox, several observational studies have been conducted to test the potential association between HT and levels of CAC. The results of these studies are mixed. Two separate studies did not find a significant difference between those taking and not taking HT. In these studies, however, there were differences in CAC by levels of certain risk factors, such as body mass index42 and lipoproteins,43 within each of the HT groups. Conversely, others have shown the reported use of HT44 and duration of HT use45,46 to be associated with less CAC. Moreover, an analysis separate from the WHI-CACS study showed that women ages between 50 and 59 years at baseline and who were randomized to receive CEE had significantly lower odds of the presence and extent of CAC that was independent of multiple CVD and socioeconomic risk factors.26
What about the potential interaction between oophorectomy and HT for coronary artery disease? Higano et al47 found less clinical CHD among women who had bilateral oophorectomy and received HT than among women who had bilateral oophorectomy but had not received HT. Furthermore, results from the Dutch Nurse Cohort study indicate that among women who had a history of bilateral oophorectomy, those who never used HT had nearly a threefold higher risk of incident CHD than women who had used HT after oophorectomy.48 The results of our study are in accord with these previous findings and support the premise that the status of HT in women with a history of bilateral oophorectomy is clinically important for assessing CHD risk.
Classic histologic studies have demonstrated that calcium is deposited during the process of atherosclerosis.4,5,49 These calcified lesions can be detected in many vascular beds6,7 and quantified by CT.28 Previous studies have demonstrated calcium in the coronary arteries to be a significant and independent predictor of future CVD events.13,14,17,19,50 Moreover, among asymptomatic individuals the addition of the CAC score to the Framingham risk score can improve the risk prediction for future CHD events.20 Therefore, CAC is gaining support as a novel marker for identifying individuals at elevated risk of myocardial infarction and adding significant predictive ability to existing risk stratification algorithms.51
The prevalence and distribution of CAC in the WHI-CACS cohort (47%) are comparable with other reported samples of similar age. For example, the prevalence of CAC among white women with an average age of 63.1 years in the Multi-Ethnic Study of Atherosclerosis was 44.6%.52 Additionally, in the Multi-Ethnic Study of Atherosclerosis and among white women, the risk factors that were significantly associated with CAC included age, body mass index, low-density lipoprotein and high-density lipoprotein cholesterol, current and previous smoking, hypertension, and cholesterol medication use. We also found significant associations for age, body mass index, high cholesterol, smoking status, and hypertension. The concordance in findings between the WHI-CACS and other studies on the prevalence of CAC, as well as the risk factors for CAC, indicate that the WHI-CACS cohort is, in general, representative of national community-based samples of women.
Although the WHI-CACS study was conducted using women enrolled in a randomized clinical trial, the current analysis was observational and cross-sectional in nature. Therefore, there could be residual confounding affecting the results. We have attempted to address this issue by considering as many potential confounding variables as possible in the analysis. Also, as the measures for CAC were obtained at the end of the WHI-CEE trial, there is the possibility of survival bias. As the presence and extent of CAC is significantly associated with incident morbidity and mortality, the bias in this case would result in an attenuation of the magnitude of the effect. For some of the analyses, the number of women available was relatively small. However, the finding of a significant association between bilateral oophorectomy and CAC among those with no history of HT before WHI enrollment was clinically relevant and robust across several analyses. Finally, a large number of analyses were conducted, raising the issue of multiple comparisons. Although this may be an issue for the marginal associations, the magnitude of the association between bilateral oophorectomy and CAC among those who did not use HT before WHI enrollment was relatively large and statistically significant.
CONCLUSIONS
In summary, among women in their 60s who did not have a history of HT use before enrollment in the WHI, those with a history of bilateral oophorectomy had significantly higher odds of the presence and extent of CAC than women who had no history of oophorectomy, independent of CEE randomization assignment. Among women with bilateral oophorectomy, those who used HT before WHI enrollment did not have higher odds of any CAC, whereas HT use within 5 years of oophorectomy was associated with a lower prevalence of CAC. These findings are consistent with the thesis that the estrogen deficiency associated with bilateral or unilateral/partial oophorectomy is related to an increased burden of calcified plaque in the coronary arteries that can be countered by the use of HT. These results are also consistent with previous findings and indicate that medical professionals providing health care to women should carefully evaluate the reproductive history for oophorectomy status and previous HT use when assessing the risk of coronary artery disease.
Acknowledgments
We gratefully acknowledge the dedication of investigators and staff at the WHI clinical centers, the WHI-CACS centers, the WHI Clinical Coordinating Center, and the National Heart, Lung, and Blood Institute (NHLBI) Program Office. Most importantly, we are indebted to the WHI participants for their extraordinary commitment to women’s health research. The Women’s Health Initiative Coronary Artery Calcium Research Group, Program Office, NHLBI, Bethesda, MD: Jacques E. Rossouw, Shari Ludlam. Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, WA: Barbara B. Cochrane, Julie R. Hunt. CT Reading Center: Wake Forest University, Winston-Salem, NC: J. Jeffrey Carr, Chris O–Rourke, Lining Du, Suzanne Pillsbury, Caresse Hightower, Robert Ellison, Joshua Tan. Clinical Centers: Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, Maureen Magnani, David H. Noble, Tony Dellicarpini; Brigham and Women’s Hospital, Harvard Medical School, Boston, MA: JoAnn Manson, Maria Bueche, Ann D. McGinnis, Frank J. Rybicki; Brown University, Providence, RI: Charles B. Eaton, Gretchen Sloane; Emory University, Atlanta, GA: Lawrence S. Phillips, Vicki Butler, Margaret Huber, Jane Vitali; George Washington University Medical Center, Washington, DC: Judith Hsia, Claire LeBrun, Ron Palm, Donna Embersit; Kaiser Permanente Center for Health Research, Portland, OR: Evelyn Whitlock, Kathy Arnold; Kaiser Permanente Division of Research, Oakland, CA: Steve Sidney, Virginia Cantrell; Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen, Cindy Feltz; MedStar Research Institute/Howard University, Washington, DC: Barbara V. Howard, Asha Thomas-Geevarghese, Gerrye Boggs, James S. Jelinick; Northwestern University, Chicago/Evanston, IL: Philip Greenland, Annette Neuman, Grace Carlson-Lund, Susan M. Giovanazzi; Stanford Prevention Research Center, Stanford, CA: Marcia L. Stefanick, Sue Swope; The Ohio State University, Columbus, OH: Rebecca Jackson, Kim Toussant; University of Alabama at Birmingham, Birmingham, AL: Cora E. Lewis, Penny Pierce, Cathy Stallings; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende, Sandy Goel, Rosemary Laughlin; University of California at Davis, Sacramento, CA: John Robbins, Sophia Zaragoza, Denise Macias, Dennis Belisle; University of California at Los Angeles, Los Angeles, CA: Lauren Nathan, Barbara Voigt, Jonathan Goldin, Michael Woo; University of California at San Diego, LaJolla/Chula Vista, CA: Robert D. Langer, Matthew Allison, Xi Lien, C. Michael Wright; University of Cincinnati, Cincinnati, OH: Margery Gass, Susie Sheridan; University of Iowa, Iowa City/Davenport, IA: Jennifer G. Robinson, Deborah Feddersen, Kathy Kelly-Brake, Jennifer Carroll; University of Massachusetts/Fallon Clinic, Worcester, MA: Judith Ockene, Linda Churchill; University of Medicine and Dentistry of New Jersey, Newark, NJ: Norman L. Lasser, Barbara Miller, Pierre D. Maldjian, Jacques Pierre-Louis; University of Miami, Miami, FL: Joel Fishman, Mary Jo O’Sullivan; Diann Fernandez; University of Minnesota, Minneapolis, MN: Karen L. Margolis, Cindy L. Bjerk, Charles Truwit, Julie A. Hearity; University of North Carolina, Chapel Hill, NC: W. Brian Hyslop, Kelley Darroch, Carol Murphy, Gerardo Heiss; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller, Daniel Edmundowicz, Diane Ives; University of Tennessee, Memphis, TN: Karen C. Johnson, Suzanne Satterfield, Stephanie A. Connelly, Elizabeth L. Jones; University of Texas Health Science Center, San Antonio, TX: Robert Brzyski, Melissa Anne Nashawati, Susan Torchia, Angela Rodriguez, Ruben Garza, Paul Nentwich; University of Wisconsin, Madison, WI: Gloria E. Sarto, Lynn Broderick, Nancy K. Sweitzer. The Women’s Health Initiative Investigator Group, Program Office, NHLBI, Bethesda, MD: Barbara Alving, Jacques E. Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, WA:Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker; Medical Research Labs, Highland Heights, KY: Evan Stein; University of California at San Francisco, San Francisco, CA: Steven Cummings.Clinical Centers: Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller; Baylor College of Medicine, Houston, TX: Jennifer Hays; Brigham and Women’s Hospital, Harvard Medical School, Boston, MA: JoAnn Manson; Brown University, Providence, RI: Annlouise R. Assaf; Emory University, Atlanta, GA: Lawrence Phillips; Fred Hutchinson Cancer Research Center, Seattle, WA: Shirley Beresford; George Washington University Medical Center, Washington, DC: Judith Hsia; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA: Rowan Chlebowski; Kaiser Permanente Center for Health Research, Portland, OR: Evelyn Whitlock; Kaiser Permanente Division of Research, Oakland, CA: Bette Caan; Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen; MedStar Research Institute/Howard University, Washington, DC: Barbara V. Howard; Northwestern University, Chicago/Evanston, IL: Linda Van Horn; Rush Medical Center, Chicago, IL: Henry Black; Stanford Prevention Research Center, Stanford, CA: Marcia L. Stefanick; State University of New York at Stony Brook, Stony Brook, NY: Dorothy Lane; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Alabama at Birmingham, Birmingham, AL: Cora E. Lewis; University of Arizona, Tucson/Phoenix, AZ: Tamsen Bassford; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende; University of California at Davis, Sacramento, CA: John Robbins; University of California at Irvine, CA: F. Allan Hubbell; University of California at Los Angeles, Los Angeles, CA: Howard Judd; University of California at San Diego, LaJolla/Chula Vista, CA: Robert D. Langer; University of Cincinnati, Cincinnati, OH: Margery Gass; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Hawaii, Honolulu, HI: David Curb; University of Iowa, Iowa City/Davenport, IA: Robert Wallace; University of Massachusetts/Fallon Clinic, Worcester, MA: Judith Ockene; University of Medicine and Dentistry of New Jersey, Newark, NJ: Norman Lasser; University of Miami, Miami, FL: Mary Jo O’Sullivan; University of Minnesota, Minneapolis, MN: Karen Margolis; University of Nevada, Reno, NV: Robert Brunner; University of North Carolina, Chapel Hill, NC: Gerardo Heiss; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; University of Tennessee, Memphis, TN: Karen C. Johnson; University of Texas Health Science Center, San Antonio, TX: Robert Brzyski; University of Wisconsin, Madison, WI: Gloria E. Sarto; Wake Forest University School of Medicine, Winston-Salem, NC: Denise Bonds; Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI: Susan Hendrix.
Funding/Support: The National Heart, Lung, and Blood Institute, US Department of Health and Human Services, funds the Women’s Health Initiative (WHI), program and provided support for the WHI-Coronary Artery Calcium Study ancillary study. Wyeth provided study pills (active and placebo) for the WHI-Conjugated Equine Estrogens trial but had no other role in the study.
Footnotes
Financial disclosure: None reported.
REFERENCES
- 1.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 2.Hsia J, Barad D, Margolis K, et al. Usefulness of prior hysterectomy as an independent predictor of Framingham risk score (The Women’s Health Initiative) Am J Cardiol. 2003;92:264–269. doi: 10.1016/s0002-9149(03)00621-0. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 4.Stary HC. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol. 2001;88:16E–19E. doi: 10.1016/s0002-9149(01)01713-1. [DOI] [PubMed] [Google Scholar]
- 5.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 6.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 7.Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–866. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 8.Wong ND, Sciammarella M, Arad Y, et al. Relation of thoracic aortic and aortic valve calcium to coronary artery calcium and risk assessment. Am J Cardiol. 2003;92:951–955. doi: 10.1016/s0002-9149(03)00976-7. [DOI] [PubMed] [Google Scholar]
- 9.Sun K, Takasu J, Yamamoto R, et al. Assessment of aortic atherosclerosis and carotid atherosclerosis in coronary artery disease. Jpn Circ J. 2000;64:745–749. doi: 10.1253/jcj.64.745. [DOI] [PubMed] [Google Scholar]
- 10.Sutton-Tyrrell K, Kuller LH, Matthews KA, et al. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160:407–416. doi: 10.1016/s0021-9150(01)00591-3. [DOI] [PubMed] [Google Scholar]
- 11.Allison M, DiTomasso D, Criqui M, Langer R. Renal artery calcium: relationship to systemic calcified atherosclerosis. Circulation. 2006;113:e357. doi: 10.1177/1358863x06073449. [DOI] [PubMed] [Google Scholar]
- 12.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 13.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253–1260. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 14.Georgiou D, Budoff MJ, Kaufer E, et al. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol. 2001;38:105–110. doi: 10.1016/s0735-1097(01)01364-x. [DOI] [PubMed] [Google Scholar]
- 15.Guerci AD, Arad Y, Agatston A. Predictive value of EBCT scanning. Circulation. 1998;97:2583–2584. doi: 10.1161/01.cir.97.25.2583. [DOI] [PubMed] [Google Scholar]
- 16.Keelan PC, Bielak LF, Ashai K, et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–417. doi: 10.1161/hc2901.093112. [DOI] [PubMed] [Google Scholar]
- 17.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 18.O’Malley PG, Taylor AJ, Jackson JL, Doherty TM, Detrano RC. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am J Cardiol. 2000;85:945–948. doi: 10.1016/s0002-9149(99)00906-6. [DOI] [PubMed] [Google Scholar]
- 19.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 20.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 21.Writing Group for the Women’s Health Initiative I. Effects of conjugate equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 22.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 suppl):S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 25.Hsia J, Langer RD, Manson JE, et al. Women’s Health Initiative I. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 27.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 28.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 29.Oliver MF, Boyd GS. Effect of bilateral ovariectomy on coronary-artery disease and serum-lipid levels. Lancet. 1959;2:690–694. doi: 10.1016/s0140-6736(59)92129-4. [DOI] [PubMed] [Google Scholar]
- 30.Rivin AU, Dimitroff SP. The incidence and severity of atherosclerosis in estrogen-treated males, and in females with a hypoestrogenic or a hyperestrogenic state. Circulation. 1954;9:533–539. doi: 10.1161/01.cir.9.4.533. [DOI] [PubMed] [Google Scholar]
- 31.Robinson RW, Higano N, Cohen WD. Increased incidence of coronary heart disease in women castrated prior to the menopause. Arch Intern Med. 1959;104:908–913. doi: 10.1001/archinte.1959.00270120064010. [DOI] [PubMed] [Google Scholar]
- 32.Wuest JH, Jr, Dry TJ, Edwards JE. The degree of coronary atherosclerosis in bilaterally oophorectomized women. Circulation. 1953;7:801–809. doi: 10.1161/01.cir.7.6.801. [DOI] [PubMed] [Google Scholar]
- 33.Parrish HM, Carr CA, Hall DG, King TM. Time interval from castration in premenopausal women to development of excessive coronary atherosclerosis. Am J Obstet Gynecol. 1967;99:155–162. doi: 10.1016/0002-9378(67)90314-6. [DOI] [PubMed] [Google Scholar]
- 34.Khurana C, Rosenbaum CG, Howard BV, et al. Coronary artery calcification in black women and white women. Am Heart J. 2003;145:724–729. doi: 10.1067/mhj.2003.99. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer KM, Nordstrom CK, Bairey Merz CN, Dwyer JH. Carotid wall thickness and years since bilateral oophorectomy: the Los Angeles Atherosclerosis Study. Am J Epidemiol. 2002;156:438–444. doi: 10.1093/aje/kwf051. [DOI] [PubMed] [Google Scholar]
- 36.Bukovsky I, Halperin R, Schneider D, Golan A, Hertzianu I, Herman A. Ovarian function following abdominal hysterectomy with and without unilateral oophorectomy. Eur J Obstet Gynecol Reprod Biol. 1995;58:29–32. doi: 10.1016/0028-2243(94)01969-e. [DOI] [PubMed] [Google Scholar]
- 37.Chan CCW, Ng EHY, Ho P-C. Ovarian changes after abdominal hysterectomy for benign conditions. J Soc Gynecol Invest. 2005;12:54–57. doi: 10.1016/j.jsgi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Cooper GS, Thorp JM. FSH levels in relation to hysterectomy and to unilateral oophorectomy. Obstet Gynecol. 1999;94:969–972. doi: 10.1016/s0029-7844(99)00429-9. [DOI] [PubMed] [Google Scholar]
- 39.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. Br J Obstet Gynaecol. 2005;112:956–962. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 40.Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol. 1997;146:870–880. doi: 10.1093/oxfordjournals.aje.a009204. [DOI] [PubMed] [Google Scholar]
- 41.Writing Group for the Women’s Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 42.Schisterman EF, Gallagher AM, Bairey Merz CN, et al. The association of hormone replacement therapy and coronary calcium as determined by electron beam tomography. J Womens Health Gend Based Med. 2002;11:631–638. doi: 10.1089/152460902760360577. [DOI] [PubMed] [Google Scholar]
- 43.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the healthy women study. Arterioscler Thromb Vasc Biol. 1999;19:2189–2198. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 44.Budoff M, Chen G, Hunter C, et al. Effects of hormone replacement on progression of coronary calcium measured by electron beam tomography. J Womens Health. 2005;14:410–417. doi: 10.1089/jwh.2005.14.410. [DOI] [PubMed] [Google Scholar]
- 45.Akhrass F, Evans AT, Wang Y, et al. Hormone replacement therapy is associated with less coronary atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2003;88:5611–5614. doi: 10.1210/jc.2003-031008. [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin VV, Hoff JA, Rich S. Relation between hormone replacement therapy in women and coronary artery disease estimated by electron beam tomography. Am Heart J. 1997;134:1115–1119. doi: 10.1016/s0002-8703(97)70033-4. [DOI] [PubMed] [Google Scholar]
- 47.Higano N, Robinson RW, Cohen WD. Increased incidence of cardiovascular disease in castrated women. Two-year follow-up studies. N Engl J Med. 1963;268:1123–1125. doi: 10.1056/NEJM196305162682007. [DOI] [PubMed] [Google Scholar]
- 48.Lokkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89 Suppl 2:28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 50.Alexopoulos D, Toulgaridis T, Davlouros P, Christodoulou J, Sitafidis G, Vagenakis AG. Prognostic significance of coronary artery calcium in asymptomatic subjects with usual cardiovascular risk. Am Heart J. 2003;145:542–548. doi: 10.1067/mhj.2003.169. [DOI] [PubMed] [Google Scholar]
- 51.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 52.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]