Abstract
In previous studies the cerebellar interpositus nucleus, but not the hippocampus, was shown to be necessary both for initial learning and retention and for long-term retention of the standard delay eyeblink conditioned response (CR). However, in the trace eyeblink CR procedure, the hippocampus is also necessary for initial learning and retention, but not for long-term retention. Here we evaluate the role of the interpositus nucleus in both initial learning and retention, and in long-term retention of the trace eyeblink CR, using muscimol infusion to reversibly inactivate the interpositus nucleus. For the short-term study, there were 2 subgroups, the first sequentially passed through acquisition, inactivation, and reacquisition phases, whereas the second subgroup went through inactivation, acquisition, and inactivation phases. For the long-term study, the rabbits acquired the conditioned response (CR) and then rested for a month. Next, they were distributed into 2 subgroups: with or without retention training, and finally went through inactivation and reacquisition phases. The results showed that the pre-learning IP nucleus inactivation prevented the acquisition of the trace CR, whereas the post-learning inactivation reversibly abolished the expression of both the short- and long-term CR.
Keywords: Eyeblink conditioning, interpositus nucleus, short term memory, long term memory, inactivation
Introduction
In the attempt to find a memory trace, one of the most widely used behavioral paradigms is eyeblink conditioning (EBC), which involves the paired presentation of a conditioning stimulus (CS) i.e. tone or light and an unconditioning stimulus (US) i.e. airpuff to the eyes or periorbital shock to evoke a conditioned response (CR) (Gormezano, Kehoe & Marshall, 1983). In delay EBC, the CS comes first, followed by the US, and both are terminated at the same time. Evidence strongly supports the view that the permanent storage sites of the memory trace are in the cerebellum. The cerebellum and its associated brainstem circuitry appear to be sufficient to mediate and maintain the delay conditioned response memory. Indeed, it appears that the cerebellar anterior interpositus nucleus (IP) ipsilateral to the training eye is necessary for acquisition, short and long term retention, and expression of the memory (Christian & Thompson, 2003; 2005; Clark et al., 1984; Krupa et al., 1993; 1996; Krupa & Thompson, 1995; 1997; Lavond & Steinmetz, 1989; McCormick et al., 1982; Yeo et al., 1985a).
On the other hand, trace EBC, where there is a gap between CS offset and US onset, appears to require the interaction between cerebellum and other brain areas. The hippocampus was shown to be essential for acquisition of trace, but not delay, eyeblink conditioning as well as the cerebellum (Moyer et al., 1990; Solomon et al., 1986; Woodruff-Pak, et al., 1985); moreover, studies with humans indicated that medial temporal lobe amnestics cannot learn the long trace eyeblink procedure (but can learn the standard delay procedure) and normal subjects showed much better trace learning if they are aware of the contingencies (Clark & Squire, 1998, 1999), suggesting that trace conditioning can be considered as a simple model of hippocampal-dependent declarative memory (Christian & Thompson, 2003). However, the involvement of the hippocampus is critical only during the acquisition and for time limited retention (Kim et al., 1995), raising questions about the nature of the interaction between cerebellum and hippocampus. Weiss and Disterhoft (1996) proposed that the hippocampus helps to potentiate the effect of the CS at pontine nuclei, facilitating CS and US integration within the cerebellum. There is considerable empirical support for this view that forebrain structures can act to potentiate processing of the CS via the pontine nuclei (Bao et al., 2000; Knowlton & Thompson, 1988; 1992; Knowlton et al., 1993; Steinmetz et al., 1986; 1987; Tracy et al., 1998).
Evidence suggests that other brain areas such as the caudal anterior cingulate gyrus (cAC), the basal ganglia and thalamus may be involved in trace conditioning as well (Weible et al., 2003). Lesions of the cAC impair acquisition of trace CRs (Weible et al., 2000), and single neuron recording data indicate that the cAC is robustly involved only early in training, suggesting an attentional role during the acquisition process (Weiss & Disterhoft, 1996). This may be mediated through the connection of cAC with the basal forebrain cholinergic system (Everitt & Robbins, 1997; Sarter et al., 2005; Weiss et al., 2006). The cAC then could indirectly affect the hippocampal circuit via thalamo-cortical, stratal-cortical or claustral-cortical projections, and directly affect the pontine nuclei, the CS relay station. Weible et al. (2007) demonstrated a direct connection between the cAC and the lateral pons.
A fundamental issue for trace EBC is the location(s) of the long-term permanent store of the memory trace. To date, evidence shows that hippocampus and frontal cortex play critical roles in initial acquisition but not in long-term retention (at least not the hippocampus, Kim et al., 1995). So where are the long term memories stored? The cerebellum is the obvious possibility. At least one study, in rat, indicates that the frontal cortex may also play a key role in long term storage of the trace EBC (Takehara et al., 2003). Interestingly, the cerebellar cortex seems less critical for trace EBC than for delay EBC (Kishimoto et al., 2001a,b,c; Kishimoto & Kano, 2006; Miyata et al., 2001; Woodruff-Pak et al., 2006). Here we test the possibility that the cerebellar IP is necessary for both short and long term retention of the trace EBC. Actually, Woodruff-Pak et al. (1985) reported earlier that the IP is necessary for short term retention of trace EBC, so we simply replicate these findings here.
Experiment I (short-term study)
Methods
Subjects
Ten male New Zealand White rabbits (2.2 – 2.7 kg) were obtained from a local USDA-licensed supplier, housed individually in the Hedco Neuroscience vivarium on a 12-hr light-dark cycle, and provided ad lib access to food and water. All procedures and treatments of the animals were in full accordance with the American Psychological Association Guidelines for Ethical Conduct in the Care and Use of Animals and the National Institute of Health regulations.
Surgery and apparatus
One week after delivery, rabbits underwent aseptic surgery for implanting a cannula and attaching a head stage. The animals received preoperative injections of ketamine (60 mg/kg) and xylazine (8 mg/kg), and anesthesia was maintained throughout the procedure with a mixture of 1–3% halothane in oxygen. After anesthetization, the rabbit was placed in a stereotaxic frame, and the skull was adjusted so that lamda was 1.5 mm (± 0.1 mm) lower than bragma. Then, a cannula was stereotaxically implanted into the left interpositus (IP) nuclei of the cerebellum (14.25 mm below, 0.7 mm anterior, and 5.1 mm lateral to lamda), and it was fixed to the skull with dental cement. Each animal received analgesic postoperatively every 12 hrs for 2 days (buprenorphine hydrochloride, 0.02 mg/kg).
Behavioral training
After one week of recovery, the behavioral training began with habituation. All rabbits were restrained in a Plexiglas box and kept in training chambers, and the potentiometer and air nozzle were secured to the animal’s head stage. During the habituation period, the animals were given no stimuli in the training chamber for one hour for each of 2 days. After that they were distributed into 2 subgroups (SMS and MSM) according to the sequence of infused substances. In general, they were trained daily with a 500 ms trace eyeblink conditioning procedure, thirty minutes after the 0.5 μl, IP nucleus infusion of either muscimol (0.01 M; a GABA A receptor agonist) during the inactivation phase or physiological saline during the acquisition phase, depending on the phase sequences in each subgroup. The training procedure consisted of 13 blocks of 9 trials (total 117 trials), and each block was composed of 1 (a 250-ms, 85-dB, 1-kHz tone) CS alone trial, 1 (a 100-ms, 3-psi, left corneal airpuff) US alone trial, and 7 pairings of a CS and a US with a 500 ms gap between the two stimuli. Inter trial intervals were randomly varied from 20 to 40 s. The first subgroup (SMS) sequentially received saline for 8 days (acquisition), muscimol for 4 days (inactivation) and finally saline for 8 days (reacquisition), and the second group (MSM) was infused with muscimol for 8 days (inactivation), saline for 8 days (acquisition), followed by muscimol for 4 days (inactivation), corresponding to phase I, II, and III respectively in each subgroup. The learning criterion was 8 CRs in any block of 9 trials.
Behavioral analysis
The nictitating membrane (NM) movements were measured by a minitorque potentiometer connected with a nylon stitch at left nictitating membrane via a thread lead. Voltage changes were measured and recorded by an IBM PC system controlled by FORTH program (David Lavond, University of Southern California, Los Angeles, CA).
NM movement recorded time was 1500 ms (pre-CS period: −250 – 0 ms; CS onset: 0 ms; CR period: 50 – 250; Trace period: 250 – 750; US onset: 750 ms; UR period: 750 – 850 ms). The amplitude of conditioned eyeblink response (CR) and unconditioned eyeblink response (UR) were analyzed by FORTH program. On paired trails, CRs were defined as NM movements of ≥ 0.5 mm prior to US onset, but at least 50 ms after CS onset to eliminate any nonassociative responses. URs were the movements ≥ 0.5 mm after US onset. On CR alone trials, any movement (≥ 0.5 mm) within the trial period was counted as a CR. The percentage of CR (CR%) was the ratio of the number of CRs to the number of valid trials.
Histology
After the end of the experiments, all animals were lesioned at the locus of the drug infusion cannula by applying a 0.1 mAcurrent for 10 sec. Next, they were sacrificed by ear vein injection of pentobarbital, and perfused intracardially with physiological saline and a fixation solution of 10% formalin. Brains were removed and then embedded in gelatin solution. The 80 μm coronal sections were taken and stained with cresyl violet to localize the point of infusion.
Results
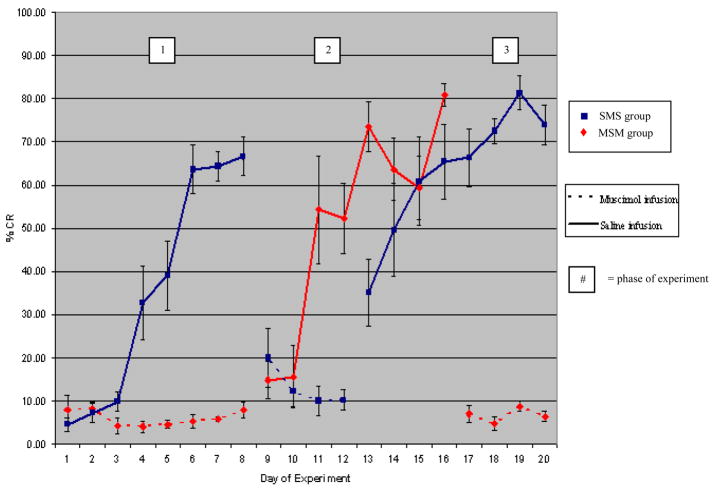
Figure 1 shows the mean CR percentage in the SMS (N = 5) and MSM (N = 4) groups in the short-term study. The data from one animal in MSM group was removed from the analysis because it had brain infection and died before the end of the experiment. In phase I (the first 8 days of experiments in both groups), the rabbits in SMS group, which were infused with saline, learned normally and were able to reach the criterion following 4 days of training, following which the CR expression continued to increase (M = 35.84, SD = 28.85). In contrast, IP nucleus inactivation by muscimol completely prevented learning of the rabbits in MSM group (M =6.83, SD = 4.51). Comparing CR% in SMS group with that in MSM group, analysis of variance showed a highly significant difference between two groups, F(1,70) = 31.66, p <.001. The analysis also revealed highly significant main effect for day of training in SMS group, F(7,32) = 19.30, p <.001, but no significant main effect in MSM group, F(7,24) = 0.87, p >.05.
Figure 1.
The effect of IP nucleus inhibition on short-term memory for trace eyeblink conditioning. SMS, saline-muscimol-saline infusion group; MSM, muscimol-saline-muscimol infusion group. Solid lines = saline infusion; dotted lines = muscimol infusion. Muscimol infusion completely prevents acquisition and completely abolishes retention of the short-term trace CR memory. The numbers in the white boxed refer to the three phases of the study. See text.
In phase II, (the second 4 days in SMS group and 8 days in MSM group), the IP nucleus inactivation reduced CR expression significantly in SMS group, and there was no significant difference between day of training (M = 12.44, SD = 8.52), F(3,16) = 0.16, p >.05. The statistical analysis confirmed that there was strongly significant main effect between phase I and II of SMS group, F(1,58) = 12.52, p =.001. Follow-up planned contrasts were conducted and showed that the sum of the data in phase II (since there was no main effect between day of training) differed significantly from the performance in phase I only during day 4th- day 8th of the training, F(8,51) = 28.15, p <.05. However, in MSM group, the release of the IP nucleus inactivation allowed the animals to learn CRs significantly over 8 days of training (M = 51.23, SD = 30.05), F(7,24) = 5.87, p <.001, and the analysis showed the highly significant main effect between phase I and II of SMS group, F(1,62) = 68.36, p <.001. Moreover, planned contrast analysis indicated that not until the 3rd day of training in phase II, animals did not show any significant learning differing from the that in phase I, F(8,55) = 30.91, p <.001. These data indicated that the IP nucleus inactivation prevented the animals from acquiring the new trace memory during phase I of MSM group, and also abolished CR expression of the previously learned memory during phase II of the SMS group. But it must be noted that the MSM group, when first trained without muscimol (starting day 9) learned faster than initial learning by the SMS group (on day 3 of training, the SMS group showed 10% CR and the MSM group showed 54% CR).
In phase III, (the final 8 days of the SMS group and 4 days of the MSM group), in the SMS group, although CR% was totally blocked during the IP nucleus inactivation, the CR% increased markedly upon the removal of inactivation (M = 62.82, SD = 24.17). The statistical analysis showed a significant main effect for day of training during phase III, F(7,32) = 2.69, p <.05, and there were apparently significant difference both between phase I and phase III, and between phase II and phase III, F(1,78) = 20.55, p <.001 and F(1,58) = 81.23, p <.001, respectively. Moreover, the contrast analysis indicated that the significant difference between phase II and phase III started since the 1st day of training in phase III, F(8,51) = 17.14, p <.05, compared to the 4th day of training in earlier case of phase I suggesting a saving of memory at the beginning of phase III may be that the muscimol infusions in phase II interfered with a consolidation process at the end of phase I. In the MSM group, muscimol abolished the learned CR (M = 6.57, SD = 4.10), and there was also no significant main effect for day of training during phase III, F(3,12) = 0.88, p >.05. As expected, analysis of variance showed no significant main effect between phase I and III, but highly significant main effect between phase I and II, F(1,46) = 0.04, p >.05 and F(1,46) = 34.66, p <.001, respectively. This effect was similar to the effect of muscimol during phase II of the SMS group. The locations of the cannula tips are shown in Figure 2.
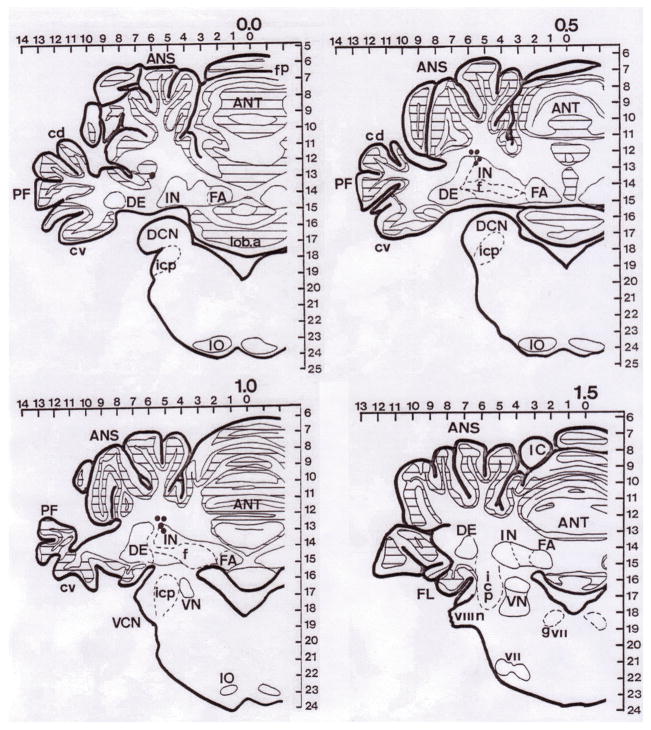
Figure 2.
Histological reconstruction of marking interpositus nucleus (IP) lesions on sections from the short-term study. ANS = ansiform lobule; ANT = anterior lobe; PF = paraflocculus; IN = interpositus nucleus; f = fibers; DE = dentate nucleus; FA = fastigial nucleus; cv = ventral crus; icp = inferior cerebellar peduncle; IO = inferior olive; VN = vestibular nucleus; VCN = ventral cochlear nucleus. Each dot represents the tip of infusion cannular seen in each brain slice. Due to the localization technique, the some lesions could be found in more than one slice.
Experiment II (long-term study)
Methods
Subjects
Ten male New Zealand White rabbits (2.2 2.7 kg) were obtained and kept in the same environment.
Surgery and apparatus
The same techniques and devices were applied.
Behavioral training
After one week of recovery, the animals underwent the same preparation, habituation and training procedures. The only difference was the phase sequence during the experiment, and it began with 8 days of acquisition training with the physiological saline infusion. Those animals achieving the learning criteria (8 CRs in any block of 9 trials) were selected and kept in their cages (rest) for a month. On the next day after the rest period, the animals were distributed into 2 subgroups: the retention training (+RT) group in which the animals received a day of training after the rest period, and the no retention training (−RT) group in which there was no retraining. The animals in both groups were then trained daily after being sequentially infused with muscimol for 2 days (days 40 and 41 in figure 3) (inactivation), and then with saline for 8 days (reacquisition).
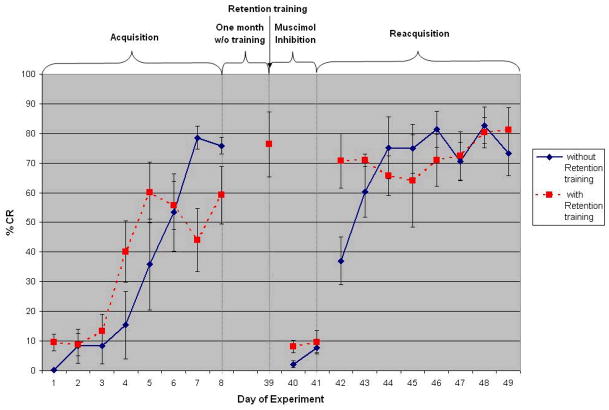
Figure 3.
The effect of IP nucleus inhibition on long-term memory for trace eyeblink conditioning. Both groups trained identically with no infusions for 8 days of initial acquisition and then given one month of rest. The “with retention training” group were then given one day of additional training and the “without retention training” group were not. Both groups were then given two days of training with muscimol infusion (days 40 & 41) and then given eight days of saline infusion. Muscimol infusion in the IP nucleus completely abolishes long-term retention of the trace eyeblink CR memory.
Behavioral analysis
The same techniques were applied.
Histology
The same techniques were applied.
Results
Figure 3 shows the mean CR percentage of +RT group (N = 4) and −RT group (N = 4) in the long term study. Only eight out of ten rabbits in this group achieved the criterion. Data showed that the expression of the trace conditioned response was reversibly abolished when the IP nuclei were inactivated by muscimol and the long rest period did not effect the expression of conditioned responses. With analysis of variance calculated from each phase of training (acquisition, inactivation, and reacquisition), there was no significant main effect between the two groups in all phases; acquisition phase (F(1,48) = 0.20, p >.05), inactivation phase (F(1,12) = 2.59, p >.05), and reacquisition phase (F(1,48) = 0.40, p >.05), suggesting that both groups represented the same sample. In the acquisition phase, the analysis revealed a highly significant main effect for day of training, F(7,48) = 17.81, p <.001, and a significant interaction between group and day of training, F(7,48) = 2.71, p <.05, showing the animals in both groups learned significantly over the course of acquisition training.
In the inactivation phase, the IP nucleus inactivation by muscimol dramatically abolished the long-term trace CR. The analysis of variance of both groups showed no significant main effect for day of training, F(1,12) = 2.12, p >.05, and no significant interaction between group and day of training, F(1,12) = 0.77, p >.05. In both −RT and +RT groups, the inactivation phase differed significantly from the activation phase, F(1,38) = 5.99, p <.05 and F(1,38) = 8.82, p <.01, respectively. The planned analysis showed that the difference occurred between the inactivation phase and the acquisition phase only during day 5th – 8th in −RT group, and day 4th – 8th in +RT group, F(8,31) = 16.89, p <.05 and F(8,31) = 10.98, p <.05. Moreover, the analysis of variance showed the highly significant difference between the inactivation phase of +RT group and the retention day, F(1,10) = 72.31, p <.001. The contrast analysis also showed the retention day differed significantly from the acquisition phase only on day 1st – 4th and day 7th of training, F(8,27) = 8.47, p <.05, suggesting that the resting period did not have any effect on the amount of the stored memory.
In the reacquisition phase, the animals from both groups showed the sudden increase of CR% on the first day of reacquisition phase and quickly reached very high levels of CR%, indicating marked savings of memory after the inactivation phase. The analysis of both group showed no significant main effect for day of training, F(7,48) = 2.09, p >.05, and no significant interaction between group and day of training, F(7,48) = 1.65, p >.05. However, on the first day of reacquisition (day 42), the group that had retention training showed 70% CRs but the group without retention training showed only 38% CR. Obviously, the analysis of both −RT and +RT group showed highly significant difference between the reacquisition phase and the inactivation phase, F(1,38) = 83.31, p <.001 and F(1,38) = 113.71, p <.001, respectively. To compare the rate of learning between acquisition and reacquisition phases for each group, for the −RT group, analysis of variance revealed a highly significant main effect for phases, F(1,63) = 69.94, p <.001; a significant main effect for day of training, F(7,63) = 12.51, p <.001; and a significant interaction between phase and day, F(7,63) = 4.80, p <.001. In the +RT group, analysis showed a highly significant main effect for phase, F(1,63) = 71.20, p <.001; a significant main effect for days of training, F(7,63) = 4.66, p <.001; and a significant interaction between phase and day, F(7,63) = 2.93, p <.05. Therefore, the above data indicate that the IP nuclei are essential for the expression of the long-term trace eyeblink conditioning memory. The locations of the cannula tips are shown in Figure 4.
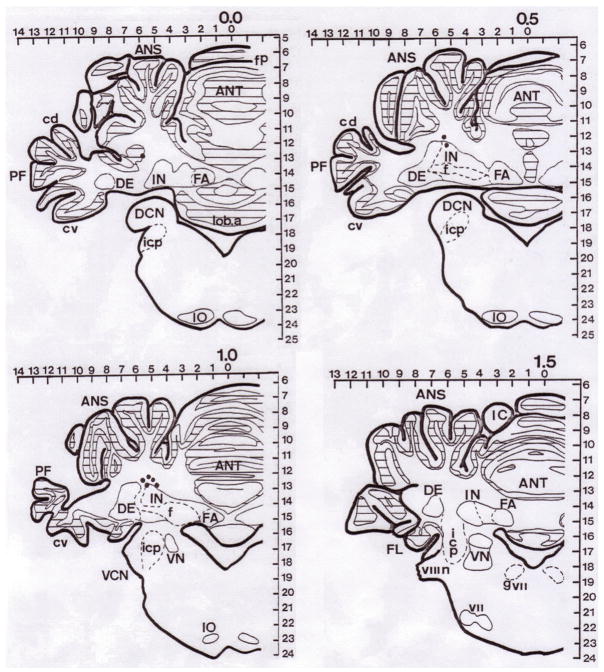
Figure 4.
Histological reconstruction of marking lesions of interpositus nucleus (IP) infusion sites on cerebellar brain sections from the long-term study (abbreviations as in Figure 2). Each dot represents the tip of infusion cannular seen in each brain slice. Due to the localization technique, the some lesions could be found in more than one slice.
Discussion
In classical conditioning, a causal relationship is formed between two or more separate stimuli; however, the temporal relationships of the stimuli vary greatly in nature and the brain has to cope with this variation. In general, brain areas respond similarly to the stimuli in both delay and trace eyeblink conditioning procedures. For example, hippocampus and the IP nucleus show learning-induced increases in activity preceding and predicting the occurrence and temporal form of the learned conditioned response in both delay and trace paradigms (Berger & Thompson, 1978; Green & Arenos, 2007; McCormick et al., 1982; Solomon et al., 1986). However, the brain areas necessary for each kind of memories are different, at least in part.
In the standard delay eyeblink conditioning procedure where there is no gap between CS and US, it appears that only the cerebellum and its associated circuits are necessary for the acquisition and maintenance of this kind of memory. It is clear that the cerebellar interpositus nucleus is necessary and the cerebellar cortex plays a critically important role in normal learning of delay EBC (see review by Christian & Thompson, 2003; Nores et al., 2000; Thompson & Krupa, 1994; Woodruff-Pak & Steinmetz, 2000; Yeo & Hesslow, 1998). It is our view (not shared by all) that the essential long-term memory trace for delay EBC is stored in the interpositus nucleus (Christian & Thompson, 2003; 2005; Kleim et al., 2002; Weeks et al., 2007).
For trace eyeblink conditioning, less is known about the circuitry. Although we might assume that at least the CS and US pathways are shared by both trace and delay eyeblink conditioning procedures, more brain circuits are required in order to acquire the trace memory e.g. hippocampus and forebrain. The hippocampal lesion abolished only the recently formed, not remotely formed, trace memory (Kim et al., 1995; Takehara et al., 2003). Moreover, Kotani et al., 2003, reported that decerebrate guinea pigs could learn the trace eyeblink conditioning procedure, although they required more training than normal animals. These data suggested that the loci where the association is formed may be in the cerebellar circuitry. However it is still not known to what extent forebrain structures may be involved in the long-term storage of the memory trace for the trace eyeblink CR (Takahara et al., 2003; Woodruff-Pak and Disterhoft, 2008). The data reported here show clearly that the IP is necessary for long-term memory (expression) of the trace CR. However, there are suggestions in our data that structures other than the cerebellum may play some role. Thus, in experiment 1 (Figure 1), the group first trained with muscimol (MSM) showed savings in subsequent acquisition compared to the group initially trained without muscimol (SMS). See also the difference between the two groups on day 42 in experiment 2.
But, the cerebellar cortex, surprisingly, seems not to play an important role in acquisition of trace EBC, in marked contrast to its role in delay EBC (Woodruff-Pak and Disterhoft, 2008). Several studies of mutant mice involving altered functions of neurons in cerebellar cortex show impairments in delay EBC but not in trace EBC. Thus, mutant mice deficient in phospholipase Cβ4 (PLCβ4) in Purkinje neurons in rostral cerebellar cortex show marked impairment in cerebellar cortical long-term depression (LTD) and in standard delay conditioning, in contrast to wild-type controls, but no impairment in the acquisition of the trace EBC (Kishimoto et al., 2001a; Miyata et al., 2001). By the same token, mice lacking the glutamate subunit δ2 (glu R δ 2 −1− ) show impairments in cerebellar cortical LTD and in delay but not trace EBC (Kishimoto et al., 2001b,c). The CB1 cannabinoid receptor knock-out mice showed the severely impaired performance in the delay EBC, but showed normal performance in the trace EBC (Kishimoto & Kano, 2006). In a similar vein, deletion of a sodium channel gene (scn 8A; Nav 1.6) which resulted in a 10-fold reduction in spontaneous firing and halved the maximum firing rates selectively in Purkinje neurons, markedly impairs delay EBC (and acquisition of the Morris Water Maze) but not trace EBC (Woodruff-Pak et al., 2006). All these findings argue that normal mechanisms of cerebellar LTD and normal firing patterns are important for normal learning of delay EBC but not trace EBC. In an imaging study, patients with pure cerebellar cortex lesions, albeit not all cerebellar cortex, did not show impairment in the acquisition of trace eyeblink conditioning, but patients with an IP lesion did show the impairment (Gerwig et al., 2006).
In the present experiment, muscimol, which is a reversible GABA A receptor agonist, inactivates the IP nucleus neurons upon infusion. Inactivation of the IP nucleus prevented learning, and upon removal of inactivation, animals showed no CRs (anterograde amnesia). Further, IP inactivation reversibly abolished expression of the previously learned memory, both short- and long-term (retrograde amnesia). Since the IP nucleus inactivation caused both prevention of learning and abolition of the learned response, it could be argued that the IP nucleus is a storage site where the association between the CS and the US is formed, not only for delay, but also for trace eyeblink conditioning. However, as noted above, this does not preclude the possibility that one or more forebrain regions might also be necessary for long-term storage of the trace memory.
Computational network models of eyeblink conditioning that focus on the cerebellum, particularly “real-time” models, can account for many aspects of standard delay conditioning (e.g., Gluck et al., 1990; 2001; Medina et al., 2000; Medina & Mauk, 2000; Moore et al., 1989) but fail to account adequately for behavior in the trace procedure. As noted above, the data indicate that when the gap between the stimuli the trace interval exceeds some species-specific length, the cerebellum is no longer able to form the association between CS and the US (Woodruff-Pak et al., 2006). Several top down models explicitly propose that the hippocampus functions as a buffer in trace conditioning (Levy & Rodriguez, 2001; Yamasaki & Tanaka, 2005), a possibility suggested earlier by Weiss et al. (1996). Interestingly, one top down model (Gluck & Myers, 1993) concluded that the trace, per se, is not critical, rather it is the long duration between CS onset and US onset, in either the trace or delay procedure that is critical. Indeed, hippocampal lesions in rat impair the acquisition of very long delay conditioning (Beylin et al., 2006).
Acknowledgments
This research was supported in part by the National Institute of Health Grant AG023742 and funds from the University of Southern California.
References
- Bao S, Chen L, Thompson RF. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behavioral Neuroscience. 2000;114(2):254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Berger TW, Thompson RF. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response. I. The hippocampus. Brain Research. 1978;145:323–346. doi: 10.1016/0006-8993(78)90866-1. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shores TJ. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiology of Learning and Memory. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning & Memory. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Christian KM& Thompson RF. Long-term storage of an associative memory trace in the cerebellum. Behavioral Neuroscience. 2005;119(2):526–537. doi: 10.1037/0735-7044.119.2.526. [DOI] [PubMed] [Google Scholar]
- Clark GA, McCormick DA, Lavond DG, Thompson RF. Effects of lesions of the cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses. Brain Research. 1984;291:125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Human eyeblink classical conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychological Science. 1999;10:14–18. [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Haerter K, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Thilmann AF, Gizewski ER, Timmann D. Trace eyeblink conditioning in human subjects with cerebellar lesions. Experimental brain research. 2006;170:7–21. doi: 10.1007/s00221-005-0171-2. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Allen MT, Myers CE, Thompson RF. Cerebellar substrates for error-correction in motor conditioning. Neurobiology of Learning and Memory. 2001;76:314–341. doi: 10.1006/nlme.2001.4031. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers CE. Hippocampal mediation of stimulus representation: A computational theory. Hippocampus. 1993;3:491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Reifsnider ES, Thompson RF. Adaptive signal processing and the cerebellum: Models of classical conditioning and VOR adaptation. In: Gluck MA, Rumelhart DE, editors. Neuroscience and Connectionist Models. Lawrence Erlbaum Associates; Hillsdale, N.J: 1990. pp. 131–185. [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiology of Learning and Memory. 2007;87:269–284. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109(2):195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Hirono M, Sugiyama T, Kawahara S, Nakao K, Kishio M, Katsuki M, Yoshioka T, Kirino Y. Impaired delay but normal trace eyeblink conditioning in PLC_4 mutant mice. Neuroreport. 2001a;12:2919–2922. doi: 10.1097/00001756-200109170-00033. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y& Kano M. Endogenous Cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. Journal of Neuroscience. 2006;26(34):8829. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Fujimichi R, Mori H, Mishina M, Kirino Y. Impairment of eyeblink conditioning in GluR_2-mutant mice depends on the temporal overlap between conditioned and unconditioned stimuli. The European journal of neuroscience. 2001b;14:1515–1521. doi: 10.1046/j.0953-816x.2001.01772.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical eyeblink conditioning in glutamate receptor subunit _ 2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. The European journal of neuroscience. 2001c;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Freeman JHJr Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D. Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of Sciences. 2002;99(20):13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson JK, Thompson RF. Projections form the auditory cortex to the pontine nuclei in the rabbit. Behavioural brain research. 1993;56(1):23–30. doi: 10.1016/0166-4328(93)90019-m. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson RF. Microinjections of local anesthetic into the pontine nuclei reduce the amplitude of the classically conditioned eyeblid response. Physiology and Behavior. 1988;43(6):855–857. doi: 10.1016/0031-9384(88)90389-7. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson RF. Conditioning using a cerebral cortical conditioned stimulus is dependent on the cerebellum and brain stem circuitry. Behavioral Neuroscience. 1992;106(3):509–517. doi: 10.1037//0735-7044.106.3.509. [DOI] [PubMed] [Google Scholar]
- Kotani S, Kawahara S, Kirino Y. Trace eyeblink conditioning in decerebrate guinea pigs. The European journal of neuroscience. 2003;17:1445–1454. doi: 10.1046/j.1460-9568.2003.02566.x. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260(5110):989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Inactivation of the superior cerebellar peduncle blocks expression but not acquisition of the rabbit’s classically conditioned eye-blink response. Proceedings of the National Academy of Sciences. 1995;92(11):5097–5101. doi: 10.1073/pnas.92.11.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learning & Memory. 1997;3(6):545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Weng J, Thompson RF. Inactivation of brainstem motor nuclei blocks expression but not acquisition of the rabbit’s classically conditioned eyeblink response. Behavioral Neuroscience. 1996;110(2):219–227. doi: 10.1037//0735-7044.110.2.219. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural brain research. 1989;33(2):113–64. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Levy WB, Rodriguez P. A model of hippocampal activity in trace conditioning: where’s the trace. Behavioral Neuroscience. 2001;115:1224–1238. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Clark GA, Lavond DG, Thompson RF. Initial localization of the memory trace for a basic form of learning. Proceedings of the National Academy of Sciences. 1982;79(8):2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. Journal of Neuroscience. 2000;20(14):5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nature Neuroscience Review. 2000;3(Suppl):1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- Miyata M, Kim HT, Hashimoto K, Lee TK, Cho SY, Jiang H, Wu Y, Jun K, Wu D, Kano M, Shin HS. Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C _4 mutant mice. The European journal of neuroscience. 2001;13:1945–1954. doi: 10.1046/j.0953-816x.2001.01570.x. [DOI] [PubMed] [Google Scholar]
- Moore JW, Desimong NE, Barthier JE. Adaptive timed conditioned responses and the cerebellum: a neural network approach. Biological cybernetics. 1989;62:17–28. doi: 10.1007/BF00217657. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Nores WL, Medina JF, Steele PM, Mauk MM. Relative contributions of the cerebellar cortex and cerebellar nucleus to eyelid conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning: Animal models. Vol. 2. Kluwer Academic Publishers; Boston: 2000. pp. 205–228. [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain research. Brain research reviews. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences. 1987;84(10):3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS:I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100(6):878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. Journal of Neuroscience. 2003;23(30):9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Krupa DJ. Organization of memory traces in the mammalian brain. Annual Review of Neuroscience. 1994;17:519–49. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- Tracy J, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the ponto-cerebellar CS pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112(2):267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Weeks AC, Connor S, Hinchcliff R, Leboutillier JC, Thompson RF, Petit TL. Eye-blink conditioning is associated with changes in synaptic ultrastructure in the rabbit interpositus nuclei. Learning & Memory. 2007;14(6):385–389. doi: 10.1101/lm.348307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behavioral Neuroscience. 2000;114(6):1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Activity profiles of single neurons in supragenual cingulated cortex during trace eyeblink conditioning in the rabbit. Journal of Neurophysiology. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience. 2007;145(1):288–302. doi: 10.1016/j.neuroscience.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Weiss C& Disterhoft JF. Eyeblink conditioning, motor control, and the analysis of limbic-cerebellar interactions. Behavioral and Brain Sciences. 1996;19(3):479–481. [Google Scholar]
- Weiss C, Weible AP, Galvez R, Disterhoft JF. Forebrain-cerebellar interactions during learning. Cellscience reviews. 2006;3(2):1–31. [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends in Neurosciences. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Nav1.6) in Purkinje neurons impairs learning in morris water maze and delay but not trace eyeblink classical conditioning. Behavioral Neuroscience. 2006;120:229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: Abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Research. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning. Boston: Kluwer Academic Publishers; 2000. [Google Scholar]
- Yamazaki T, Tanaka S. A neural network model for trace conditioning. International journal of neural systems. 2005;15:23–30. doi: 10.1142/S0129065705000037. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. lesion of the cerebellar nuclei. Experimental Brain Research. 1985;60(1):87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hesslow G. Cerebellum and conditioned reflexed. Trends in Cognitive Sciences. 1998;9:322–330. doi: 10.1016/s1364-6613(98)01219-4. [DOI] [PubMed] [Google Scholar]