Abstract
The use of live recombinant attenuated Salmonella vaccines (RASV) synthesizing Yersinia proteins is a promising approach for controlling infection by Yersinia species. In this study, we constructed attenuated Salmonella strains which synthesize a truncated form of LcrV, LcrV196 and evaluated the immune response and protective efficacy elicited by these strains in mice against two other major species of Yersinia: Yersinia pseudotuberculosis and Yersinia enterocolitica. Surprisingly, we found that the RASV strain alone was sufficient to afford nearly full protection against challenge with Y. pseudotuberculosis, indicating the likelihood that Salmonella produces immunogenic cross-protective antigens. In contrast, lcrV196 expression was required for protection against challenge with Y. enterocolitica. As LcrV has been previously shown to provide protection against Yersinia pestis, these findings indicate that a single vaccine may provide protection against the three human pathogenic species of Yersinia.
Keywords: Recombinant Attenuated Salmonella Vaccine, LcrV, Yersinia
1. Introduction
The genus Yersinia contains three species pathogenic for humans: Yersinia pestis, Yersinia enterocolitica and Yersinia pseudotuberculosis [1, 2]. Y. pestis is the causative agent of plague. Y. enterocolitica and Y. pseudotuberculosis are food-borne pathogens found widely in the environment and are a common cause of animal infections, affecting several mammalian and avian species [3–5]. In humans, the infections are initiated by consumption of contaminated food or water leading to gastrointestinal symptoms ranging from diarrhea to acute mesenteric lymphadenitis [2, 6]. After oral ingestion, the bacteria move to the small intestine and invade the intestinal epithelium via interactions with the specialized M cells overlying the Peyer’s patches. This interaction is mediated by the inv and ail genes [7, 8]. Once established, they colonize the underlying lymph tissues [9]. Occasionally, Y. enterocolitica but not Y. pseudotuberculosis may spread to the spleen and liver, causing systemic disease [6].
Strong molecular evidence supports the fact that Y. pestis recently diverged from Y. pseudotuberculosis and is therefore closely related [10]. Y. enterocolitica has evolved into a more heterogeneous group, classified into 6 biogroups [11, 12] including biotype 1B, associated with human infection. Biotype 1B includes the most virulent serotype O8, primarily isolated in North America [2, 13].
The three human pathogenic Yersinia species harbor the 70-kb low calcium response plasmid that encodes essential virulence factors including the type III secretion system and the adherence protein YadA [14, 15] (not functional in Y. pestis [16]). LcrV is a secreted, multifunctional protein that is central to the activity of the type III secretion system apparatus. LcrV affects effector secretion by binding the negative regulator LcrG and in conjunction with YopB and YopD, is essential for the translocation of effectors into eukaryotic cells [17]. LcrV is highly immunogenic, inducing a protective immune response, and is therefore a component of most plague vaccines [18]. It is highly conserved among the Yersiniae, although some protein polymorphism has been described which is especially evident in Y. enterocolitica strains [19, 20]. As a result of this polymorphism, there are two distinct types of LcrV, LcrV-YenO8, derived from Y. enterocolitica serotype O8 and LcrV-Yps, which includes LcrV from Y. pestis, Y. pseudotuberculosis and other Y. enterocolitica serotypes [19]. While the two LcrV types differ at a number of amino acids across the protein, the most notable difference lies in a hypervariable region between aa 225 and 232 that includes a 9 aa insertion in the LcrV-YenO8 group. One exception is the O8 strain Y. enterocolitica 8081, which synthesizes an LcrV protein that is more like LcrV-Yps across aa 225–232 [19]. Monospecific antisera against the two types of LcrV used in passive immunization studies, protected mice against challenge with strains expressing the homologous LcrV, but not strains expressing LcrV from the other group [19]. In addition, LcrV contributes to pathogenicity via TLR2-mediated IL-10 induction [21]: LcrV-YenO8 of Y. enterocolitica causes TNF-αsuppression in macrophages in a CD14 and TLR2-dependant manner by inducing IL-10 [22–24]. However, the TLR2-LcrV interaction seems to play less of a role in Y. pestis and Y. pseudotuberculosis pathogenicity [25–27]. Administration of TNF-α and INF-γ can promote survival after Y. pestis challenge [28].
Live attenuated Salmonella strains were first developed as vaccines to prevent disease caused by Salmonella infections of both humans and animals [29, 30]. Subsequently, genetically modified attenuated Salmonella strains were constructed for delivery of heterologous antigens. Orally administered Salmonella vaccines offer a variety of advantages over traditional vaccines, including stimulation of a strong mucosal response that is important for protection against pathogens that colonize and/or enter the body through mucosal surfaces, stimulation of a systemic response, needle-free delivery and a relatively low cost of production.
Ongoing research in our laboratory has been directed toward improving the immunogenicity and stability of Salmonella delivery strains and methods of antigen expression. To maintain the plasmid expressing an antigen of interest and avoid the use of antibiotics, a balanced-lethal host-vector system has been designed. In this system, the gene encoding aspartate β-semialdehyde dehydrogenase (asd) is used to maintain plasmids co-expressing the protective antigens in Δasd Salmonella strains [31, 32]. We have shown previously that secretion of a protective pneumococcal antigen, PspA, to the periplasm of an attenuated Salmonella strain enhances the immune response and protective efficacy of the vaccine compared to a strain that expresses, but does not secrete PspA [33]. We have designed a number of secretion vectors that rely on fusing an antigen of interest to secretion signals from β-lactamase, including pYA3493, which is designed to enable an amino-terminal fusion between the first 35 aa of the β-lactamase gene, bla, (bla-SS) and an antigen of interest [34] and pYA3620, which in addition to the amino-terminal bla-SS fusion creates a C-terminal fusion with the C-terminal 22 aa of bla (bla-SS-CT) [35, 36]. The β-lactamase C-terminus is required for secretion of native β-lactamase [37], and, in some cases, can significantly improve heterologous antigen secretion [35, 36].
Immunization of mice with attenuated Salmonella expressing a β-lactamase signal sequence fusion to the truncated lcrV gene, lcrV196, induced a strong serum IgG response against LcrV and protected the immunized mice against challenge with virulent Y. pestis [38]. The purpose of the present study is to investigate the immunogenicity and protective efficacy of orally administered recombinant attenuated Salmonella vaccines (RASV) synthesizing the Y. pestis LcrV196 protein against challenge with virulent Y. pseudotuberculosis and Y. enterocolitica. In addition, we compared the effects of two different signal sequence fusion proteins that direct the antigen to the periplasmic space and supernatant with a construct that resulted in cytoplasmic expression.
2. Materials and methods
2.1 Bacterial Strains and growth media
Y. enterocolitica strain 8081, serotype O8, was kindly provided by V. Miller. Y. pseudotuberculosis PB1/+ and Y. enterocolitica strain WA were kindly provided by R. Perry. Yersinia strains were routinely grown in heart infusion broth (Difco, Detroit, MI) at 28°C.
Salmonella enterica serovar Typhimurium strain χ8501 (hisG Δcrp-28 ΔasdA16) was used as the vaccine vector in all studies [34]. Escherichia coli strain χ6212 (F− λ− φ80 Δ(lacZYA-argF) endA1 recA1 hsdR17 deoR thi-1 glnV44 gyrA96 relA1 ΔasdA4) [34] was used for cloning. E. coli strain BL21(DE3) (F− ompT hsdSB (rB− mB−) gal dcmλ (DE3)) was used for expressing proteins for purification. Bacteria were grown in LB broth [39], or on LB agar or MacConkey agar supplemented with 1% lactose. Diaminopimelic acid (DAP) was included in the growth media at 100 μg/ml when necessary for growth of non-complemented asd strains and for plasmid stability tests.
2.2 Construction of plasmids pYA3839, pYA3840 and pYA3841
As a first step in vaccine construction, the lcrV DNA sequence obtained from Y. pestis KIM was codon-optimized for expression in Salmonella. We identified codons in lcrV that are used with less than a 2% frequency in highly expressed Salmonella genes [40]. These codons were then modified to correspond to more frequently used codons in Salmonella. The codon-modified truncated lcrV sequence, corresponding to aa131-aa326 (lcrV196) cloned in pUC19, was previously described [38].
For construction of pYA3839 a 616 bp PCR fragment encoding the codon-optimized, lcrV196 sequence was cloned into Asd+ plasmid pYA3342 [34]. The primers used for PCR were 5′-GACCATGGGAATCGATGATGATATTTTGAAAGTG and 5′-CCCAAGCTTTCATTTACCAGACGTGTCATCGAG. These primers also encode an NcoI or HindIII site (underlined), respectively. The lcrV196 gene was amplified using pUC19-lcrV196 as the template, digested with NcoI and HindIII, and ligated into the corresponding sites of pYA3342.
A similar strategy was used to clone codon-optimized lcrV196 into the Asd+ secretion vector pYA3493 [34] to create pYA3840 using primers GCTCTAGAGAATTCATCGATGATGATATTTTGAAAGTG and CCCAAGCTTTCATTTACCAGACGTGTCATCGAG, which contain EcoRI andHindIII sites, respectively (underlined). The PCR product amplified from pUC19-lcrV196, was digested with EcoRI and HindIII and the 613-bp fragment was ligated into the EcoRI and HindIII sites of plasmid pYA3493. This created an in-frame fusion between the first 35 aa of β-lactamase and the amino-terminal amino acid of LcrV196, creating bla-SS-lcrV196. Of the 35 aa derived from β-lactamase, the first 23 aa constitute a sec-dependent signal sequence. Upon secretion, this sequence is cleaved, leaving the first 12 aa of mature β-lactamase fused to LcrV196.
The construction of plasmid pYA3841 was described previously [38]. This plasmid encodes the same bla-SS-lcrV196 fusion as pYA3840 and also contains the C-terminal 22 amino acids of β-lactamase fused to the C-terminus of LcrV196, creating bla-SS-lcrV196-CT.
All three plasmids were electroporated into Salmonella strain χ8501. The DNA sequence of the lcrV196 inserts in each was confirmed to be correct by DNA sequence analysis (Arizona State University, USA). Plasmid stability in strain χ8501 was performed as previously described [34].
2.3 SDS PAGE and western blot analysis
To evaluate antigen expression, Salmonella strain χ8501 harboring either pYA3342, pYA3493, pYA3620, pYA3839, pYA3840 or pYA3841 was cultured in LB broth at 37°C; Y. pestis KIM, Y. pestis CO92, Y. pseudotuberculosis PB1/+, Y. enterocolitica 8081 were cultured in HIB broth at 28°C. The cultures were harvested when the cultures reached an optical density at 600 nm (OD600) of 0.8. The bacteria were harvested by centrifugation at 10,000 × g and the pellets resuspended in Laemmli sample buffer containing 2% 2-mercaptoethanol. To evaluate secreted proteins, culture supernatants were passed through a 0.22μm filter and precipitated overnight with 20% TCA (v/v). After centrifugation, the TCA pellet was resuspended in cold PBS, and acetone precipitated. The precipitates were washed with acetone, resuspended in PBS and stored at −20°C.
The proteins were separated by SDS-polyacrylamide gel electrophoresis as previously described [41] and transferred onto nitrocellulose sheets (Biorad, Hercules, CA) using a semi-dry system with Tris buffer (48 mM Tris, pH 9.2, 39 mM glycine, 1.3 mM SDS, 20% methanol). After overnight blocking at 4°C with 3% BSA in TBST (10 mM Tris, pH 8, 150 mM NaCl, 0.05% Tween 20), recombinant proteins were selectively identified by western blot using rabbit anti-LcrV followed by alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma). In some experiments sera from mice immunized with a S. Typhimurium vaccine strain (χ8501) was used as the primary antibody followed by alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma). Antibody complexes were detected with the NBT-BCIP liquid substrate (Amresco). The experiments were performed three times.
2.4 Immunization of mice and challenge studies
Female Swiss Webster mice, 6–8 weeks of age, were purchased from Charles River (Wilmington, MA). Mice were deprived of food and water for 4 h prior to immunization and re-supplied 30 min after. S. Typhimurium strains were grown in LB broth to an OD600 of 0.9 and concentrated to 5 × 1010 CFU/ml in phosphate-buffered saline containing 0.01% gelatin (BSG)[42]. Groups of mice were orally immunized with 20 μl of RASV suspensions on days 0 and 10. Challenge was performed 28 days after the second immunization (day 38). For oral challenge, a freshly prepared culture of Y. pseudotuberculosis strain pB1/+, Y. enterocolitica 8081 or WA grown in heart infusion broth (HIB) at 28°C was used. Blood samples were collected on days 0, 21, 32, and at the end of the experiment. Mice were observed daily and mortality was recorded for 35 days after the challenge. The surviving animals were euthanized at the end of the experiment to obtain blood samples for serological analysis. The challenge experiments were performed twice, except for challenge with Y. enterocolitica WA, which was performed once.
2.5 Colonization of mouse tissues
Mice were immunized with 1 – 2 ×109 CFU of χ8501(pYA3620) (control), or one of the three strains expressing lcrV196; χ8501(pYA3839) (lcrV196), χ8501(pYA3840) (bla-SS-lcrV196) or χ8501(pYA3841) (bla-SS-lcrV196-CT). Three mice from each group were euthanized by asphyxiation with CO2 at day 3, 7, 10 and 15. The Peyer’s patches, spleen and part of the liver were aseptically taken from each mouse and homogenized in PBS using a PowerGen 125 S1 homogenizer (Fisher Scientific, Pittsburgh, PA). Dilutions of these samples were spread onto LB and McConkey-1% lactose plates. The plates were incubated overnight at 37°C. Colonies were counted to determine the number of CFU recovered from each organ. This experiment was performed twice.
2.6 Enzyme-linked immunosorbent assay (ELISA)
Nunc Immunoplate Maxisorb F96 plates (Nalge Nunc. Rochester, NY, USA) were coated with purified His6-tagged full length LcrV (500ng/well) or S. Typhimurium LPS (100ng/well) (Sigma, L2262) and incubated overnight at 4°C. The expression and purification of His6-tagged LcrV encoded by plasmid pHT-V has been previously described [43]. The plates were washed three times with PBS containing 0.1% Tween 20, blocked with PBS containing 10% (v/v) Sea BLOCK Blocking buffer (Pierce) for 1 h at 37°C and then washed three times with PBS. Pooled sera from the mice in each group were diluted in PBS and added to the plates with a dilution of 1:800, except for the IgG1 analysis, where the dilution was 1:400. After 1 h incubation at 37°C, the plates were washed and incubated for 1 h at 37°C sequentially with biotin-conjugated goat anti-mouse IgG(H+L), anti-mouse IgG1, anti-mouse IgG2a, (SouthernBiotech) (1:5000) and streptavidin conjugated to alkaline phosphatase (SouthernBiotech) (1:4000) with washings in between. Plates were then incubated with the chromogenic substrate, p-nitrophenyl phosphate (Sigma), for 10 min and the reaction was stopped by the addition of 2 M H2SO4. The optical density at 405 nm was measured with a Labsystems Multiskan MCC/340. The experiment was performed twice.
2.7 Statistical analysis
Statistical significance was determined by the χ2 test, with P<0.05 considered to be statistically significant.
3. Results
3.1 Expression of lcrV196 in RASV
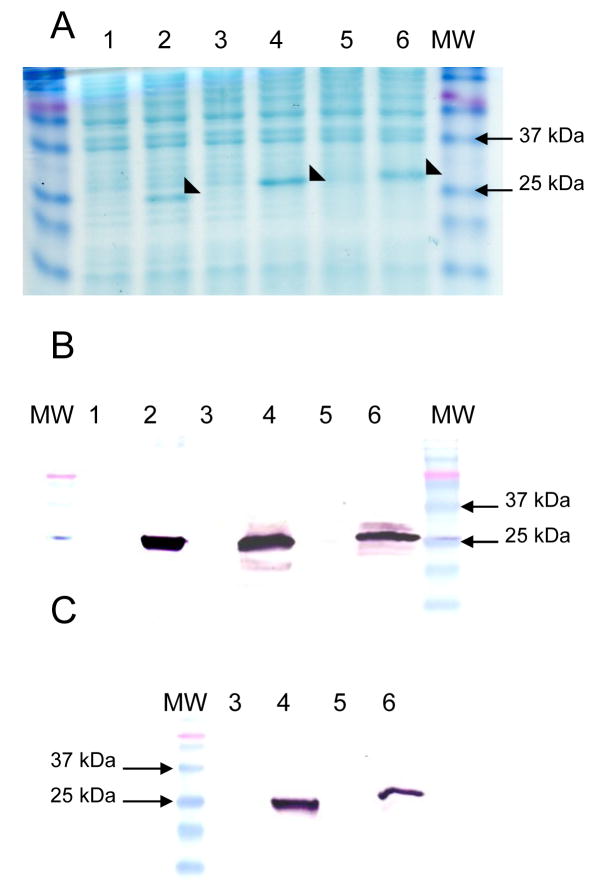
Expression of lcrV196 was evaluated in each RASV χ8501 strain by Coomassie brilliant blue-staining of SDS-PAGE gels and by western blot analysis (Fig. 1). RASV strainsχ8501(pYA3839), χ8501(pYA3840), χ8501(pYA3841) produced similar amounts of cell-associated LcrV (Fig. 1A, 1B). Strains carrying the empty vector plasmids did not produce any protein that reacted with anti-LcrV antibody (Fig. 1B). Plasmids pYA3840 and pYA3841 encode bla-SS-lcrV196 and bla-SS-lcrV196 -CT fusions that we expect will direct LcrV to the periplasm and supernatant. The supernatant fractions from χ8501(pYA3840) and χ8501(pYA3841) contained protein that reacted with the anti-LcrV antibody (Fig. 1C), indicating that the β-lactamase signal sequence was directing antigen secretion as expected. Supernatant fractions from strains carrying empty vector plasmids or plasmid pYA3839 in which lcrV is not fused to a secretion signal sequence, did not react with the anti-LcrV antibody (Fig. 1C and data not shown).
Figure 1.
Evaluation of LcrV in whole cells and culture supernatants. The presence of LcrV was observed in whole cells separated on Coomassie brilliant blue-stained SDS-polyacrylamide gels (A). The presence of LcrV was evaluated by western blots of whole cells (B) and culture supernatants (C) probed with rabbit anti-LcrV polyclonal antibodies. Lanes: (1) χ8501(pYA3342), (2) χ8501(pYA3839) (lcrV196), (3) χ8501(pYA3493), (4) χ8501(pYA3840) (bla-SS-lcrV196), (5) χ8501(pYA3620), (6) χ8501(pYA3841) (bla-SS-lcrV196-CT). MW: molecular mass markers. The predicted molecular weights of LcrV196 and LcrV196 fusion proteins are 23.5 kDa (pYA3839), 26.3 kDa (pYA3840) and 29 kDa (pYA3841).
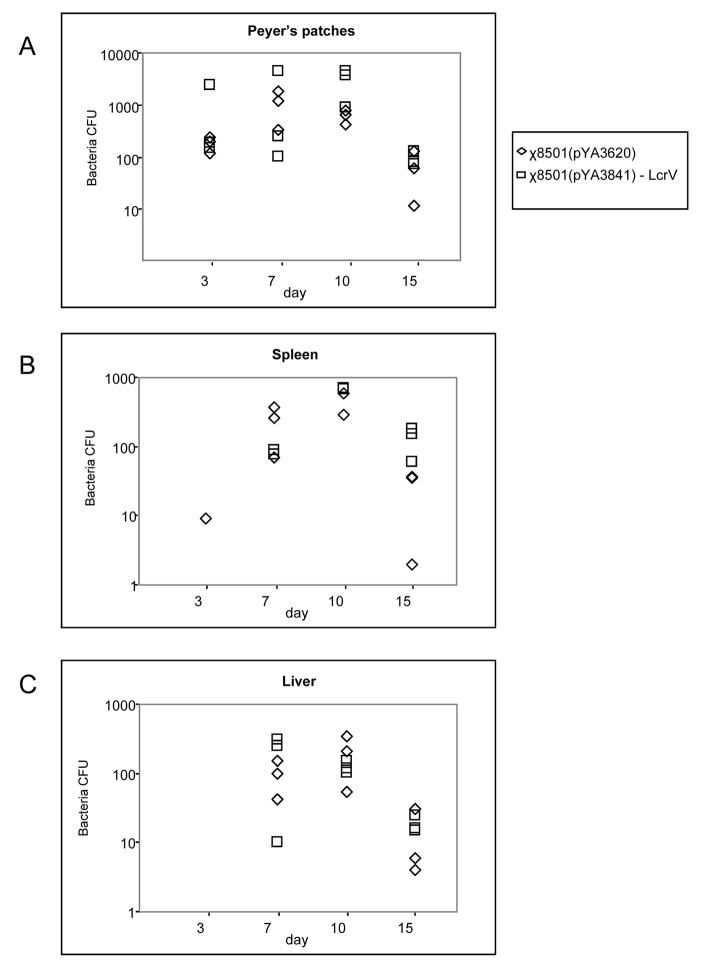
3.2 Colonization of mice by attenuated Salmonella vaccine strains
Attenuated S. Typhimurium strain χ8501 carrying the empty vector plasmid pYA3620 or pYA3841 encoding bla-SS-lcrV196-CT, was evaluated for the ability to colonize mouse spleen, liver and Peyer’s patches (Fig. 2). We did not observe any significant differences in colonization between strains. Similar results were obtained with χ8501(pYA3839) (lcrV196) and χ8501(pYA3840) (bla-SS-lcrV196).(data not shown).
Figure 2.
Persistence of S. Typhimurium strains χ8501(pYA3841) (bla-SS-lcrV196-CT) and χ8501(pYA3620) in Peyer’s patches (A), spleens (B), and livers (C) of orally immunized mice. Results are expressed as individual values recovered from the tissues of each mouse at days 3, 7, 10 and 15 (3 animals per time point).
3.3 Anti-LcrV and anti-Salmonella LPS body responses in immunized mice
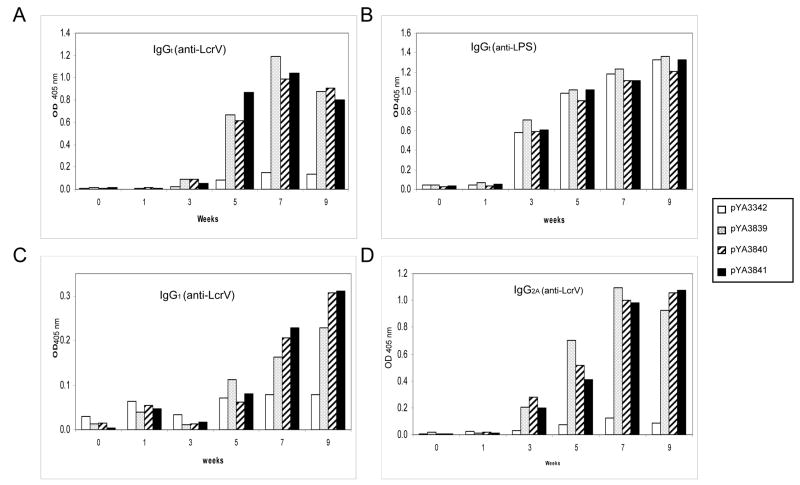
The serum IgG responses from immunized mice were analyzed by ELISA against the recombinant full length LcrV protein (Fig. 3A). No anti-LcrV IgG was detected in mice receiving the control strain χ8501(pYA3342). Mice orally immunized with strains expressing lcrV196, χ8501(pYA3839)(lcrV196), χ8501(pYA3840) (bla-SS-lcrV196) or χ8501(pYA3841) (bla-SS-lcrV196-CT), developed a strong immune response against LcrV. The anti-rLcrV response was not detected until after the second immunization (day 10) and increased over time until week 9, when the levels of anti-LcrV IgG dropped slightly. All the immunized mice developed a strong serum IgG response against S. Typhimurium LPS with no significant differences between groups (Fig. 3B).
Figure 3.
Serum IgG responses in orally immunized mice. Mice were immunized with lcrV196-expressing attenuated S. Typhimurium strains χ8501(pYA3839) (lcrV196), χ8501(pYA3840) (bla-SS-lcrV196) and χ8501(pYA3841) (bla-SS-lcrV196-CT). Strain χ8501(pYA3342) that does not express lcrV196 served as a negative control. Panels: (A) total anti-LcrV IgG, (B) total anti-LPS IgG, (C) anti-LcrV IgG1, (D) anti-LcrV IgG2a.
We also evaluated sera for anti-LcrV IgG isotypes IgG1 (Fig. 3C) and IgG2a (Fig. 3D). The levels of IgG2a were greater than IgG1 levels at all time points, indicating induction of a predominantly Th1 response by all strains expressing lcrV196. Taken together, these results indicate that all three strains expressing lcrV196 induce similar serum IgG immune responses.
3.4 Protection of mice immunized against Y. pseudotuberculosis challenge
After our initial evaluation of serum IgG responses, we chose one strain, χ8501(pYA3841) (bla-SS-lcrV196-CT) for further analysis. In two separate experiments, we immunized groups of 10 mice with either χ8501(pYA3620) (control) or χ8501(pYA3841). Mice were boosted on day 10 and orally challenged 28 days later with either 2.3 × 107 CFU (5 mice) or 2.5 × 108 CFU (5 mice) of Y. pseudotuberculosis PB1/+ in the first experiment and 1.8 × 108 CFU or 2.0 × 109 CFU in the second experiment. Mortality was recorded for 35 days after challenge. In preliminary experiments we determined that the oral LD50 of Y. pseudotuberculosis PB1/+ is 1 × 107 CFU (data not shown).
Our results in the first experiment (Table 1) showed that all mice immunized with the strain expressing lcrV196, χ8501(pYA3841) (bla-SS-lcrV196-CT) were protected against challenge at both doses, while only 2 of 5 and 1 of 5 mice receiving BSG buffer alone survived challenge with low or high doses of Y. pseudotuberculosis, respectively. Surprisingly, 4 of 5 mice given χ8501(pYA3620), which does not express lcrV196 and did not elicit an immune response against LcrV (Fig. 3) were protected in both the low and high-dose challenge groups. When we repeated the experiment, all 10 non-immunized mice succumbed to challenge, while all the mice immunized with either χ8501(pYA3620) or χ8501(pYA3841) (bla-SS-lcrV196-CT) survived, regardless of the challenge dose (Table 1). These results indicate that vaccination with attenuated Salmonella strain χ8501 is sufficient to provide significant protection against Y. pseudotuberculosis challenge (P<0.05), regardless of whether it synthesizes LcrV.
TABLE 1.
Effect of immunization with RASVs followed by oral Y. pseudotuberculosis challengea.
| Experiment 1 | total | Experiment 2 | total | |||
|---|---|---|---|---|---|---|
| Challenge dose (CFU) | 2.3×107 | 2.5×108 | 1.8×108 | 2.0×109 | ||
| χ8501(pYA3841) (bla-SS-lcrV196-CT) | 5/5b | 5/5 | 10/10* | 5/5 | 5/5 | 10/10*** |
| χ8501(pYA3620) | 4/5 | 4/5 | 8/10** | 5/5 | 5/5 | 10/10*** |
| BSG control | 2/5 | 1/5 | 3/10 | 0/5 | 0/5 | 0/10 |
Animals were vaccinated twice at 10 day intervals and challenged with Yersinia pseudotuberculosis strain pB1/+, 28 days after the last immunization.
Number of surviving animals at 35 days after challenge/number of animals challenged.
The LD50 of PB1+ in non immunized BALB/c mice is 1 × 107 (data not shown).
P<0.01,
P<0.05, or
P<0.001 compared to the BSG group.
3.5 Serum antibodies against S. Typhimurium proteins that cross-react with Yersinia proteins
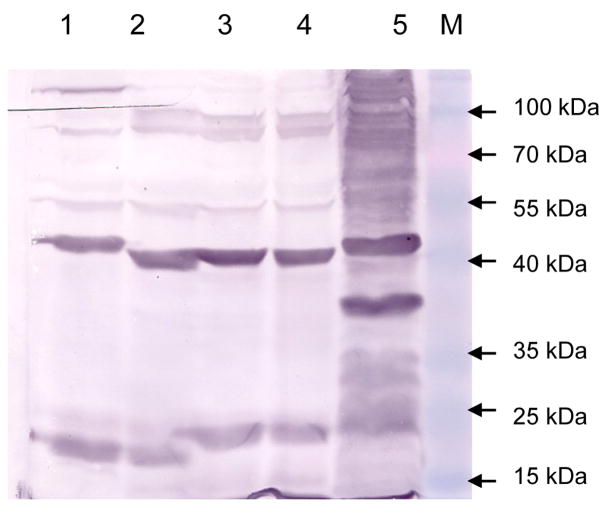
Based on the above results, it is likely that S. Typhimurium strain χ8501 produces one or more antigens that induce antibodies that are cross-reactive with Y. pseudotuberculosis. To evaluate whether Salmonella produces a cross-reactive protein, we performed western blot on whole cell extracts from two strains of Y. pestis, one strain of each of Y. pseudotuberculosis and Y. enterocolitica and the S. Typhimurium vaccine vector strain χ8501. Membranes were probed with anti-sera obtained from mice immunized with the control strain χ8501(pYA3620). The resulting western blot revealed a number of bands, including ones at 17 kDa, 25 kDa and 65 kDa that reacted strongly with the serum (Fig. 4).
Figure 4.
Western blot performed on Salmonella and Yersinia cells. Blots were probed with mouse anti-S. Typhimurium strain χ8501 sera. Lanes: (1) Y. pestis KIM, (2) Y. pestis CO92, (3) Y. pseudotuberculosis PB1/+,(4) Y. enterocolitica 8081, (5) S. Typhimurium χ8501. MW: molecular mass markers.
3.6 Efficacy of χ8501(pYA3841) (bla-SS-lcrV196-CT) against challenge with Y. enterocolitica
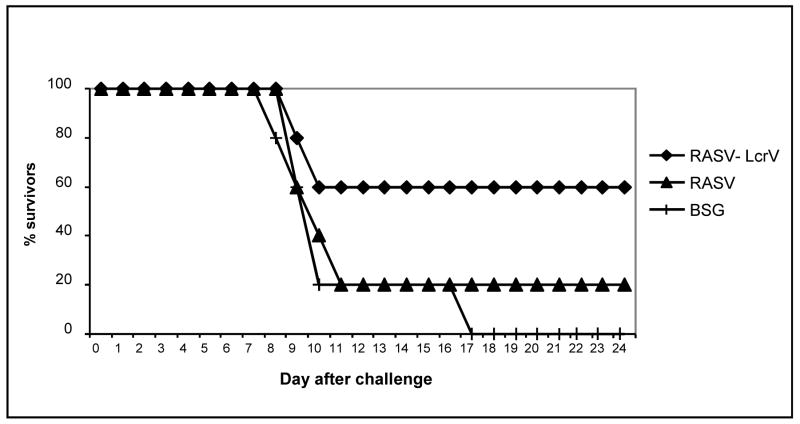
We also evaluated our vaccine for the ability to protect against another important enteric species, Y. enterocolitica. Since we had observed significant cross-protection against Y. pseudotuberculosis using a dose as high as 100 LD50s, we decided to use an equally high dose to evaluate protection against Y. enterocolitica, as this would allow us to test the limits of protection provided by the vaccine and maximize the differences between groups immunized with χ8501(pYA3841), expressing lcrV196 and groups immunized with Salmonella alone. Groups of five mice were orally immunized on days 0 and 10, orally challenged with 1.3 × 109 CFU of Y. enterocolitica 8081 and monitored for 24 days. The oral LD50 of Y. enterocolitica 8081 is 1 × 107 CFU. We monitored the serum immune response and found that the levels of anti-LcrV IgG in mice immunized with χ8501(pYA3342) (negative control strain used in this experiment) or χ8501(pYA3841) was similar to what we observed in our previous experiments (Fig. 3 and data not shown). Sixty per cent of mice immunized with χ8501(pYA3841), the strain synthesizing LcrV196, survived challenge, while only 20% of the mice immunized with the empty vector strain χ8501(pYA3620) were protected against Y. enterocolitica strain 8081 (Fig 5). All of the BSG-immunized mice succumbed to challenge. These results indicate that the vaccine expressing lcrV196 elicited a protective immune response and, unlike the results for Y. pseudotuberculosis, LcrV synthesis was required to achieve significant protection against Y. enterocolitica challenge (P<0.05).
Figure 5.
Survival of mice orally immunized with χ8501(pYA3620) or χ 8501(pYA3841) (bla-SS-lcrV196-CT) or mice mock-immunized with BSG, followed by challenge with 1.3 × 109 CFU of Y. enterocolitica 8081. P<0.05 for mice immunized with χ8501(pYA3841) compared to the other two groups. This experiment was performed twice with similar results.
To evaluate the effect of our vaccine to protect against an O8 strain synthesizing LcrV-YenO8, we orally challenged 2 groups of 5 immunized mice and 5 control mice with 6.9 × 108 CFU of Y. enterocolitica WA, which has an oral LD50 of 6.4 × 106 CFU. All of the mock-immunized mice succumbed to challenge. Forty per cent of the mice immunized with χ8501(pYA3841), survived challenge, while 20% of mice immunized with the empty vector strain χ8501(pYA3620) were protected. The level of protection in immunized mice was not significantly different from the controls (P>0.05).
4. Discussion
LcrV has been shown to be a protective antigen against Y. pseudotuberculosis and Y. enterocolitica challenge [19, 44, 45]. In a recent study, mice immunized intranasally with Lactobacillus lactis engineered to secrete LcrV derived from Y. pseudotuberculosis were protected against both oral and systemic challenges, although no protection against Y. pestis challenge was observed [46]. In previous work, we showed that immunization of mice with χ8501(pYA3841), an attenuated S. Typhimurium strain expressing lcrV196, protected mice against Y. pestis challenge [43] In this work, we demonstrate that this same strain can induce a protective immune response against the two enteric pathogens Y. pseudotuberculosis and Y. enterocolitica 8081 (Table 1 and Fig. 5). Immunized animals developed a serum IgG response against LcrV (Fig. 3) that was primarily Th1-based, as is typical for Salmonella vaccines, whereas animals immunized with the control strain did not produce anti-LcrV antibodies. The immune response elicited in mice immunized with χ8501(pYA3841) (bla-SS-lcrV196-CT) afforded significant protection against oral challenge with 100 LD50s of a Y. enterocolitica 08 strain 8081 (Fig. 5). The fact that we used a truncated protein as the immunogen confirms that the central region of LcrV contains the principal epitope(s) needed for protection against human pathogenic Yersiniae as seen by others [44, 47].
LcrV is polymorphic and strains expressing this antigen can be divided into 2 different groups [19]. One group consists of Y. pestis, Y. pseudotuberculosis, and the 03 serotype of Y. enterocolitica. The second group contains additional amino acids within the hypervariable region of LcrV and is represented by most serotype 08 strains of Y. enterocolitica. The Y. enterocolitica 8081 strain used in this study, while being serotype 08, has an LcrV more closely related to that of the 03 strains compared to other O:8 strains like WA and NTC10938 that are more divergent. Antisera against LcrV from Y. pestis or the Y. enterocolitica 03 serotype protects against Yersiniae expressing a related LcrV protein but not against 08 serotypes and vice versa [19, 44]. Consistent with these findings, vaccine strain χ8501(pYA3841), expressing Y. pestis lcrV, provided only a low level of protection against challenge with Y. enterocolitica strain WA that was not statistically significant.
However Schmidt et al. found that active immunization with LcrV from an O8 serotype of Y. enterocolitica did protect against Y. pseudotuberculosis infections while protection via passive immunity required higher levels of antisera [45]. A more recent analysis of the LcrV hypervariable hot spot at amino acids 41–60 provided a basis for regrouping all of the O:8 strains into a single group whereas Y. pestis and Y. pseudotuberculosis belong to another group [20]. This sequence is related directly to the level of IL-10 expression: strain 8081 belongs to the most virulent group, where TLR2-mediated up-regulation of IL-10 is the strongest [20]. Interestingly, the virulence of Y. enterocolitica strains in this virulent group diminishes after alteration of the TLR2 binding site, whereas no significant difference in virulence has been observed for Y. pestis [26]. Therefore, the IL-10 response seems play a greater role in the virulence of Y. enterocolitica. Based on these results, it may be necessary to remove the immunomodulatory residues of LcrV to obtain a safer and more efficient vaccine. Presumably, residues 31-57 of LcrV interact with TLR2 receptor to suppress the innate immune response [20, 23]. In our studies, we used RASV expressing a fragment of the lcrV gene encoding the 131-327 residues, without the TLR2 interacting sequence but with the central part of the protein that contributes to protection. However, Overheim [48] and Abramov [49] observed that over sequences on LcrV in the C-terminal end of the protein could also be involved in the up-regulation of IL-10.
Since our LcrV was derived from Y. pestis and therefore would be expected to protect against the 8081 strain of Y. enterocolitica, it will be interesting to to evaluate an lcrV mutant where this sequence has been modified to determine if it protects against other O8 serotypes of Y. enterocolitica including strain WA. If so, it may be possible to develop a single vaccine against all of the medically important Yersinia.
Perhaps the most surprising finding in this study is that mice immunized with the control strain, χ8501(pYA3620) were fully protected against challenge with up to 100 LD50s of Y. pseudotuberculosis (Table 1). The basis of this protection is not clear. However, we performed a similar experiment with a different attenuated S. Typhimurium strain that did not express a Yersinia antigen and that was less invasive than χ8501, based on the strains ability to colonize Peyer’s patches, spleen and liver (data not shown). In that experiment, we found that only 50% of the mice immunized with this strain were protected, indicating that colonization of lymphoid tissues is important for induction of cross-protective immunity.
Immune sera from mice immunized with the S. Typhimurium control strain reacted with a number of proteins from all three Yersinia species tested (Fig. 4). Despite this, when mice immunized with the control strain, χ8501(pYA3620) were challenged with 100 LD50s of Y. enterocolitica strains 8081 or WA (Fig. 5), the level of cross-protection provided by the Salmonella vector strain was not statistically significant (P>0.05), unlike what we observed for Y. pseudotuberculosis challenge (Table 1). There are several genetic loci present in Y. enterocolitica that promote intestinal colonization and survival, including the cellulose biosynthetic operon (cel) and the hydrogenase loci (hyf), that are absent from Y. pseudotuberculosis [12]. The cel operon may be involved in production of an extracellular matrix whose presence increases retention time in the gut. The hydogenase loci provides Y. enterocolitica with the ability to utilize locally generated hydrogen as an energy source, a characteristic essential for gut colonization [50] and, in addition, it may serve a role in osmoprotection. The absence of these and other loci in Y. pseudotuberculosis could influence the enteric environment occupied by the two Yersiniae [12]. Exactly how these niche differences affect the cross-protective efficacy of Salmonella is not clear and is an interesting question for future study.
Our long-term goal is to develop a Salmonella vaccine strain expressing multiple Yersinia antigens to protect against all pathogenic Yersinia species. Combining the results reported here with those obtained in our previous work, we have demonstrated that a single attenuated S. Typhimurium strain expressing truncated lcrV can protect mice against challenge with Y. pestis, Y. enterocolitica and Y. pseudotuberculosis. The low level of protection observed in immunized mice against Y. enterocolitica challenge, in particular strain WA, is an important concern that will be addressed in future studies. We are currently developing new means to attenuate and enhance the immunogenicity of Salmonella vaccine strains and are evaluating additional Yersinia antigens to improve the protective efficacy of our vaccines against these important pathogens.
Acknowledgments
We thank S. Straley for providing plasmid pHT-V. J. Kilbourne is thanked for her expert assistance with animal experiments. This work was supported by National Institutes of Health grant 5R01 AI057885.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clin Microbiol Rev. 1997 Jan;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottone EJ. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997 Apr;10(2):257–76. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottone EJ. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1999 Apr;1(4):323–33. doi: 10.1016/s1286-4579(99)80028-8. [DOI] [PubMed] [Google Scholar]
- 4.Slee KJ, Button C. Enteritis in sheep, goats and pigs due to Yersinia pseudotuberculosis infection. Aust Vet J. 1990 Sep;67(9):320–2. doi: 10.1111/j.1751-0813.1990.tb07814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukushima H, Gomyoda M. Intestinal carriage of Yersinia pseudotuberculosis by wild birds and mammals in Japan. Appl Environ Microbiol. 1991 Apr;57(4):1152–5. doi: 10.1128/aem.57.4.1152-1155.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carniel E, Mollaret HH. Yersiniosis. Comp Immunol Microbiol Infect Dis. 1990;13(2):51–8. doi: 10.1016/0147-9571(90)90516-v. [DOI] [PubMed] [Google Scholar]
- 7.Wren BW. The Yersiniae--a model genus to study the rapid evolution of bacterial pathogens. Nat Rev Microbiol. 2003;1(1):55–64. doi: 10.1038/nrmicro730. [DOI] [PubMed] [Google Scholar]
- 8.Simonet M, Falkow S. Invasin expression in Yersinia pseudotuberculosis. Infect Immun. 1992 Oct;60(10):4414–7. doi: 10.1128/iai.60.10.4414-4417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grutzkau A, Hanski C, Hahn H, Riecken EO. Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990 Sep;31(9):1011–5. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chain PS, Carniel E, Larimer FW, Lamerdin J, Stoutland PO, Regala WM, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 2004 Sep 21;101(38):13826–31. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MT, Prentice MB, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001 Oct 4;413(6855):523–7. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 12.Thomson NR, Howard S, Wren BW, Holden MT, Crossman L, Challis GL, et al. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2006 Dec 15;2(12):e206. doi: 10.1371/journal.pgen.0020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wauters G, Kandolo K, Janssens M. Revised biogrouping scheme of Yersinia enterocolitica. Contrib Microbiol Immunol. 1987;9:14–21. [PubMed] [Google Scholar]
- 14.Heise T, Dersch P. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3375–80. doi: 10.1073/pnas.0507749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson KJ, Bouton AH. Yersinia pseudotuberculosis adhesins regulate tissue-specific colonization and immune cell localization in a mouse model of systemic infection. Infect Immun. 2006 Nov;74(11):6487–90. doi: 10.1128/IAI.00718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988 Aug 11;334(6182):522–4. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 17.Sarker MR, Neyt C, Stainier I, Cornelis GR. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998 Mar;180(5):1207–14. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titball RW, Williamson ED. Vaccination against bubonic and pneumonic plague. Vaccine. 2001 Jul 20;19(30):4175–84. doi: 10.1016/s0264-410x(01)00163-3. [DOI] [PubMed] [Google Scholar]
- 19.Roggenkamp A, Geiger AM, Leitritz L, Kessler A, Heesemann J. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect Immun. 1997 Feb;65(2):446–51. doi: 10.1128/iai.65.2.446-451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sing A, Reithmeier-Rost D, Granfors K, Hill J, Roggenkamp A, Heesemann J. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16049–54. doi: 10.1073/pnas.0504728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker RR. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen) Infect Immun. 2003 Jul;71(7):3673–81. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reithmeier-Rost D, Bierschenk S, Filippova N, Schroder-Braunstein J, Sing A. Yersinia V antigen induces both TLR homo- and heterotolerance in an IL-10-involving manner. Cell Immunol. 2004 Sep–Oct;231(1–2):63–74. doi: 10.1016/j.cellimm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, et al. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002 Oct 21;196(8):1017–24. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sing A, Roggenkamp A, Geiger AM, Heesemann J. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J Immunol. 2002 Feb 1;168(3):1315–21. doi: 10.4049/jimmunol.168.3.1315. [DOI] [PubMed] [Google Scholar]
- 25.Reithmeier-Rost D, Hill J, Elvin SJ, Williamson D, Dittmann S, Schmid A, et al. The weak interaction of LcrV and TLR2 does not contribute to the virulence of Yersinia pestis. Microbes Infect. 2007 Jul;9(8):997–1002. doi: 10.1016/j.micinf.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Pouliot K, Pan N, Wang S, Lu S, Lien E, Goguen JD. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect Immun. 2007 Jul;75(7):3571–80. doi: 10.1128/IAI.01644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auerbuch V, Isberg RR. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect Immun. 2007 Jul;75(7):3561–70. doi: 10.1128/IAI.01497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima R, Brubaker RR. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993 Jan;61(1):23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germanier R, Fürer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–8. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 30.Germanier R, Fürer E. Immunity in experimental salmonellosis. II. Basis for the avirulence and protective capacity of gal E mutants of Salmonella typhimurium. Infect Immun. 1971 Dec;4(6):663–73. doi: 10.1128/iai.4.6.663-673.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galan JE, Nakayama K, Curtiss R., III Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990 Sep 28;94(1):29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama K, Kelly SM, Curtiss R., III Construction of of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned gene in a Salmonella vaccine strain. Biotechnology. 1988;6:693–7. [Google Scholar]
- 33.Kang HY, Curtiss R., III Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol Med Microbiol. 2003 Jul 15;37(2–3):99–104. doi: 10.1016/S0928-8244(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 34.Kang HY, Srinivasan J, Curtiss R., III Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect Immun. 2002 Apr;70(4):1739–49. doi: 10.1128/IAI.70.4.1739-1749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xin W, Wanda SY, Li Y, Wang S, Mo H, Curtiss R., III Analysis of type II secretion of recombinant pneumococcal PspA and PspC in a Salmonella enterica serovar Typhimurium vaccine with regulated delayed antigen synthesis. Infect Immun. 2008 Jul;76(7):3241–54. doi: 10.1128/IAI.01623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zekarias B, Mo H, Curtiss R., III Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin Vaccine Immunol. 2008 May;15(5):805–16. doi: 10.1128/CVI.00457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshland D, Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–60. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- 38.Branger CG, Fetherston JD, Perry RD, Curtiss R., III Oral vaccination with different antigens from Yersinia pestis KIM delivered by live attenuated Salmonella Typhimurium elicits a protective immune response against plague. Adv Exp Med Biol. 2007;603:387–99. doi: 10.1007/978-0-387-72124-8_36. [DOI] [PubMed] [Google Scholar]
- 39.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosjean H, Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- 41.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 42.Curtiss R, III, Charamella LJ, Berg CM, Harris PE. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–50. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fields KA, Nilles ML, Cowan C, Straley SC. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999 Oct;67(10):5395–408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motin VL, Nakajima R, Smirnov GB, Brubaker RR. Passive immunity to Yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect Immun. 1994 Oct;62(10):4192–201. doi: 10.1128/iai.62.10.4192-4201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt A, Schaffelhofer S, Muller K, Rollinghoff M, Beuscher HU. Analysis of the Yersinia enterocolitica 0:8 V antigen for cross protectivity. Microb Pathog. 1999 Apr;26(4):221–33. doi: 10.1006/mpat.1998.0268. [DOI] [PubMed] [Google Scholar]
- 46.Daniel C, Sebbane F, Poiret S, Goudercourt D, Dewulf J, Mullet C, et al. Protection against Yersinia pseudotuberculosis infection conferred by a Lactococcus lactis mucosal delivery vector secreting LcrV. Vaccine. 2009 Jan 7; doi: 10.1016/j.vaccine.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Hill J, Leary SE, Griffin KF, Williamson ED, Titball RW. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect Immun. 1997 Nov;65(11):4476–82. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overheim KA, Depaolo RW, Debord KL, Morrin EM, Anderson DM, Green NM, et al. LcrV plague vaccine with altered immunomodulatory properties. Infect Immun. 2005 Aug;73(8):5152–9. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abramov VM, Khlebnikov VS, Vasiliev AM, Kosarev IV, Vasilenko RN, Kulikova NL, et al. Attachment of LcrV from Yersinia pestis at dual binding sites to human TLR-2 and human IFN-gamma receptor. J Proteome Res. 2007 Jun;6(6):2222–31. doi: 10.1021/pr070036r. [DOI] [PubMed] [Google Scholar]
- 50.Maier RJ, Olczak A, Maier S, Soni S, Gunn J. Respiratory hydrogen use by Salmonella enterica serovar Typhimurium is essential for virulence. Infect Immun. 2004 Nov;72(11):6294–9. doi: 10.1128/IAI.72.11.6294-6299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]