Abstract
Objective
Auditory temporal processes in quiet are impaired in Auditory Neuropathy (AN) similar to normal hearing subjects tested in noise. N100 latencies were measured from AN subjects at several tone intensities in quiet and noise for comparison with a group of normal hearing individuals.
Methods
Subjects were tested with brief 100 ms tones (1.0 kHz 100 dB to 40 dB SPL) in quiet and in continuous noise (90 dB SPL). N100 latency and amplitude were analyzed as a function of signal intensity and audibility.
Results
N100 latency in AN in quiet was delayed and amplitude was reduced compared to the normal group; the extent of latency delay was related to psychoacoustic measures of gap detection threshold and speech recognition scores, but not to audibility. Noise in normal hearing subjects was accompanied by N100 latency delays and amplitude reductions paralleling those found in AN tested in quiet. Additional N100 latency delays and amplitude reductions occurred in AN with noise.
Conclusions
N100 latency to tones and performance on auditory temporal tasks were related in AN subjects. Noise masking in normal hearing subjects affected N100 latency to resemble AN in quiet.
Significance
N100 latency to tones may serve as an objective measure of the efficiency of auditory temporal processes.
Keywords: Temporal processes, Dys-synchrony, Deafferentation, Noise masking, Psychoacoustics, Hearing impairment
1. Introduction
Auditory neuropathy (AN) describes patients with dysfunction of the auditory nerve in the presence of preserved cochlear outer hair-cell receptor functions (Starr et al., 1996). The sites of involvement in the auditory periphery include auditory nerve (Starr al., 2003), or the inner hair cells and their synapses with auditory nerve fibers (Rodríquez-Ballesteros et al., 2008; Roux et al., 2006). The diagnosis of AN is based primarily on physiological measures of function of auditory nerve and brainstem pathways, cochlear outer hair cells, and auditory middle-ear muscles. The criteria include: (1) absence or marked abnormalities of auditory brainstem responses (ABRs) beyond that expected for the degree of hearing loss; (2) preserved outer hair-cell activity including otoacoustic emissions (OAEs) and/or cochlear microphonics (CMs); and (3) absence of acoustic and preserved, non-acoustic middle-ear muscle reflexes (Berlin et al., 2003; Starr et al., 1996; 2001).
Adult AN patients typically complain of an impaired ability to understand speech especially in the presence of noise (Rance et al., 2004; Sininger and Oba, 2001; Zeng and Liu 2006). The auditory processes that contribute to the speech perceptual deficits appear related to abnormal temporal and masking functions (Vinay and Moore, 2007; Zeng et al., 1999; 2005). Rance et al. (2008) identified in some Freidreich's ataxia, a mitochondrial disorder affecting degeneration of auditory nerves but not outer hair cells (Spoendlin, 1976), that speech perceptual errors involve stop consonants (e.g., /t/ vs. /d/) distinguished by voice onset times, a temporal cue, but not fricatives (e.g., /s/ vs. /f/) distinguished by spectral cues. Speech perception in other etiologies of AN are necessary to define if these defects are also present.
Our understanding of AN will benefit from a knowledge based on etiology, such as specific gene mutations, and the relationship of the auditory nerve disorder to other clinical findings. For instance, temporal bone studies of adult AN patients have shown loss of auditory nerve fibers, demyelination of remaining fibers, and normal numbers and morphology of both outer- and inner-hair cells (Bahmad et al., 2007; Hallpike et al., 1980; Spoendlin, 1974; Starr et al., 2003). These findings are consistent with some post-synaptic disorders of auditory nerve as being part of generalized disorders affecting both peripheral and other cranial nerves. A pre-synaptic form of AN is now identified due to mutations of the OTOF (otoferlin) gene affecting neuro-transmitter release (Rodríguez-Ballesteros et al., 2003; 2008; Roux et al., 2006, Varga et al., 2003; 2006). Temporal bones in this condition have not yet been examined to determine whether there are long-term effects of pre-synaptic disorders on the viability of either inner hair cells or auditory nerve fibers.
AN subjects, in spite of abnormal or absent ABRs, show auditory N100 and P200 cortical sensory potentials to tones (Kraus et al., 1993; Rance et al., 2002; Satya-Murti et al., 1983; Starr et al., 1996; 2003; 2004), speech signals (Kraus et al., 2000; Narne and Vanaja, 2008), and to silent gaps in continuous noise (Michalewski et al., 2005). These cortical potentials typically were delayed in latency sometimes by as much as 60 ms or more compared to normal hearing subjects. In contrast, Rance et al., (2002) showed cortical potentials were absent in 50% of young children with AN and the absence of these potentials was related to impaired speech perception. Early studies of auditory cortical potentials to tones in normal hearing subjects showed that N100 latency was remarkably stable over a wide range of intensities (e.g., 70 - 30 dB SL, Rapin et al., 1966) whereas changes of rise time, a temporal cue, resulted in striking delays of N100 latency (Onishi and Davis, 1968). Moreover, auditory cortical single unit data in experimental animals reveal that the latency of response is sensitive to temporal rather than intensity cues (Phillips, 1990). These human and animal results suggest that the N100 latency delays observed in AN subjects may reflect altered auditory nerve activity encoding temporal cues.
In the study reported below, tones were presented in quiet and in continuous noise to evoke cortical potentials. The quiet mode served as a “favorable” listening condition whereas the noise mode served as a “difficult” listening condition. The ability of AN subjects to understand speech as mentioned earlier is abnormally affected by background noise. Noise activates auditory neurons and reduces their responsiveness to other signals, a phenomenon known commonly as “the line-busy effect” (Derbyshire and Davis, 1935; Powers et al., 1995). In addition, noise can specifically interfere with neural synchrony independent of a change of responsiveness (Miller et al., 1987).
Of the many potentials that can be recorded from the scalp to acoustic stimuli (see Picton et al., 1977), we focused here on the N100 cortical potential for testing AN subjects. Our working hypotheses included that: (1) in quiet, N100 in AN would be delayed in latency and reduced in amplitude reflecting disruption of auditory nerve activity sensitive to temporal cues, (2) in noise, AN would show additional effects on N100 latency and amplitude measures, and (3) in normal hearing subjects, N100 potentials to tones in noise would be delayed in latency and attenuated in amplitude to resemble N100 measures in AN.
2. Methods
2.1. Normal subjects
Twelve normal individuals equally divided by sex and ranging between 18 and 22 years of age (mean = 19.8) were tested. A pure-tone hearing test using a MAICO 790 audiometer was used to screen each subject. Average hearing thresholds (between 0.5 - 8.0 kHz) were within normal ranges (<10 dB) for both left and right ears.
2.2. AN subjects
Eight AN subjects (3 males, 5 females) were tested, seven ranged from 18 to 33 years (mean = 25.3), and one older female subject 60 years of age. Each AN subject was assigned an identification number (ID#), #1 through #8. Audiological, psychophysical, neurophysiological test results, and clinical details for the AN group are summarized in Table 1. Four of the eight AN individuals were participants in other published studies and their subject ID# is included in Table 1 for reference.
Table 1.
Auditory neuropathy subject Information
| AN Subject ID |
AN Code1 |
Age | Sex | Ear Tested |
PTA Low |
PTA High |
1.0 kHz Threshold nHL |
N100 Detection nHL |
|---|---|---|---|---|---|---|---|---|
| #1 | AN13 | 33 | F | Left | 52 | 10 | 40 | 40 |
| #2 | AN7 | 27 | M | Left | 45 | 18 | 50 | 10 |
| #3 | AN2 | 60 | F | Right | 45 | 30 | 25 | 30 |
| #4 | AN16 | 18 | F | Left | 35 | 20 | 50 | 50 |
| #5 | AN10 | 26 | M | Left | 30 | 5 | 30 | 30 |
| #6 | AN29 | 26 | F | Right | 27 | 5 | 15 | 20 |
| #7 | AN28 | 26 | F | Left | 12 | 25 | 20 | 20 |
| #8 | AN30 | 21 | M | Right | 15 | 13 | 10 | 10 |
| Mean or Count | 29.6 | 5F-3M | 5L-3R | 32.6 | 15.8 | 30.0 | 26.3 |
| Subject ID | AN Code1 | Psycho-acoustic Gap (ms) | Speech Quiet/Noise | N100 Latency (ms) at 30 dB SL | N100 Amplitude (μV) at 30 dB SL | ABR (Wave V) | Auditory Neuropathy2 | Special Features |
|---|---|---|---|---|---|---|---|---|
| #1 | AN13 | 11.0 | 60/38 SNR=15 | 143 | -5.9 | Absent | Unspecified | |
| #2 | AN7 | 7.7 | 85/DNT | 138 | -3.6 | Abnormal (6.6 ms) | Unspecified | |
| #3 | AN2 | 15.0 | 19/3 SNR=15 | 179 | -1.8 | Absent | Type I | Vestibular |
| #4 | AN16 | 12.0 | 16/DNT | 147 | -15.7 | Absent | Unspecified | |
| #5 | AN10 | 5.0 | 76/51 SNR=10 | 117 | -3.7 | Absent | Unspecified | |
| #6 | AN29 | 7.1 | 21/DNT | 157 | -1.7 | Absent | Type I | Optic |
| #7 | AN28 | 5.4 | 95/DNT | 126 | -2.7 | Absent | Type I | Optic |
| #8 | AN30 | 5.8 | 98/40 SNR=0 | 113 | -3.5 | Abnormal (6.4 ms) | Type II | Temperature Sensitive |
| Mean or Count | 8.6 | 58.8/33.0 | 140 | -4.8 | 6 Absent | Type I: 3 | ||
| 2 Abnormal | Type II: 1 | |||||||
| Unspecified:4 |
ABR = auditory brainstem response. The ABRs were tested on separate occasions whereas psychoacoustic measures were performed on the same day as evoked potential testing. PTA = pure tone average. SNR = signal-to-noise ratio.
AN identification codes for subjects tested previously from published studies in our laboratory are included for cross reference. (Note the exception to the subject cross-reference codes in Michalewski, et al., 2005; subjects 2, 3, 4, and 5 in that study correspond to AN7, AN2, AN16, and AN10, respectively.)
Type I AN = post-synaptic; Type II AN = pre-synaptic; Unspecified = type not defined. DNT = did not test.
The results of diagnostic physiological tests were entirely consistent with the diagnosis of AN (Starr et al., 1996). Neural components of the ABRs were absent in six subjects, or abnormal in two subjects consisting of only a Wave V of delayed latency beyond that expected for the degree hearing loss. Outer hair cell receptor measures including otoacoustic emissions (OAEs) and cochlear microphonics (CMs) were normal in all subjects. Brainstem acoustic reflexes involving middle ear muscles were absent in all of the AN subjects.
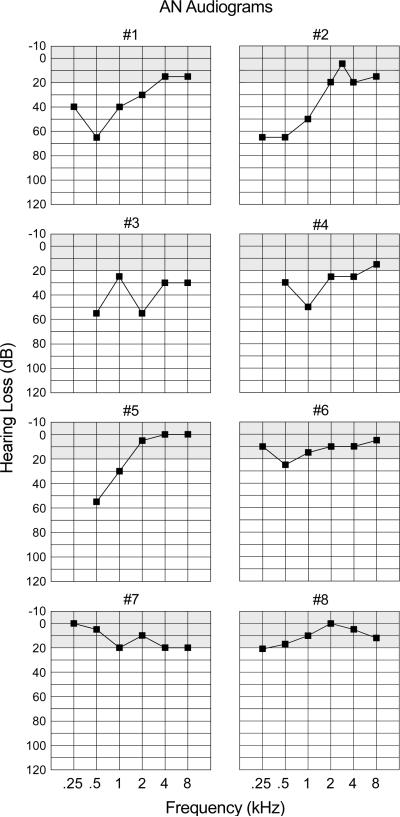
Audiograms for each AN subject are provided in Fig. 1. Audiometric thresholds were normal (20 dB or less) for three subjects (#6, #7, and #8) and were mildly- to moderately-elevated for the lower frequencies for five subjects (#1 through #5). The average threshold for the AN subjects at 1.0 kHz was elevated to 31.3 dB nHL compared to 5.6 dB nHL for the normal hearing group.
Figure 1.
Pure tone audiograms are shown for each auditory neuropathy subject. The audiograms correspond to the ear used (Table 1) for testing auditory evoked potentials.
Psychoacoustic gap detection thresholds were moderately elevated (mean = 8.6 ms) compared to normal hearing subjects (approximately 3 ms at high sensation levels, Zeng et al., 2005).
Speech perception scores were measured in seven of the eight AN subjects by the percent of correctly identified keywords in daily sentences (refer to Zeng and Liu, 2006 for testing details) and in one subject (#7) by results from the referring audiologist to spondee words in quiet. Normal hearing listeners typically score close to 100% in quiet but hearing-impaired listeners tend to score lower and are particularly susceptible to noise (e.g., Bench et al., 1979; Gifford et al., 2008). There was a moderate impairment in seven of the eight AN subjects tested in quiet (mean = 58.8%; normal 100%). For AN subjects, accuracy for speech recognition scores in quiet was associated with gap detection thresholds (r = -0.73, p < 0.05).
We classified the disruption of auditory nerve function to a disorder of the auditory nerve (post-synaptic AN) in three subjects (#3, #6, and #7); #3 had accompanying peripheral and vestibular neuropathies due to MPZ gene mutation (Starr et al., 2003); subjects #6 and #7 were twins with accompanying optic neuropathies. The disorder is pre-synaptic in #8 who had a temperature sensitive hearing loss (see Starr et al., 1998) due to compound heterozygous mutation of OTOF (Varga et al., 2003, 2006). This subject was tested when afebrile. The remaining four AN subjects (#1, #2, #4, and #5) did not have peripheral or cranial nerve abnormalities and they were classified as unspecified for site of the disorder. Details of the procedures used to characterize neurological, audiological, and physiological measures of the AN subjects are more fully described in Starr et al. (1996) and Zeng et al. (2005).
2.3. Subject recruitment
Normal subjects were young adults recruited from the university community. AN subjects are part of a research cohort either self-referred or referred by professional colleagues (see Acknowledgments). Internal review board (IRB) approval for the study was obtained prior to testing. Each subject signed an informed consent following university IRB guidelines for testing human subjects and each subject was paid for participating in the study.
2.4. Auditory stimuli
Auditory stimuli consisted of tones in quiet and tones mixed in a background of continuous noise. Pure 1.0 kHz tones of 100 ms duration were generated (16-bit, 44.1 kHz sampling rate) for seven intensity levels in 10 dB steps from 40 to 100 dB SPL. Audiometric thresholds measured at 1.0 kHz for normal hearing subjects in our laboratory average approximately 35 dB SPL in quiet. Individual tones were windowed (10 ms Hamming) at onset and offset to reduce spectral splatter. Tones were separately mixed with broadband “white” noise (within ± 0.3 dB from 30.0 Hz to 20.0 kHz) at 90 dB SPL. This noise level was relatively loud (90 dB SPL) but not uncomfortable and accommodated the wide range of hearing loss anticipated for the AN subjects. All subjects could detect the 1.0 kHz tone even at negative signal-to-noise ratios (SNRs) since only noise energies within the 1.0 kHz critical band affected the detection of the tone. Adding brief tones to continuous noise instead of noise bursts was used to avoid possible component overlap of brain potentials to bursts and tone onsets. Sound stimuli were generated with Cool Edit Pro (Syntrillium) and were converted from .wav-to-.snd files for playback on a PC-based Neuroscan stimulation system. Sounds were delivered monaurally from a shielded-transducer (Etymotic Type ER-3A MN) connected through a 25 cm plastic tube to a foam insert within the ear canal. In the normal subjects, only the right ear was tested since there were no threshold differences between the ears; for AN subjects, the ear with the lower threshold at 1.0 kHz was tested. Tones in quiet and tones in noise were presented at a regular 1.4 sec interval; the order of tone presentation was randomly determined in quiet and in noise. Periodic measurements of stimulus output levels delivered from the transducer were checked with a B&K 2260 sound level meter.
2.5. Experimental conditions
Tones in quiet and tones in noise were presented in separate passive and active listening conditions in a sound-attenuating electrically-shielded chamber. In the passive conditions, subjects fixated a point on a CRT monitor directly ahead; in the active conditions, subjects again fixated their gaze, but also pressed a reaction time (RT) button as quickly as they could whenever a tone was detected. Thus, there were four conditions randomly ordered among subjects: tones in quiet (passive, active) and tones in noise (passive, active). A testing condition was completed when at least 50 stimuli at each tone level were presented (approximately 10 min). A practice sequence for each condition was presented to the subject before recording was started to verify that the instructions for each stimulus condition were understood. The ongoing EEG was continuously monitored during recording for signs of slowing reflecting drowsiness in the passive conditions, and disruptive muscle activity and movement-related artifact in the active conditions. Testing was paused until recording adjustments could be made. On-line averages were computed to each stimulus to monitor for any other unwanted artifacts developing in the potentials. A short break of 5 -10 minutes occurred between conditions.
2.6 Recording and analysis
Scalp recordings were made from 8-mm diameter Ag/AgCl (sintered) electrodes at midline sites Fz, Cz, and Pz referenced to linked mastoids. Ocular movements were monitored from electrodes located above and below the right eye. A ground electrode was attached at the forehead. A PC-based Neuroscan recording system (Scan) with SynAmps (biological amplifiers) was used to collect the EEG data. Amplifier bandpass was set between DC and 100 Hz. Ongoing EEG was digitized (500 Hz), displayed, and stored to disk. Off-line, the digitized records were adjusted for DC drift and were corrected for ocular artifact with Scan system software. The records were epoched off-line from coded stimuli to include a 100 ms pre-stimulus baseline period and an 800 ms post-stimulus period. Separate averages were computed to each tone sorted by loudness level (40 -100 dB SPL) in the passive conditions and correct responses in the active conditions.
Individual averages were low-pass filtered (Butterworth) with an upper cutoff frequency of 30 Hz (12 dB/octave slope). Peak latencies to N100 were measured from stimulus onset to peak maximum; peak amplitudes were computed relative to the average pre-stimulus voltage to peak maximum.
2.6.1. Normal subjects
Peak measures were separately evaluated using procedures based on a general linear model (GLM). A multi-factor design (one between, four repeated) was used to analyze evoked potential measures by sex, active and passive conditions, tones in quiet and noise, intensity level, and electrode site (2 × 2 × 2 × 7 × 3 design) with Greenhouse-Geiser correction. Post-hoc comparisons of the means for cortical and behavioral measures were tested with the Tukey-Kramer multiple comparison procedure. The significance level was set at p < 0.05; values of the test statistic and level of significance are reported.
In quiet, normal subjects displayed N100 potentials for the entire stimulus range (40 to 100 dB SPL). The presence of noise however affected identification of N100 potentials in the averaged potentials evoked to tones. At 60 dB SPL and below (SNR ≥-30 db), cortical potentials to tones in noise were no longer detected in the averages for normal subjects. At 70 dB SPL (SNR = -20 dB), N100 peaks were identified in nine of the 12 subjects and assigned zero amplitude if absent; correspondingly, peak latency was scored as missing. Since cortical responses could not be identified to 60 dB SPL tones in noise and below, the GLM analysis of tones in quiet vs. tones in noise was conducted only on the intensity levels from 70 to 100 dB SPL (SNR = -20 to +10 dB). A separate ANOVA analysis over the entire intensity range (40 - 100 dB SPL) was performed for tones in quiet conditions to evaluate latency and amplitude effects.
Behavioral measures of accuracy and reaction time were analyzed using analysis of variance (ANOVA) procedures for repeated measures. Factors included gender, condition (tones in quiet, in noise), and intensity level (dB SPL). Accuracy was assessed as the percentage of correctly detected tones (in quiet and in noise) over the 40 - 100 dB SPL intensity range. Reaction time measures to correct button presses were computed at each intensity level. In noise, a no response was scored as missing and accuracy as zero.
2.6.2. AN subjects
Each of the AN subjects performed the tones in quiet and tones in noise in the passive conditions but because of time limitations and availability, only four of the eight subjects were able to complete all four conditions (passive and active). We will present here only the evoked potential results from the passive conditions with the single exception of the behavioral results (accuracy and reaction time) from the normal hearing subjects in quiet and noise. The order of the conditions was random whether two or four conditions were tested. Group comparisons between AN (n = 8) and normal hearing group (n = 12) to tones in quiet and tones in noise (passive conditions) were evaluated using independent t-tests (for unequal n) at separate intensity levels, and dependent t-tests for measures within the AN group. False discovery rate (FDR) procedures (Curran-Everett, 2000) were used in the evaluation of the multiple comparisons based on t-values; the FDR was set at 0.05. At least five AN subjects were required for group comparisons with the normal hearing subjects. Group tests in noise resulted in comparing only the intensities at 80, 90, and 100 dB SPL. Linear fit procedures were used to evaluate slope measures of mean latency and amplitude as a function of intensity. Correlation procedures were used to examine the relationship in AN between psychoacoustic measures of gap detection, speech, PTA for lower frequencies (0.5, 1.0 and 2.0 kHz), PTA for higher frequencies (4.0, 6.0, and 8.0 kHz), threshold at 1.0 kHz, and N100 latency and amplitude (Table 1). N100 latency and amplitude to thresholds at 1.0 KHz tones were adjusted for audibility differences between subjects. Recordings from the Cz electrode site are shown in the figures and graphs involving evoked potentials.
3. Results
3.1. Grand averages
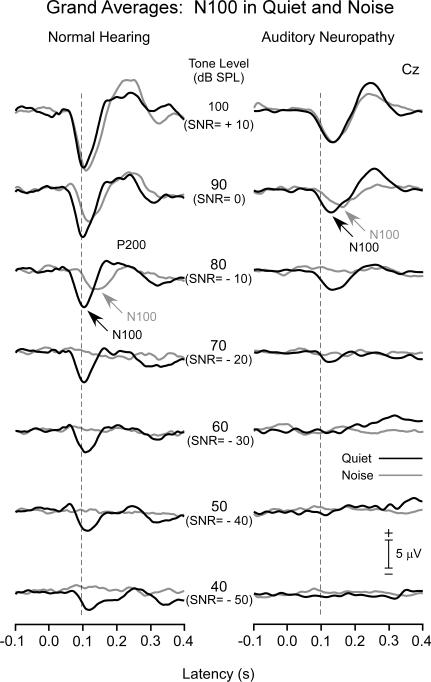
The overlayed grand averages in quiet and in noise for normal hearing (left panel) and AN groups (right panel) are shown in Fig. 2 for the passive conditions. In addition to N100, other recorded cortical components included a P200 followed by a late negative wave at approximately 300 ms. Additionally, a late positive potential appeared in active conditions (not shown) sharing properties common to P300 (see review by Polich, 2007). Measures of latency and amplitude of these latter components are not presented here.
Figure 2.
Grand averages are shown for the normal hearing (left panel) and AN subjects (right panel) to tones in quiet and in noise. For the normal group, N100 latencies were relatively constant in quiet from 100 to 70 dB SPL but lengthened accompanied by reduced amplitudes to lower signal levels; in noise, N100 latencies were noticeably delayed at 90 dB SL (SNR = 0) and reduced in amplitude compared to quiet. AN subjects followed a similar pattern of latency and amplitude effects in quiet and noise as the normal hearing group. Note however that AN potentials were delayed and reduced in amplitude starting from 100 dB SPL (SNR = +10) compared to the normal group.
3.1.1. Normal hearing subjects
In quiet, distinct N100 potentials to tones were identifiable for all subjects extending from 100 dB down to 40 dB SPL with only a slight increase in N100 latency starting approximately at 60 dB SPL. The N100 component was of broad duration (>200 ms) at 40 and 50 dB SPL. In the presence of noise, N100 potentials to tones were progressively delayed and amplitudes reduced compared to tones in quiet starting at 90 dB SPL (SNR= 0). By 70 dB SPL (SNR= -20), N100 was identified in nine of the 12 subjects, and by 60 dB SPL (SNR= -30) in none of the subjects. The rapid disappearance of N100 with reduced levels was attributed to the relatively high level of the continuous noise.
3.1.2. AN subjects
The general waveform morphology of the AN group was similar to the normal hearing subjects but with noticeable differences. N100 latencies starting at 100 db SPL were prolonged and additionally delayed at lower intensities. N100 amplitudes were reduced in amplitude from 100 dB SPL and deceased with lower intensities. In the presence of noise, N100 latency to tones lengthened compared to tones in quiet and the magnitude of the delay increased as SNR was reduced.
3.2. N100 measures in quiet and noise
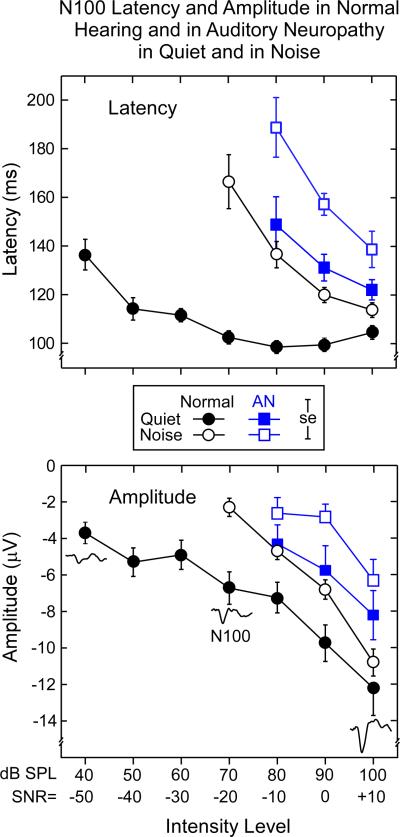
N100 latency and amplitude means for the normal hearing and AN groups in quiet and noise are shown for comparison in Fig. 3.
Figure 3.
Mean N100 latencies and amplitudes for the normal hearing and AN groups to tones in quiet (db SPL) and tones in noise as a function of intensity level. Note in the upper panel that N100 latencies are prolonged for the AN group in quiet and noise relative to the normal hearing subjects. N100 amplitudes (lower panel) were reduced in AN in quiet and noise compared to the normal group. ± 1.0 SE is indicated. (Increasing N100 potentials are plotted downwards shown by the sample potentials.)
3.2.1. N100 latencies: Normal hearing
The upper panel of Fig. 3 shows the mean N100 latencies in quiet were relatively stable at higher intensities (70-100 dB SPL) and became delayed at lower intensities. In noise, N100 latency was progressively delayed as the SNR decreased from +10 to -20.
ANOVA analysis over the 70 to 100 dB range confirmed significant N100 latency delays resulting from the presence of noise (quiet vs. noise; F = 203, p < 0.001), tone intensity (F = 18.6, p < 0.001), and an interaction of these factors (F = 161.6, p < 0.001). Active and passive conditions were not statistically different (F < 1, ns). We detail below the interaction between quiet and noise and intensity level for the passive conditions. The remaining ANOVA factors of gender (F < 1, ns) and electrodes (F = 1.2, ns) did not attain significant levels.
Mean N100 latencies in quiet ranged between 102.5 ms and 104.6 ms for intensities between 70 and 100 dB SPL, respectively. In quiet, post-hoc tests indicated no latency differences between levels from 70 dB to 100 dB SPL. A linear fit of the means (70 - 100 dB SPL) indicated that the slope was not different from zero (slope: 0.06 ms/dB SPL, r = 0.31, F < 1, ns). In contrast, N100 latencies in noise were delayed compared to quiet and ranged from 113.8 ms at 100 dB to 166.6 ms at 70 dB SPL. Post-hoc tests showed that the mean N100 latencies in noise increased at each intensity level from 100 dB down to 70 dB SPL. The slope (linear fit) in noise was significantly different from zero (slope: -1.75 ms/dB, r = 0.96, F = 21.6, p < 0.04). The potentials in noise were significantly longer than in quiet at each level between 100 - 70 dB SPL.
The separate in quiet ANOVA analysis over the range between 40 - 100 dB SPL (intensity level, F = 28.4, p < 0.001) found only that the latencies at 40 dB and 50 dB SPL were significantly delayed compared to the higher intensity levels (70 dB SPL and above) in post-hoc tests.
3.2.2. N100 latencies: AN subjects
Overall, N100 latencies in AN subjects were longer than normal hearing in quiet and were further prolonged in noise (upper panel, Fig. 3).
In quiet, N100 latencies in AN were significantly prolonged compared to normal hearing at 80 dB SPL (t = 4.2, p < 0.003), 90 dB SPL (t = 5.7, p < 0.001), and 100 dB SPL (t = 3.8, p < 0.001). In noise, AN latency delays were also significantly longer than normal hearing in noise at 80 dB SPL (SNR -10), 90 dB SPL (SNR = 0), and 100 dB SPL (SNR +10) (t = 2.2, p < 0.03; t = 4.6 p < 0.002, and t = 3.0, p < 0.02, respectively).
The above results show that the AN group displayed N100 latency functions in quiet that were similar to the normal hearing group in noise. Moreover, N100 latency in noise was additionally delayed in AN consistent with further disruption of auditory nerve activity that may contribute to the difficulty with noise experienced in AN than normal hearing subjects (Rance et al., 2008). For the AN group, the slope functions in quiet and in noise were not different from each other over the range of 80 to 100 dB SPL (F = 6.6, p = 0.12, ns). AN slopes (quiet and noise) were not different from the slope of the normal hearing group in noise (AN in quiet vs. normal hearing in noise, F <1, p = 0.6, ns; AN in noise vs. normal hearing in noise F = 1.5, p = 0.3, ns). Inspection of the latencies of the normal hearing subjects in noise were remarkably similar to the latencies of the AN group in quiet (upper panel, Fig. 3). Statistical tests revealed that there were no differences between the groups (100 dB SPL: t = -1.6, p = 0.12, ns; 90 dB SPL: t = -1.9, p = 0.07, ns; 80 dB SPL: t = 1.1, p = 0.30, ns) suggesting that noise in normal hearing subjects may simulate perceptual conditions similar to what AN experience in quiet.
3.2.3. N100 amplitudes: Normal hearing
The bottom panel of Fig. 3 shows the mean N100 amplitudes the normal hearing and AN group tested in quiet and in noise. Note that larger amplitude N100 potentials are plotted downwards to correspond to the negative-going direction portrayed in the averaged waveforms. In the normal hearing group, N100 amplitudes were reduced to low- compared to high-intensities both in quiet and in noise, as well as being reduced overall in noise compared to quiet. Linear slope functions fitted to means in quiet (slope: -0.20 μV/dB) and in noise (slope: -0.27 μV/dB) were not different (F = 3.7, p = 0.13, ns) over the 70 to 100 dB SPL range. ANOVA amplitude differences were found between tones in quiet and tones in noise (F = 16.8, p < 0.002), intensity level (F = 90.0, p < 0.001), and electrode (F = 45.3, p < 0.001). In quiet, amplitudes were significantly reduced at each level from 100 to 80 dB SPL; in noise, amplitudes were reduced significantly at each level between 100 to 70 dB SPL. The electrode effect indicated N100 potentials were larger over the Fz and Cz sites than for Pz, with no differences between Fz and Cz. Further, a sex effect (F = 5.4, p < 0.04) showed that amplitudes for females were overall larger, by approximately 1.7 μV, than for males. Amplitude differences between passive and active conditions (not shown) were without significant effect (F = 1.5, ns).
For the separate in quiet ANOVA analysis, N100 amplitudes (intensity level, F = 36.1, p < 0.001) between 40 - 60 dB SPL were significantly smaller than at the higher 90 and 100 dB SPL intensity levels in post-hoc tests.
3.2.4. N100 amplitudes: AN subjects
Overall the AN group had smaller N100 amplitudes in quiet than the normal hearing subjects which were further reduced in amplitude in the presence of noise (bottom panel, Fig. 3). In quiet, the AN group had smaller potentials than normal hearing but only the difference at 90 dB SPL (SNR = 0) attained significance (t = 2.4, p = 0.03). In noise, N100 amplitudes were smaller in AN than normal hearing subjects with significant differences at 100 dB SPL (SNR +10) and 90 dB SPL (SNR = 0) intensity levels (t = 3.6, p < 0.002; t = 3.2, p < 0.004, respectively).
The slopes in quiet and noise were not different for either the AN group (all F-values < 1, ns; 80 to 100 dB SPL), or the normal hearing group (all F-values < 1, ns; 70 to 100 dB SPL). There were also no differences in slopes comparing the AN group to the normal hearing group in quiet, or in noise (all F-values < 1, ns, over the 80 - 100 dB SPL range).
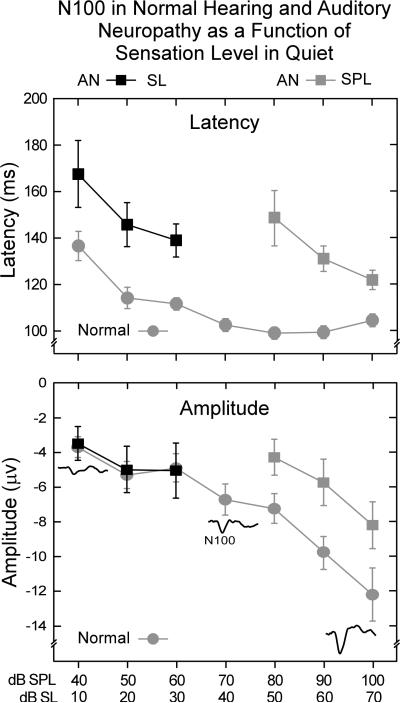
3.3. Audibility in the AN subjects
Figure 4 shows mean N100 latencies and amplitudes in quiet adjusted for audibility or sensation level (SL). Hearing thresholds were measured in dB nHL and converted to SL relative to the signal levels dB SPL. The normal hearing group was included for comparison. The delayed latencies of the AN group measured as SPL (upper panel, Fig. 4) still showed significant delays of N100 latency after adjustment for audibility at 10, 20, and 30 dB SL (t = 2.7, p < 0.015; t = -3.5, p < 0.003; t = -3.8, p < 0.001, respectively). However when amplitudes were adjusted for audibility (bottom panel, Fig. 4) no group differences were evident.
Figure 4.
Mean N100 latencies and amplitudes of the AN group to tones as a function of intensity adjusted for audibility (SL); measures for dB SPL are included for comparison. N100 latencies shown (upper panel) are prolonged in AN relative to the normal hearing group as a function of audibility (SL). In contrast, N100 amplitudes (bottom panel) for the AN group did not differ from the normal hearing group as a function of audibility (SL). ± 1.0 SE is indicated.
3.4. Influence of threshold elevation in individual AN subjects
3.4.1. N100 latency
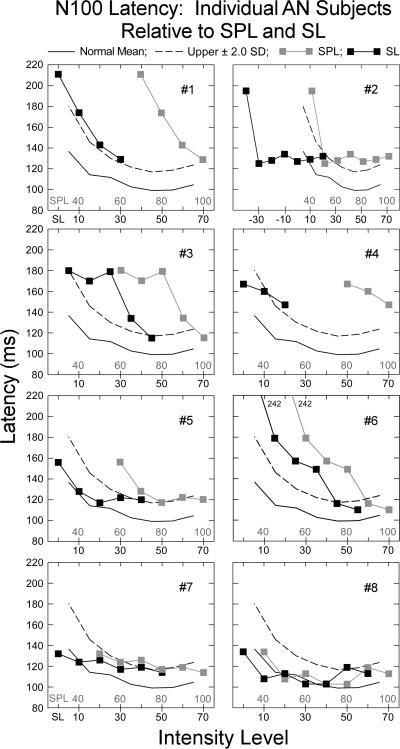
We examined how differences in individual AN hearing thresholds at 1.0 kHz contributed to the abnormality of N100 latency. Figure 5 shows the mean N100 latency for each AN subject as a function of both absolute tone intensity (40 -100 dB SPL) and sensation level (10 - 70 dB SL) in quiet. The upper ± 2.0 SD limit for the normal hearing subjects in quiet is indicated by the dashed line for each AN subject.
Figure 5.
Individual N100 latencies to tones in quiet for each auditory neuropathy subject as a function of intensity (SPL) and audibility (SL). The normal mean (solid line) and upper ± 2.0 SD limit of normal (dashed line) are shown. For absolute signal intensity (dB SPL), seven of the eight subjects (#8 is the exception) had abnormally delayed N100 latencies defined as 50% or more of the intensities tested falling beyond the upper limit of normal. When the data were adjusted for audibility, four of the seven subjects (#1, #3, #4, and #6) maintained abnormal N100 latencies. Subject #2 was not included as N100 thresholds were 30 dB lower than behavioral thresholds.
N100 latency for each AN subject was considered as “abnormal” if the latency was at, or beyond, the normal upper ± 2.0 SD limit in 50% or more of the intensities tested. We found N100 latency to be “abnormal” as a function of SPL in seven AN subjects (#1 - #7) and normal in one, #8. When N100 latency was defined as a function of audibility or sensation level (SL), the findings of abnormality were reduced to four AN subjects (#1, #3, #4, and #6). AN subject #2 was not included in this analysis since N100 potentials were present to tones that were 30 dB below behavioral threshold. For both normal hearing and the other AN subjects, N100 potentials to 1.0 kHz tones were identified within 10 dB of their audiometric thresholds to 1.0 kHz. AN subject #2, the exception, showed N100 potentials to 1.0 kHz from 10 to 70 dB nHL. Audiometric thresholds were 50 dB nHL, an intensity 40 dB higher than that evoking N100. AN subject #2 has a steep up-sloping audiogram (Fig. 1) with approximately a 45 dB threshold difference between 1.0 and 3.0 kHz. In this subject, we also tested cortical potentials to 3.0 kHz tones and found N100 to intensities within 5 dB of the normal audiometric threshold in keeping with results from the other AN and control subjects tested at 1.0 kHz. For this subject, the effects of noise masking on threshold for low (1.0 kHz) and high (4.0 kHz) tone frequencies also differed. There was relatively little change of threshold at 1.0 kHz as noise intensity increased whereas, at 4.0 kHz, the expected 1/1 ratio of threshold shift in noise was observed (see AN #7 in Figs. 7 and 8 in Zeng et al., 2005). These results indicate that cortical and behavioral measures of threshold can differ in AN. In our prior study of AN cortical potentials to temporal gaps in noise (Michalewski et al., 2005), four of twelve AN subjects did not show an N100 in a passive condition but did so when engaged in an active button pressing condition whenever they “heard” the gap. The engagement of auditory cortex in processing acoustic stimuli in certain AN subjects appears to be more variable than normal hearing subjects in both passive and active test conditions.
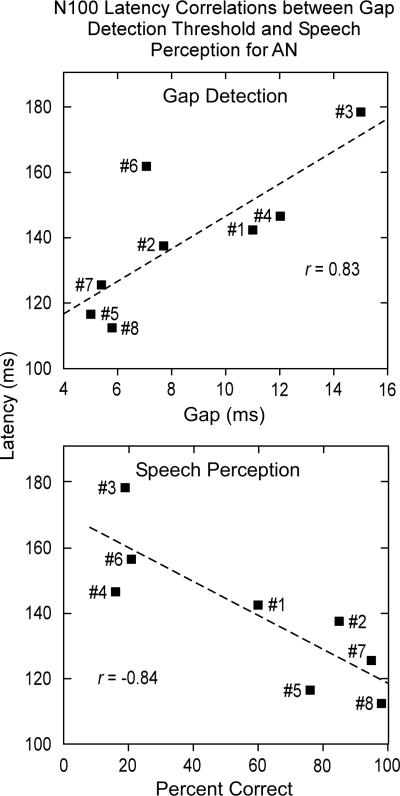
Figure 7.
Correlations between N100 latency (at 30 dB SL) and gap detection (upper panel) and between N100 latency and speech scores (bottom panel) for the eight AN subjects are shown. Gap and speech correlations were significant suggesting that measures of N100 latency are sensitive to temporal processes. The linear regression line (dashed) is indicated.
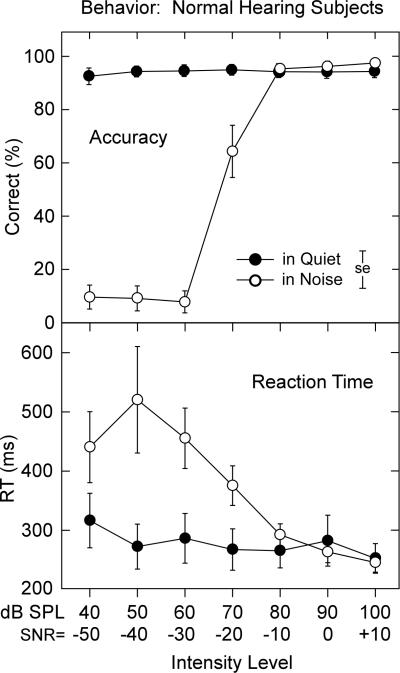
Figure 8.
Mean accuracy (upper panel) and mean RT (lower panel) for the normal hearing subjects in quiet and continuous noise. Note the drop in accuracy in noise compared to quiet. Reaction times in quiet were stable across intensity but increased in noise at approximately the same level (80 db SPL, SNR = -10) where accuracy decreased. ± 1.0 SE is indicated.
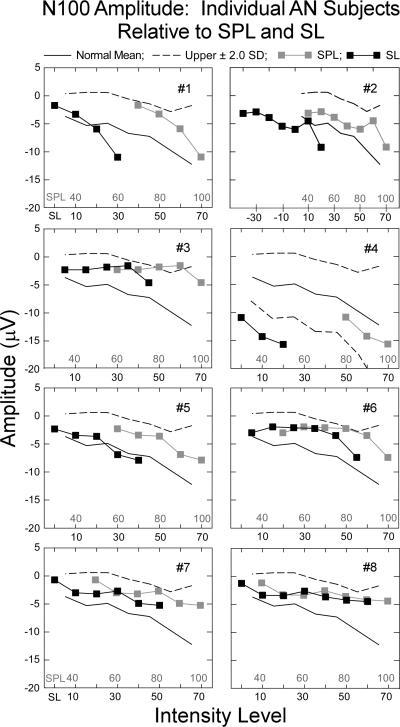
3.4.2. N100 amplitude
Mean N100 amplitudes for individual AN subjects in quiet as a function of both absolute tone intensity (40 -100 dB SPL) and sensation level (threshold to 70 dB SL) are shown in Fig. 6. The upper ± 2.0 SD limit for the normal group in quiet is indicated by the dashed lines for each AN subject. N100 amplitudes were within normal ranges for seven of the eight subjects as a function of either SPL or SL. AN subject #4 showed abnormally elevated amplitudes of N100 considered as a function of audibility but not absolute intensity.
Figure 6.
Individual mean amplitudes for each auditory neuropathy subject for tones in quiet as a function of intensity (SPL) and audibility (SL). The normal mean (solid line) and upper ± 2.0 SD limit of normal (dashed line) are shown. All but one AN subject had amplitudes that were within the normal range. The exception, #4, had amplitudes that exceeded normal limits as a function of audibility (SL); upper and lower ±2.0 SD limits are included for this subject.
3.5. Relation of N100 latencies and amplitudes to psychoacoustic measures of gap thresholds, and speech perception
The three AN subjects (#5, #7, and #8) with normal N100 latencies had gap thresholds (5.0, 5.4, and 5.8 ms, respectively) at the upper end of the normal range (± 2.0 SD limits for normal subjects are 1.6 and 5.2 ms). Their speech recognition scores in quiet were also normal (#5 = 76%, #7 = 95%, and #8 = 98%). The four AN subjects with abnormally delayed N100 latencies (#1, #3, #4, and #6) had abnormal gap detection thresholds (11.0, 15.0, 12.0, and 7.1 ms, respectively) and impaired speech scores (60%, 19%, 16%, and 21%, respectively). Subject #2, the individual with N100 potentials below audiometric threshold, had abnormal gap detection thresholds of 7.7 ms and impaired speech recognition scores of 85%.
The relationship of N100 latency at 30 dB SL to measures of gap threshold, speech recognition in quiet, and PTA (low and high) for AN in quiet were tested. Significant correlations between N100 latency and measures of gaps (r = 0.83, p < 0.01) and speech in quiet (r = -0.84, p < 0.01) were found and are shown in Fig. 7. In contrast, correlations between N100 latency and measures of PTA (low r = 0.57; high r = 0.41) and 1.0 kHz threshold (r = 0.13) were low and not significant. The relation between amplitude measures of N100 at 30 dB SL and these same psychoacoustic tests were not significantly related to gap detection (r = 0.09), speech recognition (r = 0.32), PTA (low r = 0.07; high r = 0.08), or 1.0 kHz threshold (r = 0.60).
N100 latencies in the group of AN subjects in this report were sensitive to psychoacoustic measures of temporal cues but not audibility and suggest a strong correspondence of the latency of N100 to temporally related perceptions.
3.6. Behavioral results: Normal hearing subjects
Accuracy for the detection of tones in quiet was high (>93%) to tones from 40 to 100 dB SPL and remained at these levels in the presence of noise with SNRs of +10, 0, and -10. Performance in noise declined rapidly when the SNR was -20 or below. Analysis confirmed the effects for condition (quiet vs. noise, F = 118.4, p < 0.001), tone intensity (F = 159.0, p < 0.001), and the interaction between quiet and noise and tone intensity (F = 146.1, p < 0.001).
Reaction times in quiet were relatively stable (300 ms) and increased slightly at the lowest intensities (40 dB SPL). In noise, RTs increased rapidly only when tones were 70 dB SPL (SNR = 20) or below. Significant factor effects for condition (quiet vs. noise, F = 6.12, p < 0.04), intensity level (F = 12.3, p < 0.001), and a quiet and noise by tone intensity level interaction (F = 11.2, p < 0.001) were evident. In quiet, only RTs at 40 dB SPL were significantly prolonged compared to the 70, 80, and 100 dB SPL levels. In 90 dB SPL noise, RTs for SNRs of -50, -40, and -30 were significantly longer than at SNR 0 or +10 dB; the larger standard errors among SNRs for -50, -40, and -30 dB most likely reflect, with only 10% accuracy, guesses. Sex was not a significant factor for any of the behavioral measures of accuracy or RT.
4. Discussion
The results of the present study demonstrate that auditory cortical N100 potentials to 1.0 kHz tones are prolonged in latency and reduced in amplitude in AN compared to normal hearing subjects. The latency but not amplitude changes in AN were related to their psychoacoustic measures of auditory temporal processing (threshold for silent gaps in noise, speech recognition). Comparable N100 changes develop in normal hearing subjects when tones were presented with background noise or when tones were of low intensity presented in quiet. We consider below the roles of neural synchrony and magnitude of auditory nerve responses to the latency and amplitude of auditory cortical N100 in normal hearing and AN.
4.1 N100 latency and auditory temporal processes in normal hearing
Onishi and Davis (1968) in an early study on human auditory cortical potentials showed N100 latency in normal hearing to be sensitive to temporal cues of the acoustic stimulus. N100 latency was shortest with fast stimulus rise times and lengthened as rise times were extended. Further, these latency effects were enhanced at low stimulus intensities. In contrast, when rise time was held constant, changing stimulus intensity had little influence on N100 latency except to intensities near threshold (Onishi and Davis, 1968; Rapin et al., 1966). In the present study, N100 latency in normal hearing subjects was relatively constant (100 -120 ms) over a wide range of intensity (50 to 100 dB SPL) and lengthened to 140-160 ms only when tones were of low intensity (40 dB SPL, or 10 dB SL). N100 latencies approaching 180 ms have been reported for normal hearing subjects to intensities close to behavioral threshold (Lütkenhöener and Klein, 2007).
Psychoacoustic measures of auditory temporal processes such as gap detection threshold and language comprehension show a similar sensitivity to signal intensity. The threshold for detecting gaps is typically 3 - 5 ms at “comfortable” loudness levels (e.g., 30 - 40 dB SL) but increases to approximately 30 ms when intensities are reduced to 10 dB SL (see Zeng et al., 2005). Similarly, speech recognition scores in normal hearing that are > 90% when tested at comfortable loudness levels (circa 40 - 60 dB SL) then decrease when intensities are reduced to levels at which the spoken words are first identified as “speech.”
The diminished temporal-perceptual acuity to low- compared to highsignal intensities may be related to effects of signal intensity on both the synchrony of auditory nerve discharges, and the rate and numbers of neural units activated. The experiments of Rose and his colleagues (Rose et al., 1967; 1971) of auditory nerve activity in monkey demonstrated that the timing of auditory nerve fiber discharges to low frequency tones is synchronous with the phase of the corresponding individual acoustic pressure waves. Neural synchrony is evident at signal intensities lower than those causing changes of discharge rates. We suggest that in AN, auditory nerve synchrony is disrupted to provide the basis for changes of both auditory cortical activity (N100 latency) and auditory temporal perceptions (e.g., gap thresholds, speech). These same physiological and psychoacoustic changes relating to the processing of temporal cues may develop in normal subjects when signals are presented at low intensity or at high intensities in the presence of noise. At the level of the brainstem, neural synchrony is reflected by the frequency following responses (Worden and Marsh, 1968), or FFR, recorded from scalp electrodes that reproduce the waveform of low-frequency acoustic stimuli. The FFR was absent in one of the first AN subjects published (Starr et al., 1991). Temporal synchrony at cortical levels appears to be represented by the latency of initial discharge of cortical neurons to changes in signal rise time or envelope (Biermann and Heil, 2000). Phase locking at the cortex appears to be restricted to rates of stimulus change below approximately 50 Hz (Middlebrooks, 2008).
4.1.1 N100 latency and auditory temporal processes: Individual AN subjects
The latency of N100 in AN subjects was compared to two behavioral measures of auditory temporal processing: (1) threshold for detecting brief silence gaps in noise and (2) speech recognition scores. Gap thresholds in the AN group ranged from 5 - 15 ms (mean = 8.6 ms) and were above the 5 ms upper range found in normal hearing subjects to “comfortably loud” test signals (Zeng et al., 2005). Those AN subjects with normal N100 latencies had normal gap thresholds, and normal speech recognition scores. Those AN subjects with abnormally delayed N100 latencies had abnormal gap thresholds and abnormal speech recognition scores. Correlations between N100 latency and both gap thresholds and speech scores were significant. In contrast, correlations between N100 latency and audiometric threshold (high- and low-frequency pure tone averages as well as threshold at 1.0 kHz) were not significant. N100 latencies in the present study were delayed and gap thresholds are increased in normal hearing subjects when signal intensities were lowered close to threshold (< 20 dB SL) (Zeng et al., 2005). The results from the present study are consistent with earlier observations that psychoacoustic measures of temporal processes (e.g., gap thresholds, temporal modulation transfer functions) are significantly correlated with speech perception (Rance et al., 2004; Zeng et al., 1999). These results suggest that N100 latency may serve as a reliable objective measure of auditory temporal processing.
4.2. N100 amplitude in normal hearing and AN
N100 amplitude in AN was lower than in normal hearing subjects over a range of signal intensities (dB SPL). However, when AN amplitudes were adjusted relative to audibility or sensation level, there were no significant group amplitude differences between AN and the normal subjects. Only one subject, #4, was identified with amplitudes that were increased beyond the normal 95% confidence limits as a function of audibility. The finding of “normal” or increased N100 amplitudes in AN subjects is unexpected since the number of auditory nerve fibers is reduced in the temporal bones of AN subjects that have come to autopsy. Auditory nerve activity could also be reduced in pre-synaptic disorders involving neurotransmitter release. These normal or heightened cortical N100 amplitudes may represent central auditory pathway compensation to disrupted auditory nerve functions as has been described in experimental animals with lesions of the auditory nerve (Salvi et al., 2000). AN subject #3 is of particular interest in support of this hypothesis as the temporal bone of the affected mother showed profound loss of auditory nerve fibers (Starr et al., 2003).
4.3. Noise masking in normal hearing and AN
Continuous noise masking in normal hearing subjects led to significant latency delays and reduction of amplitudes of auditory N100 resembling N100 changes in AN subjects tested in quiet. Noise masking in normal hearing subjects affects auditory temporal processes involved in speech recognition and thresholds for gap detection resembling the impairments experienced by AN tested in quiet (see Whiting et al., 1998).
One of the peripheral mechanisms involved in noise masking is its effect in activating auditory nerve fibers to interfere with synchronous responses to other signals, a phenomenon commonly referred to as “the line-busy effect.” The interference has been shown to be enhanced for auditory nerve fibers with “best” frequencies distant from the stimulus tone's frequency resulting in an overall decrease in the numbers of auditory nerve fibers that respond synchronously (Miller et al., 1987).
Auditory neuropathy subjects tested in quiet behave as if they were in a “noisy” environment. Compared to normal hearing subjects, AN thresholds for 1.0 kHz tones are elevated by approximately 20 dB over a wide range of background noise levels (Zeng et al., 2005). Moreover, identification of spondees in noise for AN subjects requires a higher SNR (approximately 10 dB) compared to normal hearing subjects (Rance et al., 2007). The masking functions in both of these studies showed marked individual variability between AN subjects and may account for clinical reports that the ability of AN subjects to understand speech both in quiet and in noise vary widely (Sinninger and Oba, 2001).
The concept of “noise” as a factor relating auditory temporal processing disorders in AN to those accompanying noise masking in normal hearing is superficially attractive but difficult to substantiate. We favor the idea that the disrupted auditory nerve activity in AN actually provides “background neural noise” that competes with those nerves that still respond somewhat synchronously.
We suggest that the desynchronous auditory nerve activity in AN is a likely source of “neural noise” that, in effect, competes with activity from those nerves that still respond somewhat synchronously. This model suggests that for AN subjects, acoustic signals presented in quiet, would be represented centrally similarly to that found in normal hearing subjects to acoustic signals presented in noise. The finding that the latency delays of N100 in AN to signals presented in quiet were similar to N100 delays recorded from normal hearing subjects in noise lends support to this possibility. Moreover, for AN, the addition of environmental noise is accompanied by further N100 delays and increasing difficulty to discriminate temporal cues compared to normal hearing subjects presented with the same degree of noise masking.
5. Translational Implications
The results from the present study provide evidence that auditory nerve synchrony may be reflected by the latency of auditory cortical N100. The latency of auditory N100 in AN was related to perceptual measures of impaired auditory temporal processes including speech perception and threshold for detecting silent gaps in noise but not to audibility measures. We suggest that the definition of N100 latency could provide objective measures of disrupted auditory nerve activity in infants, children, and those adults whose auditory temporal processing abilities may be difficult to assess by behavioral measures.
Acknowledgements
We would like to thank Drs. Jon Shallop, Mayo Clinic Rochester, Chuck Berlin, Kresge Hearing Research Laboratory, and Yvonne Sininger, University of California, Los Angeles for referring candidate subjects with auditory neuropathy. We also thank Tin T. Nguyen for assisting in testing the normal hearing subjects.
The authors appreciate the comments of Drs. Hillel Pratt and Lenny Kitzes on early versions of the manuscript. This research was supported by grant DC-02618 from the National Institutes of Health.
This research was supported by grant DC-02618 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahmad F, Jr, Merchant SN, Nadol JB, Jr, Tranebjaerg L. Otopathology in Mohr-Tranebjaerg syndrome. Laryngoscope. 2007;117:1202–1208. doi: 10.1097/MLG.0b013e3180581944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench J, Kowal A, Bamford J. The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. Br J Audiol. 1979;13:108–112. doi: 10.3109/03005367909078884. [DOI] [PubMed] [Google Scholar]
- Biermann S, Heil P. Parallels between timing of onset responses of single neurons in cat and of evoked magnetic fields in human auditory cortex. J Neurophysiol. 2000;84:2426–2439. doi: 10.1152/jn.2000.84.5.2426. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood L, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony: Diagnosis and Management. Ment Retard Dev Disabil Res Rev. 2003;9:225–231. doi: 10.1002/mrdd.10084. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:1–8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Davis H, Bowers C, Hirsh SK. Relations of the human vertex potential to acoustic input: Loudness and masking. J Acoust Soc Am. 1968;43:431–438. doi: 10.1121/1.1910849. [DOI] [PubMed] [Google Scholar]
- Derbyshire AJ, Davis H. The action potentials of the auditory nerve. Am J Physiol. 1935;113:476–504. [Google Scholar]
- Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Audiol Neurootol. 2008;13:193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- Hallpike CS, Harriman DG, Wells E. A case of afferent neuropathy and deafness. J Laryngol Otol. 1980;94:945–964. doi: 10.1017/s0022215100089696. [DOI] [PubMed] [Google Scholar]
- Hellstrom LI, Schmiedt RA. Rate/level functions of auditory-nerve fibers in young and quiet-aged gerbils. Hear Res. 1991;53:217–222. doi: 10.1016/0378-5955(91)90055-e. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Ferre J, Hoeppner J, Carrell T, Sharma A, Nicol T. Mismatch negativity in the neurophysiologic/behavioral evaluation of auditory processing deficits: A case study. Ear Hear. 1993;14:223–234. doi: 10.1097/00003446-199308000-00001. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunningham J, Kind CD, Koch DB, Nicol TG, Mcgee TJ, Stein LK, Wright BA. Consequences of neural asynchrony: A case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütkenhöener B, Klein JS. Auditory evoked field at threshold. Hear Res. 2007;228:188–200. doi: 10.1016/j.heares.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Nguyen TT, Kong Y-Y, Zeng FG. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005;116:669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Auditory cortex phase locking to amplitude-modulated cochlear implant pulse trains. J Neurophysiol. 2008;100:76–91. doi: 10.1152/jn.01109.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Barta PE, Sachs MB. Strategies for the representation of a tone in background noise in the temporal aspects of the discharge patterns of auditory-nerve fibers. J Acoust Soc Am. 1987;81:665–679. doi: 10.1121/1.394835. [DOI] [PubMed] [Google Scholar]
- Narne VK, Vanaja CS. Speech identification and cortical potentials in individuals with auditory neuropathy. Behav Brain Functions. 2008;4:15. doi: 10.1186/1744-9081-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S, Davis H. Effects of duration and rise time of tone bursts on evoked V potentials. J Acoust Soc Am. 1968;44:582–591. doi: 10.1121/1.1911124. [DOI] [PubMed] [Google Scholar]
- Phillips DP. Neural representation of sound amplitude in the auditory cortex: Effects of noise masking. Behav Brain Res. 1990;37:197–214. doi: 10.1016/0166-4328(90)90132-x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Woods DL, Baribeau-Braun J, Healey TMG. Evoked potential audiometry. J Otolaryngol. 1977;2:90–119. [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers NL, Salvi RJ, Wang J, Spongr V, Qiu CX. Elevation of auditory threshold by spontaneous cochlear oscillations. Nature. 1995;375:585–587. doi: 10.1038/375585a0. [DOI] [PubMed] [Google Scholar]
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9:1–43. doi: 10.1177/108471380500900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical evoked potentials in children with auditory neuropathy. Ear Hear. 2002;23:239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear. 2004;25:34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- Rance G, Barke E, Mook M, Dowell R, Rincon A, Garratt R. Speech perception in noise for children with auditory neuropathy/dys-synchrony type hearing loss. Ear Hear. 2007;28:351–360. doi: 10.1097/AUD.0b013e3180479404. [DOI] [PubMed] [Google Scholar]
- Rance G, Fava R, Baldock H, Chong A, Barker E, Corben L, Delatycki MB. Speech perception ability in individuals with Freidreich ataxia. Brain. 2008;131:2002–2012. doi: 10.1093/brain/awn104. [DOI] [PubMed] [Google Scholar]
- Rapin I, Schimmel H, Tourk LM, Krasnegor NA, Pollak C. Evoked responses to clicks and tones of varying intensity in waking adults. Electroencephalogr Clin Neurophysiol. 1966;21:335–344. doi: 10.1016/0013-4694(66)90039-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ballesteros M, del Castillo FJ, Martín Y, Moreno-Pelayo MA, Morera C, Prieto F, Marco J, Morant A, Gallo-Terán J, Morales-Angulo C, Navas C, Trinidad G, Tapia MC, Moreno F, del Castillo I. Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF) Hum Mutat. 2003;22:451–456. doi: 10.1002/humu.10274. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ballesteros M, Reynoso R, Olarte M, Villamar M, Morera C, Santarelli R, Arslan E, Medá C, Curet C, Völter C, Sainz-Quevedo M, Castorina P, Ambrosetti U, Berrettini S, Frei K, Tedín S, Smith J, Cruz Tapia M, Cavallé L, Gelvez N, Primignani P, Gómez-Rosas E, Martín M, Moreno-Pelayo MA, Tamayo M, Moreno-Barral J, Moreno F, del Castillo I. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum Mutat. 2008;29:823–831. doi: 10.1002/humu.20708. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferline, defective in human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Rose JE, Hind JE, Anderson DJ, Brugge JF. Some effects of stimulus intensity on response of auditory nerve fibers in the squirrel monkey. J Neurophysiol. 1967;30:769–793. doi: 10.1152/jn.1971.34.4.685. [DOI] [PubMed] [Google Scholar]
- Rose JE, Hind JE, Anderson DJ, Brugge JF. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1971;34:685–699. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Sininger Y, Oba S. Patients with auditory neuropathy: Who are they and what can they hear? In: Sininger Y, Starr A, editors. Auditory Neuropathy: A new Perspective on Hearing Disorders. Singular; San Diego, CA: 2001. pp. 15–35. [Google Scholar]
- Satya-Murti S, Wolpaw JR, Cacace AT, Schaffer CA. Late auditory evoked potentials can occur without brain stem potentials. Electroencephalogr Clin Neurophysiol. 1983;56:304–308. doi: 10.1016/0013-4694(83)90255-9. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Optic and cochleovestibular degenerations in hereditary ataxias. II. Temporal bone pathology in two cases of Freidreich's ataxia with vestibule-cochlear disorders. Brain. 1974;97:41–48. doi: 10.1093/brain/97.1.41. [DOI] [PubMed] [Google Scholar]
- Starr A. A classification of auditory neuropathy: Lessons from patients, physiology, and genetics. In: Kaga K, Starr A, editors. Neuropathies of the auditory and vestibular eighth cranial nerves. Springer; Japan: 2009. pp. 3–9. [Google Scholar]
- Starr A, McPherson D, Patterson JV, Don M, Luxford WM, Shannon R, Sininger Y, Tonokawa LT, Waring M. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain. 1991;114:1157–1180. doi: 10.1093/brain/114.3.1157. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton T, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Dereberry MJ, Oba S, Michalewski HJ. Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear. 1998;19:169–179. doi: 10.1097/00003446-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng F-G, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (tyr145→Ser) Brain. 2003;126:1604–1619. doi: 10.1093/brain/awg156. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Nguyen T, Michalewski HJ, Oba S, Abdala C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brainstem potentials activity) in auditory neuropathy. Ear Hear. 2001;22:91–99. doi: 10.1097/00003446-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser) Brain. 2003;126:1604–1619. doi: 10.1093/brain/awg156. [DOI] [PubMed] [Google Scholar]
- Starr A, Isaacson B, Michalewski HJ, Zeng FG, Kong YY, Beale P, Paulson GW, Keats B, Lesperance MM. A dominantly inherited progressive deafness affecting distal auditory nerve and hair cells. J Assoc Res Otolaryngol. 2004;5:411–426. doi: 10.1007/s10162-004-5014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Zeng FG, Michalewski HJ, Moser T. Perspectives on auditory neuropathy: Disorders of inner hair cell, auditory nerve, and their synapse. In: Dallos P, Oertel D, editors. The Senses: A comprehensive reference. Vol 3. Elsevier; Amsterdam: 2008. pp. 397–412. [Google Scholar]
- Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ. Nonsyndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet. 2003;40:45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga R, Avenarius MR, Kelley PM, Keats BJ, Berlin CI, Hood LJ, Morlet TG, Brashears SM, Starr A, Cohn ES, Smith RJ, Kimberling WJ. OTOF mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. J Med Genet. 2006;43:576–581. doi: 10.1136/jmg.2005.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay, Moore BC. Ten(HL)-test results and psychophysical tuning curves for subjects with auditory neuropathy. Int J Audiol. 2007;46:39–46. doi: 10.1080/14992020601077992. [DOI] [PubMed] [Google Scholar]
- Warden FG, Marsh JT. Frequency-following (microphonic-like) neural responses evoked by sound. Electroencephalogr Clin Neurophysiol. 1968;25:42–52. doi: 10.1016/0013-4694(68)90085-0. [DOI] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR, Syapin CL. Hunan auditory brainstem response to temporal gaps in noise. J Speech Lang Hear Res. 2001;44:737–750. doi: 10.1044/1092-4388(2001/058). [DOI] [PubMed] [Google Scholar]
- Whiting KA, Martin BA, Stapells DR. The effects of broadbran noise masking on cortical event-related potentials to speech sounds /ba/ and /da/ Ear Hear. 1998;19:218–231. doi: 10.1097/00003446-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger YS, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93:3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49:367–380. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]