Abstract
Purpose
To search for the genetic cause of juvenile open-angle glaucoma (JOAG) in a Caucasian family and to perform genotype/phenotype correlation studies in the kindred.
Methods
Six members of a three-generation family originating from Uzbekistan and now living in the Middle East were recruited from one large clinic in Israel. Ophthalmologic investigations comprised of visual field assessments, intraocular pressure measurements, optic disc evaluation, and gonioscopy. Medical charts were obtained to date the onset of glaucoma and to evaluate aggressivity of the trait. We screened the myocilin gene (MYOC, OMIM 601652) by direct genomic sequencing of its three exons in all family members.
Results
JOAG segregated as an autosomal dominant trait in four members of the family. The proband, a 14-year-old girl, had been diagnosed with juvenile open-angle glaucoma at 12 years old. Her mother, maternal aunt, and maternal grandfather all had JOAG that started at an early age. The disorder progressed rapidly even under optimal medical treatment, and all four patients had to undergo trabeculectomy. One missense mutation, Y371D (1111t→g, Tyr [Y] 371 Asp [D]), was identified. This mutation cosegregated with the disorder in all affected members and was absent in 200 Caucasian controls. The Y371D MYOC mutation has not been reported before. One cousin of the proband was a silent heterozygotic carrier of the mutation and was still asymptomatic at nine years of age.
Conclusions
We identified a novel mutation (Y371D) in MYOC from a Caucasian family who presented with an aggressive form of JOAG that required early trabeculectomy. Genetic screening of the MYOC mutation was beneficial in predicting one asymptomatic heterozygotic carrier.
Introduction
Open-angle glaucoma (OAG) is the most frequent form of glaucoma, accounting for more than half of all cases [1]. The increased frequency of OAG among relatives of patients with this condition indicates that its susceptibility is influenced by genetic factors. Ocular hypertension (OHT) above 21 mmHg is considered a major risk factor for OAG. According to the age of onset and aggressivity, OAG is divided into juvenile-onset OAG (JOAG) and adult-onset OAG. When associated with OHT, this form of OAG is known as primary open-angle glaucoma (POAG). JOAG has an earlier age of onset, between 10 and 35 years of age, and usually presents with high intraocular pressure (IOP), visual field loss, and optic disc damage. JOAG often requires early surgical treatment [2].
JOAG is typically inherited as an autosomal dominant trait whereas adult-onset POAG is considered a complex genetic trait [3,4]. More than 20 genetic loci have been mapped for POAG [3,4]. Among these, 14 genetic loci, designated GLC1A to GLC1N, have been defined for JOAG and/or POAG in family-based linkage studies of several pedigrees [3-10].
Five loci contribute to JOAG while the others exclusively account for adult-onset POAG [3,4]. Three of these five loci, 1q21-q31 (myocilin gene [MYOC], GLC1A), 9q22, and 20p12, have been confirmed in several studies [3,4]. Two additional studies have identified one novel locus on chromosome 15q22-q24 in one JOAG family and another on chromosome 5q in a different JOAG family [11,12].
Myocilin (MYOC) at GLC1A has been established as a direct cause of glaucoma [13]. In spite of intensive investigation, its function remains unknown. MYOC is primarily mutated in patients with JOAG [13,14]. Approximately 10%–20% of all JOAG cases are caused by mutations in MYOC [15].
MYOC consists of three exons with lengths of 604, 126, and 782 base pairs (bp) and encodes a 504 amino acid polypeptide [16]. Among 73 reported MYOC mutations, 63 (86.3%) are located in exon 3 (Myocilin allele-specific phenotype database), suggesting that the olfactomedin-like domain is important for POAG pathogenesis [17,18]. More than 50% of these mutations cause early onset severe glaucoma (JOAG) whereas a few cause late-onset POAG or even normal tension glaucoma (NTG) [19]. In Caucasians, mutations in MYOC account for as many as 36% of the families with JOAG but only for 2%–4% of sporadic patients with POAG [20,21]. We herein report a novel MYOC missense mutation in a Caucasian family that causes a characteristic JOAG phenotype spanning three generations.
Methods
We investigated six members of a family originating from Uzbekistan and now living in the Middle East. When a patient has been diagnosed before moving to the Middle East, we obtained their ocular examination charts from the previous hospitals or clinics where they were first investigated.
Mutation analysis of MYOC was performed at the Laboratory of Ocular Genetics and Genomics (Québec City, Canada). Genomic DNA was extracted using the Puregene DNA isolation protocol (QIAGEN, Mississauga, Ontario, Canada) from whole blood drawn by venipuncture. Three MYOC amplicons were obtained by polymerase chain reaction (PCR) using the primer pairs described in a previously published study [21]. The myocilin genes were screened for sequence alterations by PCR and direct sequencing as previously reported [22] using an Applied Biosystems Prism 3730xl DNA Analyzer automated sequencer (Applied Biosystems Inc, Foster City, CA). Sequence data were analyzed using the Staden preGap4 and Gap4 programs [23]. Each familial proband was screened for mutations in all three exons of MYOC. Sequencing of the mutation was performed on both DNA strands.
Results
Phenotypic studies
A 14-year-old girl presented with progressive primary open-angle glaucoma in our glaucoma clinic two years ago when she was 12 years old. She was diagnosed at age 10 and treated with maximal topical therapy. Her visual acuity was then 20/20 in both eyes. Intraocular pressure was 24 and 28 mmHg in the right and left eyes, respectively. The anterior segment was normal on slit-lamp examination, and the angle was wide open on gonioscopy. The cup to disc ratio was 0.7 and 0.8 in the right and left eyes, respectively. Visual field testing revealed damage to both eyes. The left eye had upper and lower arcuate scotomas, and the right eye had superior arcuate scotoma and inferior nasal step. Trabeculectomy with mitomycin C and 5-fluorouracil was performed in her left eye at the age of 12 years, and then three months later, the procedure was performed in her right eye. She had refractive hypotony after both procedures and needed a second procedure for revision of the filter in both eyes. Today, at 14 years old, her visual acuity is 20/30 in the right eye and 20/20 in the left eye. Intraocular pressure (IOP) is 7 mmHg in the right eye and 10 mmHg in the left. She has large and raised filtering blebs. The cup to disc ratio has improved to 0.5 in both eyes, and there is also an improvement in the visual fields (this may seem quite unusual but possible in children) with lower nasal arcuate scotoma in the right eye and inferior nasal step scotoma in the left eye.
Her mother who is 32 years old, the mother’s sister who is 36 years old, and the father of these two women also suffer from progressive open-angle glaucoma. None of the patients had any other ocular or systemic abnormalities. The aunt of the proband, subject II-2, was recently admitted to our department as an emergency with end stage glaucoma in her only seeing (right) eye. Her visual acuity was 20/40, and the IOP was 50 mmHg. She was known to have juvenile glaucoma since the age of 16 in both eyes and was treated with topical and systemic medications to lower intraocular pressures. She became blind in her left eye after glaucoma surgery 12 years ago at the age of 24. She recently underwent uncomplicated trabeculectomy in her right eye, and today her visual acuity is 20/40 and intraocular pressure is 7 mmHg with a large diffuse filtering bleb and nearly total cup to disc ratio. Her visual field in her right eye was severely affected with only the central 10° remaining (tubular vision). The proband’s maternal aunt daugther (subject III-2), who is a nine-year-old, is currently healthy (Table 1).
Table 1. Phenotypic status of Israeli family members affected by OAG.
| Subject Number | Family member | Age today | Age at onset | Highest IOP OD/OS | Gonioscopy | VF–24–2 2009 | MD OD/OS | PSD OD/OS | VA-2009 | CD ratio OD/OS 2009 | IOP today OD/OS 2009 | Age of surgery: OD/OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-1 |
grandfather |
55 |
16 |
39/40 |
no data |
No data |
No data |
No data |
No data |
No data |
No data |
No surgery |
| I-2 |
grandmother |
61 |
- |
22/22 |
no data |
No data |
No data |
No data |
No data |
No data |
No data |
- |
| II-1 |
mother |
32 |
18 |
No data |
open |
- |
- |
- |
NLP/NLP |
1/1 |
No data |
No surgery |
| II-2 |
aunt |
36 |
16 |
50/50 |
open |
OD- tubular vision 10 ° |
No parameters (stimulus V) |
No parameters (stimulus V) |
OD-20/40 OS-NLP |
0.9/1 |
10/14 |
36/24 |
| III-1 |
proband |
14 |
10 |
24/28 |
open |
OD-lower arcuate scotoma OS- inferior nasal step |
−7.18/-7.91 |
4/6.52 p<0.5% |
OD-20/30 OS-20/20 |
0.5/0.7 |
11/12 |
12/12 |
| III-2 | cousin | 9 | Still asymptomatic | 16/16 Feb 2007- the date of the last exam | open | No data | No data | No data | No data | normal | No data | No surgery |
OD: right eye, OS: left eye. IOP: intraocular pressures, Age at onset in years, VF: visual fields, MD- mean deviation, PSD: pattern standard deviation, VA: visual acuity, CD: cup to disc ratio, IOP: intraocular pressure.
The cousin was found to be a heterozygotic carrier, and she is currently healthy at nine years of age. She will be followed up closely and treated if necessary. We were unable to examine additional family members as the family had lost contact with the husbands of the sisters and immigrated to Israel alone.
Genotypic studies
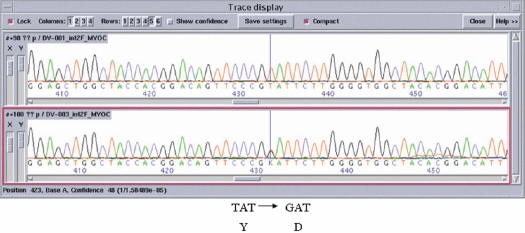
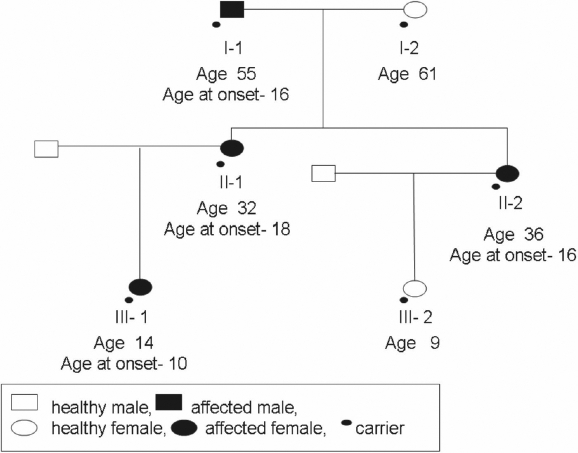
After screening all three exons of MYOC, a single T to G transition in exon 3 at position 1111t→g was detected in the coding sequence of MYOC (see Figure 1, which compares the normal and the mutated DNA sequences by ABI tracing). This transition changes the amino acid at position 371. As this transition was absent in more than 200 control persons (400 chromosomes) coming from all parts of the world and as this variation cosegregated with the disorder within the family, this Y371D change represents a mutation in exon 3. This mutation is novel since it has not been reported before as far as we know (see Myocilin allele-specific phenotype database). Figure 2 shows the segregation of the mutation in the family. This mutation causes autosomal dominant glaucoma.
Figure 1.
Sequence of the region of the mutation. MYOC sequence electropherogram is shown of a wild-type unaffected subject (top panel) and of a heterozygous patient carrying the myocilin mutation, Y371D (lower panel). Nucleotides and predicted amino acid changes are indicated under the electropherogram. The vertical line points to the Y371D mutation.
Figure 2.
Segregation of the Y371D glaucoma-causing MYOC mutation in an Israeli pedigree. The phenotypic status of each subject is as described in the box and corresponds to Table 1. Heterozygotic carriers of the mutation are depicted by a small black dot under their own respective sign.
Discussion
We report a novel mutation (Y371D) in MYOC from a Caucasian family who presented with progressive open-angle glaucoma requiring early trabeculectomy. Specific mutations have been described in different population groups [24-29]. Cys433Arg, which is thought to be the most prevalent mutation in the Brazilian population, is associated with higher IOP and greater vertical cup/disc ratio when compared to patients without this mutation [28]. In Caucasian populations originating from Europe, the most frequently identified MYOC mutation is Gln368STOP, which has been reported in 1.65% of probands with POAG and has been associated with older-onset POAG and a lower level of IOP elevation [19,22,30].
As investigations of the molecular causes of glaucoma are now being undertaken in populations living in the Middle-East, it is envisaged that mutations not yet reported will be discovered in disease-causing genes. In this regard, the Y371D MYOC mutation is novel as far as we know. Interestingly, since this mutation was observed in a Caucasian family originating from Uzbekistan, it should be observed in other regions of the world.
Determining the clinical characteristics associated with particular MYOC mutations are essential to establish good prognosis and to initiate the most appropriate therapy. Phenotype/genotype correlation studies clearly established that patients carrying the MYOC Gly246Arg, Pro370Leu, or Tyr437His mutation displayed a severe clinical presentation appearing at a young age in children or in teenagers whereas those harboring the Gln368Stop mutation show a mild clinical presentation appearing at middle age or old age [24]. On the other hand, a few MYOC mutations exhibit variable expressivity of the phenotype. For instance, Wirtz et al. [31] described a family with an intermediate phenotype between juvenile and adult onset glaucoma with a MYOC Asp380His mutation while Morissette et al [32]. reported that the phenotypes associated with the MYOC Lys423Glu ranged from juvenile-onset to adult-onset open-angle glaucoma and showed either aggressive or mild phenotypes.
We present a family with open-angle glaucoma, which progressed aggressively. The aggressiveness of the glaucoma led us to perform filtering surgery in two members of this family (proband and her aunt) in a relatively short period of follow-up and at a younger age compared with most cases of open-angle glaucomas. The mother of the proband was already blind from glaucoma when first examined in our clinic.
The primary mechanism by which the MYOC Y371D mutation causes glaucoma may involve misfolding and intracellular sequestration of the mutant protein within the trabecular meshwork cell, thereby altering cell-mediated processes that control aqueous humor outflow. Indeed, several studies demonstrated that mutations occurring within the vicinity of amino acid 371 impeded secretion of heterodimers and multimers made of mutant myocilin polypeptides from interacting with their wild-type counterpart [33,34]. In particular, transfection experiments using COS-7 and human trabecular meshwork cells showed that myocilin mutant proteins, G364V, G367R, P370L, and D380A, were not secreted and remained within the intracellular milieu when studied at 37 °C [33,35].
Our phenotype/genotype correlation study on five patients clearly demonstrated here that the Y371D MYOC mutation caused juvenile onset glaucoma with a characteristic phenotype that includes onset in the second decade of life, usually high intraocular pressures, and a rapidly progressive OAG disease. We also described a sixth potential patient, a nine year-old carrier who is still asymptomatic. The gain of this report is in predicting the high probability that this asymptomatic cousin will become affected as the mutation is fully (100%) penetrant. Finally, observation of the reported mutation when screening for myocilin variations should help in managing other patients and their families treated for progressive open-angle glaucoma. This report emphasizes the importance of taking a good family history when investigating new glaucoma patients.
Acknowledgments
V.R. was supported by La Fondation des Maladies de l’Oeil and the Fonds de la Recherche en Santé du Québec (FRSQ) Health Vision Research Network.
References
- 1.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variation in the prevalence of primary open angle glaucoma; the Baltimore Eye Survey. JAMA. 1991;266:369–75. [PubMed] [Google Scholar]
- 2.Society EUGS. Terminology and guidelines for glaucoma. www.eugs.org; 3rd ed. Italy: Dogma; 2008. [Google Scholar]
- 3.Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: A review. Exp Eye Res. 2009;88:837–44. doi: 10.1016/j.exer.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiggs JL. Genetic etiologies of glaucoma. Arch Ophthalmol. 2007;125:30–7. doi: 10.1001/archopht.125.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 6.Stoilova D, Child A, Trifan OC, Crick RP, Coakes RL, Sarfarazi M. Localization of a locus (GLC1B) for adult-onset primary open angle glaucoma to the 2cen-q13 region. Genomics. 1996;36:142–50. doi: 10.1006/geno.1996.0434. [DOI] [PubMed] [Google Scholar]
- 7.Wirtz MK, Samples JR, Kramer PL, Rust K, Topinka JR, Yount J, Koler RD, Acott TS. Mapping a gene for adult-onset primary open-angle glaucoma to chromosome 3q. Am J Hum Genet. 1997;60:296–304. [PMC free article] [PubMed] [Google Scholar]
- 8.Sarfarazi M, Child A, Stoilova D, Brice G, Desai T, Trifan OC, Poinoosawmy D, Crick RP. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. Am J Hum Genet. 1998;62:641–52. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtz MK, Samples JR, Rust K, Lie J, Nordling L, Schilling K, Acott TS, Kramer PL. GLC1F, a new primary open-angle glaucoma locus, maps to 7q35-q36. Arch Ophthalmol. 1999;117:237–41. doi: 10.1001/archopht.117.2.237. [DOI] [PubMed] [Google Scholar]
- 10.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult onset primary open angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–9. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 11.Wang DY, Fan BJ, Chua JK, Tam PO, Leung CK, Lam DS, Pang CP. A genome-wide scan maps a novel juvenile-onset primary open-angle glaucoma locus to 15q. Invest Ophthalmol Vis Sci. 2006;47:5315–21. doi: 10.1167/iovs.06-0179. [DOI] [PubMed] [Google Scholar]
- 12.Pang CP, Fan BJ, Canlas O, Wang DY, Dubois S, Tam PO, Lam DS, Raymond V, Ritch R. A genome-wide scan maps a novel juvenile-onset primary open angle glaucoma locus to chromosome 5q. Mol Vis. 2006;12:85–92. [PubMed] [Google Scholar]
- 13.Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR. Genetic dissection of myocilin glaucoma. Hum Mol Genet. 2004;13 Spec No 1:R91–102. doi: 10.1093/hmg/ddh074. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clarke MS, Schwartz AL, Downs CA, Vollrath D, Richards JE. Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol. 2000;130:165–77. doi: 10.1016/s0002-9394(00)00536-5. [DOI] [PubMed] [Google Scholar]
- 15.Fingert JH, Héon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 16.Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–9. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- 17.Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, Chua JK, Fan DS, Liu Y, Lam DS. TIGR/MYOC gene sequence alterations in individuals with and without primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2002;43:3231–5. [PubMed] [Google Scholar]
- 18.Bruttini M, Longo I, Frezzotti P, Ciappetta R, Randazzo A, Orzalesi N, Fumagalli E, Caporossi A, Frezzotti R, Renieri A. Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Arch Ophthalmol. 2003;121:1034–8. doi: 10.1001/archopht.121.7.1034. [DOI] [PubMed] [Google Scholar]
- 19.Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med. 1998;338:1022–7. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 20.Kanagavalli J, Pandaranayaka E, Krishnadas SR, Krishnaswamy S, Sundaresan P. A review of genetic and structural understanding of the role of myocilin in primary open angle glaucoma. Indian J Ophthalmol. 2004;52:271–80. [PubMed] [Google Scholar]
- 21.Allingham RR, Wiggs JL, De La Paz MA, Vollrath D, Tallett DA, Broomer B, Jones KH, Del Bono EA, Kern J, Patterson K, Haines JL, Pericak-Vance MA. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2288–95. [PubMed] [Google Scholar]
- 22.Faucher M, Anctil JL, Rodrigue MA, Duchesne A, Bergeron D, Blondeau P, Côté G, Dubois S, Bergeron J, Arseneault R, Morissette J, Raymond V, Québec Glaucoma Network Founder TIGR/myocilin mutations for glaucoma in the Québec population. Hum Mol Genet. 2002;11:2077–90. doi: 10.1093/hmg/11.18.2077. [DOI] [PubMed] [Google Scholar]
- 23.Staden R. The Staden sequence analysis package. Mol Biotechnol. 1996;5:233–41. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- 24.Funayama T, Mashima Y, Ohtake Y, Ishikawa K, Fuse N, Yasuda N, Fukuchi T, Murakami A, Hotta Y, Shimada N, Glaucoma Gene Research Group. The Glaucoma Gene Research Group. SNPs and interaction analyses of noelin 2, myocilin, and optineurin genes in Japanese patients with open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:5368–75. doi: 10.1167/iovs.06-0196. [DOI] [PubMed] [Google Scholar]
- 25.Aung T, Yong VH, Chew PT, Seah SK, Gazzard G, Foster PJ, Vithana EN. Molecular analysis of the myocilin gene in Chinese subjects with chronic primary-angle closure glaucoma. Invest Ophthalmol Vis Sci. 2005;46:1303–6. doi: 10.1167/iovs.04-1163. [DOI] [PubMed] [Google Scholar]
- 26.Michels-Rautenstrauss K, Mardin C, Wakili N, Jünemann AM, Villalobos L, Mejia C, Soley GC, Azofeifa J, Ozbey S, Naumann GO, Reis A, Rautenstrauss B. Novel mutations in the MYOC/GLC1A gene in a large group of glaucoma patients. Hum Mutat. 2002;20:479–80. doi: 10.1002/humu.9092. [DOI] [PubMed] [Google Scholar]
- 27.Puska P, Lemmelä S, Kristo P, Sankila EM, Järvelä I. Penetrance and phenotype of the Thr377Met Myocilin mutation in a large Finnish family with juvenile- and adult-onset primary open-angle glaucoma. Ophthalmic Genet. 2005;26:17–23. doi: 10.1080/13816810590918208. [DOI] [PubMed] [Google Scholar]
- 28.Povoa CA, Malta RF, Rezende Mde M, de Melo KF, Giannella-Neto D. Correlation between genotype and phenotype in primary open angle glaucoma of Brazilian families with mutations in exon 3 of the TIGR/MYOC gene. Arq Bras Oftalmol. 2006;69:289–97. doi: 10.1590/s0004-27492006000300002. [DOI] [PubMed] [Google Scholar]
- 29.Fan BJ, Leung DY, Wang DY, Gobeil S, Raymond V, Tam PO, Lam DS, Pang CP. Novel myocilin mutation in a chinese family with juvenile-onset open-angle glaucoma. Arch Ophthalmol. 2006;124:102–6. doi: 10.1001/archopht.124.1.102. [DOI] [PubMed] [Google Scholar]
- 30.Baird PN, Richardson AJ, Mackey DA, Craig JE, Faucher M, Raymond V. A common disease haplotype for the Q368STOP mutation of the myocilin gene in Australian and Canadian glaucoma families. Am J Ophthalmol. 2005;140:760–2. doi: 10.1016/j.ajo.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Wirtz MK, Samples JR, Choi D, Gaudette ND. Clinical features associated with an Asp380His Myocilin mutation in a US family with primary open-angle glaucoma. Am J Ophthalmol. 2007;144:75–80. doi: 10.1016/j.ajo.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morissette J, Clépet C, Moisan S, Dubois S, Winstall E, Vermeeren D, Nguyen TD, Polansky JR, Côté G, Anctil JL, Amyot M, Plante M, Falardeau P, Raymond V. Homozygotes carrying an autosomal dominant TIGR mutation do not manifest glaucoma. Nat Genet. 1998;19:319–21. doi: 10.1038/1203. [DOI] [PubMed] [Google Scholar]
- 33.Gobeil S, Letartre L, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: Integrity of N-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006;82:1017–29. doi: 10.1016/j.exer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Gobeil S, Rodrigue MA, Moisan S, Nguyen TD, Polansky JR, Morissette J, Raymond V. Intracellular sequestration of hetero- oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci. 2004;45:3560–7. doi: 10.1167/iovs.04-0300. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]