Abstract
Objective
Otoacoustic emissions and the speech-evoked auditory brainstem response are objective indices of peripheral auditory physiology and are used clinically for assessing hearing function. While each measure has been extensively explored, their interdependence and the relationships between them remain relatively unexplored.
Methods
Distortion product otoacoustic emissions (DPOAE) and speech-evoked auditory brainstem responses (sABR) were recorded from 28 normal-hearing adults. Through correlational analyses, DPOAE characteristics were compared to measures of sABR timing and frequency encoding. Data were organized into two DPOAE (Strength and Structure) and five brainstem (Onset, Spectrotemporal, Harmonics, Envelope Boundary, Pitch) composite measures.
Results
DPOAE Strength shows significant relationships with sABR Spectrotemporal and Harmonics measures. DPOAE Structure shows significant relationships with sABR Envelope Boundary. Neither DPOAE Strength nor Structure is related to sABR Pitch.
Conclusions
The results of the present study show that certain aspects of the speech-evoked auditory brainstem responses are related to, or covary with, cochlear function as measured by distortion product otoacoustic emissions.
Significance
These results form a foundation for future work in clinical populations. Analyzing cochlear and brainstem function in parallel in different clinical populations will provide a more sensitive clinical battery for identifying the locus of different disorders (e.g., language based learning impairments, hearing impairment).
Keywords: otoacoustic emissions, cochlea, speech-evoked auditory brainstem response, speech encoding
Introduction
Distortion product otoacoustic emissions (DPOAE) and speech-evoked auditory brainstem responses (sABR) are objective measures of peripheral auditory physiology. These tools are used in both clinical and research applications, often in tandem for differential diagnosis. It is important, therefore, to understand issues related to their overlap and independence. The current study is our maiden attempt to explore these relationships in normal hearing young adults. The underlying objective is to examine and document the links between cochlear and brainstem function and ultimately improve the clinical power of these instruments by using them together for specific clinical purposes.
Otoacoustic Emissions
Otoacoustic emissions (OAEs) are signals generated in the cochlea that are detectable in the ear canal (Kemp, 1979; 1978). These acoustic signals are considered a byproduct of physiological processes necessary for normal hearing, specifically outer hair cell function (Brownell, 1982). Otoacoustic emissions can be generated spontaneously (SOAEs) and can also be evoked by clicks (transient-evoked otoacoustic emissions, TEOAEs), single tones (stimulus-frequency otoacoustic emissions, SFOAEs), or tone pairs (distortion product otoacoustic emissions, DPOAEs).
Emissions evoked by tone pairs, or DPOAEs, are equally popular in the clinic and the laboratory. They are measured by stimulating the cochlea simultaneously with two pure tones (f1 and f2, f1 < f2). Distortion products at various frequencies arithmetically-related to the stimulus frequencies are generated in the cochlea. The DPOAE at 2f1-f2 is the most robust in human ears under certain stimulus conditions and is used routinely in clinical practice. DPOAEs such as the one at 2f1-f2 are lower in frequency than the stimulus tones making their characteristic frequency (CF) place apical to f1 and f2 on the basilar membrane (BM). There is now irrefutable evidence that for apical DPOAEs, the signal measured in the ear canal is a mixture of two components, one from the overlap region between the traveling wave patterns of the stimulus tones and the other from the CF region of the DPOAE itself (Mauermann et. al., 1999a; Talmadge et al., 1999). In many current theories of OAE generation, these two DPOAE components are modeled to arise from fundamentally different mechanisms resulting in significantly different phase-frequency functions of each component (Shera and Guinan, 1999). The phase of the overlap component (also called wave-fixed or distortion component in the literature) is relatively invariant with frequency. On the other hand, the phase of the DP CF component (also called the place-fixed or the reflection component in the literature) varies rapidly with frequency.
As these two components with different phase gradients are mixed in the ear canal, the interaction between them causes a pattern of alternating maxima and minima in the level-frequency function known as fine structure (Dhar et al., 2002). The presence of fine structure in a given ear reflects the presence of the two components and their relative magnitudes determine the depth of fine structure. Two equal components would lead to the deepest fine structure while complete domination by either component would result in little or no fine structure. There is initial evidence that fine structure characteristics could be a more sensitive indicator of alterations in cochlear status than the currently-used metric of overall level of DPOAEs (Wagner et al., 2008). Thus, the origin of fine structure in basic mechanical properties of the cochlea makes it interesting to examine its relationship with other physiological phenomena in the auditory system. Here we report such an exploration of the relationship between distortion product fine structure and speech-evoked brainstem responses.
Speech-evoked Auditory Brainstem Response
The auditory brainstem, a conglomerate of nuclei belonging to the efferent and afferent auditory systems, receives and processes the output of the cochlea en route to the higher centers of auditory processing. The function of the brainstem nuclei can be assessed using stimulus-evoked electrophysiology. Evoked brainstem responses, often using click stimuli, can be diagnostic of clinical populations because of their temporal precision. When evoked by a periodic stimulus, such as speech or music, a frequency-following response (FFR) results. The FFR is driven by neural phase locking and reflects the fundamental periodicity of the stimulus and its harmonics. It is likely generated in the inferior colliculus and lateral lemniscus (Hoormann et al., 1992; Marsh et al., 1970; Moushegian et al., 1973; Smith et al., 1975; Worden and Marsh, 1968). There is also a growing body of literature showing that the human brainstem response is malleable with lifelong linguistic (Krishnan et al., 2005; Swaminathan et al., 2008) and musical (Wong et al., 2007; Musacchia et al., 2007; Kraus et al., in press; Strait et al., 2009) experience, as well as short-term auditory training (Song et al. 2008; Russo et al. 2005).
The speech-evoked brainstem response to a consonant-vowel syllable, such as the voiced syllable [da] used in this study, contains both an onset, similar to the click-evoked response, due to the initial noise burst marking the onset of the consonant, and an FFR corresponding to the periodic, voiced formant transition. In the response, the acoustic properties of the stimulus are represented by discrete response peaks representing both transient events in the stimulus, such as voicing onset, and a sustained frequency-following response (FFR) to the fundamental periodicity (i.e., glottal pulsing) of the vowel.
Latency delays in transient sABR peaks have been found in children with reading impairments relative to normal learning children (Banai et al., in press; Banai et al., 2005; Cunningham et al., 2001; Johnson et al., 2005; King et al., 2002; Wible et al., 2004) and they are particularly affected by the stimulus presentation rate and background noise (Basu et al., in press; Wible et al., 2004). The FFR peaks track the fundamental frequency (F0) of the stimulus; yet, the raw peak latencies are also likely modulated by the high frequency content of the stimulus (Johnson et al., 2008; Hornickel et al., 2009b) which is important for determining phonemic identity. The latencies of FFR peaks have been shown to differ depending on ear of stimulation (Hornickel et al., 2009a) and their timing is related to reading ability (Banai et al., in press; Banai et al., 2005).
Frequency-domain analyses of the sABR reveal energy at the fundamental frequency and harmonics of the voiced syllable. Measures of harmonics have been found to differ for right and left ear presentation (Hornickel et al., 2009a), between reading impaired and normal learning children (Cunningham et al., 2001; Johnson et al., 2005; Wible et al., 2004), and to be significantly correlated with measures of reading ability (Banai et al., in press). These effects are likely due to the importance of harmonics in determining and distinguishing speech sounds. In contrast, measures of F0 representation have not been found to be significantly related to reading (Banai et al., in press), nor does its encoding differ between reading impaired and normal learning children (Johnson et al., 2005; Kraus and Nicol, 2005; Wible et al., 2004) or ear of stimulation (Hornickel et al. 2009a).
While there is a vast literature on DPOAEs and brainstem responses, little is known about the relationship between these measures despite their widespread use in the assessment of hearing. Only a few studies have examined both measures in the same subjects (Cone-Wesson et al., 2000; Elsisy and Krishnan, 2008; Oswald et al., 2006; Purcell et al., 2006) and even fewer have related the function of both despite their common relationship to clinical populations and efferent control (de Boer and Thornton, 2008; Hall, 1992; Russo et al., 2005; Song et al., 2008). The current study proposes to identify relationships between DPOAEs and speech-evoked brainstem responses recorded in normal hearing young adults. Deeper understanding of the aspects that do and do not overlap between the two responses will allow for a more detailed knowledge of hearing function and better inform clinical practice.
Methods
Participants
Participants were 28 adults (ages 19–30, mean = 25; 17 women) who were right handed. All participants had normal (less than 20 dB HL) audiometric thresholds for octaves from 250–8000 Hz, no conductive loss as evidenced by a lack of an air-bone threshold gap, and normal click-evoked brainstem response, as measured by wave V latency. All OAE and brainstem results reported are from the right ear.
Procedure
In order to encourage participants to remain as still as possible, they were allowed to watch a movie of their choice during data collection. In the case of the sABR, the movie soundtrack was played at ~40 dB SPL in soundfield, while only subtitles were presented for the DPOAE recording. The two test sessions occurred within 4 months of each other and often on the same day. All participants were monetarily compensated for their time. The Internal Review Board of Northwestern University approved all procedures.
DPOAE recording and processing
Level and phase estimates of DPOAEs were obtained at closely spaced frequencies (2-Hz apart) using stimulus tones (f1 and f2, f2 > f1, f2/f1=1.22) swept in frequency over a (2f1-f2) range of 500 to 10200 Hz. The stimulus tones presented at 65 and 55 dB SPL, respectively, were swept in frequency at the rate of 8 seconds per octave below 6 kHz and 24 seconds per octave above 6 kHz, while keeping the frequency ratio between them constant. Signal generation and recording were controlled by an Apple Macintosh computer and custom software via a MOTU 828MkII Firewire Audio Interface. Signals generated by the MOTU were passed through a Behringer headphone amplifier and delivered to the ear canal from MBQuartz speakers coupled through an Etymotic ER10B probe. The ER10B microphone was used to pick up the signal in the ear canal which was amplified by the ER10B pre-amplifier and stored on disk for analysis. Digitization and recording used a sampling rate of 44100 Hz with a 24 bit converter. The level and phase of the resultant DPOAE at 2f1-f2 were estimated using a least-squares-fit algorithm. The raw data were screened to have a minimal signal-to-noise ratio of 6 dB before analysis. Data points where the noise floor was above 0 dB SPL were also rejected. The preliminary output of this analysis was fitted with a smoothing function before maxima and minima were identified using change in slope direction. A threshold of −10 dB SPL was set following careful inspection of the data and DPOAE levels were normalized to this threshold, yielding positive values for average DPOAE level. Two composite measures were then created, Strength and Structure (Figure 1). These new measures were created specifically to give us a condensed metric to use in comparison with measures of brainstem function and to the best of our knowledge have not been used previously in the literature.
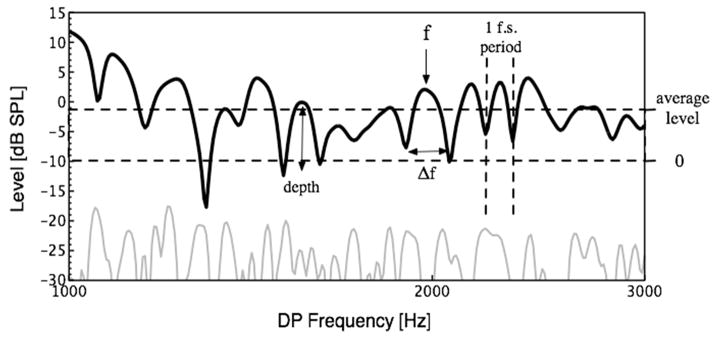
Figure 1.
A typical DPOAE response from a normal-hearing adult subject, with all fine structure variables defined. The black line indicates the ear-canal response, while the gray line shows the level of the noise floor. Depth is defined by the difference in level between a maximum and the geometric mean of its surrounding minima. Fine structure spacing is characterized by the ratio between f, the center frequency, and Δf, the distance in Hz between adjacent minima. Average DPOAE level is normalized to a −10dB SPL threshold, yielding positive values for all data points.
Strength
The measure Strength used here is the product of the overall level (in dB) normalized to a threshold of −10 dB SPL over the 1–3 kHz frequency range1 of the DPOAE and the average fine structure depth (in dB) over the same range. Thus ears with greatest overall DPOAE level and distinct fine structure had largest values of Strength. Lack of fine structure could be offset by large overall DPOAE level in a given ear. Similarly, small overall DPOAE level could be offset by exceptionally deep fine structure. Because of the normalization procedure described above, all participants had positive Strength scores.
Structure
The second measure of DPOAE used here is termed Structure. It is calculated by dividing the number of fine structure periods identified in the 1–3 kHz range by the average spacing over that range. Spacing was computed by dividing the absolute spacing in Hz between adjacent fine structure periods by the center-frequency. Because the phase of the DPOAE component from the overlap region of the stimulus tones does not vary substantially with frequency (Dhar and Shaffer, 2004; Mauermann et al., 1999a; b), the spacing of fine structure periods in a given ear is dependent on the slope of the phase of the DP CF component. Specifically, ears with the steepest slope of the phase of the DP CF component have the most closely spaced fine structure periods, and therefore the highest Structure values.
Brainstem response recording and processing
The voiced [da] stimulus is a 40 ms synthesized speech syllable, containing a release burst and voiced formant transition (Figure 2, top left). It was produced in KLATT (Klatt, 1980) with a fundamental frequency (F0) that linearly rises from 103 to 125 Hz with voicing beginning at 5 ms and an onset release burst during the first 10 ms. While the utterance is short, and there is no steady-state vowel, the [da] is perceived as a consonant-vowel syllable.
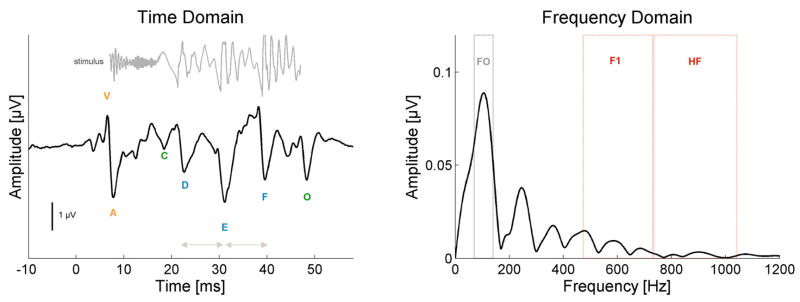
Figure 2.
Schematic of the brainstem response to the speech syllable [da] in the time (left) and frequency (right) domains. The time domain waveform of the stimulus is plotted in gray above sABR waveform. The stimulus is time-shifted 8 ms in order to facilitate its visual comparison with the response by accounting for the neural conduction lag. Onset measures (V, A latencies) are labeled in orange. Spectrotemporal (D, E, F latencies) elements are labeled in blue. Envelope Boundary measures (C, O latencies) are labeled in green. Pitch measures (F0 amplitude, E–D, F–E inter-peak latencies) are labeled in grey and Harmonic measures (F1, HF amplitudes) are labeled in red.
Stimuli were presented through an insert earphone (ER-3; Etymotic Research, Elk Grove Village, IL) at 80.3 dB SPL using alternating polarities at 10.9 Hz. Responses were recorded with a vertical montage of three Ag-AgCl electrodes (central vertex (Cz), forehead ground, and ipsilateral earlobe reference). Responses were recorded with the Bio-logic Navigator Pro system, BioMARK module (Bio-logic Systems Corp., a Natus Company, Mundelein, IL). Responses were online bandpass filtered from 100–2000 Hz (12 dB/octave) and digitally sampled at 6855 Hz. 6000 artifact-free trials were collected in two blocks and averaged using a 74.67 ms time window (−15.8 ms pre-stimulus). Trials with artifact exceeding +/− 23 μV were excluded from the average.
Data analyses followed published reports using a similar stimulus and recording parameters. The characteristic seven peaks of the response (V, A, C, D, E, F, O, Figure 2, left, bottom) were manually identified by the experimenter, and confirmed by a second rater. See Russo et al., 2004 and Johnson et al., 2005 for an in depth review of these peaks. Peaks V, A, D, E and O were 100% detectable in all subjects. Peak C was not detectable in one subject, and peak F was likewise absent in another subject. Spectral analyses using fast Fourier transforms over the time range 21.9–40.6 ms (Figure 2, right), encompassing D, E and F, were computed using routines coded in Matlab 7 (The MathWorks, Inc., Natick, MA). Five composite measures of the sABR to [da] are used in this paper. In order to create them, Z scores of each constituent measure were calculated ([sample−mean]/standard deviation) and the composite was an average of the constituent z scores, i.e., (Zmeasure1 + Zmeasure2 + …ZmeasureN)/N.
Onset
A composite was created from the latencies of the two onset peaks, V and A, which mark the onset of sound, and are comparable to the click-evoked peak V and Vn.
Spectrotemporal
This composite was created from the latencies of peaks D, E, and F, which arise in response to the fundamental periodicity of the stimulus (glottal pulsing in the case of speech), but are also affected by the higher harmonic information in the speech signal.
Envelope Boundary
The latencies for peaks C and O were combined to form the Envelope Boundary composite. Peak C marks the start of voicing while peak O is thought to signal the end of voicing, and the two bookend the periodic portion of the response, thus forming the boundary of the response to the stimulus envelope. While the term envelope may be used to describe the response over a range of temporal scales, in the present study it refers to that associated with the voiced portion of the stimulus.
Pitch
Average spectral amplitude was calculated for a range encompassing the fundamental frequency (F0), 103–120 Hz. Pitch is a composite of the amplitude at F0 and the interpeak intervals between peaks D and E, and E and F. These interpeak intervals mimic the fundamental periodicity of the stimulus and are suggestive of F0 encoding. While other aspects of speech are certainly important for the perception of pitch, we focus here on the fundamental frequency which has major contributions to the percept (Cruttenden, 1997).
Harmonics
The Harmonics measure is a composite of the average spectral energy from two frequency bands: first formant (F1) 455–720 Hz, and high frequency (HF) 721–1154 Hz. F1 includes the harmonics of the stimulus that make up the most prominent frequencies of the first formant range. The HF range is composed of harmonics between the first and second formants (F1 and F2, respectively). Because F2 and higher formants are above the phaselocking limits of the brainstem, no higher frequency ranges were included. See Table 1 for the means of all individual measures.
Table 1.
Composite variables and the means of their component measures.
| Composite | Measure | Mean (standard deviation) |
|---|---|---|
| Harmonics | F1 | 0.732 (0.34)μV |
| HF | 0.343 (0.14)μV | |
| Spectrotemporal | D | 22.915 (0.80)ms |
| E | 31.068 (0.62)ms | |
| F | 39.520 (0.42)ms | |
| Onset | V | 6.718 (0.26)ms |
| A | 7.644 (0.37)ms | |
| Envelope | C | 18.525 (0.64)ms |
| O | 48.392 (0.52)ms | |
| Pitch | D-E | 8.153 (0.88)ms |
| E-F | 8.467 (0.57)ms | |
| F0 | 5.605 (1.67)μV | |
| Strength | normalized amplitude | 12.294 (5.03)dB |
| depth | 5.520 (0.98)dB | |
| Structure | fine structure periods | 12.607 (7.20) |
| frequency spacing | 10.570 (3.05)Hz | |
Statistical Analyses
In order to determine relationships between the OAE measures of Strength and Structure and the five sABR composites, while also taking into account relatedness among the brainstem measures, two multivariate regressions were run in SPSS (SPSS Inc., Chicago, IL). While the ratio of cases (28) to independent variables (5 composite sABR measures) was small, the measures were normally distributed, did not show evidence of collinearity, and inspection of the residual plots indicated the data met the assumptions of normality, linearity, and homoscedasticity (Tabachnick and Fidell, 2007). Brainstem composites were added to the model using the Enter method and then removed from the model using a backward Stepwise method if they did not significantly contribute to prediction of the variance in the dependent measure (p < 0.1). Additionally, Pearson’s correlations among the composites and the constituent measures were conducted in SPSS for additional support of multiple regression results. If we were to correct for multiple comparisons by adopting an alpha level of 0.01, the majority of conducted correlations would not be significant, however, the pattern of significant results with an alpha of 0.05 indicates that the brainstem and OAE measures are indeed moderately to strongly related in absolute terms.
Results
Relationships between DPOAE Strength and sABR measures
The strongest model predicting variance in DPOAE Strength comprised Spectrotemporal and Harmonics composite measures only (R = 0.611, adjusted R2 = 0.323, F(2, 27) = 7.432, p < 0.01). No other brainstem measures significantly contributed unique variance to the predictive model (see Figure 3, which schematically shows overlap among all the measures and indicates R2 values for each pairing). The Spectrotemporal and Harmonics composite sABR measures were weighed similarly in the model with standardized β coefficients of −0.341 and 0.381, respectively. The negative standardized coefficient for Spectrotemporal indicates that individuals with greater DPOAE Strength also demonstrated earlier sABR peak latencies. Harmonics’ positive standardized coefficient suggests that greater DPOAE Strength was related to increased spectral amplitude at the F1 and HF frequency ranges in the sABR. See Figure 4 for the plot of Strength against Harmonics, its best predictor.
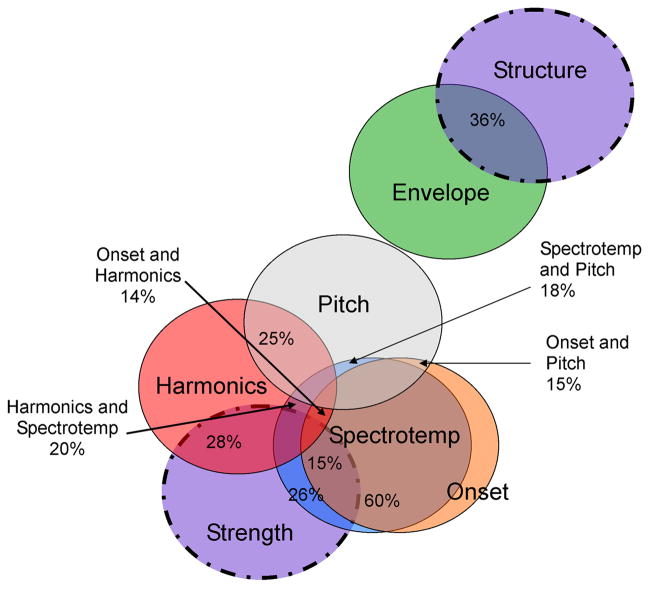
Figure 3.
Venn diagram of the relationships between composite sABR and DPOAE measures. sABR circles are solid; DPOAE circles are dashed. The percentage overlap between the variables (R2; bivariate relationships) is marked. While all measures are in some minor way related, and all circles in the figure should be touching, any overlap not reported was less than 0.5%. Spectrotemporal was shortened to Spectrotemp for space constraint reasons. Note that only a weak relationship was observed between the two DPOAE composite measures, Strength and Structure (R2 = 0.0025).
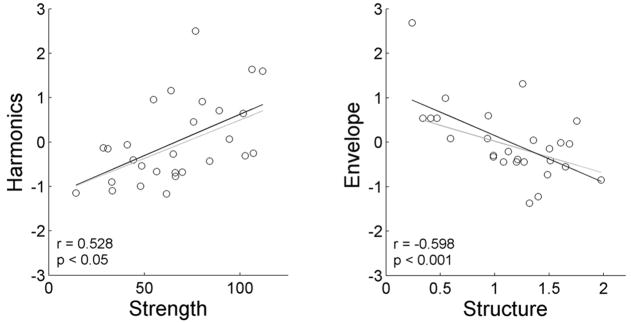
Figure 4.
Relationships between DPOAE measures and their predictors. (A) DPOAE Strength is positively related to its strongest predictor, Harmonics, while (B) Structure is negatively related to its strongest predictor, Envelope Boundary. All brainstem composite measures are plotted as Z-scores. Black lines (and reported statistics) are regressions using all data points, grey lines are the regressions omitting individuals with Z > 2.
Relationships between DPOAE Structure and sABR measures
In the predictive model of DPOAE Structure, only Envelope Boundary was a significant predictor (R = 0.587, adjusted R2 = 0.319, F(1,27) = 13.662, p = 0.001, Figure 3). The Envelope Boundary composite standardized coefficient was −0.587, suggesting increases in Structure were related to decreases in the latencies of the Envelope Boundary. See Figure 4 for the plot of Structure against Envelope Boundary.
sABR Pitch and Onset are not unique predictors of either DPOAE measure
The brainstem Onset and Pitch composites did not significantly contribute to either model. The inclusion of Onset in the Strength model was predicted based on previously established relationships between brainstem response latency and factors affecting stimulus integrity, such as hearing impairment and stimulus level (Hall, 1992). Onset was found to be related to Strength (r = −0.391, p < 0.05), but did not contribute significantly to the predictive model. The overlap in variance between Onset and Spectrotemporal composites was found to be quite large and this great overlap likely forced the exclusion of sABR Onset from the DPOAE Strength model because the measure did not predict any unique variance beyond that predicted by Spectrotemporal (see Figure 3). Similarly, Pitch was somewhat related to Harmonics, Onset, and Spectrotemporal (see Figure 3), but was not significantly correlated with Strength or Structure (r = 0.199, p = n.s.; r = −0.234, p = n.s., respectively). These results indicate that encoding of the fundamental frequency is not captured by the DPOAE measures utilized in this study.
Discussion
The results of the current study show clear and significant relationships between speech-evoked auditory brainstem responses and cochlear function assessed via distortion product otoacoustic emissions. In exploring these relationships we have organized the data to represent specific aspects of brainstem and cochlear function. Two DPOAE (Strength and Structure) and five sABR (Onset, Spectrotemporal, Harmonics, Envelope Boundary, Pitch) composite measures were created. Relationships with the DPOAE measures were found for brainstem measures Spectrotemporal, Harmonics, and Envelope Boundary, but not uniquely for Onset nor Pitch.
Relationships between DPOAE Strength and sABR measures
DPOAE Strength reflects the mechanical activity of the basilar membrane, with a higher Strength value arguably signifying a stronger cochlear amplifier. Strength was found to be related to the latency of transient and spectrotemporal elements, and the amplitude of the harmonics composite of F1 and HF as well as the individual measures. Relationships between spectral amplitudes and DPOAE Strength are greatest for the HF range, followed by the F1 range, and lastly the F0 range, which showed a weak relationship (see Figure 3).
The range of harmonics through HF represent spectral content roughly up to, but not including, the second formant of the [da], and are encoded through phase locking. A healthier cochlea, arguably represented by greater DPOAE Strength, leads to greater cochlear activity at the formant frequencies possibly resulting in reduced latency of peaks D, E, and F. At lower frequencies, physiological noise increases at the cochlear apex and neural synchrony decreases. This could lead to the decreasing correlation between Harmonics and Strength observed here. This trend also suggests that as the stimulus frequency increases and approaches the phase-locking limits of the brainstem, the contribution of cochlear mechanics increases in importance, implying that the relationship between DPOAE Strength and spectral amplitude would only continue to strengthen for frequencies higher than those analyzed here.
The progression of increasing overlap between Strength and F0, F1, and HF may also be due to the increasing similarity in frequency range between the measures. Perhaps if OAE responses at the F1 range and the F0 ranges (i.e., less than 1 kHz) were more reliable (i.e., significantly above the noise floor), the same type of relationship as Strength and HF would be exhibited for DPOAE and brainstem measurements from comparable frequency ranges.
Peak latencies of the sABR Spectrotemporal composite were also found to be correlated with DPOAE Strength. While the peaks that comprised the former occur roughly at the fundamental periodicity of the stimulus, the absolute latencies of the peaks are modulated by spectrotemporal movement of formant transitions between a consonant and vowel (Johnson et al, 2008). If the latency of the peaks is modulated by the robustness of harmonic encoding, as we believe it is, then more defined mechanical activity on the BM, as suggested by increased Strength, would lead to more robust harmonic encoding, and result in greater effects on peak latency.
Relationships between sABR Onset (as well as click-evoked peak V) and DPOAE Strength are predicted given the previously established clinical relationship of response latency increasing with hearing loss and decreasing with increasing stimulus level (Hall, 1992). Healthier cochleae with more active amplification processes will arguably have greater mechanical activity resulting in greater DPOAE Strength. Our results indicate that these ears also tend to demonstrate reduced latencies of the onset-related brainstem waves. The inverse relationship between stimulus level and latency as well as the direct relationship between hearing loss and latency are usually explained on the basis of latency being driven by the most basal activity on the basilar membrane. However, the relationship between DPOAE Strength and sABR Onset latency may not be as straightforward. A more active cochlear amplifier leads to greater mechanical activity in a more limited area along the basilar membrane as the mechanical response is more sharply tuned (Robles and Ruggero, 2001). Thus earlier onset latencies are either a result of more synchronized neural activity from this limited area of the basilar membrane or both DPOAE Strength and sABR latency are driven by a different, but common mechanism.
Relationships between DPOAE Structure and sABR measures
Structure reflects both the presence and spacing of DPOAE fine structure. The presence of a DPOAE component from the 2f1-f2 region of the cochlea results in fine structure. The steeper the phase slope of this DPOAE component the closer the peaks of DPOAE fine structure and the greater the Structure value in a given ear. Structure was found to be correlated with brainstem Envelope Boundary measures only.
The Envelope Boundary composite was composed of peak C and O latencies. DPOAE Structure was correlated with the sABR Envelope Boundary and the two constituent peak latencies (C and O) individually. Peak C is thought to signal the onset of voicing in the speech stimulus, while peak O corresponds to the cessation of voicing (Kraus and Nicol, 2005). Together these response peaks demarcate the most prominent envelope cues in the stimulus. Envelope cues are important for speech perception (Shannon et al., 1995), and are especially crucial for speech recognition in cochlear implant users. Fine structure spacing in DPOAEs is inversely related to the steepness of the slope of the phase of the DP CF component. A steeper phase/frequency function of stimulus frequency OAEs has been shown to be related to psychophysical tuning measured using forward masking (Shera et al., 2002). However, this finding has proven to be highly controversial and is being actively debated in the literature (Siegel et al., 2005). The relationship between Structure and Envelope Boundary may be even more complicated to interpret as the neural generators of the low-frequency response characteristics classified as Envelope Boundary may be higher in the auditory midbrain or the cortex.
The mutual exclusivity of relationships with Strength and Structure suggests that they assess different constructs. Strength reflects the gain of the cochlear amplifier while Structure represents the relative equality of multiple DPOAE components. It appears that the gain of the cochlea is important for brainstem encoding of higher frequency information, crucial for distinguishing phonemes, while the phase of the DPOAE component from the DP CF region is related to the encoding of stimulus envelope by the brainstem.
sABR Pitch is not related to either DPOAE measure
Unlike the other brainstem measures, sABR Pitch was not found to contribute to the predictive models for the DPOAE measures or correlate with them individually. This is in line with previous work which found Pitch to be distinct from brainstem timing and harmonics (Russo et al., 2004; Kraus and Nicol, 2005; Hornickel et al. 2009a), and that only the latter are impaired in reading-impaired children (Banai et al., 2005; Banai et al., in press; Cunningham et al., 2001; Johnson et al., 2005; King et al., 2002; Wible et al., 2004).
While this dissociation may explain the results of the current study, the insignificant relationships with Pitch could also result from difficulties in recording OAEs in the frequency range of the F0. Physiological noise is high at typical speech fundamental frequency ranges and OAE responses tend to be below, or close to, the noise floor. Future work using OAE recording techniques that can overcome the physiological noise in this frequency range may be instructive.
Role of the Efferent System
Both OAE and brainstem measures are influenced by the efferent system. In our measurements of DPOAEs, there was no induced activation of the efferent system. However, there is evidence that the stimuli used to evoke OAEs themselves activate the efferent olivocochlear system and cause a change in OAE magnitude and phase (Guinan et al., 2003). These effects are operational in the tens-of-milliseconds time range and are arguably mediated at the level of the brainstem via the superior olivary complex (Guinan, 2006). The general effect is a reduction in DPOAE magnitude in the first few milliseconds after the stimuli are activated (Guinan, 2006). In contrast, the effect of the efferent system on brainstem measures is arguably more long term and mediated by higher centers in the cortico-thalamic pathway, via corticofugal mechanisms (Suga and Ma, 2003). Given the two contrasting time lines of efferent activity, we argue that the relationships between brainstem and OAE measures are not merely manifestations of the same efferent phenomenon affecting the peripheral auditory system.
Conclusion
The results of the present study show that certain aspects of the speech-evoked auditory brainstem responses to speech (Harmonics, Spectrotemporal, Envelope Boundary) are related to, or covary with, cochlear function as measured by Strength and Structure of DPOAEs. As such, these results form a foundation for future work in clinical populations, patients with hearing loss, children with language-based learning impairments, and patients with speech in noise perception deficits. Brainstem responses in children with language-based learning impairments have delays in timing and reductions in harmonic encoding but normal pitch encoding (Banai et al., in press; Banai et al., 2005; Basu et al., in press; Cunningham et al., 2001; Johnson et al., 2005; King et al., 2002; Wible et al., 2004) and it is possible that the relationships found in the current study could vary as a function of language (e.g., reading) ability and listening in noise. Similarly, alterations in these relationships may be observed in the aging system, as well as other clinical conditions. Analyzing cochlear and brainstem function in parallel in different clinical populations will provide a more sensitive clinical battery for identifying the locus of different disorders. It is also possible that these relationships can be enhanced with proper auditory training (de Boer and Thornton, 2008).
Acknowledgments
Work reported here was supported by grants DC008420 and DC01510 from the NIH/NIDCD. We wish to thank the members of the Auditory Research Lab and the Auditory Neuroscience Lab, specifically Judy H. Song for her help with data collection and other aspects of this work. We also thank Professor Steven Zecker for his advice on appropriate statistical treatment of these data.
Footnotes
In preliminary analyses DPOAE data between 1 and 3 kHz, which encompasses the sABR [da]’s second and third formants, demonstrated the strongest relationship with the brainstem measures. All results reported in this manuscript use DPOAE data between the frequencies of 1 and 3 kHz.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banai K, Skoe E, Nicol T, Zecker S, Kraus N. Reading and Subcortical Auditory Function. Cereb Cortex. doi: 10.1093/cercor/bhp024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Nicol T, Zecker SG, Kraus N. Brainstem timing: implications for cortical processing and literacy. J Neurosci. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Kraus N. The dynamic brainstem: implications for APD. In: McFarland D, Cacace A, editors. Current Controversies in Central Auditory Processing Disorder. San Diego: Plural Publishing Inc; 2008. pp. 269–289. [Google Scholar]
- Basu MKA, Weber-Fox C. Brainstem correlated of temporal auditory processing in children with specific language impairment. Dev Sci. doi: 10.1111/j.1467-7687.2009.00849.x. in press. [DOI] [PubMed] [Google Scholar]
- Brownell WE. Cochlear transduction: an integrative model and review. Hear Res. 1982;6:335–360. doi: 10.1016/0378-5955(82)90064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone-Wesson B, Vohr BR, Sininger YS, Widen JE, Folsom RC, Gorga MP, Norton SJ. Identification of neonatal hearing impairment: infants with hearing loss. Ear Hear. 2000;21:488–507. doi: 10.1097/00003446-200010000-00012. [DOI] [PubMed] [Google Scholar]
- Cruttenden A. Intonation. Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol. 2001;112:758–767. doi: 10.1016/s1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci. 2008;28:4929–4937. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S, Shaffer LA. Effects of a suppressor tone on distortion product otoacoustic emissions fine structure: why a universal suppressor level is not a practical solution to obtaining single-generator DP-grams. Ear Hear. 2004;25:573–585. doi: 10.1097/00003446-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Dhar S, Talmadge CL, Long GR, Tubis A. Multiple internal reflections in the cochlea and their effect on DPOAE fine structure. J Acoust Soc Am. 2002;112:2882–2897. doi: 10.1121/1.1516757. [DOI] [PubMed] [Google Scholar]
- Elsisy H, Krishnan A. Comparison of the acoustic and neural distortion product at 2f1-f2 in normal-hearing adults. Int J Audiol. 2008;47:431–438. doi: 10.1080/14992020801987396. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW. Handbook of auditory evoked responses. xi. Boston: Allyn and Bacon; 1992. p. 871. [Google Scholar]
- Hoormann J, Falkenstein M, Hohnsbein J, Blanke L. The human frequency-following response (FFR): normal variability and relation to the click-evoked brainstem response. Hear Res. 1992;59:179–188. doi: 10.1016/0378-5955(92)90114-3. [DOI] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Kraus N. Subcortical Laterality of Speech Processing. Audiol Neurootol. 2009a;14:198–207. doi: 10.1159/000188533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Subcortical Differentiation of Voiced Stop Consonants: Relationships to Reading and Speech in Noise Perception. Association for Research in Otolaryngology Midwinter Meeting; Baltimore, MD. 2009b. [Google Scholar]
- Johnson KL, Nicol TG, Kraus N. Brain stem response to speech: a biological marker of auditory processing. Ear Hear. 2005;26:424–434. doi: 10.1097/01.aud.0000179687.71662.6e. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Nicol TG, Zecker SG, Bradlow A, Skoe E, Kraus N. Brainstem encoding of voiced consonant-vowel stop syllables. Clin Neurophysiol. 2008;119:2623–2635. doi: 10.1016/j.clinph.2008.07.277. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Arch Otorhinolaryngol. 1979;224:37–45. doi: 10.1007/BF00455222. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- King C, Warrier CM, Hayes E, Kraus N. Deficits in auditory brainstem pathway encoding of speech sounds in children with learning problems. Neurosci Lett. 2002;319:111–115. doi: 10.1016/s0304-3940(01)02556-3. [DOI] [PubMed] [Google Scholar]
- Klatt DH. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–995. [Google Scholar]
- Kraus N, Nicol T. Brainstem origins for cortical ‘what’ and ‘where’ pathways in the auditory system. Trends Neurosci. 2005;28:176–181. doi: 10.1016/j.tins.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kraus N, Skoe E, Parbery-Clark A, Ashley R. Experience-induced malleability in neural encoding of pitch, timbre and timing: implications for language and music. Ann N Y Acad Sci: Neurosciences and Music III. 2009;1169:543–557. doi: 10.1111/j.1749-6632.2009.04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Xu Y, Gandour J, Cariani P. Encoding of pitch in the human brainstem is sensitive to language experience. Brain Res Cogn Brain Res. 2005;25:161–168. doi: 10.1016/j.cogbrainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Marsh JT, Worden FG, Smith JC. Auditory frequency-following response: neural or artifact? Science. 1970;169:1222–1223. doi: 10.1126/science.169.3951.1222. [DOI] [PubMed] [Google Scholar]
- Mauermann M, Uppenkamp S, van Hengel PW, Kollmeier B. Evidence for the distortion product frequency place as a source of distortion product otoacoustic emission (DPOAE) fine structure in humans. I. Fine structure and higher-order DPOAE as a function of the frequency ratio f2/f1. J Acoust Soc Am. 1999a;106:3473–3483. doi: 10.1121/1.428200. [DOI] [PubMed] [Google Scholar]
- Mauermann M, Uppenkamp S, van Hengel PW, Kollmeier B. Evidence for the distortion product frequency place as a source of distortion product otoacoustic emission (DPOAE) fine structure in humans. II. Fine structure for different shapes of cochlear hearing loss. J Acoust Soc Am. 1999b;106:3484–3491. doi: 10.1121/1.428201. [DOI] [PubMed] [Google Scholar]
- Moushegian G, Rupert AL, Stillman RD. Laboratory note. Scalp-recorded early responses in man to frequencies in the speech range. Electroencephalogr Clin Neurophysiol. 1973;35:665–667. doi: 10.1016/0013-4694(73)90223-x. [DOI] [PubMed] [Google Scholar]
- Musacchia G, Sams M, Skoe E, Kraus N. Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proc Natl Acad Sci U S A. 2007;104:15894–15898. doi: 10.1073/pnas.0701498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald JA, Rosner T, Janssen T. Hybrid measurement of auditory steady-state responses and distortion product otoacoustic emissions using an amplitude-modulated primary tone. J Acoust Soc Am. 2006;119:3886–3895. doi: 10.1121/1.2197789. [DOI] [PubMed] [Google Scholar]
- Purcell DW, Van Roon P, John MS, Picton TW. Simultaneous latency estimations for distortion product otoacoustic emissions and envelope following responses. J Acoust Soc Am. 2006;119:2869–2880. doi: 10.1121/1.2191616. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Nicol T, Musacchia G, Kraus N. Brainstem responses to speech syllables. Clin Neurophysiol. 2004;115:2021–2030. doi: 10.1016/j.clinph.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Nicol TG, Zecker SG, Hayes EA, Kraus N. Auditory training improves neural timing in the human brainstem. Behav Brain Res. 2005;156:95–103. doi: 10.1016/j.bbr.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Jr, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci U S A. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH, Cerka AJ, Recio-Spinoso A, Temchin AN, van Dijk P, Ruggero MA. Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. J Acoust Soc Am. 2005;118:2434–2443. doi: 10.1121/1.2005867. [DOI] [PubMed] [Google Scholar]
- Smith JC, Marsh JT, Brown WS. Far-field recorded frequency-following responses: evidence for the locus of brainstem sources. Electroencephalogr Clin Neurophysiol. 1975;39:465–472. doi: 10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Song JH, Skoe E, Wong PC, Kraus N. Plasticity in the Adult Human Auditory Brainstem following Short-term Linguistic Training. J Cogn Neurosci. 2008;20:1892–1902. doi: 10.1162/jocn.2008.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D, Skoe E, Kraus N, Ashley R. Musical experience and neural efficiency: effects of training on subcortical processing of vocal expressions of emotion. Eur J Neurosci. 2009;29:661–668. doi: 10.1111/j.1460-9568.2009.06617.x. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Swaminathan J, Krishnan A, Gandour JT. Pitch encoding in speech and nonspeech contexts in the human auditory brainstem. Neuroreport. 2008;19:1163–1167. doi: 10.1097/WNR.0b013e3283088d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Boston: Allyn and Bacon; 2007. [Google Scholar]
- Talmadge CL, Long GR, Tubis A, Dhar S. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J Acoust Soc Am. 1999;105:275–292. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Wagner W, Plinkert PK, Vonthein R, Plontke SK. Fine structure of distortion product otoacoustic emissions: its dependence on age and hearing threshold and clinical implications. Eur Arch Otorhinolaryngol. 2008;265:1165–1172. doi: 10.1007/s00405-008-0593-0. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biol Psychol. 2004;67:299–317. doi: 10.1016/j.biopsycho.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wong PC, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci. 2007;10:420–422. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden FG, Marsh JT. Frequency-following (microphonic-like) neural responses evoked by sound. Electroencephalogr Clin Neurophysiol. 1968;25:42–52. doi: 10.1016/0013-4694(68)90085-0. [DOI] [PubMed] [Google Scholar]