Abstract
Purpose
O-linked carbohydrates (O-glycans) contribute to the hydrophilic character of mucins in mucosal tissues. This study aimed to identify the repertoire of O-glycans in the tear film, and the glycosyltransferases associated with their biosynthesis, in normal subjects and patients with non-Sjögren’s dry eye.
Methods
Human tear fluid was collected from the inferior conjunctival fornix. O-glycans were released by hydrazinolysis, labeled with 2-aminobenzamide, and analyzed by fluorometric, high-performance liquid chromatography (HPLC) coupled with exoglycosidase digestions. O-glycan structures identified in tears were related to potential biosynthetic pathways in human conjunctival epithelium using a glycogene microarray database. Lectin-binding analyses were performed using agglutinins from Arachis hypogaea, Maackia amurensis, and Sambucus nigra.
Results
The O-glycan profile of human tears consisted primarily of core 1 (Galβ1-3GalNAcα1-Ser/Thr)-based structures. Mono-sialyl O-glycans represented approximately 66% of the glycan pool, being α2-6-sialyl core 1 the predominant O-glycan structure in human tears (48%). Four families of glycosyltranferases potentially related to the biosynthesis of these structures were identified in human conjunctiva. These included thirteen polypeptide-GalNAc-transferases (GALNT), the core 1 β-3-galactosyltransferase (T-synthase), three α2-6-sialyltransferases (ST6GalNAc), and two α2-3-sialyltransferases (ST3Gal). No significant differences in total amount of O-glycans were detected between tears of normal subjects and dry eye patients, by HPLC and lectin blot. Likewise, no differences in glycosyltransferase expression were found by glycogene microarray.
Conclusions
This study identifies the most common mucin-type O-glycans in human tears and their expected biosynthetic pathways in ocular surface epithelia. Patients with non-Sjögren’s dry eye show no alterations in composition and amount of O-glycans in the tear fluid.
Keywords: dry eye, glycosyltransferase, mucin, O-glycan, tear film
INTRODUCTION
The tear film covering the ocular surface epithelia consists of two distinct layers, an outer lipid layer produced primarily by meibomian glands, and an inner layer containing a mixture of mucin and lacrimal fluid, adjacent to the glycocalyx of apical epithelial cells.1 The mucin component of the tear film contains both secreted and cell-surface-associated mucins,2 the latter being constitutively shed from the apical glycocalyx of the ocular surface epithelia. The goblet-cell-specific MUC5AC is the major secreted mucin in tears, whereas MUC1, MUC4 and MUC16 constitute the major cell-surface-associated mucins.2
Structurally, both types of mucins are large and very densely glycosylated proteins. O-glycosylation is the predominant posttranscriptional modification of mucins, conferring upon them unique physico-chemical properties required for their functions at the ocular surface. These functions include hydrophilicity and an antiadhesive character that provide boundary lubrication and protection against pathogen invasion.3–5 Mucin-type O-linked glycosylation is initiated by a family of enzymes known as UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases (GALNT) that transfer α-N-acetylgalactosamine (GalNAc) to Ser/Thr residues in the protein backbone to form the Tn-antigen. The Tn-antigen can be elongated by other glycosyltransferases to generate a series of inner core O-linked glycans. To date, eight different core structures have been described, with core 1 (Galβ1-3GalNAcα1-Ser/Thr)—synthesized by core 1 β-3-galactosyltransferase (T-synthase)—being among the most common on mucins.6 Core structures can be further modified by addition of other carbohydrates, such as poly-lactosamine, and may be terminated by blood group or tissue antigens, or by sialic acid and sulfate. The composition and sequence of carbohydrates in the mucin O-glycan chain is governed by cell type and tissue-specific expression of mucin-type glycosyltransferases. Recent work by Royle et al. has elegantly demonstrated the structure of mucin O-glycans isolated from conjunctival tissue,7 but the detailed nature of mucin O-glycans in tears (where they may interact with lectin-like proteins or microorganisms) and the glycosyltransferases associated with their biosynthesis remain mostly uncharacterized.
Altered mucin O-glycosylation has been frequently observed with several pathologies, including Tn syndrome, IgA nephropathy, cystic fibrosis, inflammatory bowel disease, and cancer.8–12 In the eye, several reports have described alterations in the carbohydrate composition of the apical glycocalyx of conjunctival epithelial cells in patients with dry eye. These include a reduction in lectin and antibody binding to cell-surface carbohydrate epitopes such as sialic acid and core 1, and alteration in the distribution of glycosyltransferases involved in mucin-type O-glycosylation.13–17 As determined by rose bengal staining, alteration in cell surface mucin O-glycosylation in aqueous-deficient dry eye correlates with epithelial damage at the ocular surface.15 It remains unknown, however, whether the O-glycosylation of tear film mucins is altered in dry eye and if so, whether it contributes to an increased susceptibility to ocular surface damage. This study was designed to determine the profile of O-glycans in the human tear film, as well as to analyze the expression of mucin-type O-glycosyltransferases involved in their biosynthesis in normal subjects and patients with dry eye.
MATERIALS AND METHODS
Subject Selection
This study was performed in accordance with the Declaration of Helsinki and The Schepens’s Institutional Review Board. Informed consent was obtained from all participants. All prospective subjects completed an institutional review board-approved questionnaire regarding the presence, type, and frequency of their dry eye symptoms.
Two groups of subjects were studied and included individuals for whom the expression of glycogene mRNA at the ocular surface epithelia has been previously analyzed.18 The first group consisted of 9 normal subjects (7 females and 2 males) with an age range between 30–55 years (average 40±10), who had no dry eye symptoms, no eye diseases, and no history of eye surgery or contact lens wear. The second group consisted of 8 patients with non-Sjögren’s dry eye (7 females and 1 male) with an age range between 30–55 years (average 41±9), who fit the inclusion criteria for non-Sjögren’s dry eye. These criteria are (1) moderate to severe dry eye symptoms, (2) Schirmer I reading of less than 8 mm, without anesthesia, (3) tear breakup time of less than 5 seconds, and (4) positive corneal and conjunctival staining with rose bengal. None of the study subjects was receiving topical or systemic medications, and none had history of ocular surgery and/or contact lens wear, ocular allergies, diabetes, Sjögren’s syndrome, or other autoimmune diseases. Subjects primarily included women, who are known to have a higher prevalence of dry eye.19 All subjects were blood type O to avoid variation in terminal glycosylation.20,21
Sample Collection
Tear fluid and conjunctival epithelium were collected from normal subjects and non-Sjögren’s dry eye patients as previously described.22 Briefly, tear washes were collected from the inferior fornix of each eye by micropipette after instillation of 60 μl of sterile saline onto the unanesthetized ocular surface. Tear samples were centrifuged for 30 minutes at 14,000 rpm at 4°C, and the protein concentration determined using a protein assay reagent kit (MicroBCA; Pierce, Rockford, IL). A drop of topical anesthetic (0.5% proparacaine HCl, Alcaine; Alcon, Inc., Fort Worth, TX) was then applied to the eye. A sterile disc of nitrocellulose filter paper 10 mm in diameter was placed on the temporal bulbar conjunctiva for 15 seconds, and the disc was carefully transferred into a tube (Eppendorf, Fremont, CA) containing RNA extraction reagent (TRIzol; GibcoBRL, Grand Island, NY). Conjunctival RNA was used in a parallel study to determine the overall expression of glycogenes in the human conjunctival epithelium by microarray.18 For a complete list of glycogenes expressed in the conjunctival epithelium of normal subjects and non-Sjögren’s dry eye patients view the public database http://www.functionalglycomics.org/glycomics/publicdata/microarray.jsp, ref. MAEXP_310_052307).
Release of O-glycans by Hydrazinolysis and Fluorescent Labeling
The soluble fractions of tear samples after centrifugation were pooled in groups to allow detection by fluorometric, high-performance liquid chromatography (HPLC). These included two normal groups, containing protein pooled from 4 and 5 subjects, and two dry eye groups, containing protein pooled from 4 subjects each. Tear samples (300 μg of total protein) and bovine fetuin (1 mg; Sigma-Aldrich, St. Louis, MO) in reaction vials were lyophilized for 24 hours. Hydrazinolysis was performed using the GlycoRelease™ glycan hydrazinolysis kit (Prozyme, San Leandro, CA). Briefly, O-glycans were released by incubation at 60°C for 6 hours with anhydrous hydrazine. Excess hydrazine was removed by evaporation, and the released O-glycans were re-N-acetylated and separated from peptide-derived material using Glycoclean™ R cartridges (Prozyme). The samples were then eluted in water and lyophilized prior to labeling.
O-glycans were fluorescently labeled with 2-aminobenzamide (2-AB) using the Signal™ 2-AB labeling kit (Prozyme). Briefly, released O-glycans were dissolved in 5 μl of a freshly prepared solution containing 5 mg 2-AB and 6 mg sodium cyanoborohydride in 150 μl acetic acid and 350 μl dimethyl sulfoxide. The mixture was incubated at 65°C for 3 hours in a heating block. For subsequent analysis by HPLC, excess dye and other labeling reagents were removed using a Glycoclean S cartridge (Prozyme). The 2-AB-labeled O-glycans were eluted in water, lyophilizated, and stored at −20°C wrapped in foil, until analysis.
Fluorometric HPLC Analysis
2-AB-labeled O-glycans were analyzed by fluorometric normal-phase HPLC using a Zorbax NH2 analytical column (4.6 × 250 mm; Agilent Technologies, Palo Alto, CA) connected to a Bio-LC chromatography system (Dionex Corporation; Sunnyvale, CA). Samples were eluted in 50 mM formic acid, pH 4.4, and acetonitrile, following gradient conditions previously described.23 The total amount of O-glycans in normal and dry eye samples was expressed in pmol after normalization of fluorescence intensity values to that obtained for a 2-AB-labeled core 1 O-glycan standard (Prozyme).
Exoglycosidase Digestions
2-AB-labeled O-glycans were subject to exoglycosidase digestions with 1 U/ml α2-3-sialidase from Streptococcus pneumoniae (NAN1, Prozyme) and 1.5 U/ml α2-3,6,8-sialidase from Arthrobacter ureafaciens (ABS, Prozyme). Digestions were carried out in 50 mM sodium phosphate, pH 6.0, for 16 hours at 37°C. To remove enzymes prior to HPLC analyses, samples were passed through protein-binding Micropure-EZ filters (Millipore, Bedford, MA).
Lectin Blot Analysis
Tear fluid (7.5 μg of total protein) was diluted in Laemmli buffer and separated by 1% (wt/vol) agarose gel electrophoresis, as described by Thornton et al.24 For lectin blot, proteins were transferred to nitrocellulose membranes (Millipore) by vacuum. Membranes were then blocked with 1% polyvinylpyrrolidone in 0.1% Tween-Tris-buffered saline, pH 7.5, for 1 hour, and incubated with biotin-labeled peanut agglutinin (PNA, 25 μg/mL) to the mucin-associated T-antigen epitope, Sambucus nigra agglutinin (SNA, 100 μg/mL) to terminal α2-6 sialic acid, and Maackia amurensis agglutinin (MAA, 100 μg/mL) to terminal α2-3 sialic acid for 1.5 hours at room temperature. Membranes were developed with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and lectin binding visualized using chemiluminescence (SuperSignal West Pico; Pierce). Films were scanned and densitometric analysis was performed using Kodak Digital Science 1D Image Analysis software (New Haven, CT).
Statistical Analysis
Statistical comparisons of HPLC data were performed using InStat3 software (GraphPad Software, La Jolla, CA). P values were determined using the unpaired t test with Welch correction. For microarray data, statistical comparisons were performed using analysis of variance (ANOVA). A value of p< 0.05 was considered statistically significant.
RESULTS
Identification of Mucin-type O-glycans in Normal Tear Fluid
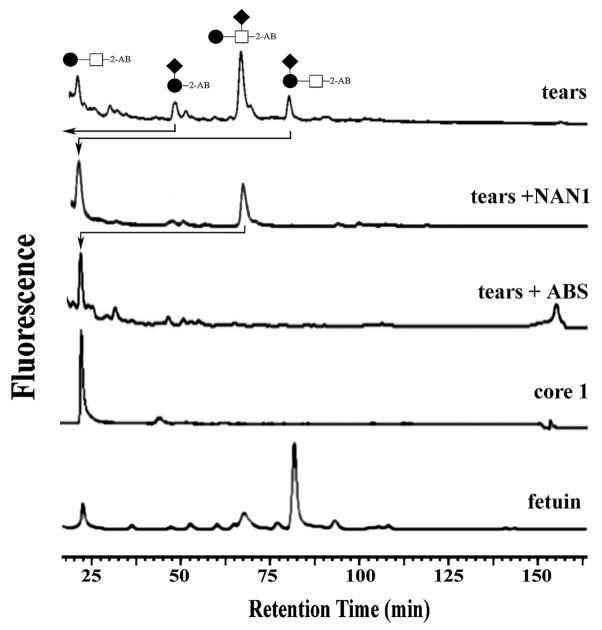
Mucin-type O-glycans in normal tear fluid were fluorescently labeled and analyzed by normal-phase fluorometric HPLC (Fig. 1). The identity of each O-glycan structure in the chromatographic profile was assigned by comparison to retention times of the core 1 standard and O-glycans derived from bovine fetuin. Three major O-glycan structures were detected in normal tear film and included core 1 (Galβ1-3GalNAc), α2-6 sialyl core 1 (Galβ1-3[NeuNAcα2-6]GalNAc), and α2-3 sialyl core 1 (NeuNAcα2-3Galβ1-3GalNAc). In addition to these structures, α2-3 sialyl galactose (NeuNAcα2-3Gal), a peeling product from the α2-3 sialyl core 1,7 was also detected. Analyses of the relative amounts of structures revealed that mono-sialylated core 1 constituted 66% of the O-glycan pool in tears. α2-6 sialyl core 1 was the most abundant O-glycan in tears (48%), followed by core 1 (16%), α2-3 sialyl galactose (10%), and α2-3 sialyl core 1 (8%).
Figure 1.
HPLC elution profile of mucin O-glycans in normal tear fluid. Tear samples containing 300 μg of total protein were subjected to hydrazinolysis. O-glycans were fluorescently labeled with 2-aminobenzamide (2-AB) and analyzed by fluorometric normal-phase HPLC. O-glycan structures were identified by comparison of their retention times with those of the core 1 standard and fetuin. The top panel shows a representative O-glycan profile of normal tear fluid. Panels below show the O-glycan profile after exoglycosidase digestion with α2-3-sialidase (NAN1) and α2-3,6,8-sialidase (ABS). Symbols represent galactose (●), N-acetylgalactosamine (□), and N-acetylneuraminic acid (◆).
The identities of the different structures in the chromatogram were further confirmed by exoglycosidase digestions (Fig. 1). The NAN1 sialidase, specific for α2-3 sialic acid linkages, hydrolyzed the α2-3 sialyl core 1 structure to yield core 1 alone. Similarly, the peeling product α2-3 sialyl galactose was also hydrolyzed after NAN1 treatment. The α2-6 sialyl core 1, on the other hand, was resistant to NAN1, but was cleaved by ABS, a generic sialidase that removes α2-3,6,8-linked sialic acids (α2-8 sialic acids are not found in mono-sialylated structures, but mainly in di- or tri-sialylated O-glycans25).
Pathways of Mucin O-glycosylation at the Ocular Surface
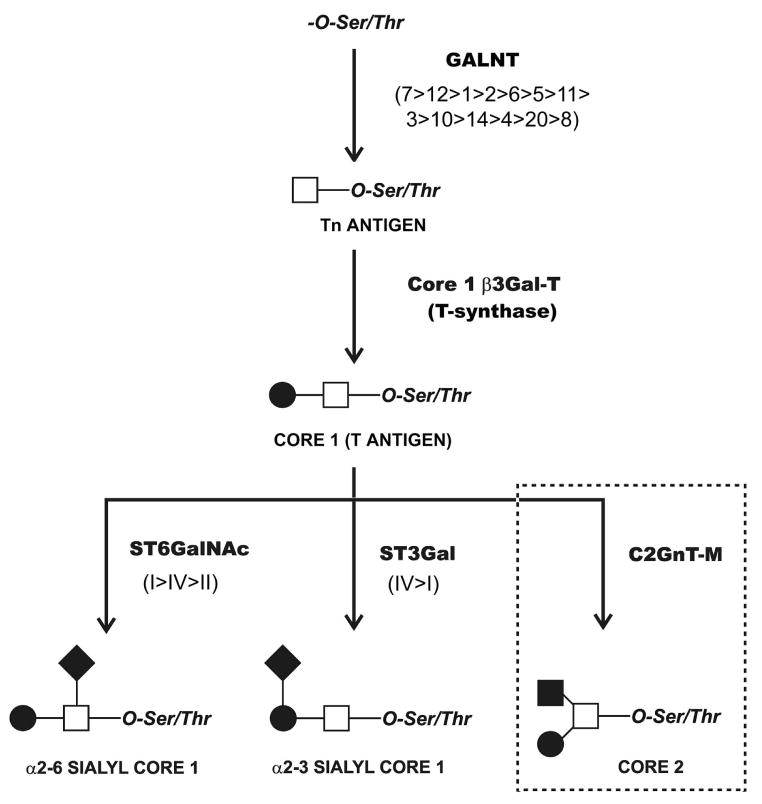
Analyses of the O-glycan profile in normal tears provided the basis to delineate the pathways of mucin-type O-glycosylation at the ocular surface epithelia, taking advantage of public data on the expression of glycosyltransferases identified by glycogene microarray in conjunctival epithelium. Four families of glycosyltranferases potentially associated with the biosynthesis of O-glycan structures identified in normal tears were detected in human conjunctiva (Fig. 2). These included thirteen UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (GALNT1-8, 10-12, 14 and 20), the core 1 β-3-galactosyltransferase (core 1 β3Gal-T or T-synthase), three α2-6-sialyltransferases (ST6GalNAc-I, II and IV) and two α2-3-sialyltransferases (ST3Gal-I and IV). In addition to these, six additional sialic acid glycosyltranfersases were detected by microarray—three α2-6-sialyltransferases (ST6GalNAc-III, V and VI), and three α2-3-sialyltransferases (ST3Gal-III, V and VI). However, acceptor substrate specificity assays have shown that these transferases act preferentially to non-mucin substrates.26,27
Figure 2.
Pathways of O-glycosylation on the ocular surface epithelia. Glycosyltransferases potentially involved in the biosynthesis of mucin-type O-glycans present on tear mucins were identified using a public microarray database, as described in Materials and Methods. These included thirteen polypeptide GalNAc-transferases (GALNT) involved in the initiation of mucin-type O-glycosylation, the core 1 β-3-galactosyltransferase (T-synthase) involved in the addition of galactose to the Tn antigen, three α2-6-sialyltransferases (ST6GalNAc), and two α2-3-sialyltransferases (ST3Gal). Core 2 (dotted line) in conjunctival epithelial cells7 is synthesized by core 2 β1,6 N-acetylglucosaminyltransferase (C2GnT-M). The relative levels of expression of individual glycosyltransferase isoforms are indicated in parenthesis. Symbols represent galactose (●), N-acetylgalactosamine (□), N-acetylglucosamine (■), and N-acetylneuraminic acid (◆).
In addition to the O-glycan structures detected in tear fluid in the present study, a recent report showed core 2 (Galβ1-3(GlcNAcβ1-6)GalNAc) O-glycans in protein extracts from human conjunctival epithelium.7 Core 2 is synthesized by β1,6 N-acetylglucosaminyltransferases acting on core 1. Analysis of the microarray data revealed two enzymes with β1,6 N-acetylglucosaminyltransferase activity in conjunctiva; the mucin-type core 2 β1,6 N-acetylglucosaminyltransferase (C2GnT-M), and the I-branching β1,6 N-acetylglucosaminyltransferase. C2GnT-M is implicated in the formation of core 2, core 4, and the blood group I structure, whereas the I-branching transferase catalyzes the formation of blood group I.28–31 Since blood group I was not detected on mucin-type O-glycans from human conjunctival epithelium or tear fluid, the I-branching transferase was not included as a component in the proposed pathways of mucin-type O-glycosylation at the ocular surface (Fig. 2).
Mucin-type O-glycosylation in Patients with Dry Eye
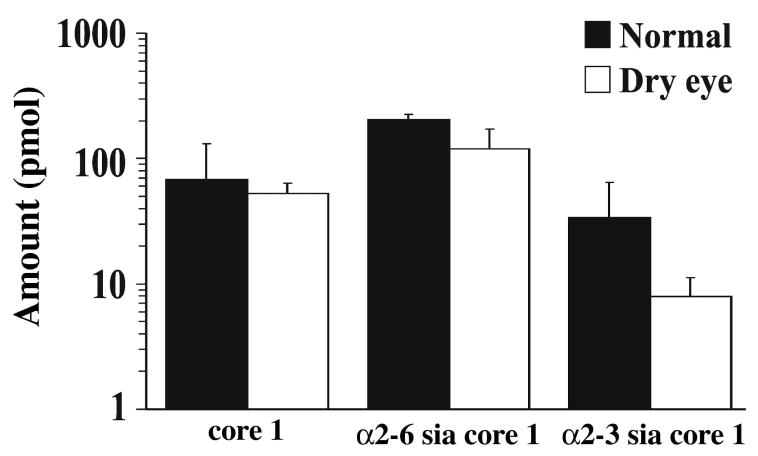
By fluorometric HPLC, the chromatographic profile of O-glycans from tear samples of dry eye patients was similar to that from normal subjects; core 1 and mono-sialylated core 1 O-glycans were detected in the chromatograms from both normal and dry eye samples (data not shown). Quantitative analysis revealed no differences in the amount of core 1 structures between both groups (Fig. 3). In these experiments, α2-3 sialyl core 1 exhibited a downward trend in dry eye, but the difference was not statistically significant.
Figure 3.
Comparison of O-glycan content in tear fluid of normal subjects and dry eye patients. Tear samples containing 300 μg of total protein were analyzed by fluorometric normal-phase HPLC. The total amount of O-glycans was expressed in pmol after normalization of fluorescence intensity values to that obtained for a 2-AB-labeled core 1 O-glycan standard. No differences were observed in the amount of core 1 structures between normal subjects and dry eye patients. The decrease in α2-3 sialyl core 1 observed in dry eye patients was not statistically significant.
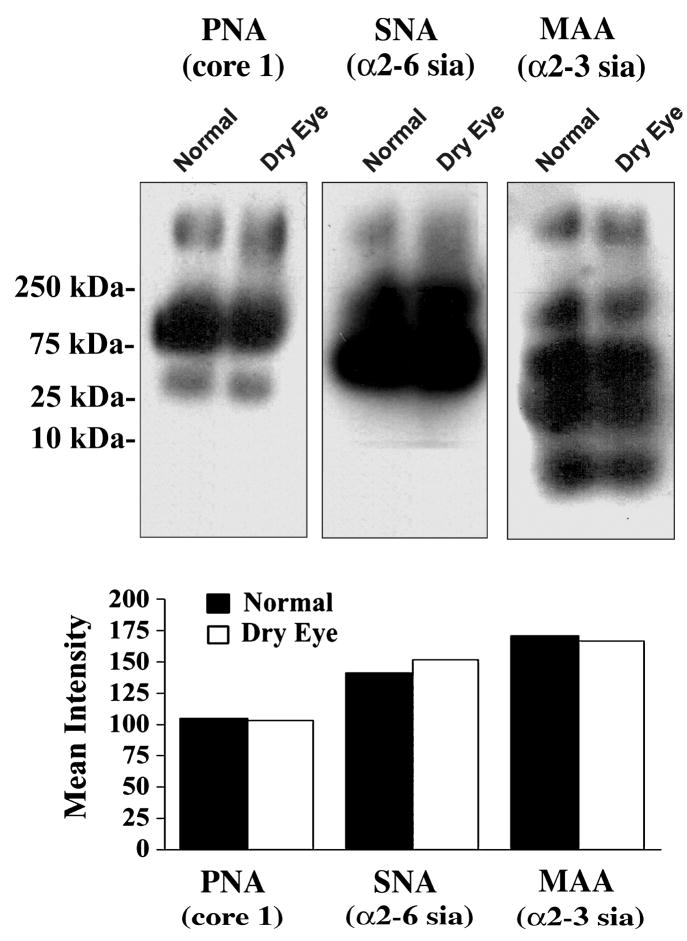
Lack of alteration of glycan structures in the tear fluid of dry eye patients was further confirmed by lectin blot after separation of the tear proteins in agarose gels (Fig. 4). These analyses revealed several bands with different electrophoretic mobilities, which correspond to different glycoproteins present in human tear fluid. In normal samples, PNA bound to core 1 structures on a high-molecular-weight band that exhibited an electrophoretic migration typical of mucins (>250 kDa). Binding to low-molecular weight glycoproteins was also detected, and could correspond to proteolytically degraded mucins or to other O-linked glycoproteins present in tear fluid. PNA binding in non-Sjögren’s dry eye samples was comparable in both mobility and intensity to that observed in normal samples (Fig. 4). SNA and MAA also revealed the presence of several bands corresponding to tear glycoproteins carrying either individual or combined α2-3 and α2-6 sialic acid-terminated glycans. SNA and MAA can recognize sialic acid epitopes in both N-glycans and O-glycans, as compared to PNA, which primarily recognizes O-glycans. The mobility and intensity of the SNA and MAA bands was comparable in normal and dry eye samples.
Figure 4.
Lectin binding to glycoproteins in tear fluid of normal subjects and dry eye patients. Samples containing 7.5 μg of total protein were separated by 1% agarose gel electrophoresis and blotted to nitrocellulose membranes. Glycan content was assessed using biotin-labeled PNA to the mucin-associated T-antigen epitope, SNA to terminal α2-6 sialic acid, and MAA to terminal α2-3 sialic acid. The position of the molecular weight markers is indicated to the left. As determined by densitometry (lower panel), no differences in lectin binding were detected between tear samples from normal subjects and dry eye patients.
The expression of glycosyltranferases in the conjunctival epithelium of patients with dry eye was analyzed using a microarray database, as reported in Materials and Methods. As shown in Table 1, no significant differences were observed in the overall expression of glycosyltranferases associated with the biosynthesis of O-glycan structures identified in the tear fluid of normal and dry eye subjects.
Table 1.
Glycosyltransferases associated to mucin O-glycosylation expressed in the conjunctival epithelium of normal subjects and patients with non-Sjögren’s dry eye.*#
| Common name | Accession number | Normal subjects | Dry eye patients | p value |
|---|---|---|---|---|
| GALNT1 | NM_020474.2 | 185.37 | 149.53 | >0.05 |
| GALNT2 | NM_004481 | 135.23 | 139.47 | >0.05 |
| GALNT3 | NM_004482.2 | 61.70 | 66.20 | >0.05 |
| GALNT4 | NM_003774 | 39.37 | 32.83 | >0.05 |
| GALNT5 | NM_014568.1 | 72.70 | 82.30 | >0.05 |
| GALNT6 | NM_007210.2 | 106.20 | 99.87 | >0.05 |
| GALNT7 | NM_017423.1 | 280.57 | 292.47 | >0.05 |
| GALNT8 | NM_017417.1 | 9.10 | 9.20 | >0.05 |
| GALNT10 | NM_198321.2 | 56.97 | 53.20 | >0.05 |
| GALNT11 | NM_022087.1 | 65.23 | 54.90 | >0.05 |
| GALNT12 | NM_024642.1 | 222.17 | 180.30 | >0.05 |
| GALNT14 | NM_024572.1 | 56.63 | 64.97 | >0.05 |
| GALNT20 | NM_022479 | 28.40 | 41.70 | >0.05 |
| Core 1 β3Gal-T | NM_020156.1 | 67.00 | 75.97 | >0.05 |
| ST3Gal-I | NM_003033.1 | 44.37 | 41.10 | >0.05 |
| ST3Gal-IV | NM_006278.1 | 299.17 | 291.17 | >0.05 |
| ST6GalNAc-I | NM_018414.2 | 251.30 | 241.00 | >0.05 |
| ST6GalNAc-II | NM_006456.1 | 34.20 | 23.33 | >0.05 |
| ST6GalNAc-IV | NM_014403.1 | 39.47 | 30.07 | >0.05 |
| C2GnT-M | NM_004751.1 | 1089.97 | 1260.17 | >0.05 |
Data on glycogene expression is available at http://www.functionalglycomics.org/glycomics/publicdata/microarray.jsp, ref. MAEXP_310_052307.
Expression values represent the geometric mean of intensities corresponding to 9 normal subjects and 9 dry eye patients as described by Mantelli et al.18
DISCUSSION
Mucin-type O-glycans have been ascribed multiple functions in mucosal secretions, which include hydration and protection of underlying epithelial cells, providing resistance to protease degradation, and trapping and removing bacteria via specific receptor sites within the O-glycan chains of the mucin.32 In this study, we have determined the structure and relative amounts of mucin-type O-glycans in human tear fluid, as well as expression of glycosyltransferases involved in their biosynthesis in conjunctival epithelium, in normal subjects, and in patients with non-Sjögren’s dry eye.
Our findings on the composition of O-glycans in human normal tears are comparable to those reported recently by Royle et al.7 on human mucins extracted from human conjunctival tissue; core 1-based structures are the major mucin-type O-glycans at the ocular surface. It is of interest, however, to note three major differences between tissue-associated O-glycans in conjunctiva and those found in tears. First, the conjunctival epithelium contains core 2-based structures, including galactosyl core 2 and di α2-3 sialyl galactosyl core 2. Second, α2-3 sialyl core 1 is the predominant O-glycan in human conjunctival tissue (47.4%), whereas in tears the prominent O-glycan is α2-6 sialyl core 1 (48%). And third, disialyl core 1 is present in conjunctival mucin but not in tears. These discrepancies could be due to several factors. First, Royle et al. evaluated O-linked glycans in high-molecular-weight fractions of mucin isolates (representing undegraded mucin) from conjunctival tissue,7 whereas our study included all O-linked glycans in tears. Second, differences in cellular trafficking might influence the O-glycosylation profiles in mucins. Engelmann et al. have shown that breast cancer epithelial cells transfected with membrane-bound and secretory-recombinant MUC1 produce a transmembrane form that contains mainly sialylated core 1 structures and a secreted form containing predominantly neutral core 2 O-glycans.33 Third, it is also possible that specific mucin-type O-glycans in conjunctival tissue are associated to intracellular or cell surface glycoproteins and, therefore, not secreted into the tear film. And fourth, it is possible that the difference in O-glycan composition between conjunctival epithelium and tear film is due to degradation by glycosidase activity in tears (Matthews E., et al. IOVS 2001;42:ARVO Abstract #1432, p. S264).34 It is exciting to speculate that the different O-glycan composition observed in epithelial cells and tear fluid confers to mucins unique roles at specific sites. For example, tear mucin bearing α2-6 sialyl core 1 could compete for binding to Pseudomonas aeruginosa pili and, therefore, prevent bacterial attachment to α2-6 sialyl receptors present on the corneal epithelial surface.35,36
Analysis of a microarray database of human conjunctival epithelium allowed us to propose potential pathways for mucin-type O-glycosylation at the ocular surface. Enzymes involved in the initiation of mucin-type O-glycosylation in human conjunctiva included 13 GALNT isoforms (GALNT1-8, 10-12, 14, and 20). A previous study using Taqman real-time RT-PCR revealed 15 isoforms in human conjunctiva.37 These included, in addition to those reported in this study (with the exception of GALNT20, which was not evaluated previously), GALNT15, and low levels of GALNT9 and GALNT13. Data quality parameters, such as background to noise ratios, could be responsible for the lack of correlation in the expression of GALNT9, 13, and 15 between the two methods.38 In addition to GALNT, six α2-6 sialyltransferases (ST6GalNAc-I, II, III, IV, V and VI) and five α2-3 sialyltransferases (ST3Gal-I, III, IV, V and VI) were identified by microarray. In vitro analysis of substrate specificity has revealed, however, that not all sialotransferases act on mucin-type substrates. ST6GalNAc-I,-II and IV mediate the transfer of sialic acid with an α2-6-linkage to GalNAc residues O-glycosidically attached to Ser/Thr, whereas ST6GalNAc-III,-V and VI catalyze the addition of sialic acid residues onto gangliosides.26 As for the α2-3 sialyltransferases, ST3Gal-I and IV have been shown to act on Galβ1-3GalNAc present on glycoproteins and glycolipids,39,40 therefore, constituting potential candidates to synthesize α2-3 sialyl core 1 in conjunctival mucin. By contrast, ST3Gal-III exhibits preference for Galβ1-3GlcNAc structures;41 the ST3Gal-VI mainly uses Galβ1-4GlcNAc as an acceptor substrate;42 and the ST3Gal-V only acts on glycolipids.43 Enzymatic assays using suitable substrates for the glycosyltransferases identified in this study would provide valuable information toward further characterization of the acceptor specificity and kinetic mechanisms of these enzymes.
Several studies have evaluated the expression of carbohydrate epitopes in dry eye, both in the tear fluid and the ocular surface epithelial glycocalyx. Using HPLC and the CA 19-9 ELISA test, Garcher and Nakamura have shown a decrease in sialic acid and sialyl-Lea in the tear fluid of dry eye patients.44,45 This decrease, however, could be attributed to changes to glycolipids and N-linked glycans present in the tear film—no sialyl-Lea was detected in our analysis of tear O-glycans or in conjunctival mucin O-glycans.7 In our hands, no changes were detected in the total amount of mucin O-glycans in tears of dry eye patients, using HPLC. Similarly, no differences were detected in PNA, SNA and MAA binding to tear glycoproteins in dry eye, indicating lack of alterations in core 1 O-glycosylation and glycoprotein sialylation in the disease. In the epithelial glycocalyx, several authors have reported changes in the distribution of mucin-associated O-glycans. These included alteration in (i) core 1, as determined by PNA staining,16 (ii) O-acetyl sialylation on MUC16, as determined by H185 antibody binding,13,15,17 and (iii) sialylation of MUC1, as determined by KL-6 antibody staining.46 These alterations in distribution suggest a compensatory attempt by the ocular surface epithelium to preserve a wet-surfaced phenotype in dry eye by modifying mucin-type O-glycosylation on membrane-associated mucins on the epithelial glycocalyx. Our results suggest that these alterations in carbohydrate distribution on the epithelial glycocalyx in dry eye do not result in major changes in O-glycan composition and amount in the tears of these patients.
We sought to identify genes with altered expression in dry eye patients by analyzing microarray data from conjunctival samples collected by impression cytology. The results revealed no differences in mRNA expression of mucin-type glycosyltransferases between normal subjects and dry eye patients. These results are in agreement with those obtained by Imbert et al. using real-time RT-PCR, showing no alterations in GALNT expression in conjunctival epithelium harvested by brush cytology in patients with aqueous-deficient dry eye.37 In these studies, however, the conjunctiva—a stratified epithelium containing both goblet and not-goblet cells—was homogenized previous to analysis. Using immunofluorescence, our laboratory has reported changes in the cell-type and cell-layer distribution of GALNT in pathologically keratinized conjunctival epithelia.14 Although no localization studies have been performed with other mucin-type glycosyltransferases, we hypothesize that alteration in the local distribution of these enzymes, not in their overall expression, might correlate with altered cell surface O-glycosylation and epithelial damage.
In summary, this study provides data on the composition of mucin-type O-glycans in human tear film, and on the expression of mucin-type O-glycosyltransferases associated with their biosynthesis in conjunctival epithelium. Using a combination of techniques that included HPLC, lectin-blot, and glycogene microarray analysis, no detectable differences in mucin-type O-glycosylation in tears or in mucin-type O-glycosyltransferase expression in conjunctiva, were found between non-Sjögren’s dry eye patients and normal subjects.
Acknowledgments
The authors thank Drs. Stefano Bonini, Audrey Chan, Magdalena Cortes, Pedram Hamrah, and Alessandra Micera for providing human tear samples.
Financial support: This work was supported by the National Eye Institute of the National Institutes of Health, EY014847 (PA), and a postdoctoral fellowship from the Alfonso Martin Escudero Foundation in Spain (AG).
References
- 1.Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res. 2001;73:281–289. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- 2.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–657. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 4.Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argueso P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:197–203. doi: 10.1167/iovs.07-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricciuto J, Heimer SR, Gilmore MS, Argueso P. Cell surface O-glycans limit Staphylococcus aureus adherence to corneal epithelial cells. Infect Immun. 2008;76:5215–5220. doi: 10.1128/IAI.00708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockhausen I. O-linked chain glycosyltransferases. Methods Mol Biol. 2000;125:273–293. doi: 10.1385/1-59259-048-9:273. [DOI] [PubMed] [Google Scholar]
- 7.Royle L, Matthews E, Corfield A, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 8.Bodger K, Halfvarson J, Dodson AR, et al. Altered colonic glycoprotein expression in unaffected monozygotic twins of inflammatory bowel disease patients. Gut. 2006;55:973–977. doi: 10.1136/gut.2005.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 10.Xia B, Royall JA, Damera G, Sachdev GP, Cummings RD. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology. 2005;15:747–775. doi: 10.1093/glycob/cwi061. [DOI] [PubMed] [Google Scholar]
- 11.Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217. doi: 10.1016/j.semnephrol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Berger EG. Tn-syndrome. Biochim Biophys Acta. 1999;1455:255–268. doi: 10.1016/s0925-4439(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 13.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16:1219–1228. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 15.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- 16.Versura P, Maltarello MC, Cellini M, Caramazza R, Laschi R. Detection of mucus glycoconjugates in human conjunctiva by using the lectin-colloidal gold technique in TEM. II. A quantitative study in dry-eye patients. Acta Ophthalmol (Copenh) 1986;64:451–455. doi: 10.1111/j.1755-3768.1986.tb06952.x. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, Maeda N, Kiritoshi A, Hamano T, Shimomura Y, Tano Y. Expression of a mucin-like glycoprotein produced by ocular surface epithelium in normal and keratinized cells. Am J Ophthalmol. 1997;124:751–757. doi: 10.1016/s0002-9394(14)71691-5. [DOI] [PubMed] [Google Scholar]
- 18.Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. Glycogene expression in conjunctiva of patients with dry eye: Downregulation of Notch ignaling. Invest Ophthalmol Vis Sci. 2008 Nov 14; doi: 10.1167/iovs.08-2734. (Epub ahead of print) URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19011014. [DOI] [PMC free article] [PubMed]
- 19.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 20.Garcher C, Bara J, Bron A, Oriol R. Expression of mucin peptide and blood group ABH- and Lewis-related carbohydrate antigens in normal human conjunctiva. Invest Ophthalmol Vis Sci. 1994;35:1184–1191. [PubMed] [Google Scholar]
- 21.Watanabe H, Gipson IK. Detection of blood group differences in human corneal epithelium using a monoclonal antibody and lectins. Arch Ophthalmol. 1994;112:667–673. doi: 10.1001/archopht.1994.01090170111032. [DOI] [PubMed] [Google Scholar]
- 22.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- 23.Royle L, Mattu TS, Hart E, et al. An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal Biochem. 2002;304:70–90. doi: 10.1006/abio.2002.5619. [DOI] [PubMed] [Google Scholar]
- 24.Thornton DJ, Khan N, Sheehan JK. Separation and identification of mucins and their glycoforms. Methods Mol Biol. 2000;125:77–85. doi: 10.1385/1-59259-048-9:077. [DOI] [PubMed] [Google Scholar]
- 25.Angata K, Suzuki M, McAuliffe J, Ding Y, Hindsgaul O, Fukuda M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct alpha 2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J Biol Chem. 2000;275:18594–18601. doi: 10.1074/jbc.M910204199. [DOI] [PubMed] [Google Scholar]
- 26.Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P. The human sialyltransferase family. Biochimie. 2001;83:727–737. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 27.Ishii A, Ohta M, Watanabe Y, et al. Expression cloning and functional characterization of human cDNA for ganglioside GM3 synthase. J Biol Chem. 1998;273:31652–31655. doi: 10.1074/jbc.273.48.31652. [DOI] [PubMed] [Google Scholar]
- 28.Bierhuizen MF, Maemura K, Fukuda M. Expression of a differentiation antigen and poly-N-acetyllactosaminyl O-glycans directed by a cloned core 2 beta-1,6-N-acetylglucosaminyltransferase. J Biol Chem. 1994;269:4473–4479. [PubMed] [Google Scholar]
- 29.Yeh JC, Ong E, Fukuda M. Molecular cloning and expression of a novel beta-1, 6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J Biol Chem. 1999;274:3215–3221. doi: 10.1074/jbc.274.5.3215. [DOI] [PubMed] [Google Scholar]
- 30.Beum PV, Cheng PW. Biosynthesis and function of beta 1,6 branched mucin-type glycans. Adv Exp Med Biol. 2001;491:279–312. doi: 10.1007/978-1-4615-1267-7_19. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto M, Tan S, Mori N, Cheng H, Cheng PW. Mucin biosynthesis: molecular cloning and expression of mouse mucus-type core 2 beta1,6 N-acetylglucosaminyltransferase. Glycobiology. 2007;17:994–1006. doi: 10.1093/glycob/cwm068. [DOI] [PubMed] [Google Scholar]
- 32.Varki A. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. p. 653. [PubMed] [Google Scholar]
- 33.Engelmann K, Kinlough CL, Muller S, et al. Transmembrane and secreted MUC1 probes show trafficking-dependent changes in O-glycan core profiles. Glycobiology. 2005;15:1111–1124. doi: 10.1093/glycob/cwi099. [DOI] [PubMed] [Google Scholar]
- 34.Kitaoka M, Nakazawa M, Hayasaka S. Lysosomal enzymes in human tear fluid collected by filter paper strips. Exp Eye Res. 1985;41:259–265. doi: 10.1016/s0014-4835(85)80015-4. [DOI] [PubMed] [Google Scholar]
- 35.Aristoteli LP, Willcox MD. The adhesion of Pseudomonas aeruginosa to high molecular weight human tear film species corresponds to glycoproteins reactive with Sambucus nigra lectin. Exp Eye Res. 2006;83:1146–1153. doi: 10.1016/j.exer.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Hazlett L, Rudner X, Masinick S, Ireland M, Gupta S. In the immature mouse, Pseudomonas aeruginosa pili bind a 57-kd (alpha 2–6) sialylated corneal epithelial cell surface protein: a first step in infection. Invest Ophthalmol Vis Sci. 1995;36:634–643. [PubMed] [Google Scholar]
- 37.Imbert Y, Jumblatt MM, Foulks GN, Couzin EG, Steele PS, Young WW., Jr Expression in human ocular surface tissues of the GalNAc-transferases that initiate mucin-type O-glycosylation. Cornea. 2006;25:1193–1199. doi: 10.1097/01.ico.0000240099.16420.17. [DOI] [PubMed] [Google Scholar]
- 38.Morey JS, Ryan JC, Van Dolah FM. Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online. 2006;8:175–193. doi: 10.1251/bpo126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitagawa H, Paulson JC. Cloning of a novel alpha 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem. 1994;269:1394–1401. [PubMed] [Google Scholar]
- 40.Lee YC, Kojima N, Wada E, et al. Cloning and expression of cDNA for a new type of Gal beta 1,3GalNAc alpha 2,3-sialyltransferase. J Biol Chem. 1994;269:10028–10033. [PubMed] [Google Scholar]
- 41.Kitagawa H, Paulson JC. Cloning and expression of human Gal beta 1,3(4)GlcNAc alpha 2,3-sialyltransferase. Biochem Biophys Res Commun. 1993;194:375–382. doi: 10.1006/bbrc.1993.1830. [DOI] [PubMed] [Google Scholar]
- 42.Okajima T, Fukumoto S, Miyazaki H, et al. Molecular cloning of a novel alpha2,3-sialyltransferase (ST3Gal VI) that sialylates type II lactosamine structures on glycoproteins and glycolipids. J Biol Chem. 1999;274:11479–11486. doi: 10.1074/jbc.274.17.11479. [DOI] [PubMed] [Google Scholar]
- 43.Ishii A, Ohta M, Watanabe Y, et al. Expression cloning and functional characterization of human cDNA for ganglioside GM3 synthase. J Biol Chem. 1998;273:31652–31655. doi: 10.1074/jbc.273.48.31652. [DOI] [PubMed] [Google Scholar]
- 44.Garcher C, Bron A, Baudouin C, Bildstein L, Bara J. CA 19–9 ELISA test: a new method for studying mucus changes in tears. Br J Ophthalmol. 1998;82:88–90. doi: 10.1136/bjo.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura Y, Yokoi N, Tokushige H, Kinoshita S. Sialic Acid in human tear fluid decreases in dry eye. Jpn J Ophthalmol. 2004;48:519–523. doi: 10.1007/s10384-004-0111-x. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi Y, Kao WW, Kohno N, et al. Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest Ophthalmol Vis Sci. 2004;45:2212–2217. doi: 10.1167/iovs.03-0988. [DOI] [PubMed] [Google Scholar]