Abstract
Excess fatty acid accumulation in non-adipose tissues is a hallmark of metabolic disease. When elevated lipid levels exceed the cell’s capacity to store or utilize fatty acids, a lipotoxic-response is elicited, characterized by destruction of organelle membranes, activation of stress pathways, and apoptosis. This review focuses on the mechanisms by which lipid overload causes non-adipose cell death and contributes to the pathogenesis of obesity and diabetes.
What is lipotoxicity?
The prevalence of obesity, metabolic syndrome, and diabetes worldwide is a significant health problem due to serious medical complications that include cardiovascular disease and renal failure. In these diseases, elevated serum triglycerides (TAG) and free fatty acids (FFAs) cause lipid accumulation in non-adipose tissues, including the pancreas, heart, liver, kidney and blood vessel wall. While FFAs and their metabolites play a key role in membrane structure, intracellular signaling and energy homeostasis, accumulation of excess lipid in these organs causes cell dysfunction and cell death. This process, termed lipotoxicity, has been implicated in β-cell loss during the progression of type 2 diabetes, and in the pathogenesis of diabetic complications through loss of cardiomyocytes, hepatocytes, renal parenchymal cells, and endothelial cells.
Lipotoxicity has been studied in animal models, which support a link between ectopic lipid accumulation, cell death, and organ dysfunction. In rodent models with impaired leptin signaling, elevated circulating FFA levels precede the onset of diabetes and heart failure and are associated with steatosis and subsequent apoptosis of β-cells and cardiac myocytes, respectively (Lee et al., 1994; Zhou et al., 2000). Dietary and pharmacological interventions that decrease lipid accumulation mitigate the progression to diabetes and heart failure, suggesting a causal role for lipotoxicity in the pathogenesis of metabolic disease. While the applicability of findings from leptin-deficient models to human obesity and diabetes has been debated, lipid accumulation and cell death in non-adipose tissues of murine models of diet-induced obesity are consistent with a role for lipid accumulation in the pathogenesis of organ dysfunction (Park et al., 2005). In each of these models, however, it has been difficult to distinguish complications that result from excess lipids alone versus the combination of hyperlipidemia, hyperglycemia and insulin resistance. Insight into the specific consequences of non-adipose tissue lipid overload has come from transgenic animal models in which lipid uptake into tissues has been increased by expression of proteins that facilitate lipid delivery, despite normal systemic glucose and FFA metabolism. For example, overexpression of lipoprotein lipase or long chain acyl CoA synthetase causes lipid accumulation in the heart and results in heart failure (Chiu et al., 2001; Yagyu et al., 2003), supporting the hypothesis that lipids contribute to cardiac dysfunction in metabolic diseases.
Recent studies extend the link between ectopic lipid accumulation in the myocardium and heart failure to human metabolic diseases. For example, in obese subjects, intramyocellular triglyceride can be detected non-invasively and is associated with early abnormalities of cardiac function (Szczepaniak et al., 2003). Furthermore, in endstage heart failure, histological examination reveals lipid accumulation in hearts from diabetic and obese patients (Sharma et al., 2004).
To gain insight into mechanisms of lipid-induced cell death, investigators have studied the response of cultured cells to supplementation of growth media with excess FFAs complexed to albumin. In a dose-dependent manner, long-chain saturated FFAs (i.e., palmitate) induce apoptosis in a variety of established cell lines and primary cell types, including endothelial cells, fibroblasts, pancreatic β-cells, hepatocytes, and myocytes. This response is enhanced by high glucose to model the pathophysiological hyperglycemia that often accompanies hyperlipidemia (i.e., glucolipotoxicity) (El-Assaad et al., 2003). While excess FFAs induce many cellular signaling and metabolic pathways (e.g., insulin signaling), we will focus in this review on insights into the mechanisms of lipotoxic cell death. Oxidized lipoproteins and free cholesterol can also induce cell death, but consideration of these other lipid mediators is beyond the scope of this minireview.
Lipid Metabolites
Cell death is induced by supplementation of culture media with long-chain saturated FFAs at concentration ranges that model pathophysiological conditions, but similar concentrations of unsaturated long-chain FFAs are well tolerated (de Vries et al., 1997; Listenberger et al., 2003; Maedler et al., 2003). Co-treatment with saturated and unsaturated long-chain FFAs rescues cells from lipotoxicity. While both saturated and unsaturated exogenous long-chain FFAs are directed to the mitochondria for β-oxidation and to the endoplasmic reticulum (ER) for complex lipid synthesis, only long-chain saturated fatty acyl CoAs serve as substrates for de novo ceramide synthesis, a specificity that is dictated by the enzymes serine palmitoyltransferase and ceramide synthase.
Ceramide is a lipid second messenger involved in initiation of apoptosis by stimuli such as heat shock and ionizing radiation. Increased ceramide levels are observed in pancreatic islets of ZDF rats and in hearts of MHC-ACS mice (Chiu et al., 2001; Shimabukuro et al., 1998). In vitro studies have demonstrated that, under lipotoxic-conditions, increased de novo ceramide synthesis is a result of increased substrate availability (Listenberger et al., 2001; Shimabukuro et al., 1998). Thus, ceramide may contribute to the specificity of saturated long-chain FFAs in inducing cell death. Nonetheless, inhibiting de novo ceramide synthesis prevents lipotoxicity in β-cells, but not fibroblasts, suggesting that cell type-specific metabolic channeling of FFAs may be important.
Long-chain FFAs are also key precursors in phospholipid biosynthesis. Supplementation of rat cardiomyocytes with palmitate leads to remodeling of phosphaditic acid from a species containing one saturated and one unsaturated acyl chain to dipalmitoyl phosphatidic acid, which is not abundant under physiological conditions (Ostrander et al., 2001). This species is a poor substrate for cardiolipin biosynthesis, leading to depletion of cellular cardiolipin, disruption of the mitochondrial inner membrane, and release of cytochrome c. Saturated FFAs may also cause alterations in other mitochondrial membrane phospholipids and production of reactive oxygen species (ROS), leading to mitochondrial dysfunction. Profound alterations in ER membrane phospholipids also occur under lipotoxic conditions. In metabolic labeling studies, 3H-palmitate is rapidly incorporated into phosphatidylcholine in the ER, leading to a significant increase in the saturation of ER membrane phospholipids relative to untreated cells (Borradaile et al., 2006b). These changes are followed by ER swelling and escape of the protein-folding chaperones Grp78 and PDI to the cytosol, suggesting that saturated FFAs compromise ER membrane structure and integrity.
Oxidative Stress
Under physiological conditions, ROS participate in cellular signaling and overall, cellular ROS levels are kept in check by enzymatic scavengers (e.g., dismutases and peroxidases) and redox-sensitive modulators (e.g., vitamin E and glutathione). In pathophysiolgical conditions, ROS generation may exceed cellular antioxidant defenses, resulting in oxidative damage of proteins, lipids, and DNA. Oxidative stress can impair membrane integrity, organelle function, and regulation of gene expression, thereby contributing to cell death.
Both human and animal studies suggest that oxidative stress is involved in the response to lipid overload. DNA and protein oxidation products, pro-oxidants, and glutathione disulfide are elevated in the serum and β-cells of type 2 diabetics with hyperlipidemia. Similarly in the ob/ob, db/db and AY mouse models, and in streptozotocin-treated mice, markers of oxidative stress are increased concomitant with lipid accumulation. Organ-specific overexpression of enzymatic scavengers (e.g., superoxide dismutase) or systemic administration of antioxidants (e.g., α-lipoic acid) improves end organ function in diabetic and transgenic lipotoxicity mouse models (Lee et al., 2006; Ye et al., 2004). In cell culture models of lipotoxic stress, supplementation of growth media with palmitate produces ROS and treatment with antioxidants inhibits FFA-induced caspase-3 activation, ER dysfunction, and cell death (Borradaile et al., 2006a; Listenberger et al., 2001). Together, these findings are consistent with a role for oxidative stress in the pathogenesis of lipotoxicity.
ER Stress
The ER is central to the regulation of both lipid and protein metabolism. Conditions that disrupt ER homeostasis or misfolded mutant proteins activate the ER stress response. This initially inhibits protein synthesis to prevent further overloading of the ER and induces chaperones to aid in the refolding of misfolded proteins. However, prolonged or extreme ER stress overwhelms the cell, triggering activation of pro-apoptotic genes (e.g., CHOP) and inducing oxidative stress.
Lipids can also initiate ER stress. Supplementation of cultured fibroblasts, myoblasts, and β-cells with saturated FFAs in vitro leads to alterations in ER structure and function that precede activation of the ER stress response, suggesting that lipid incorporation into this organelle may contribute to initiation of ER stress (Borradaile et al., 2006b; Moffitt et al., 2005). While lipid-induced ER stress may initially be cytoprotective (Cnop et al., 2007), prolonged FFA-induced ER stress promotes cell death. In the livers and adipose tissues of ob/ob and high-fat-diet-induced (HFD) mouse models of obesity, transcription of the ER chaperone Grp78 is increased and PERK is activated (Ozcan et al., 2004). Inhibition of FA-induced ER stress with chemical ER chaperones improves insulin signaling and diminishes systemic insulin resistance in ob/ob mice (Ozcan et al., 2006). In a model of non-alcoholic fatty liver disease, rats fed a high saturated FFA diet show Grp78 and CHOP induction and caspase-3 activation following lipid accumulation in the liver (Wang et al., 2006). Furthermore, knockout of the pro-apoptotic transcription factor CHOP in db/db and HFD mice increases β-cell mass and function and prevents hyperglycemia and glucose intolerance in the setting of obesity (Song et al., 2008). These studies and others have suggested that chronic lipid-induced ER stress is detrimental.
Molecular mechanisms that link lipids to stress
Emerging evidence implicates both oxidative and ER stress responses in lipotoxicity, yet distinct molecular pathways linking FFA overload to these stress responses are not well characterized. To uncover specific mechanisms, investigators have probed signaling pathways and carried out expression array analyses, proteomics, lipidomics, and genetic screens. Collectively, these studies suggest that a number of downstream pathways involving enzymes, signaling molecules, and non-coding RNAs (ncRNAs) act in concert to direct lipid-overloaded, damaged cells to die. In vivo, the functions of these mediators of lipotoxicity are amplified by signaling pathways downstream of Toll-like Receptor 4 and the insulin receptor, both of which are modulated by pathophysiological lipid levels.
There are several mechanisms through which lipotoxicity may induce oxidative stress. The FFA metabolite ceramide can activate NADPH oxidase and disrupt mitochondrial respiration, either by inducing release of cytochrome c or through interaction with mitochondrial respiratory chain complex III. However, FFA-induced ROS production can occur even in the absence of de novo ceramide biosynthesis, implicating other mechanisms (Listenberger et al., 2001). Excess FFAs also increase diacylglycerol (DAG), which activates NADPH oxidase through PKC-dependent pathways (Inoguchi et al., 2000). Inhibition of NADPH oxidase activity, by overexpression of dominant negative RAC1 or p67 mutants, blocks FFA-induced apoptosis (Cacicedo et al., 2005). Increased FFA oxidation and the attendant increases in oxidative phosphorylation also have the potential to produce singlet electrons, contributing to oxidative stress. It has also been proposed that FFA remodeling of mitochondrial membranes leads to organelle dysfunction that causes or amplifies oxidative stress. Moreover, extreme or prolonged ER stress leads to oxidative stress. Lipid-induced oxidative stress may involve a combination of these pathways.
FFAs may cause ER stress through direct effects on this organelle or by indirect activation of upstream mediators. Adverse physiochemical properties of saturated triglyceride that accumulates in the ER may disrupt the architecture of this organelle (Moffitt et al., 2005). Alternatively, remodeling of ER membrane lipids by increased saturation of ER membrane acyl chain composition is predicted to favor a thicker, less fluid bilayer structure and may profoundly alter the function of ER integral membrane proteins and membrane function (Borradaile et al., 2006a). Indirectly, lipid-induced ROS may trigger ER stress, since pre-treatment of cardiomyoblasts with the antioxidant alpha-tocopherol (vitamin E) inhibits palmitate-induced ROS generation and attenuates Grp78 and CHOP induction. Nonetheless, the precise mechanism by which oxidative stress leads to ER stress has yet to be determined.
The importance of oxidative and ER stress in the lipotoxic response is underscored by the observation that, in a genetic screen, loss-of-function mutations in genes that disrupt oxidative and ER stress inhibit lipotoxic cell death. Retroviral promoter trap mutagenesis of Chinese hamster ovary cells led to the isolation of mutants disrupted in the expression of eukaryotic elongation factor 1A-1 (eEF1A-1) and the ncRNA gadd7, both of which are induced by lipotoxic stress (Borradaile et al., 2006a; Brookheart et al., 2009). eEF1A-1 is critical for palmitate- and non-lipid-induced ER stress, possibly through its role in regulation of the length and stability of actin filaments. Gadd7 serves as a feed forward regulator of palmitate-induced and generalized oxidative stress.
Additional insights into molecular mechanisms of lipotoxicity have come from studies that compare the response of cultured cells to toxic saturated long-chain FFAs and less-toxic unsaturated FFAs. Transcript profiling of cells treated with palmitate versus oleate demonstrates significant changes in expression of many genes, although the functional role of such changes is unknown. Signaling molecules that are differentially activated by palmitate and oleate include PKC-δ and NFκB, and inhibition of these pathways prevents cell death in insulinoma and retinal pericytes, respectively (Cacicedo et al., 2005; Eitel et al., 2003). By contrast, FFA-induced β-cell death is NFκB independent (Kharroubi et al., 2004). A number of studies have implicated a role for JNK, but it remains uncertain whether this kinase is an activator or executor of the lipotoxic response.
Pathways that modulate lipotoxicity
Molecular pathways that channel FFAs to alternate fates can protect cells from lipotoxicity. Co-supplementation of cells with exogenous saturated and unsaturated FFAs or overexpression of stearoyl CoA desaturase 1 leads to more efficient incorporation of exogenous palmitate into TAG stores and prevents lipotoxic cell death (Listenberger et al., 2003; Maedler et al., 2003). Precisely why unsaturated FFAs favor TAG synthesis and storage is not known. Overexpression of DAG acyltransferase 1 (Dgat1) in skeletal muscle increases TAG stores, decreases DAG and ceramide, and restores insulin sensitivity in HFD mice (Liu et al., 2007). Conversely, Dgat1−/− fibroblasts have increased susceptibility to lipotoxicity. Together, these studies suggest that diversion of excess lipid to neutral lipid droplets is initially protective in the setting of lipotoxic stress. However, either because lipid storage capacity is exceeded, or because TAGs are eventually hydrolyzed, toxic lipids can still become available to disrupt cellular function even in the face of increased TAG stores.
The net effect of channeling excess FFAs to the mitochondria for β-oxidation is less clear. In cultured myoblasts and endothelial cells, activation of AMP kinase, which increases β-oxidation, diminishes palmitate-induced activation of NFκB, apoptosis and cell death (Borradaile et al., 2006b; Cacicedo et al., 2004). Conversely, it has been proposed that increased rates of flux oxidative phosphorylation cycles overwhelms the metabolic machinery, generating increased levels of the free radical superoxide and thus oxidative stress. Under lipotoxic conditions, incomplete β-oxidation contributes to insulin resistance and may play a role in signals for lipotoxic cell death (Koves et al., 2008). The functional state of the mitochondria is likely an important determinant of whether β-oxidation is protective or harmful in lipotoxicity.
Conclusion
An understanding of the molecular events of lipotoxic stress is now beginning to emerge from the study of animal models of diabetes and obesity and from cell culture models of lipid overload. FFAs induce oxidative and ER stress through alterations of organelle membrane structure/function, production of toxic metabolites, and activation of signaling pathways. Although important insights have been gained from simplified in vitro approaches, findings from these studies require extension to in vivo models of pathophysiology. Combining these approaches, future studies of the molecular mechanisms of lipotoxicity will provide insight into the pathogenesis of complications of diabetes and obesity.
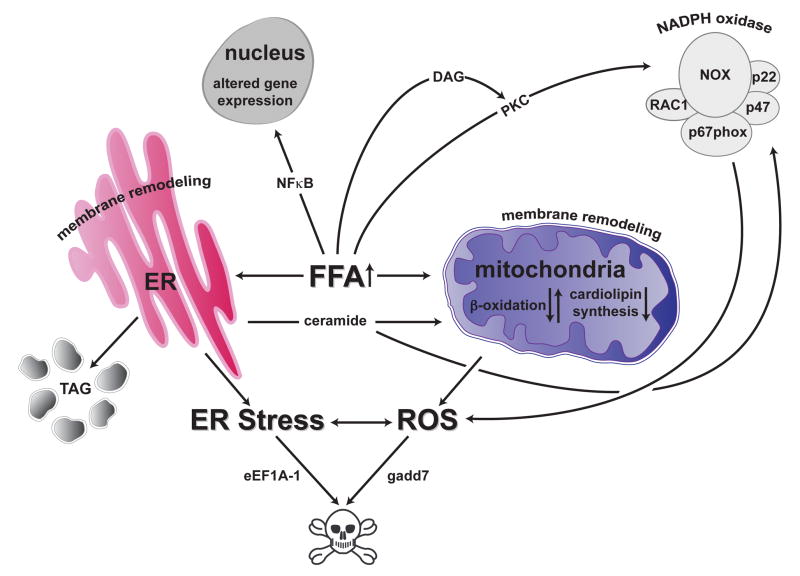
Figure 1. The lipotoxic-response.
In non-adipose cells, excess saturated FFAs induce oxidative and ER stress through lipid metabolites and signaling pathways. Dysfunction of mitochondria and the ER are key steps through which excess lipid induces cell death, whereas channeling of excess FFAs to lipid droplets is cytoprotective.
Acknowledgments
This work was supported by grants from the NIH (DK064989, DK077583, DK077577) and the Burroughs Wellcome Foundation (1005935).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borradaile NM, Buhman KK, Listenberger LL, Magee CJ, Morimoto ET, Ory DS, Schaffer JE. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol Biol Cell. 2006a;17:770–778. doi: 10.1091/mbc.E05-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006b;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and ER stress. J Biol Chem. 2009 doi: 10.1074/jbc.M806209200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem. 2007;282:3989–3997. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38:1384–1394. [PubMed] [Google Scholar]
- Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, Bretzel RG, Haring HU, Kellerer M. Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes. 2003;52:991–997. doi: 10.2337/diabetes.52.4.991. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochem Biophys Res Commun. 2006;344:446–452. doi: 10.1016/j.bbrc.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]