Abstract
Reactive oxygen species (ROS) are produced as by-products of oxidative metabolism and occur in the heart during ischemia and coronary artery reperfusion. The effects of ROS on the electrophysiological properties of intracardiac neurons were investigated in the intracardiac ganglion (ICG) plexus in situ and in dissociated neurons from neonatal and adult rat hearts using the whole-cell patch clamp recording configuration. Bath application of ROS donors, hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (t-BHP) hyperpolarized, and increased the action potential duration of both neonatal and adult ICG neurons. This action was also recorded in ICG neurons in an adult in situ ganglion preparation. H2O2 and t-BHP also inhibited voltage-gated calcium channel (VGCC) currents and shifted the current–voltage (I–V) relationship to more hyperpolarized potentials. In contrast, H2O2 increased the amplitude of the delayed rectifier K+ current in neonatal ICG neurons. In neonatal ICG neurons, bath application of either superoxide dismutase (SOD) or catalase, scavengers of ROS, prior to H2O2 attenuated the hyperpolarizing shift but not the inhibition of VGCC by H2O2. In contrast, in adult ICG neurons, application of SOD alone had no effect upon either VGCC current amplitude or the I–V relationship, whereas application of SOD prior to H2O2 exposure abolished both the H2O2-mediated hyperpolarizing shift and inhibition. These data indicate that ROS alter depolarization-activated Ca2+ and K+ conductances which underlie neuronal excitability of ICG neurons. This affects action potential duration and therefore probably modifies autonomic control of the heart during ischemia/reperfusion.
Keywords: Hydrogen peroxide, Intracardiac neuron, Ischaemia, Voltage-gated calcium channel, Delayed rectifier potassium channel
1. Introduction
Mammalian intrinsic cardiac ganglia (ICG) are composed of clusters of neurons located throughout the atrial epicardium and interatrial septum, which receive both parasympathetic (vagal) and sympathetic input (Adams and Cuevas, 2004; Parsons, 2004). Given that electrical or chemical activation of mammalian intrinsic cardiac neurons are able to modify cardiac function (Butler et al., 1990a,b), it has been proposed that the intrinsic cardiac nervous system acts as the final common pathway for the control of regional cardiac function (Horackova and Armour, 1995; Arora et al., 2000).
The location of the mammalian ICG make them susceptible to the effects of myocardial ischemia and reperfusion associated with coronary heart disease (Horackova and Armour, 1995; Armour, 1999). Each ganglionated plexus in the human heart is perfused by two or more arterial branches arising from different major coronary arteries, and the activity of ICG neurons is modified by transient coronary occlusion (Huang et al., 1993). Coronary occlusion results in lack of oxygen and metabolic substrates, as well as the accumulation of local ischemic products such as oxygen-derived free radicals. The resulting autonomic imbalance may contribute to the development of arrhythmias during acute myocardial ischemia. Disordered function of autonomic efferent neurons can also result in coronary vascular dysfunction, which in turn can lead to loss of cardiomyocyte contractile function and heart failure (Armour, 1999).
Oxygen-derived oxidants, O2−•, H2O2 and •OH−, are produced as by-products of oxidative metabolism (for review see Kourie, 1998; Dröge, 2001). Reactive oxygen species (ROS) are produced extracellularly and intracellularly in the heart during ischemia and during coronary artery reperfusion and have been implicated in cardiac dysfunction (Huang et al., 1993; O'Neill et al., 1996; Armour, 1999). The proximity of ICG to the coronary blood supply make them susceptible to the effects of ROS. Studies carried out on canine cardiac ganglia in situ have revealed that H2O2 administered to the local blood supply modifies neuronal firing activity (Thompson et al., 1998). ROS produced by the myocardium during ischemia-reperfusion have been shown to alter the firing properties of cardiac sensory neurites associated with afferent axons in vagal and sympathetic nerves (Ustinova and Schultz, 1994; Huang et al., 1995). However, the effects of ROS on membrane currents and neuronal excitability on individual neurons in mammalian ICG have not been investigated either in intact ganglia or after dissociation.
The major hypothesis of this study is that ROS, generated during myocardial ischemia and post-ischemic reperfusion, influence the electrical activity of ICG neurons via the modulation of voltage-dependent Ca2+ and K+ conductances. Given the proposed importance of the intrinsic cardiac nervous system to the control of regional cardiac function, the response of ICG neurons to ROS donors may shed light on the pathological changes affecting the ability of these cells to stabilize cardiac function. Preliminary reports of some of these results have been presented in abstract form (Whyte et al., 2003; Dyavanapalli et al., 2008).
2. Materials and methods
2.1. Electrophysiological recordings from intracardiac ganglion preparations in situ
Young adult female Wistar rats (150–220 g) were killed by stun and cervical dislocation, in accordance with current UK Home Office guidelines. The hearts were quickly excised and atria isolated and placed in cold Krebs solution. The right atrial ganglion plexus and underlying myocardium were isolated from the dorsal surface of the atria. The preparation was pinned to the Sylgard (Dow-Corning, Midland, MI) covered base of a 35 mm tissue culture dish and superfused with a bicarbonate-buffered Krebs solution comprising (in mM): NaCl 118.4, NaHCO3 25.0, NaH2PO4 1.13, CaCl2 1.8, KCl 4.7, MgCl2 1.3 and glucose 11.1, gassed with Carbogen (95% O2:5% CO2) to pH 7.4 (Rimmer and Harper, 2006). The recording chamber was continuously superfused (∼ 2 ml/min) with Krebs solution at 35 °C. The temperature of the solution was controlled by Peltier thermoelectric elements (Medical systems PDMI-2 micro incubator, NY, USA) and monitored by an independent thermistor probe in the recording chamber (Yellow Springs Instruments, Yellow Springs, OH). Intracellular recordings were made using sharp glass microelectrodes (GC120F; Harvard Apparatus Ltd., Edenbridge, UK) with ∼ 120 MΩ resistance when filled with 0.5 M KCl. Membrane voltage responses were recorded with a conventional bridge amplifier (Axoclamp 2A; Molecular Devices, Sunnyvale, CA). Signals were filtered at 20 kHz, digitized at 50 kHz and transferred to a Pentium 4 computer using an analogue-to-digital converter (Micro 1401 interface, CED, Cambridge, UK) and Spike 2 software (CED). Electrophysiological properties and responses of the neurons were analyzed using the same program. Pharmacological antagonists were bath applied at the concentrations indicated.
Brief intracellular depolarizing currents (≤ 3 ms in duration) were used to directly evoke single somatic action potentials. Action potential parameters measured were: the overshoot, rate of rise (drise/dt), rate of fall (dfall/dt) and the afterhyperpolarization (AHP) following the action potential, characterized by its amplitude and decay time. Long (500 ms) hyperpolarizing and depolarizing pulses were applied to measure time constant, and evoked discharge characteristics. Time constant (τ) was measured at small hyperpolarizing current pulses (≤ − 0.1 nA) using Spike 2 software. Membrane resistance (Rm) was calculated from the time constant, τ = Rm · Cm, where Cm is the specific membrane capacitance, assumed to be 1 µF cm− 2.
2.2. Dissociated intracardiac ganglion neurons
Neurons from neonatal (3–10 day old) and adult (> 6 weeks old) rat ICG were isolated and placed in tissue culture. The majority of neurons (> 60%) in the rat intracardiac nerve plexus are unipolar with either none or few neurites (Pauza et al., 1997). The procedures for isolation of the ICG neurons have been described previously (Xu and Adams, 1992; Hogg et al., 2001). The protocols of our experiments were approved by the University of Queensland Animal Experimentation Committee, an independent review committee, and the investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Briefly, Wistar rats were sacrificed by stunning and cervical dislocation and the heart excised and placed in Hank's balanced salt solution (HBSS). Atria were isolated and the atrial ganglion plexus located around the pulmonary veins and superior vena cava on the dorsal surface of the atria (Batulevičius et al., 2003) were dissected and transferred to HBSS containing 1.2 mg/ml collagenase (Type 2, 300 U/ml; Worthington-Biochemical, Freehold, NJ). Following 1 h digestion, the ganglia were rinsed twice in HBSS and triturated in culture medium (Dulbecco's modified Eagle medium containing 10% (v/v) fetal calf serum and 1% (v/v) penicillin–streptomyocin) (Gibco — Invitrogen, Melbourne, Australia) using a fine-bore Pasteur pipette. The dissociated neurons were plated on laminin-coated coverslips and incubated at 37 °C in a 95% O2:5% CO2 atmosphere for 12–24 h.
2.3. Electrophysiological recording
Membrane voltage and current were recorded in a whole-cell (dialyzed or perforated) patch-clamp configuration using an Axopatch 200A patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). Voltage and current protocols were applied using pClamp software (Molecular Devices). Signals were filtered at 5 kHz, digitized at 20 kHz and transferred to a Pentium PC using a Digidata 1200 A/D interface (Molecular Devices).
In voltage-clamp (dialyzed whole-cell) experiments to record depolarization-activated calcium channel currents, Ba2+ was used as the charge carrier and the extracellular solution contained (in mM): 143 TEACl, 5 BaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, adjusted to pH 7.4 with TEAOH. Pipettes were filled with solution containing (in mM): 135 CsCl, 1 MgCl2, 14 triphosphocreatinine, 3.6 MgATP, 50 µl/ml creatinine phosphokinase and 10 HEPES, adjusted to pH 7.2 with CsOH. The osmolarity of the extra- and intracellular solutions was monitored by a vapor pressure osmometer (Wescor 5500, Logan, UT) and were in the range 295–310 mOsm/kg.
In experiments to record outward K+ currents (voltage clamp) and action potential firing (current clamp), cells were recorded using the perforated-patch recording configuration. Cells were superfused with extracellular solution containing (in mM): 140 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 7.7 glucose and 10 HEPES-NaOH, pH 7.2. Action potential duration (APD) at 0 mV was measured in the absence and presence of ROS donors bath applied at maximally effective concentrations. The concentrations tested (100–1000 µM) were similar to those shown previously to affect the excitability of canine intrinsic cardiac neurons in situ (Thompson et al., 1998). Total outward K+ currents were recorded in the presence of 300 nM tetrodotoxin (TTX) to inhibit depolarization-activated Na+ currents, and delayed rectifier K+ currents were isolated by the addition of 100 µM CdCl2 to the extracellular solution to inhibit voltage-dependent Ca2+ currents and Ca2+-activated K+ currents (Xu and Adams, 1992). Pipettes were filled with solution containing (in mM): 75 K2SO4, 55 KCl, 5 MgSO4 and 10 HEPES-KOH, adjusted to pH 7.2 with N-methyl-d-glucamine. Amphotericin-B in dimethylsulfoxide was added at a final concentration of 240 µg/ml. The temperature of the superfusing solutions was controlled ± 1 °C by a Peltier thermoelectric device and monitored by a thermistor probe in the recording chamber. Recordings were made at either 22 °C or 37 °C as indicated. Data are expressed as mean ± S.E.M. of the number of observations indicated, and were compared using ANOVA and paired t-tests (SigmaStat 3.1, Systat Software Inc., San Jose, CA) with statistical significance (P value) as indicated.
All chemicals were of analytical grade. These included amphotericin-B, hydrogen peroxide (H2O2;) tert-butyl hydroperoxide (t-BHP), catalase, glibenclamide, superoxide dismutase (SOD), tetraethylammonium chloride (TEA), and tetrodotoxin (all from Sigma Chemical Co.).
3. Results
Dissociated neonatal ICG neurons were smaller in size than those from adults, and had a mean cell capacitance of 18.8 ± 3.2 pF (n = 45) compared with 52.4 ± 5.6 pF (n = 39) for adult neurons. Resting membrane potential was − 53.0 ± 1.2 mV (n = 45) in neonatal rat ICG neurons, and − 53.6 ± 1.6 (n = 39) in adult cells, which is in accordance with previously reported values (Hogg et al., 2001).
3.1. Effect of ROS donors on resting and action potentials
Under current clamp conditions, bath application of the ROS donors H2O2 or t-BHP reversibly hyperpolarized dissociated neonatal and adult ICG neurons, at both 22 °C and 37 °C (Fig. 1). At 22 °C, H2O2 and t-BHP, at a maximally effective concentration of 1 mM, hyperpolarized the neonatal and adult ICG neurons by approximately 4–10 mV (see Table 1). At 37 °C, 1 mM H2O2 and 1 mM t-BHP hyperpolarized the neonatal ICG neurons by 9 ± 1.4 mV (n = 4) and 4 ± 0.6 mV (n = 3), respectively, whereas 1 mM H2O2 hyperpolarized the adult cells by 13.2 ± 2.2 mV (n = 3). At both temperatures, membrane hyperpolarization caused by exposure to either H2O2 or t-BHP was not reversed by bath application of either 10 µM glibenclamide (n = 4) or Ba2+ (n = 3), inhibitors of KATP channels present in adult ICG neurons (Hogg and Adams, 2001).
Fig. 1.
Effect of ROS donors, H2O2 and t-BHP, on resting and action potentials in neonatal and adult rat ICG neurons. Superimposed traces of action potential discharge in neonatal (A) and adult (B) rat ICG neurons evoked by a + 200 pA current pulse from a holding potential of − 60 mV. Temperature 22 °C. The dashed line represents 0 mV. In the presence of either 1 mM H2O2 or t-BHP (light traces), ICG neurons were reversibly hyperpolarized by > 5 mV and the action potential duration increased (n ≥ 4).
Table 1.
Effect of ROS donors on resting membrane potential (RMP) and action potential duration (APD) in rat ICG neurons.
| Preparation | 1 mM H2O2 (n) |
1 mM t-BHP (n) |
|||
|---|---|---|---|---|---|
| Δ RMP (mV) | APD (% increase) | Δ RMP (mV) | APD (% increase) | ||
| Neonate ICG | 22 °C | − 7.9 ± 2.0 (4) | 16.2 ± 2.6 (4)⁎⁎ | − 5.6 ± 1.6 (4) | 11.2 ± 2.8 (4)⁎ |
| 37 °C | − 9.0 ± 1.4 (4) | – | − 4.0 ± 0.6 (3) | – | |
| Adult ICG | 22 °C | − 5.7 ± 0.9 (9) | 24.9 ± 2.8 (9)⁎⁎ | − 8.0 ± 1.2 (3) | 24.1 ± 1.8 (9)⁎⁎ |
| 37 °C | − 13.2 ± 2.2 (3) | – | – | – | |
Δ RMP, change in resting membrane potential; –, not determined; n, number of cells; ⁎P < 0.05, ⁎⁎P < 0.01.
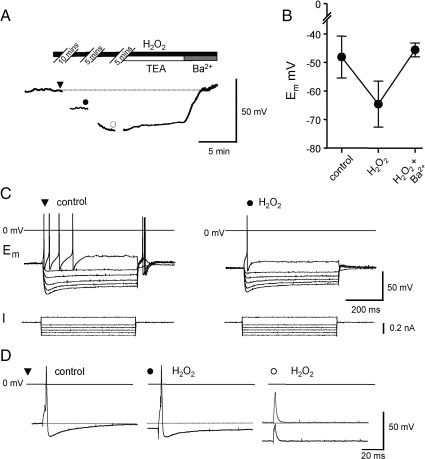
The action of the ROS donors H2O2 and t-BHP on the resting membrane potential of adult ICG neurons in the in situ preparation was in good agreement with that on dissociated ICG neurons. In the in situ ICG preparation, at 35 °C, both H2O2 and t-BHP evoked a membrane hyperpolarization. Superfusion of H2O2 (1 mM) hyperpolarized the resting membrane potential by 16.7 ± 0.4 mV (n = 9, from − 47.5 ± 2.4 mV to − 64. 2 ± 2.9 mV, P < 0.001, paired t-test.) A lower concentration of H2O2 (0.5 mM) also caused a modest hyperpolarization of 6.7 ± 1.3 mV (n = 4). Similarly, t-BHP (1 mM) produced a hyperpolarizing shift in membrane potential of − 5.5 ± 0.2 mV (n = 8, from − 51.5 ± 3.0 mV to − 57.0 ± 2.8 mV). The hyperpolarization produced by H2O2 was accompanied by a decrease in membrane resistance from 5.1 ± 0.8 kΩ cm2 to 4.0 ± 0.6 kΩ cm2 (n = 7, P < 0.05) and the membrane resistance was reduced to 0.57 ± 0.14 of control values (n = 5, P < 0.05) upon superfusion of t-BHP (1 mM) indicating that an increase in K+ conductance was most likely responsible. To test for this, tetraethylammonium (TEA, 10 mM), an inhibitor of voltage- and Ca2+-dependent K+ channels and Ba2 (1 mM), a wide-spectrum K+ channel inhibitor, were applied to preparations in which the H2O2-induced hyperpolarization had fully developed. The hyperpolarizing shift in membrane potential was partially reversed by TEA, but fully reversed by Ba2+ (see Fig. 2A). In a group of 5 neurons, the resting membrane potential was − 48.1 ± 3.3 mV in control conditions, − 64.6 ± 3.6 mV in the presence of H2O2 and − 45.6 ± 2.4 mV following superfusion of Ba2+ (see Fig. 2B). Bath application of apamin (100 nM), an SKCa channel inhibitor, iberiotoxin (100 nM), a BKCa channel inhibitor, and glibenclamide (10 µM) did not reverse the effects of H2O2 (data not shown).
Fig. 2.
Effect of H2O2 on resting and action potentials in adult rat ICG neurons in situ. (A) Representative membrane potential responses of an adult ICG neuron in situ to superfusion of H2O2 (1 mM) and subsequently co application of 10 mM TEA and 1 mM Ba2+. Temperature 35 °C. (B) Ba2+ (1 mM) reverses the membrane hyperpolarization observed in the presence of H2O2. The membrane potential, taken for a group of 5 neurons, is presented for control conditions, in the presence of H2O2 (1 mM) and following superfusion of Ba2+. (C) Voltage responses (upper traces) obtained in response to depolarizing and hyperpolarizing current pulses (+ 0.2, − 0.1 to − 0.5 nA, lower traces) are shown for control conditions and after 10 min exposure to 1 mM H2O2. (D) Representative somatic action potential traces elicited by brief depolarizing current pulses (2 ms) showing the time-dependent decrease in excitability in the presence of H2O2. The symbols denote the times at which the responses were taken in A. The membrane potential was reset to control values (grey trace) in the recording taken at 20 min to test if inexcitability was secondary to membrane hyperpolarization. Stimulus current was + 0.4 nA (▼), + 0.6 nA (●), and + 0.6 nA (○) in the absence and presence of a + 0.25 nA holding current (grey trace).
Bath application of either 1 mM H2O2 or 1 mM t-BHP increased action potential duration by 11–16% in dissociated neonatal ICG neurons (n = 8; P < 0.05) and by ∼ 25% in adult cells compared to control (n = 18; P < 0.01). Action potential duration was measured as neither H2O2 nor t-BHP appreciably changed the frequency of action potential firing in response to depolarizing current pulses in dissociated ICG neurons (Fig. 1A,B). In contrast, in the in situ preparation, the evoked response to long depolarizing current pulses was switched from predominantly multiple adapting to phasic or unresponsive by H2O2 and t-BHP (see Fig. 2C). Furthermore, t-BHP (1 mM) reduced the action potential AHP amplitude from 16.5 ± 5.1 mV (control) to 9.9 ± 4.4 mV (t-BHP; n = 10, P < 0.001) but had no significant effect on either action potential overshoot or AHP duration, as gauged by time to 50% recovery. However, the absolute amplitude of AHP (measured from 0 mV) was unchanged in the presence of t-BHP indicating the change was secondary to the membrane hyperpolarization produced by t-BHP (Fig. 2D). t-BHP reduced the maximum rate of rise and fall of the action potential to 74 ± 14% and 72 ± 6%, respectively, of its value under control conditions (n = 6; P < 0.05 max drise/dt and P < 0.005 dfall/dt paired t-test). This resulted in an increase in action potential duration (measured at 0 mV) of approximately 55%.
3.2. Inhibition of voltage-gated Ca2+ channel currents by ROS donors
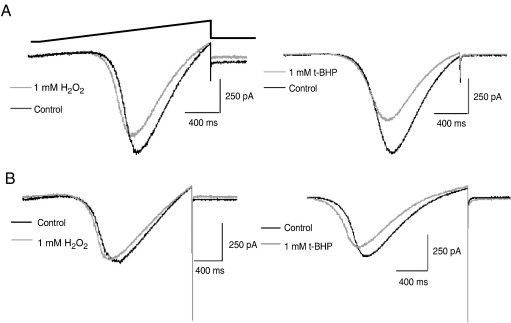
Under voltage clamp conditions, bath application of either 1 mM H2O2 or 1 mM t-BHP significantly inhibited the depolarization-activated Ca2+ channel current in both neonatal and adult ICG neurons (Fig. 3). In the presence of 300 nM TTX and using Ba2+ as the charge carrier, voltage ramps from − 70 mV to + 40 mV evoked an inward Ba2+ current which was inhibited by 14–18% and 10–16% in neonatal and adult ICG neurons, respectively, in the presence of the ROS donors (see Table 2). The whole-cell current–voltage (I–V) relationship obtained in both neonatal and adult ICG neurons was shifted to a more hyperpolarized membrane potential (Fig. 3A,B) which was reversible upon washout of H2O2 and t-BHP.
Fig. 3.
Effect of ROS donors on Ca2+ channel currents in neonatal and adult ICG neurons. Superimposed inward Ba2+ currents evoked in response to voltage ramps from − 70 mV to + 40 mV in the absence (control) and presence of 1 mM H2O2 and t-BHP (light traces) in neonatal (A) and adult (B) rat ICG neurons (n = 3–6). Temperature 22 °C.
Table 2.
Effect of ROS donors and pretreatment with SOD and catalase on voltage-gated calcium channel (VGCC) currents from neonatal and adult rat ICG neurons (22 °C).
| Preparation | % inhibition of VGCC current amplitude (n) |
|||
|---|---|---|---|---|
| 1 mM H2O2 | 1 mM t-BHP | 100 U/ml SOD | 100 U/ml catalase | |
| Neonate ICG | 13.9 ± 2.6 (8) ⁎⁎ | 17.9 ± 1.9 (5) ⁎⁎ | 12.5 ± 4.8 (4) | 13.7 ± 3.7 (4) |
| Adult ICG | 9.8 ± 1.4 (6) ⁎ | 16.5 ± 1.6 (3) ⁎⁎ | 0 (3) | ND |
ND, not determined; n, number of cells; ⁎P < 0.05, ⁎⁎P < 0.01.
3.3. Effect of pretreatment with ROS scavengers
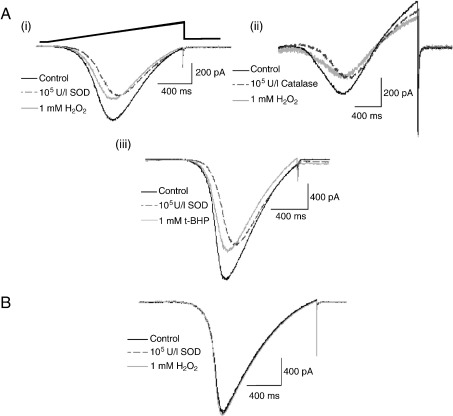
In neonatal ICG neurons, both SOD and catalase (100 U/ml) reduced non-significantly the voltage-gated Ca2+ channel (VGCC) current amplitude (Table 2) and shifted the I–V relationship to more a depolarized potential as shown in Fig. 4A. Bath application of SOD or catalase prior to exposure to either H2O2 or t-BHP attenuated the hyperpolarizing shift, but did not reverse the inhibition of the inward Ba2+ current amplitude by H2O2 and t-BHP (data not shown).
Fig. 4.
Effect of pre-application of ROS scavengers on the effects of H2O2 and t-BHP on Ca2+ channel currents in neonatal and adult rat ICG neurons. A: Superimposed inward Ba2+ currents recorded at 22 °C in response to voltage ramps from − 70 mV to + 40 mV in neonatal rat ICG neurons. Representative currents obtained in the absence (control) and presence of either 100 U/ml SOD (i) or catalase (ii) applied before and following exposure to 1 mM H2O2 (light traces). (iii) Bath application of SOD prior to exposure to 1 mM t-BHP attenuated the hyperpolarizing shift of the I–V relationship but not the inhibition of the inward Ba2+ current by t-BHP (n = 11). B: Superimposed inward Ba2+ currents obtained in the absence (control) and presence of 100 U/ml SOD before and after application of 1 mM H2O2 in adult ICG neurons (n ≥ 3). Inward currents were evoked by a 110 mV voltage ramp as illustrated in A(i).
In contrast, application of SOD (100 U/ml) alone had no significant effect upon either inward Ba2+ current amplitude (Table 2) or the I–V relationship in adult ICG neurons, but application of 100 U/ml SOD prior to exposure to either H2O2 or t-BHP abolished the hyperpolarizing shift of the I–V curve, as well as inhibition of the Ba2+ current by both ROS donors (Fig. 4B).
Considering the in situ ICG preparation, superfusion of either SOD (100 U/ml) or catalase (100 U/ml) had no significant action on membrane potential or directly evoked somatic action potential characteristics. Superfusion of SOD (100 U/ml) and H2O2 (1 mM) attenuated the membrane hyperpolarization of H2O2 in the majority of neurons studied (3/5). Similarly, the action of H2O2 was abrogated by catalase (100 U/ml) changing by − 1.8 ± 0.9 mV (n = 4) upon co-application of H2O2 and catalase (data not shown).
3.4. Potentiation of delayed rectifier K+ currents by ROS donors
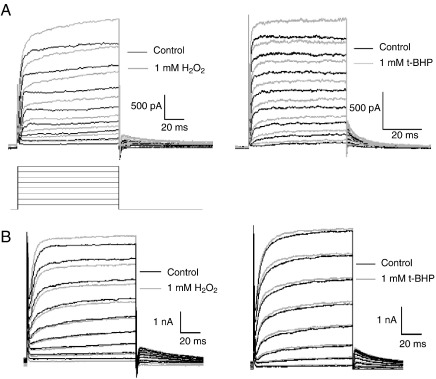
The total outward K+ current recorded in rat ICG neurons consists of both Ca2+-dependent and Ca2+-independent, delayed rectifier K+ currents, with the Ca2+-activated K+ current accounting for approximately one-third of the total outward K+ current (Xu and Adams, 1992; Xi-Moy and Dun, 1995). In the presence of either H2O2 or t-BHP (1 mM), the total outward K+ current was reduced by 12.8 ± 2.3% and 13.9 ± 1.3% compared to control (n = 4; P < 0.05) in neonatal and adult ICG neurons, respectively. The reduction in the amplitude of the total outward K+ current is largely a consequence of the inhibition of the VGCC-mediated Ca2+ influx by ROS donors. In contrast, bath application of either 1 mM H2O2 or 1 mM t-BHP increased the amplitude of the Ca2+-independent, delayed rectifier K+ current evoked by depolarizing voltage steps from − 50 to + 50 mV (Fig. 5A,B). H2O2 (1 mM) increased the delayed rectifier K+ current amplitude at + 50 mV by 15.5 ± 0.4% and 11.5 ± 1.0% compared to control (n = 3; P < 0.05) in neonatal and adult ICG neurons, respectively. Similarly, t-BHP increased the delayed rectifier K+ current amplitude at + 50 mV by 18.3 ± 2.1% (n = 3; P < 0.01) in neonatal ICG neurons and 12.9 ± 4.5% (n = 3) in adult ICG neurons. The delayed rectifier K+ current recorded in the presence of H2O2 and t-BHP was reversibly inhibited by extracellular TEA (10 mM) (data not shown).
Fig. 5.
Effect of ROS donors, H2O2 and t-BHP, on the delayed rectifier K+ current in neonatal and adult rat ICG neurons. Superimposed outward K+ currents obtained in the absence (control) and presence of 1 mM H2O2 and 1 mM t-BHP bath applied (light traces) in neonatal (A) and adult (B) rat ICG neurons (n = 5). Outward K+ currents recorded at 22 °C in the presence of 300 nM TTX and 100 µM Cd2+ extracellularly, were evoked by depolarizing voltage steps in 10 mV increments from a holding potential of − 50 mV.
4. Discussion
This is the first study to directly examine the effects of ROS donors on the electrophysiological properties of mammalian intrinsic cardiac neurons. The ROS donors H2O2 and t-BHP reversibly hyperpolarized and increased the action potential duration in dissociated neurons of both neonatal and adult rat ICG. The action of these agents on neurons was also investigated in the in situ adult ICG preparation. Dissociated neurons do not have surrounding satellite glial cells which encapsulate ICG neuronal somata (Baluk, 1995) and may release and/or take up substances which buffer or exacerbate the effects of ROS donors. Furthermore, there is accruing evidence that the expression and distribution of membrane receptors and ion channels in dissociated autonomic ganglion neurons are not necessarily the same as that in the intact ganglion (e.g. Martínez-Pinna et al., 2002; Rimmer and Harper, 2006). Therefore, in order to relate the effects of ROS on the electrophysiological properties of dissociated neurons to physiological function, it is important to complement these with studies in intact ganglion preparations. The action of H2O2 and t-BHP on the passive and active electrical properties of neurons in the intact adult ICG are in good agreement with those for dissociated adult ICG neurons. The small depolarization recorded with co application of H2O2 and Ba2+ compared with control values is consistent with the contribution of the M-current to the resting membrane potential in these neurons (Cuevas et al., 1997; Rimmer and Harper, 2004).
H2O2 and t-BHP also inhibited VGCC currents and shifted the I–V relationship to more hyperpolarized potentials. The ROS scavengers SOD and catalase inhibited the VGCC currents in neonatal ICG cells but shifted the I–V relationship to more depolarized potentials. Application of SOD or catalase to neonatal ICG cells prior to H2O2 or t-BHP exposure attenuated the hyperpolarizing shift, but not the inhibition by these ROS donors. In contrast, in adult cells, application of SOD alone had no effect upon either VGCC current amplitude or the I–V relationship. Furthermore, application of SOD prior to H2O2 treatment abolished the hyperpolarizing shift and inhibition of VGCC currents by H2O2.
Outward repolarizing K+ currents are important determinants of action potential duration and neuronal firing properties. The action potential duration in both neonatal and adult ICG neurons was increased by approximately 11–25% in the presence of either H2O2 or t-BHP but the frequency of action potential firing in response to depolarizing current pulses was unchanged. In the presence of either H2O2 or t-BHP, the total outward K+ current was reduced by ∼ 13% as a consequence of the reduced Ca2+ influx through open VGCCs. However, in contrast, H2O2 and t-BHP increased the amplitude of the Ca2+-independent, delayed rectifier K+ currents by approximately 12–18% in both neonatal and adult ICG neurons. Given that both delayed rectifier K+ currents and Ca2+-dependent K+ currents contribute to the repolarization of the action potential in rat ICG neurons (Adams and Cuevas, 2004), the action potential duration is likely to reflect the balance between the inhibition of the Ca2+-dependent K+ current and the potentiation of the delayed rectifier K+ current. We also observed the reversible prolongation of action potential duration by ROS donors in adult rat ICG neurons of the whole-mount right atrial ganglion plexus preparation using sharp microelectrode recording techniques. The prolongation of action potential duration is likely to affect transmitter release from postganglionic nerve terminals in the heart, consistent with the actions of H2O2 on quantal release and presynaptic potentiation during high frequency stimulation observed at the neuromuscular junction (Giniatullin and Giniatullin, 2003).
Although the actions of ROS on membrane conductances have not been investigated previously in mammalian intracardiac neurons, the effects of ROS on neuronal excitability have been studied in myenteric neurons in guinea-pig distal colon in situ (Wada-Takahashi and Tamura, 2000) and neurons isolated from guinea-pig intestine and grown in primary culture (Vogalis and Harvey, 2003). The majority of distal colon myenteric neurons exhibited a transient membrane hyperpolarization followed by a long-lasting depolarization in response to hypoxanthine and xanthine oxidase which was attributed to suppression and augmentation of the conductance of Ca2+-dependent K+ channels, respectively (Wada-Takahashi and Tamura, 2000). In contrast, application of sub-millimolar H2O2 to cultured intestinal neurons induced membrane hyperpolarization, and a concomitant suppression of the slow afterhyperpolarization which was attenuated by internal perfusion of catalase and glutathione (Vogalis and Harvey, 2003). Under voltage clamp conditions, the acute oxidative stress produced by H2O2 was shown to be due to the suppression of a hyperpolarization-activated inward current (Ih) and the activation of an inwardly rectifying outward current blocked by glibenclamide and TEA.
Electrophysiological studies have also shown varied effects of ROS donors on action potential amplitude and duration in guinea pig ventricular myocytes (Hayashi et al., 1989; Cerbai et al., 1991). In this system, the prolongation of action potential duration by dihydroxyfumarate-generated ROS was accompanied by a decrease in VGCC current and time-dependent outward K+ current amplitude (Cerbai et al., 1991). Similarly, H2O2 inhibited the L-type Ca2+ current but either activated an outward membrane current consistent with the ATP-sensitive K+ current (Goldhaber and Liu, 1994) or enhanced the delayed rectifier K+ current (Satoh and Matsui, 1997). However, in these experiments, no attempt was made to differentiate between the effects of ROS on the IKr (rapidly activating/deactivating) and IKs (slowly activating/deactivating) components of the delayed rectifier K+ current and the inward rectifier K+ current was unaffected (Sanguinetti and Jurkiewicz, 1990). Similarly, investigation of the effects of H2O2 (200 µM) on action potentials and underlying ionic currents in single myocytes from the ventricles of adult rat hearts using the amphotericin-perforated patch clamp method showed that H2O2 caused a marked prolongation of the action potential but no significant changes in either the Ca2+-independent transient outward K+ current (Ito) or the inwardly rectifying K+ current (IK1) (Ward and Giles, 1997). The most prominent effect of H2O2 on the ionic currents, which underlie the action potential, was a slowing of inactivation of the TTX-sensitive Na+ current. ROS produced by exposure to either H2O2 or FeSO4 and ascorbic acid has also been shown to inhibit several cloned K+ channels expressed in Xenopus oocytes, including HERG (KV11.1), HukII (KV1.4), KShIIID (KV3.3), and KShIIIC (KV4.4) but not rDRK1 (KV2.1), bEAG (KV10.1) or mIRK1 (Kir2.1) (Vega-Saenz de Miera and Rudy, 1992; Taglialatela et al., 1997). Likewise, the activity of three Shaker K+ channels (KV1.3, KV1.4, and KV1.5), one Shaw channel (KV3.4) and one inward rectifier K+ channel (IRK3) expressed in Xenopus oocytes was inhibited by photoactivation of rose bengal, a generator of ROS, whereas other K+ channel types (KV1.2, KV2.1, KV2.2, KV4.1, inward rectifiers IRK1 and ROMK1 and hIsK) were completely resistant to this treatment (Duprat et al., 1995). A recent study of the effect of H2O2 on the KV7 family demonstrated a strong enhancement of KV7.2, 7.4 and 7.5 mediated K+ currents by H2O2 (0.3 mM) due to the oxidative modification of cysteines in a triple cysteine pocket within the cytosolic S2–S3 linker (Gamper et al., 2006). Oxidative-induced enhancement of M-current induced a hyperpolarization and a reduction of action potential firing frequency in rat sympathetic neurons (Gamper et al., 2006) consistent with our observations in rat ICG neurons in situ.
In conclusion, there is considerable evidence demonstrating that cardiac autonomic regulation may be modulated by ROS, the behavior of which is dictated by the oxidative stress of the microenvironment (for review see Danson and Paterson, 2006). Our results suggest that free radicals generated during ischemia and reperfusion may contribute to electrophysiologic abnormalities in neonatal and adult rat ICG neurons by modifying resting and depolarization-activated Ca2+ and K+ conductances which underlie neuronal excitability. Changes to these membrane conductances account for the prolongation of action potential duration by ROS donors and therefore probably modify autonomic control of the mammalian heart.
Acknowledgements
This work was supported by a National Health & Medical Research Council Project Grant to D.J.A. and British Heart Foundation Project Grant No. PG/06/132/21753 to A.A.H. K.A.W. was a recipient of a Royal Society Postdoctoral Fellowship.
References
- Adams D.J., Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Ardell J.L., Armour J.A., editors. Basic and Clinical Neurocardiology. Oxford University Press; Oxford: 2004. pp. 1–60. [Google Scholar]
- Armour J.A. Myocardial ischaemia and the cardiac nervous system. Cardiovasc. Res. 1999;41:41–54. doi: 10.1016/s0008-6363(98)00252-1. [DOI] [PubMed] [Google Scholar]
- Arora R.C., Ardell J.L., Armour J.A. Cardiac denervation and cardiac function. Curr. Interv. Cardiol. Rep. 2000;2:188–195. [PubMed] [Google Scholar]
- Baluk P. Structure of autonomic ganglia. In: McLachlan E.M., editor. vol. 6. Harwood Academic Publishers; London: 1995. pp. 13–71. (Autonomic Ganglia). [Google Scholar]
- Batulevičius D., Paužienė N., Pauža D.H. Topographic morphology and age-related analysis of the neuronal number of the rat intracardiac nerve plexus. Ann. Anat. 2003;185:449–459. doi: 10.1016/S0940-9602(03)80105-X. [DOI] [PubMed] [Google Scholar]
- Butler C.K., Smith F.M., Cardinal R., Murphy D.A., Hopkins D.A., Armour J.A. Cardiac responses to electrical stimulation of discrete loci in canine atrial and ventricular ganglionated plexi. Am. J. Physiol., Heart Circ. Physiol. 1990;259:H1365–H1373. doi: 10.1152/ajpheart.1990.259.5.H1365. [DOI] [PubMed] [Google Scholar]
- Butler C.K., Smith F.M., Nicholson J., Armour J.A. Cardiac effects induced by chemically activated neurons in canine intrathoracic ganglia. Am. J. Physiol., Heart Circ. Physiol. 1990;259:H1108–H1117. doi: 10.1152/ajpheart.1990.259.4.H1108. [DOI] [PubMed] [Google Scholar]
- Cerbai E., Ambrosio G., Porciatti F., Chiariello M., Giotti A., Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- Cuevas J., Harper A.A., Trequattrini C., Adams D.J. Passive and active membrane properties of isolated rat intracardiac neurons: regulation by H- and M-currents. J. Neurophysiol. 1997;78:1890–1902. doi: 10.1152/jn.1997.78.4.1890. [DOI] [PubMed] [Google Scholar]
- Danson E.J.F., Paterson D.J. Reactive oxygen species and autonomic regulation of cardiac excitability. J. Cardiovasc. Electrophysiol. 2006;17:S104–S112. doi: 10.1111/j.1540-8167.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2001;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Duprat F., Guillemare E., Romey G., Fink M., Lesage F., Lazdunski M., Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc. Natl. Acad. Sci. USA. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyavanapalli J., Rimmer K., Harper A.A. Reactive oxygen species increases intracellular calcium, alters the electrophysiological properties and synaptic transmission in intrinsic cardiac ganglion neurons: in vitro. Circulation. 2008;118:S359. (Abstract) [Google Scholar]
- Gamper N., Zaika O., Li Y., Martin P., Hernandez C.C., Perez M.R., Wang A.Y.C., Jakke D.B., Shapiro M.S. Oxidative modification of M-type K+ channels as a mechanism of cytoprotective neuronal silencing. EMBO J. 2006;25:4996–5004. doi: 10.1038/sj.emboj.7601374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin A., Giniatullin R. Dual action of hydrogen peroxide on synaptic transmission at the frog neuromuscular junction. J. Physiol. 2003;552:283–293. doi: 10.1113/jphysiol.2003.050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber J.I., Liu E. Excitation–contraction coupling in single guinea-pig ventricular myocytes exposed to hydrogen peroxide. J. Physiol. 1994;477:135–147. doi: 10.1113/jphysiol.1994.sp020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Miyata H., Watanabe H., Kobayashi A., Yamazaki N. Effects of hydrogen peroxide on action potentials and intracellular Ca2+ concentration of guinea pig heart. Cardiovasc. Res. 1989;23:767–773. doi: 10.1093/cvr/23.9.767. [DOI] [PubMed] [Google Scholar]
- Hogg R.C., Adams D.J. An ATP-sensitive K+ conductance in dissociated neurones from adult rat intracardiac ganglia. J. Physiol. 2001;534.3:713–720. doi: 10.1111/j.1469-7793.2001.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R.C., Harper A.A., Adams D.J. Developmental changes in hyperpolarization-activated currents Ih and IK(IR) in isolated rat intracardiac neurons. J. Neurophysiol. 2001;86:312–320. doi: 10.1152/jn.2001.86.1.312. [DOI] [PubMed] [Google Scholar]
- Horackova M., Armour J.A. Role of peripheral autonomic neurones in maintaining adequate cardiac function. Cardiovasc. Res. 1995;30:326–335. doi: 10.1016/0008-6363(95)00105-0. [DOI] [PubMed] [Google Scholar]
- Huang M.-H., Ardell J.L., Hanna B.D., Wolf S.G., Armour J.A. Effects of transient coronary artery occlusion on canine intrinsic cardiac neuronal activity. Integr. Physiol. Behav. Sci. 1993;28:5–21. doi: 10.1007/BF02691196. [DOI] [PubMed] [Google Scholar]
- Huang H.-S., Pan H.-L., Stahl G.L., Longhurst J.C. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am. J. Physiol., Heart Circ. Physiol. 1995;269:H888–H901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Kourie J.I. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol., Cell Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- Martínez-Pinna J., Lamas J.A., Gallego R. Calcium current components in intact and dissociated adult mouse sympathetic neurons. Brain Res. 2002;951:227–236. doi: 10.1016/s0006-8993(02)03165-7. [DOI] [PubMed] [Google Scholar]
- O 'Neill C.A., Fu L.-W., Halliwell B., Longhurst J.C. Hydroxyl radical production during myocardial ischemia and reperfusion in cats. Am. J. Physiol., Heart Circ. Physiol. 1996;271:H660–H667. doi: 10.1152/ajpheart.1996.271.2.H660. [DOI] [PubMed] [Google Scholar]
- Parsons R.L. Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Dun N.J., Machado B.H., Pilowsky P.M., editors. Neural Mechanisms of Cardiovascular Regulation. Kluwar Academic Publishers; Dordrecht: 2004. pp. 335–356. [Google Scholar]
- Pauza D.H., Skripkiene G., Skripka V., Pauziene N., Stropus R. Morphological study of neurons in the nerve plexus on heart base of rats and guinea pigs. J. Auton. Nerv. Syst. 1997;62:1–12. doi: 10.1016/s0165-1838(96)00102-6. [DOI] [PubMed] [Google Scholar]
- Rimmer K., Harper A.A. Developmental changes in the passive and active membrane properties of rat intracardiac neurons in situ: the effects of Ba2+ and Cs+ J. Physiol. 2004;557P:PC62. [Google Scholar]
- Rimmer K., Harper A.A. Developmental changes in electrophysiological properties and synaptic transmission in rat intracardiac ganglion neurons. J. Neurophysiol. 2006;95:3543–3552. doi: 10.1152/jn.01220.2005. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M.C., Jurkiewicz N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Matsui K. Electrical and mechanical modulations by oxygen-derived free-radical generating systems in guinea-pig heart muscles. J. Pharm. Pharmacol. 1997;49:505–510. doi: 10.1111/j.2042-7158.1997.tb06832.x. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Castaldo P., Iossa S., Pannaccione A., Fresi A., Ficker E., Annunziato L. Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc. Natl. Acad. Sci. USA. 1997;94:11698–11703. doi: 10.1073/pnas.94.21.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G.W., Horackova M., Armour J.A. Sensitivity of canine intrinsic cardiac neurons to H2O2 and hydroxyl radical. Am. J. Physiol., Heart Circ. Physiol. 1998;275:H1434–H1440. doi: 10.1152/ajpheart.1998.275.4.H1434. [DOI] [PubMed] [Google Scholar]
- Usti nova E.E., Schultz H.D. Activation of cardiac vagal afferents by oxygen-derived free radicals in rats. Circ. Res. 1994;74:895–903. doi: 10.1161/01.res.74.5.895. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E., Rudy B. Modulation of K+ channels by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992;186:1681–1687. doi: 10.1016/s0006-291x(05)81602-x. [DOI] [PubMed] [Google Scholar]
- Vogalis F., Harvey J.R. Altered excitability of intestinal neurons in primary culture caused by oxidative stress. J. Neurophysiol. 2003;89:3039–3050. doi: 10.1152/jn.01005.2002. [DOI] [PubMed] [Google Scholar]
- Wada-Takahashi S., Tamura K. Actions of reactive oxygen species on AH/type 2 myenteric neurons in guinea pig distal colon. Am. J. Physiol.: Gasterointest. Liver Physiol. 2000;279:G893–G902. doi: 10.1152/ajpgi.2000.279.5.G893. [DOI] [PubMed] [Google Scholar]
- Ward C.A., Giles W.R. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J. Physiol. 1997;500:631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte K.A., Hogg R.C., Adams D.J. Reactive oxygen species modulate neuronal excitability of rat intrinsic cardiac ganglia. J. Physiol. 2003;547P:PC20. doi: 10.1016/j.autneu.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi-Moy S.X., Dun N.J. Potassium currents in adult rat intracardiac neurones. J. Physiol. 1995;486:15–31. doi: 10.1113/jphysiol.1995.sp020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.-J., Adams D.J. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J. Physiol. 1992;456:405–424. doi: 10.1113/jphysiol.1992.sp019343. [DOI] [PMC free article] [PubMed] [Google Scholar]